Abstract
Objectives:
Probiotic approaches are being considered to eliminate pathogenic microorganisms and are an alternative and promising way to combat infections by using harmless bacteria to displace pathogenic microorganisms. The aim of this study was to evaluate the effectiveness of commercially available freeze dried powdered probiotics on mutans streptococci count among 12–15 year-old Indian schoolchildren.
Materials and Methods:
The study was conducted in two phases of in-vitro (phase I) and in-vivo (phase II) study, which was a double blind, randomized and placebo controlled clinical trial. A total of 33 schoolchildren between 12–15 years were included in the study. They were randomly allocated to three groups. Group A included 11 children using freeze dried Lactobacillus acidophilus, Bifidobacterium longum, Bifidobacterium bifidum and Bifidobacterium lactis. Group B included 11 children using freeze dried lactic acid bacillus only. Group C included 11 children using placebo powder. The study was conducted over a period of three weeks and examination and sampling of the subjects were done on days 0 (baseline), seven, 14 and 21.
Results:
For both the intervention groups A and B, statistically significant reduction (P<0.05) in salivary mutans streptococci counts was recorded up to the second week.
Conclusion:
Oral administration of probiotics showed a short-term effect on reduction of mutans streptococci count and showed a preventive role in caries development.
Keywords: Probiotics, Viridans Streptococci, Dental Caries
INTRODUCTION
Probiotics are preparations of viable microorganisms, which upon ingestion exert health benefits on the host. They are among the new agents widely used for their therapeutic action [ 1 ]. The human body is home to hundreds of known bacterial species, comprising an estimated 100 trillion microorganisms. Probiotics have been deliberately incorporated during the processing of foods, because of their beneficial effects on human health [ 2 ]. Probiotics act by microbial adhesion to the target tissue, which compete with the pathogenic microbes on adhesion sites.
They should adhere to saliva-coated surfaces and resist oral environmental conditions to exert probiotic effects on the oral cavity. Once adhered, they secrete antimicrobial substances such as bacteriocins, hydrogen peroxide and organic acids, which can modify the pH and the oxidation-reduction potential facilitating the elimination of pathogenic micro-organisms. In addition, probiotics can stimulate the non-specific immunity and modulate the cellular and humoral immune response. Modification of the oral cavity environment has already been used for the management of caries, periodontal disease, oral mucosal lesions, oro-pharyngeal cancers, halitosis and reduction in the levels of Candida albicans. Oral health diseases are emerging as common health problems, amongst which dental caries is the most common infectious disease in humans [ 3 ]. In developing countries such as India, the major part of the population resides in rural areas lacking access to basic health facilities where oral health is largely neglected and dental caries is widely prevalent across all age groups especially in children. The majority of rural Indians lack regular access to routine dental care. Therefore, there is a need to identify individuals at risk and to target preventive measures and active treatment for these individuals in the form of simple, cost-effective and easily available products. Clinical studies have demonstrated that ingestion of Lactobacillus rhamnosus GG [ 4 , 5 ], L. Reuteri [ 6 ] and a lactobacilli mix, can reduce salivary mutans streptococci counts. Most of these studies have either used a dairy vehicle, lozenges [ 7 ] or chewing gums [ 8 ] as modes of administration, which are not commonly available in developing countries like India, facilitating a need to explore a cheaper and an easily available alternative for administering probiotics. The present study was conducted to investigate whether the oral administration of two commercially available probiotic preparations could reduce Streptococcus mutans count and to evaluate the relative efficacy of them.
MATERIALS AND METHODS
The present study was carried out in two phases, which included an in-vitro study followed by an in-vivo study, which was a double blind, randomized, parallel and placebo controlled clinical trial conducted to assess and compare the effect of commercially available freeze dried powdered probiotics on mutans streptococci count. Microbiological analysis was carried out in Dr. B Lal’s Institute of Biotechnology, Jaipur. The study was carried out in Govt. Secondary School, Labana, Jaipur. A total of 33 Govt. schoolchildren between 12–15 years were included in the study.
Sample size was calculated at 80% study power and α-error of 0.05. For the ratio of standard deviation to the difference of mean to be detected as 0.8, minimum sample size required for each group came to 10 subjects. It was further enhanced to 11 subjects in each group assuming 10% dropouts or attrition. Thus, the total number of subjects to be included in the study was 33.
In Phase-one/in-vitro phase, the zone of inhibition of both probiotic groups, group A-L. acidophilus, B. Longum, B. bifidum and B. Lactis (Prowel, Alkem Laboratories, Mumbai, India) and Group B-lactic acid bacillus only (Sporlac, Uni-Sankyo Ltd., Hyderabad, India) against S. mutans of six different concentrations ranging from 1.5, 2 and 2.5 million spores was determined using the cup and plate method on Mitis Salivarius Bacitracin agar with strain of lyophilized Streptococcus mutans (Hi media) ATCC 25175. The first step involved preparation of the media (Mitis Salivarius Bacitracin Agar) for primary isolation of the culture and Muller Hinton Agar for antibacterial sensitivity. Then processing of the sample for antimicrobial susceptibility testing (cup plate method) for both the probiotic groups was performed; 50 μL of the sample (S) and 50 μL of the reference R (negative control) were dispensed in the well labeled C (control) and added to the plates as soon as possible, but no longer than 15 minutes after inoculation. After the loading of C, S and R on the plate, the plate was inverted and incubated at 35°C for 16 to 18 hours. After incubation, the diameter of the zones of complete inhibition (including the diameter of the disk) was measured and recorded in millimeters. The measurements were made with a ruler on the under surface of the plate without opening the lid. The zones of growth inhibition were compared and recorded as susceptible, intermediate or resistant to each drug tested. The diameter was obtained by measuring the distance from the colony/ colonies closest to the center of the disk, then doubled and interpretation was recorded for each diameter. The colony/colonies inside the zone were picked, re-isolated, re-identified and retested in the disk-diffusion test to confirm the previous results. The presence of colonies with a zone of inhibition predicted the eventual resistance to the agent.
Phase-2/in-vivo study included 33 subjects whose baseline S. mutans levels were assessed. They were randomly divided into three groups of 11 children each. Participants of group one were instructed to use group-A probiotics. Participants of group two were instructed to use group B probiotics. Group C was instructed to use the placebo powder (Calcium Sandoz, Novartis, Mumbai, India). The study was conducted over a period of three weeks and examination and sampling of the subjects were done on days 0 (baseline), seven (one week), 14 (two weeks) and 21 (three weeks). The inclusion criteria were school children between 12–15 years, healthy children without any systemic disorder, DMFT score > 3, plaque index > 1, no history of oral prophylaxis within the past six months and no recent history of use of antimicrobial/antibacterial agents within the past three months. The exclusion criteria were schoolchildren whose parents/guardians did not give consent, subjects who were regularly using mouthwashes/ probiotic products, children who were absent on the day of examination, subjects who were using orthodontic appliances and habitual smokers.
Before scheduling the present study the ethical clearance was obtained from the Research Review Board, Jaipur Dental College to conduct the study and permission was obtained from the Principal, Govt. Secondary School, Labana. The participants whose parents signed a written informed consent form before being interviewed were included in the study. The trial was also registered retrospectively under Clinical Trials Registry of India under reference no: CTRI/2013/05/003677, dated 27/05/2013. A detailed schedule of the study was prepared well in advance. During the morning hours, schoolchildren were examined under adequate natural light. The examination of a single subject took about five minutes. The subjects were asked to refrain from oral hygiene measures for 24 hours before each recall visit. The examination was conducted in the play field of the school under natural light and the subjects were seated in such a way that maximum illumination was obtained. Strict infection control measures were used. ADA type-III examination [ 9 ] was carried out by the calibrated investigator throughout the study.
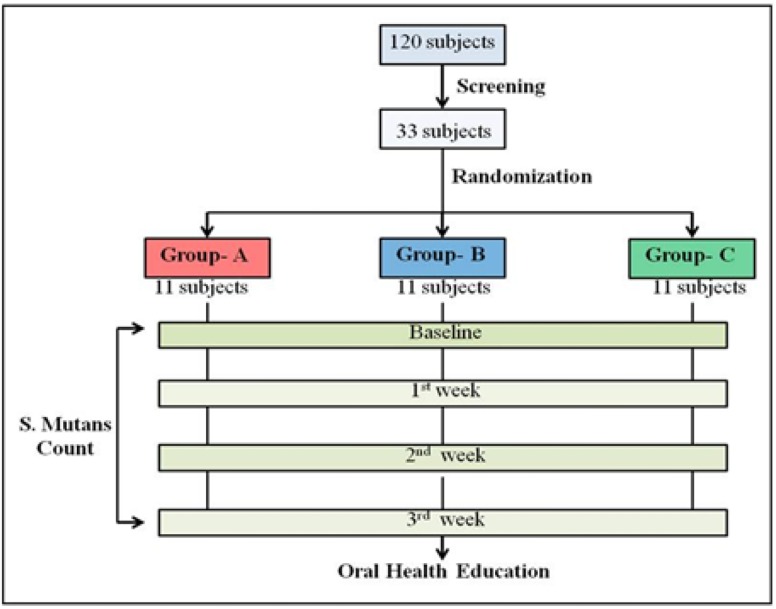
All the three powders were repackaged under sterile conditions into small transparent antistatic zip lock polythene pouches/ sachets and were individually color coded as red, blue and green. Red pouch contained group A probiotic powder containing freeze dried L. acidophilus, B. Longum, B. bifidum and B. Lactis (Prowel, Alkem Laboratories, Mumbai, India). Blue pouch contained Group B probiotic powder containing freeze dried lactic acid bacillus only (Sporlac, Uni-Sankyo Ltd., Hyderabad, India) and Green pouch contained placebo powder (Calcium Sandoz, Novartis, Mumbai, India). Six similar color coded pouches were further kept in a bigger zip lock polybag along with a stirrer, measuring jar with graduations up to 30 mL so that each study participant could use it for a period of one week until the next examination. The subjects were screened for the inclusion criteria and selected prior to the start of the study. Plaque scores were calculated for all 33 subjects by the first investigator and the subjects with varying baseline plaque scores were distributed amongst all the three groups equally using block randomization or strata of 11 subjects each. The second investigator who was blinded to the contents of the color-coded pouches carried out the allocation procedure. The color-coded pouches were distributed to the appropriate groups by the second investigator. The color-coded pouches were supplied in a regular, scheduled manner throughout the course of the study at weekly intervals. Repackaging was done just one day before the weekly recall examination to ensure the viability of the powder. The first investigator, who carried out the examination, was blinded to the allocation of study subjects to color groups and the subjects did not know which type of probiotic group they were using. The second investigator was not involved in recording of clinical parameters at any of the recall visits. Thus, the criteria of randomization and double-blinding were fulfilled. The procedure of mixing the powder with water in the measuring jar using a stirrer was demonstrated after distributing the color-coded pouches. Subjects assigned to two test groups and a placebo group, were instructed to mix each sachet in 30 mL of water with the stirrer marked on the measuring jar and rinse their mouth once daily in the morning for three minutes. During the entire study period, participants were advised to exercise their usual oral hygiene practices and abstain from using any mouthwashes. Saliva samples were collected at baseline, one week, two weeks and three weeks. All the subjects were evaluated by the same examiner throughout the study period. A pretested and validated questionnaire was used to record the information about oral hygiene practices, dietary habits, in-between meal snacking, existing dental problems and visit to a dentist. To minimize the effect of diurnal variations in the saliva, samples were collected in the morning, between 9–10 am and transferred immediately to microbiology laboratory within two hours. Saliva samples were vortexed and were then serially diluted in 10-fold steps in 0.05 M Phosphate buffer. Aliquots of 500 μL of the appropriate dilutions were cultured on the selective media for mutans streptococci, which has mitis salivarius bacitracin. All plates were cultured at 37ºC in a microaerophilic environment in 5% CO2 for 48 hours. The Streptococcus mutans count was estimated by counting the number of colony forming units (CFU) per mL of stimulated saliva. After the commencement of the study, dental health education and proper brushing techniques were taught to all the participants. A brief overview of the methodology followed is shown in Fig. 1.
Fig. 1.
Brief overview of the methodology
Qualitative data collected were summarized as mean and standard deviation (mean ± SD). Where data had extreme values, which were found unduly affecting the mean ± SD, the median was used as a measure of central tendency and inter quartile range (25th and 75th percentile) as a measure of dispersion. The mean values were compared using one-way ANOVA followed by Post hoc LSD test for comparison of independent samples. For group comparisons, Wilcoxon signed Rank test was used. All analyses were done using MedCalc v12.2.1.0.software (MedCalc, Mariakerke, Belgium). For all tests, a P-value of 0.05 or less was set at statistical significance.
RESULTS
The study was carried out from 16-01-2013 to 06-02-2013. No subject was lost to follow-up and no unintended/untoward effect was observed during the four-week follow-up period.
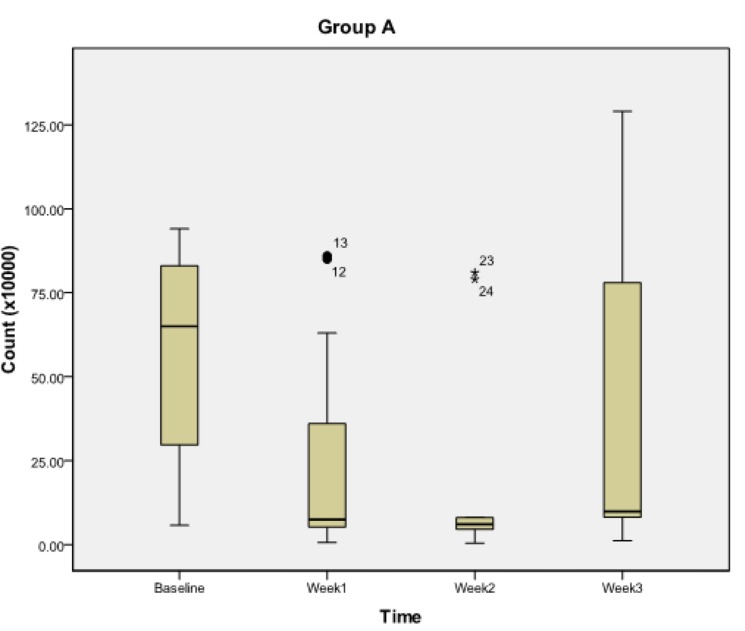
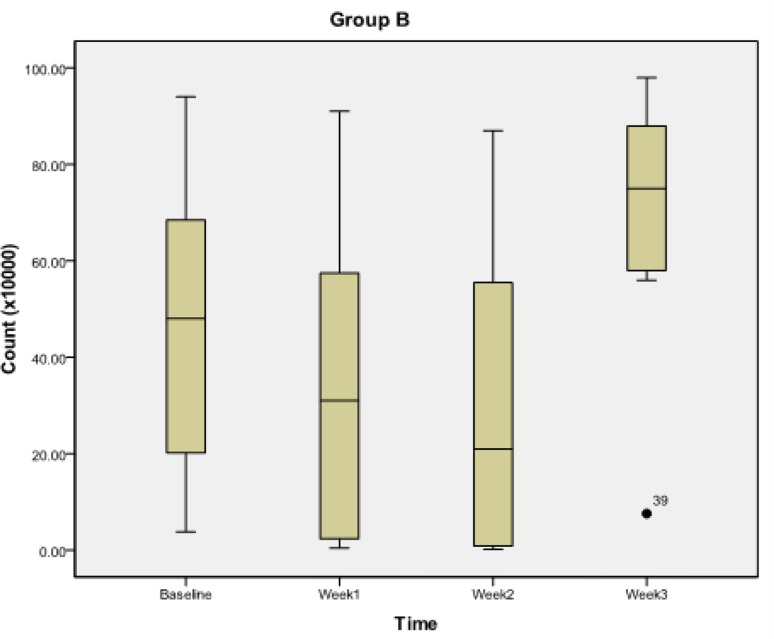
For in-vitro study (Table 1), the mean zone of inhibition for S. mutans was observed to be maximum at spore load of 2.5 million for group A probiotics. In group B, the mean zone of inhibition for S. mutans was maximum at spore load of 2 million. For Probiotic group A (Table 2, Fig. 2), the S. mutans count significantly decreased from its baseline value to its first week (P<0.018) and second week value (P<0.018), but no significant reduction was observed at third week (P>0.054) and between first week and second week (P>0.054). However, significant reduction was observed between first week and third week (P<0.018) and between third week and second week (P<0.018). For group B (Table 2, Fig. 3) the S. mutans count decreased significantly from its baseline value to its first week value (P<0.018), second week value (P<0.018) and third week value (P<0.018). Also, significant reduction was observed between second week and first week (P<0.018), between third week and first week (P<0.018) and between third week and second week (P<0.018).
Table 1.
In vitro: Zone of inhibition for Streptococcus mutans (in mm)
| Probiotics Used | Spore Load | N | Mean | Std. Deviation | ANOVA | |
|---|---|---|---|---|---|---|
| F ratio | P | |||||
| Group A | 1.5 million | 3 | 17.50 | 0.50 | 124.00 | <0.001 |
| 2 million | 3 | 18.50 | 0.50 | |||
| 2.5 million | 3 | 22.83 | 0.29 | |||
| Group B | 1.5 million | 3 | 10.17 | 0.29 | 140.44 | <0.001 |
| 2 million | 3 | 16.83 | 0.76 | |||
| 2.5 million | 3 | 12.17 | 0.29 | |||
Post hoc LSD test
Table 2.
Pairwise comparisons of Streptococcus mutans levels among different time periods.
| Group | Time Period | p * (1 st week) | p * (2nd week) | p * (3 rd week) |
|---|---|---|---|---|
| A | Baseline | <0.018 | <0.018 | >0.054 |
| 1 st week | - | >0.054 | <0.018 | |
| 2 nd week | - | - | <0.018 | |
| B | Baseline | <0.018 | <0.018 | <0.018 |
| 1 st week | - | <0.018 | <0.018 | |
| 2 nd week | - | - | <0.018 | |
| C | Baseline | <0.020 | <0.018 | <0.018 |
| 1 st week | - | >0.054 | <0.020 | |
| 2 nd week | - | - | <0.018 |
Wilcoxon Signed Rank test
Fig. 2.
Streptococcus mutans count during subsequent weekly intervals in group A
Fig. 3.
Streptococcus mutans count during subsequent weekly intervals in group B
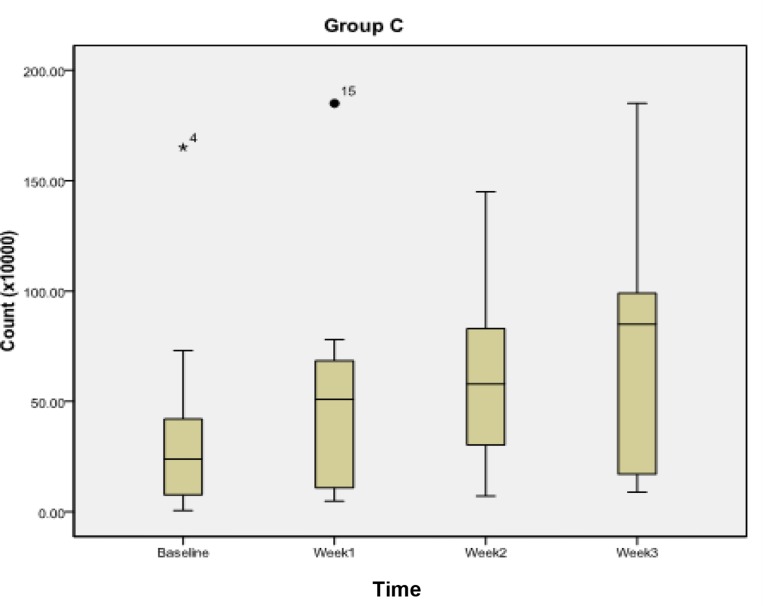
For group C or the control group (Table 2, Fig. 4) the Streptococcus mutans count did not show any significant reduction between baseline and the first week (P<0.020), second week (P<0.018) and third week (P<0.018).
Fig. 4.
Streptococcus mutans count during subsequent weekly intervals in group C
DISCUSSION
The present randomized control trial was conducted to evaluate the dental health outcomes following administration of commercially available freeze dried probiotic powder in the form of a mouthrinse at repeated weekly intervals in schoolchildren in Jaipur. A total of 120 schoolchildren were screened. Based on the inclusion and exclusion criteria, 33 schoolchildren between 12–15 years of age were included. Amongst the 33 study subjects, 19 male and 14 female subjects were randomly allocated to three groups each comprising of 11 subjects. In the present study, examination was performed at weekly intervals for a period of 21 days. The examination period of 14 days was chosen to allow comparison with other studies [ 10 , 11 ]. The period of seven days was chosen because most rapid changes in plaque formation take place during the first four to five days and the 21-day period was selected because the plaque becomes relatively stable by around the day 21 [ 12 ].
The two probiotic groups were selected based on the results of the in-vitro study (phase-1) that was conducted before the in-vivo phase. To ensure blinding and the viability of the powder, it was repackaged and color-coded as red, blue and green, one day before the weekly recall examination and distributed on the day of examination. The findings of the in-vitro study were in harmony with a study conducted by Hasslöf et al, in 2010 [ 13 ] where they compared the effect of eight commercial probiotic lactobacilli strains on growth inhibition of mutans streptococci and C. albicans and concluded that commercial probiotic lacto-bacilli inhibited the growth of reference strains and oral isolates of mutans streptococci and Candida but the capacity differed significantly between the strains. Kõll et al, in 2008 [ 14 ] used the deferred antagonism method to test the inhibitory capacity against mutans streptococci and C. albicans. The probiotic strains inhibited both the reference strains and the oral mutans streptococci isolates, except L. acidophilus. The results were consistent with the findings of Simark-Mattsson and coworkers in 2007 [ 15 ]. An in vitro study by Ahola et al, in 2002 [ 5 ] showed that Lactobacillus rhamnosus GG inhibited the colonization of streptococcal cariogenic pathogens and therefore reduced tooth decay incidence in children. The reason for this effect could be attributed to the production of bacteriocins by probiotic bacteria, which has an inhibitory effect on cariogenic pathogens [ 16 ]. An in-vitro study conducted by Haukioja et al, in 2008 described two new possible mechanisms of probiotic action in the oral cavity and showed that Lactobacillus and Bifidobacterium strains used in commercial probiotic products may affect the oral ecology by specifically preventing the adherence of other bacteria and by modifying the protein composition of the salivary pellicle [ 16 ].
In the current study, short-term administration of probiotics resulted in significant reduction of Streptococcus mutans counts, and thus signifies its preventive role against caries development. The results of the current study showed that the daily consumption of both the probiotics for two weeks reduced the salivary levels of S. mutans colony counts. For group A and group B, the S. mutans count decreased significantly from its baseline value to its first week value and even up to two weeks. The results were in accordance with the results of the study conducted by Jindal et al, in 2011 [ 11 ] where subjects who used probiotics containing Bacillus coagulans for 14 days, experienced a significant reduction in salivary S. mutans colony counts.
Similar results were achieved by Caglar et al, in 2008 [ 17 ] where salivary S. mutans levels in the probiotic test group significantly decreased. The difference in reduction of S. mutans count following 10 days of using the test medical device was significant compared to the control medical device. Several previous trials reported that the intake of lactobacilli-derived probiotic strains may reduce the counts of mutans streptococci in saliva [ 4 ]. Çaglar et al, in 2008 [ 18 ] evaluated the effects of an ice-cream containing B. lactis on S. mutans and Lactobacillus levels in the saliva and observed a significant reduction in S. mutans count, but not in Lactobacillus. However, these findings were contradictory to the findings of Lexner et al, in 2010 [ 19 ] as they could not observe any statistically significant difference in the microbial profiles or the estimated counts at baseline and follow-up, or between the two study groups after a short-term administration of milk supplemented with bacterium L. rhamnosus LB21. Also, Montalto et al, in 2004 [ 20 ] reported contradictory findings with an increase in Lactobacillus in a fluid form or in capsules. However at the third week, the S. mutans count increased but was not significantly different between baseline and third week while in group B, it increased significantly compared to the baseline values. These findings were contradictory to the study by Caglar et al, in 2006 [ 7 ] where statistically significant reduction of mutans streptococci levels was recorded after ingestion of probiotic bacteria via a straw and the tablets for three weeks, which was in contrast to the placebo controls. The variation in findings could be due to inadequate supervision and lack of interest in oral health education among rural schoolchildren. Continuous motivation, supervision and periodic reinforcement of oral hygiene practices among children are always necessary to counteract the effect of fading over a period of time, which could have been a confounding factor. It is recommended for the manufacturers to determine the physiological role, mechanisms of action, and extent of influence of probiotics on oral health using human studies targeting high-risk human populations for oral diseases and conducting further research to improve the consumer acceptance, stability, and efficacy of probiotic containing products by incorporating flavoring agents and making the products more palatable and more pleasing for the daily use.
CONCLUSION
The results of the present study showed that both the probiotic groups showed short-term mutans streptococci inhibiting properties invivo. However, the trial findings cannot be generalized to the entire population as this study was conducted on rural schoolchildren. Further randomized controlled trials on different population groups are warranted to confirm or refute the long-term effects, means of administering probiotics and the dosages needed to achieve different preventive or therapeutic purposes.
REFERENCES
- 1-. Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by micro-organisms. Science. 1965. February 12; 147 ( 3659): 747– 8. [DOI] [PubMed] [Google Scholar]
- 2-. Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol. 2006. June; 100 ( 6): 1171– 85. [DOI] [PubMed] [Google Scholar]
- 3-. Caglar E, Kargul B, Tanboga I. Bacteriotherapy and probiotics' role on oral health. Oral Dis. 2005. May; 11 ( 3): 131– 7. [DOI] [PubMed] [Google Scholar]
- 4-. Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001. Nov-Dec; 35 ( 6): 412– 20. [DOI] [PubMed] [Google Scholar]
- 5-. Ahola AJ, Yli-Knuuttila H, Suomalainen T, Poussa T, Ahlstrom A, Meurman JH, et al. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol. 2002. November; 47 ( 11): 799– 804. [DOI] [PubMed] [Google Scholar]
- 6-. Nikawa H, Makihira S, Fukushima H, Nishimura H, Ozaki Y, Ishida K, et al. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int J Food Microbiol. 2004. September 1 ; 95 ( 2): 219– 23. [DOI] [PubMed] [Google Scholar]
- 7-. Caglar E, Cildir SK, Ergeneli S, Sandalli N, Twetman S. Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand. 2006. October; 64 ( 5): 314– 8. [DOI] [PubMed] [Google Scholar]
- 8-. Caglar E, Kavaloglu SC, Kuscu OO, Sandalli N, Holgerson PL, Twetman S. Effect of Chewing Gums Containing Xylitol or Probiotic Bacteria on Salivary Mutans Streptococci and Lactobacilli. Clin Oral Investig. 2007. December; 11 ( 4): 425– 9. [DOI] [PubMed] [Google Scholar]
- 9-. Dunning JM. Principles of Dental Public Health. 4th ed. London: : Harvard University Press; , 1986. : 339 ; 173 . [Google Scholar]
- 10-. Noordin K, Kamin S. The Effect of probiotic mouthrinse on plaque and gingival inflammation. Ann. Dent. 2007; 14 ( 1): 19– 25. [Google Scholar]
- 11-. Jindal G, Pandey RK, Agarwal J, Singh M. A comparative evaluation of probiotics on salivary mutans streptococci counts in Indian children. Eur Arch Paediatr Dent. 2011. August; 12 ( 4): 211– 5. [DOI] [PubMed] [Google Scholar]
- 12-. Harris NO, Christen AG. Primary Preventive Dentistry. 4th ed. Appleton and Lange, Norwalk Connecticut; 1994: 22– 3. [Google Scholar]
- 13-. Hasslof P, Hedberg M, Twetman S, Blicks CS. Growth inhibition of oral mutans streptococci and candida by commercial probiotic lactobacilli - an invitro study. BMC Oral Health. 2010. July 2 ; 10 : 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14-. Kõll P, Mändar R, Marcotte H, Leibur E, Mikelsaar M, Hammarström L. Characterization of oral lactobacilli as potential probiotics for oral health. Oral Microbiol Immunol. 2008. April; 23 ( 2): 139– 47. [DOI] [PubMed] [Google Scholar]
- 15-. Simark-Mattsson C, Emilson CG, Håkansson EG, Jacobsson C, Roos K, Holm S. Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects. Eur J Oral Sci. 2007. August; 115 ( 4): 308– 14. [DOI] [PubMed] [Google Scholar]
- 16-. Haukioja A, Loimaranta V, Tenovuo J. Probiotic bacteria affect the composition of salivary pellicle and streptococcal adhesion in vitro. Oral Microbiol Immunol. 2008. August; 23 ( 4): 336– 43. [DOI] [PubMed] [Google Scholar]
- 17-. Caglar E, Kuscu OO, Cildir SK, Kuvvetli SS, Sandalli N. A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent. 2008. January; 18 ( 1): 35– 9. [DOI] [PubMed] [Google Scholar]
- 18-. Caglar E, Kuscu OO, Selvi Kuvvetli S, Kavaloglu Cildir S, Sandalli N, Twetman S. Short-term effect of ice-cream containing Bifidobacterium lactis Bb-12 on the number of salivary mutans streptococci and lactobacilli. Acta Odontol Scand. 2008. June; 66 ( 3): 154– 8. [DOI] [PubMed] [Google Scholar]
- 19-. Lexner MO, Blomqvist S, Dahlén G, Twetman S. Microbiological Profiles in Saliva and Supragingival Plaque from Caries-Active Adolescents Before and After a Short-Term Daily Intake of Milk Supplemented with Probiotic Bacteria-A Pilot Study. Oral Health Prev Dent. 2010; 8 ( 4): 383– 8. [PubMed] [Google Scholar]
- 20-. Montalto M, Vastola M, Marigo L, Covino M, Graziosetto R, Curigliano V, et al. Probiotic treatment increases salivary counts of lactobacilli: a double-blind, randomized, controlled study. Digestion. 2004; 69 ( 1): 53– 6. [DOI] [PubMed] [Google Scholar]