Abstract
Background:
The aim of this study was to investigate the antibiotic susceptibility pattern as well as the phenotypic and genotypic biofilm formation ability of Staphylococcus aureus isolates from patients with urinary tract infection (UTI).
Methods:
A total of 39 isolates of S. aureus were collected from patients with UTI. The antibiotic susceptibility patterns of the isolates were determined by the Kirby-Bauer disk-diffusion. We used the Modified Congo red agar (MCRA) and Microtiter plate methods to assess the ability of biofilm formation. All isolates were examined for determination of biofilm related genes, icaA, fnbA, clfA and bap using PCR method.
Results:
Linezolid, quinupristin/dalfopristin and chloramphenicol were the most effective agents against S. aureus isolates. Overall, 69.2% of S. aureus isolates were biofilm producers. Resistance to four antibiotics such as nitrofurantoin (71.4% vs. 28.6%, P=0.001), tetracycline (57.7% vs. 42.3%, P=0.028), erythromycin and ciprofloxacin (56% vs. 44%, P=0.017) was higher among biofilm producers than non-biofilm producers. The icaA, fnbA and clfA genes were present in all S. aureus isolates. However, bap gene was not detected in any of the isolates.
Conclusion:
Our findings reinforce the role of biofilm formation in resistance to antimicrobial agents. Trimethoprimsulfamethoxazole and doxycycline may be used as an effective treatment for UTI caused by biofilm producers S. aureus. Our results suggest that biofilm formation is not dependent to just icaA, fnbA, clfA and bap genes harbor in S. aureus strains.
Keywords: Urinary tract infection, Staphylococcus aureus, Biofilm formation, Antibiotic resistance
Introduction
Urinary tract infection (UTI) is one of the most common infectious diseases in humans both in the clinical and community settings. Its global incidence is estimated to be 250 million cases each year ( 1 , 2 ). Escherichia coli is the most prevalent causative organism of UTI, accounting for about 80% of bacterial isolates ( 3 , 4 ). However, involvement of Gram-positive bacteria cannot be ruled out in relation to UTI. Staphylococcus aureus is one of such agents involved in the infection that is capable of invading the urinary tract. Although S. aureus accounts to 0.5–6% of UTI, but if leave untreated infection can lead to severe life-threatening condition ( 5 , 6 ).
Emergence of multidrug-resistant (MDR) S. aureus has become an increasing health concern worldwide ( 7 ). Currently, it is estimated that bio-films are responsible for more than 65% of all nosocomial infections and 80% bacterial infections. Bacterial biofilms can play an important role in recurrent urinary tract infections and resistance to antimicrobial agents ( 8 , 9 ). In addition, the proximity of cells within the biofilm structure can facilitate genetic elements exchange and hence enhance the possible spread of genes responsible for antibiotic resistance ( 10 , 11 ). S. aureus is known to form biofilms on various surfaces. This pathogen, can invade renal tissue causing UTI by adherence to uroepithelium and formation of biofilm. Since the ability of biofilm production in S. aureus can increase resistance to commonly used antibiotics, hospitalized patients infected with this organism are at significant risk for treatment failure ( 12 , 13 ).
S. aureus biofilm formation is regulated by expression of polysaccharide intracellular adhesion (PIA), which mediates cell to cell adhesion and is encoded by the icaADBC operon ( 14 ). Moreover, surface-associated proteinaceous adhesins can contribute to the adherence, colonization and biofilm formation of S. aureus. This pathogen can express a variety of microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), such as fibronectin-binding proteins A (FnbA), clumping factors A (ClfA) and biofilm-associated protein (Bap) ( 15 , 16 ). However, the mechanism of biofilm formation and pathogenicity of S. aureus infections in the urinary tract is controversial. Recently, the study of genes involved in biofilm formation and their role in infections caused by S. aureus have attracted great interest ( 12 ).
Given the role of biofilm related genes in biofilm formation and antibiotic resistance, the need for study is more than ever. Therefore, the aim of this study was to evaluate of biofilm formation and antimicrobial resistance in S. aureus isolated from urinary tract infection.
Material and Methods
Bacteria isolates
The study was conducted with a total of 39 isolates of S. aureus among UTI patients collected at Sina Hospital, Tehran University of Medical Sciences (TUMS). S. aureus isolates were confirmed using conventional microbiological methods (Gram's stain, catalase, coagulase, DNase tests, and mannitol fermentation on mannitol salt agar [Merck, Germany]). MRSA strains were identified phenotypically using cefoxitin disk diffusion method (30 μg; MAST, UK). This method was performed according to the Clinical and laboratory standards institute (CLSI) guidelines ( 17 ).
Antibiotic susceptibility determination
The antibiotic susceptibility patterns of S. aureus isolates were determined by the Kirby-Bauer disk-diffusion method, and the results were interpreted according to CLSI guidelines ( 17 ). The antimicrobial agents (MAST, UK) tested in this study included erythromycin (15 μg), tetracycline (30 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), rifampin (50 μg), nitrofurantoin (300 μg), linezolid (30 μg), quinupristin/dalfopristin (15 μg), clindamycin (2 μg), doxycycline (30 μg), trimethoprim-sulfamethoxazole (25 μg) and gentamicin (10 μg). S. aureus ATCC 25923 was used as a standard strain.
Biofilm formation assay
Modified Congo red agar method (MCRA)
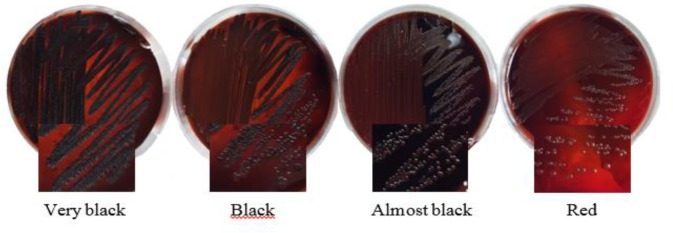
Phenotypic production of biofilm in all S. aureus isolates was assessed by culture on MCRA plates as previously explained ( 18 ). Briefly, CRA plates were prepared adding 0.8 g of Congo red (Merck, Germany) and 36 g of saccharose (Sigma, USA) to 1 liter of brain heart infusion agar (BHI agar, from Merck, Germany). The plates were incubated for 24 h at 37 °C, and subsequently over night at room temperature. The morphology of colonies was then interpreted based on colony color as red, almost black, black, and very black. Very black and black colonies were considered as strong biofilm producer strains, while almost black colors were indicative of a weak biofilm production activity. Conversely, strains with red colonies were classified as strains unable to produce biofilm.
Microtiter plate assay
Biofilm production was determined quantitatively using a modified Microtiter plate method as described previously ( 19 ). Briefly, bacterial isolates were grown in tripticase-soy broth (TSB, from Merck, Germany) with 0.5% glucose and incubated at 37 °C for overnight. Cultures were diluted 1:40 in fresh TSB-0.5% glucose. Then 200 μl of the diluted solution was added to wells of a flat-bottomed polystyrene microtitre plate and incubated for 48 hours at 37 °C. The negative control wells contained 200 μl of TSB-0.5% glucose. Wells were gently washed 3-times with phosphate-buffered saline (PBS; pH 7.2), fixed by methanol for 20 min, dried at room temperature, and then strained with 0.1% safranin. The safranin dye bound to the adherent cells was dissolved with 1 ml of 95% ethanol per well. Finally, the optical density (OD) of each well was measured at 490 nm (A490) using ELISA reader. Optical density cut-off (ODc) defined as average OD of negative control + 3× standard deviation (SD) of negative control. Formation of biofilm by strains was analyzed and categorized based on the absorbance of the safranin-stained attached cells ( Table 1 ). Staphylococcus epidermidis ATCC 35984 was used as the biofilm producer control strain.
Table 1:
Classification of biofilm formation abilities by microtiter plate method
| Cut-off value calculation | Mean of OD values results | Biofilm formation abilities |
|---|---|---|
| OD > 4×ODc | OD > 0.236 | Strong |
| 2×ODc < OD ≤4×ODc | 0.118< OD ≤ 0.236 | Moderate |
| ODc< OD ≤ 2×ODc | 0.059< OD ≤ 0.118 | Weak |
| OD ≤ 0.059 | OD ≤ 0.059 | None |
Gene pattern characterization
Genomic DNA was extracted from pure cultures using High Pure PCR Template Preparation Kit (Roche, Germany) according to the manufacturer. The purified DNA was used for PCR.
In this study, S. aureus isolates screening for icaA, fnbA, clfA and bap genes using PCR and primers described in Table 2 . PCR reaction was conducted on the final volume of 25 μl using HotStar Taq Master Mix kit (SinaClon, Iran) containing 12.5 μl of 2x HotStar Taq Master Mix (Containing 3 mM MgCl2, 0.4 mM of each dNTP and 0.08 U/μl Taq DNA polymerase in reaction buffer), 1 μl of the DNA template, 1 μl of each primer (20 pmol) and 9.5 μl of ddH2O. DNA amplification was performed in a thermocycler (Eppendorf, Hamburg, Germany) with an initial denaturation step at 95 °C for 5 min, 35 amplification cycles each with 1 min at 95 °C; 30 seconds at different temperatures for different genes ( Table 2 ); and 50 seconds at 72 °C, followed by an additional extension step of 10 min at 72 °C. The amplified products were electro-phoresed on 1% agarose gel containing 1x GelRed DNA stain (Biotium, Inc., USA).
Table 2:
Target genes and their primers used in this study
| Primer | Sequence (5′-3′) | Products sizes (bp) | Annealing (°C) | Ref. |
|---|---|---|---|---|
| icaA | Fw- CAATCAAGGCATTAAACAGGCTTC Rv- ACCTTTTCGTTTTCATTGTGCTAA |
509 | 62 | This study |
| fnbA | Fw- GATACAAACCCAGGTGGTGG Rv- TGTGCTTGACCATGCTCTTC |
191 | 55 | ( 20 ) |
| clfA | Fw- ATTCTGCTGTTAAAGGTGACACAT Rv- GTGTTGTAATTTGATCATCAGGCG |
657 | 62 | This study |
| bap | Fw- CCCTATATCGAAGGTGTAGAATTG Rv- GCTGTTGAAGTTAATACTGTACCTGC |
971 | 62 | (21) |
Statistical analysis
The relationship between biofilm formation, multidrug resistance and presence of the biofilm related genes among S. aureus isolates was evaluated by the Pearson Chi-Square test using SPSS version 21. P values less than 0.05 were considered to be significant.
Results
Antibiotic susceptibility
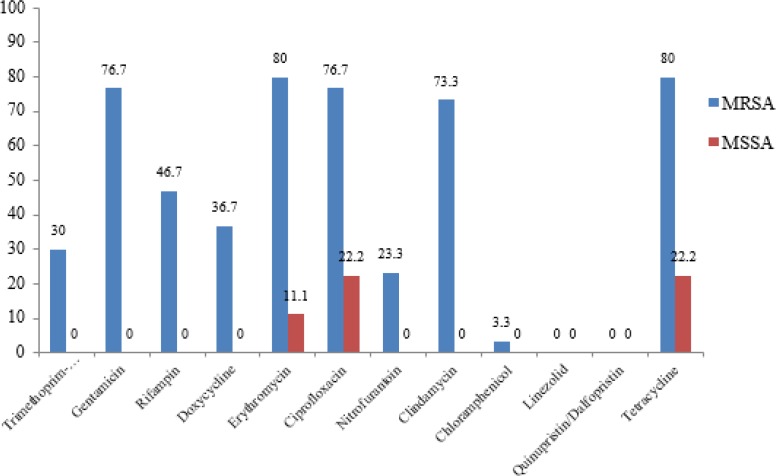
A total of 39 clinical isolates of S. aureus were collected, of which 30 and 9 isolates were MRSA and MSSA, respectively. The overall susceptibility of S. aureus isolates to antimicrobial agents was 100% for linezolid and quinupristin/dalfopristin; 97.4% for chloramphenicol; 76.9% for trimethoprim-sulfamethoxazole; 64.1% for rifampin; 43.6% for clindamycin; 41% for nitrofurantoin, doxycycline and gentamicin; 35.9% for erythromycin and ciprofloxacin; and 33.3% for tetracycline. It should be noted that 30.8% and 41% of isolates were intermediate to doxycycline and nitrofurantoin, respectively. Linezolid, quinupristin/dalfopristin and chloramphenicol were the most effective agents against S. aureus isolates. Comparison of resistance pattern of MRSA and MSSA strains to antimicrobial agents is shown in Fig. 1 .
Fig. 1:
Antimicrobial resistance pattern of S. aureus isolates (MRSA and MSSA).
Biofilm production
Biofilm formation of S. aureus isolates were studied by culturing them on Modified Congo red agar (MCRA). The numbers of different biofilm-producing isolates of MSSA and MRSA on MCRA were found to be different with varying degrees like very black, black, weak black, and red colonies ( Fig. 2 ). In this method, 48.7% and 20.5% of S. aureus isolates, respectively, showed black/very black colonies (Strong biofilm producers) and almost black (Weak biofilm producers) ( Table 3 ). Moreover, in Microtiter plate method, among the MSSA strains, 33.3% of the strains were strong, 33.3% were moderate, and 22.2% of them were found to be weakly adherent. Whereas among the MRSA strains, 16.6% of the strains were found to be strong, 26.7% were moderate, and 20% were found weakly adherent ( Table 3 ). Overall, in both methods 30.8% and 69.2% of S. aureus isolates were non-biofilm and biofilm producers, respectively. Biofilm formation abilities of MSSA strains were found to be slightly higher than those of MRSA strains. Antimicrobial resistance pattern and phenotypic biofilm formation in S. aureus isolates is shown in Table 4 . Statistical analysis showed a significant relationship between biofilm formation of S. aureus isolates and some antibiotic resistance. Resistance to four antibiotics such as nitrofurantoin (71.4% vs. 28.6%, P=0.001), tetracycline (57.7% vs. 42.3%, P=0.028), erythromycin and ciprofloxacin (56% vs. 44%, P=0.017) was higher among biofilm producers than non-biofilm producers.
Fig. 2:
Colony morphologies of S. aureus isolates on the modified Congo red agar medium
Table 3:
Biofilm production S. aureus isolates in microtiter plate and Congo red agar method
| Method | Biofilm formation | MSSA (n = 9) | Percent (%) | MRSA (n = 30) | Percent (%) | Total (n=39) | Percent (%) |
|---|---|---|---|---|---|---|---|
| Modified Congo red agar | Very black | 3 | 33.3 | 1 | 3.3 | 4 | 10.2 |
| Black | 4 | 44.5 | 11 | 36.7 | 15 | 38.5 | |
| Almost black | 1 | 11.1 | 7 | 23.3 | 8 | 20.5 | |
| Red | 1 | 11.1 | 11 | 36.7 | 12 | 30.8 | |
| Microtiter plate assay | Strong | 3 | 33.3 | 5 | 16.6 | 8 | 20.5 |
| Moderate | 3 | 33.3 | 8 | 26.7 | 11 | 28.2 | |
| Weak | 2 | 22.2 | 6 | 20 | 8 | 20.5 | |
| None | 1 | 11.1 | 11 | 36.7 | 12 | 30.8 |
Table 4:
Antimicrobial resistance pattern and phenotypic biofilm formation in S. aureus isolates
| Antibiotic | Resistance | Intermediate | Susceptible | |||
|---|---|---|---|---|---|---|
| Biofilm former | Non-biofilm former | Biofilm former | Non-biofilm former | Biofilm former | Non-biofilm former | |
| Erythromycin | 14 (56) | 11 (44) | 0 (0) | 0 (0) | 13 (92.9) | 1 (7.1) |
| Ciprofloxacin | 14 (56) | 11 (44) | 0 (0) | 0 (0) | 13 (92.9) | 1 (7.1) |
| Tetracycline | 15 (57.7) | 11 (42.3) | 0 (0) | 0 (0) | 12 (92.3) | 1 (7.7) |
| Chloramphenicol | 0 (0) | 1 (100) | 0 (0) | 0 (0) | 27 (71.1) | 11 (28.9) |
| Rifampin | 8 (57.1) | 6 (42.9) | 0 (0) | 0 (0) | 19 (76) | 6 (24) |
| Nitrofurantoin | 5 (71.4) | 2 (28.6) | 10 (62.5) | 6 (37.5) | 12 (75) | 4 (25) |
| Linezolid | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 27 (69.2) | 12 (30.8) |
| Quinupristin/Dalfopristin | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 27 (69.2) | 12 (30.8) |
| Clindamycin | 12 (54.5) | 10 (45.5) | 0 (0) | 0 (0) | 15 (88.2) | 2 (11.8) |
| Doxycycline | 5 (45.5) | 6 (54.5) | 7 (58.3) | 5 (41.7) | 15 (93.8) | 1 (6.3) |
| Trimethoprim-Sulfamethoxazole | 4 (44.4) | 5 (55.6) | 0 (0) | 0 (0) | 23 (76.7) | 7 (23.3) |
| Gentamicin | 12 (52.2) | 11 (47.8) | 0 (0) | 0 (0) | 15 (93.8) | 1 (6.3) |
Gene pattern characterization
In this study, the presence of biofilm related genes were evaluated in S. aureus isolates by PCR method. All isolates were investigated for icaA, fnbA, clfA and bap genes. The icaA, fnbA and clfA genes were present in all S. aureus isolates (100%). However, bap gene was not detected in any of the isolates. PCR- product of icaA, fnbA and clfA genes from S. aureus isolates is shown in Fig. 3 . Overall, icaA, fnbA and clfA genes were detected in all isolates, and bap gene was not found in any of the S. aureus isolates from UTI patients; therefore, we were not able to investigate relationship between biofilm formation and the presence of these genes in isolates.
Fig. 3:
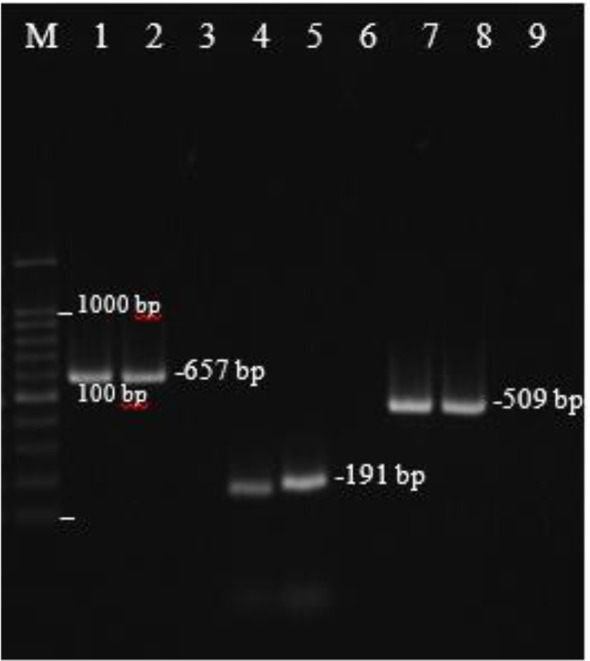
Amplification of icaA, fnbA, clfA genes from S. aureus isolates.
Lane M, DNA marker (100 bp); Lane1 and 2, clfA (657 bp); Lane 4 and 5, fnbA (191 bp);
Lane 7 and 8, icaA (509 bp); Lane 3, 6 and 9, negative controls
Discussion
S. aureus is one of the important Gram-positive bacteria involved in urinary tract infections (UTIs). Although infection caused by this pathogen included low percentage of UTI, it should not be underestimated as untreated infection because can lead to severe health threatening conditions ( 6 , 13 ). The ever-increasing emergence of antibiotic resistance in such organism has become a health concern ( 22 ). In recent years there has been an alarming increase in the prevalence of resistance to methicillin and reduced susceptibility to vancomycin in the S. aureus strains. Monitoring of antimicrobial susceptibility can lead physician for prescription of appropriate antibiotics and prevention of emergence of drug resistance ( 23 , 24 ).
In this study, susceptibility pattern of S. aureus isolated from UTI was assessed. According to results of our study, linezolid, quinupristin/dalfopristin and chloramphenicol were the most effective agents against S. aureus isolates. Whereas, high resistance of S. aureus isolates to tetracycline, ciprofloxacin and erythromycin reported in the current study (66.7%, 64.1%, and 64.1%, respectively) that is consistent with other studies ( 1 , 25 ). Antimicrobial resistance of MRSA strains to different antibiotics was significantly higher than MSSA strains. Although resistance to nitrofurantoin (59%) has been seen among our isolates, but it seems that it can be effective drugs for treatment of UTI associated with Gram-positive cocci.
Microbial cell adherence to surfaces and the development of multi-cellular communities is a key step in infection. Furthermore, bacterial biofilms can play an important role in recurrent urinary tract infections and resistance to antimicrobial agents ( 10 ). In the present study, modified Congo red agar and Microtiter plates methods used for the detection of biofilm production. The results showed that overall 30.8% and 69.2% of S. aureus isolates were non-biofilm and biofilm producers, respectively. In our study, resistance to nitrofurantoin, tetracycline, erythromycin and ciprofloxacin was significantly higher among biofilm producers than non-biofilm producers. In addition, resistance to trimethoprim-sulfamethoxazole and doxycycline was relatively less common among biofilm than non-biofilm producing isolates. Therefore, these antibiotics may be used as an effective treatment for UTI caused by biofilm producers S. aureus. Progressive increase in antimicrobial resistance of S. aureus isolates to various antibiotics in the present study may be related to increased usage of different antibiotics for treatment of UTI, as well as biofilm-forming ability of strains and acquisition of resistance genes.
In this study, although 69.2% of the S. aureus isolates produced biofilms in Microtiter plate and Congo red agar method, the icaA, fnbA, clfA genes were detected in 100% of the isolates by PCR. Other studies have also shown that despite the presence of the icaA, fnbA, clfA genes, biofilm formation may not occur in vitro ( 26 , 27 ). Although some studies have shown the role of bio-film-associated protein (Bap) in biofilm formation rarely ( 28 ), in current study the gene for the biofilm associated protein was not detected. These results correspond to previous reports on S. aureus isolates ( 29 ). Our results indicated bio-film formation regulated by several factors such as, environmental condition. Despite the presence of the ica gene, biofilm formation may not occur under in vitro conditions since S. aureus isolates are highly sensitive to environmental factors, such as the amount of glucose or glucosamine available for matrix formation ( 30 ). Furthermore, the difference between phenotypic and genotypic characterization of biofilm formation may result in heterogeneity in the genetic origins, and not because of the presence or absence of genes required for the biofilm.
Conclusion
The high level of antibiotic resistance among S. aureus causing UTI limits the use of antimicrobial agents for therapy and also the spread of MDR isolates is a threat for hospitalized patients. This study showed a significant relationship between biofilm formation of S. aureus isolates and some antibiotic resistance. So, bacterial biofilms can play an important role in resistance to antimicrobial agents. Trimethoprim-sulfamethoxazole and doxycycline may be used as an effective treatment for UTI caused by biofilm producers S. aureus. Our results suggest that biofilm formation is very complex and independent to only icaA, fnbA, clfA and bap genes harbor in S. aureus strains. Thus, molecular methods for detection of genes involved in biofilm formation are not appropriate methods for the actual biofilm phenotype under in vitro conditions.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This research was supported by Tehran University of Medical Sciences, Tehran, Iran (grant number: 10752).
References
- 1. Ranjbar R, Haghi-Ashtiani M, Jafari NJ, Abedini M. (2009). The prevalence and antimicrobial susceptibility of bacterial uropathogens isolated from pediatric patients. Iran J Public Health, 38 ( 2): 134–38. [Google Scholar]
- 2. Mobaleghi J, Salimizand H, Beiranvand S, Membari Sh, Kalantar E. (2012). Extended spectrum beta-lactamases in urinary isolates of Escherichia coli in five Iranian hospitals. Int J Infect Dis, 5: 35–6. [Google Scholar]
- 3. Taheri PA, Navabi B, Khatibi E. (2013). Frequency and susceptibility of bacteria caused urinary tract infection in neonates: eight-year study at neonatal division of bahrami children's hospital, Tehran Iran. Iran J Public Health, 42 ( 10): 1126–33. [PMC free article] [PubMed] [Google Scholar]
- 4. Kayas L, Yolbas I, Ece A, Kayas Y, Kocamaz H. (2011). Causative agents and antibiotic susceptibilities in children with urinary tract infection. J Microbiol Infect Dis, 1 ( 101): 17–21. [Google Scholar]
- 5. Tayebi Z, Seyedjavadi SS, Goudarzi M, Rahimi MK, Boromandi S, Bostanabad SZ. (2014). Frequency and antibiotic resistance pattern in gram positive uropathogenes isolated from hospitalized patients with urinary tract infection in Tehran, Iran. J Genes Microb Immun, 1 – 9 . [Google Scholar]
- 6. Vasudevan R. (2015). Emergence of UTI causing Staphylococcus aureus as a superbug: has the pathogen reduced the options of antimicrobial agents for treatment? EC Microbiol, 1: 88–112. [Google Scholar]
- 7. Havaei SA, Ohadian Moghadam S, Pourmand MR, Faghri J. (2010). Prevalence of genes encoding bi-component leukocidins among clinical isolates of methicillin–resistant Staphylococcus aureus. Iran J Public Health, 39 ( 1): 8–14. [PMC free article] [PubMed] [Google Scholar]
- 8. Choong S, Whitfield H. (2000). Biofilms and their role in infections in urology. BJU Int, 86 ( 8): 935–41. [DOI] [PubMed] [Google Scholar]
- 9. Reiter KC, Paim TGS, Oliveira CF, d'Azevedo PA. (2011). High biofilm production by invasive multiresistant staphylococci. Apmis, 119 ( 11): 776–81. [DOI] [PubMed] [Google Scholar]
- 10. Ohadian Moghadam S, Pourmand MR, Aminharati F. (2014). Biofilm formation and antimicrobial resistance in methicillin-resistant Staphylococcus aureus isolated from burn patients, Iran. J Infect Dev Ctries, 8 ( 12): 1511–17. [DOI] [PubMed] [Google Scholar]
- 11. Niveditha S, Pramodhini S, Umadevi S, KUMar S, StePheN S. (2012). The isolation and the biofilm formation of uropathogens in the patients with catheter associated urinary tract infections (UTIs). J Clin Diagn Res, 6 ( 9): 1478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ando E, Monden K, Mitsuhata R, Kariyama R, Kumon H. (2004). Biofilm formation among methicillin-resistant Staphylococcus aureus isolates from patients with urinary tract infection. Acta Med Okayama, 58 ( 4): 207–14. [DOI] [PubMed] [Google Scholar]
- 13. Soto SM. (2014). Importance of biofilms in urinary tract infections: new therapeutic approaches. Adv Biol, 543974 . [Google Scholar]
- 14. Kaur DC, Wankhede S. (2014). Biofilm formation and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus from wound infection. Asian Pac J Health Sci, 1 ( 4): 322–28. [Google Scholar]
- 15. Tang J, Chen J, Li H, Zeng P, Li J. (2013). Characterization of adhesin genes, staphylococcal nuclease, hemolysis, and biofilm formation among Staphylococcus aureus strains isolated from different sources. Foodborne Pathog Dis, 10 ( 9): 757–63. [DOI] [PubMed] [Google Scholar]
- 16. Eyoh AB, Toukam M, Atashili J, Fokunang C, Gonsu H, Lyonga EE. (2014). Relationship between multiple drug resistance and biofilm formation in Staphylococcus aureus isolated from medical and non-medical personnel in Yaounde, Cameroon. Pan Afr Med J, 17: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wayne P. (2014). Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing. 24th Inform Suppl 34 ( 1 ): M100 – S24 . [Google Scholar]
- 18. Arciola CR, Campoccia D, Gamberini S, Cervellati M, Donati E, Montanaro L. (2002). Detection of slime production by means of an optimised Congo red agar plate test based on a colourimetric scale in Staphylococcus epidermidis clinical isolates genotyped for ica locus. Biomaterials, 23 ( 21): 4233–39. [DOI] [PubMed] [Google Scholar]
- 19. Mirzaee M, Najar Peerayeh S, Ghasemian AM. (2014). Detection of icaABCD genes and biofilm formation in clinical isolates of methicillin resistant Staphylococcus aureus. Iran J Pathol, 9 ( 4): 257–62. [Google Scholar]
- 20. Tangchaisuriya U, Yotpanya W, Kitti T, Sitthisak S. (2014). Distribution among Thai children of methicillin-resistant Staphylococcus aureus lacking cna, fnbA and icaAD. Southeast Asian J Trop Med Public Health, 45 ( 1): 149–56. [PubMed] [Google Scholar]
- 21. Nemati M, Hermans K, Devriese LA, Maes D, Haesebrouck F. (2009). Screening of genes encoding adhesion factors and biofilm formation in Staphylococcus aureus isolates from poultry. Avian Pathol, 38 ( 6): 513–17. [DOI] [PubMed] [Google Scholar]
- 22. Pourmand MR, Memarian M, Hoseini M, Yazdchi SB. (2009). High prevalence of sea gene among clinical isolates of Staphylococcus aureus in Tehran. Acta Med Iran, 47 ( 5): 357–61. [Google Scholar]
- 23. Pourmand MR, Yousefi M, Salami SA, Amini M. (2014). Evaluation of expression of NorA efflux pump in ciprofloxacin resistant Staphylococcus aureus against hexahydroquinoline derivative by Real-Time PCR. Acta Med Iran, 52 ( 6): 424–429. [PubMed] [Google Scholar]
- 24. Poorabbas B, Mardaneh J, Rezaei Z, Kalani M, Pouladfar G, Alami MH, et al. (2015). Nosocomial infections: multicenter surveillance of antimicrobial resistance profile of Staphylococcus aureus and Gram negative rods isolated from blood and other sterile body fluids in Iran. Iran J Microbiol, 7 ( 3): 127–35. [PMC free article] [PubMed] [Google Scholar]
- 25. Taiwo S, Aderounmu A. (2006). Catheter associated urinary tract infection: aetiologic agents and antimicrobial susceptibility pattern in Ladoke Akintola University Teaching Hospital, Osogbo, Nigeria. Afr J Biomed Res, 9 ( 3): 141–48. [Google Scholar]
- 26. Castelani L, Pilon LE, Martins T, Pozzi CR, Arcaro JRP. (2015). Investigation of biofilm production and icaA and icaD genes in Staphylococcus aureus isolated from heifers and cows with mastitis. Anim Sci J, 86 ( 6): 340–44. [DOI] [PubMed] [Google Scholar]
- 27. Bekir K, Haddad O, Grissa M, Chaieb K, Bakhrouf A, Elgarssdi SI. (2012). Molecular detection of adhesins genes and biofilm formation in methicillin resistant Staphylococcus aureus. Afr J Microbiol Res, 6 ( 23): 4908–17. [Google Scholar]
- 28. Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penades JR. (2001). Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol, 183 ( 9): 2888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szweda P, Schielmann M, Milewski S, Frankowska A, Jakubczak A. (2012). Biofilm production and presence of ica and bap genes in Staphylococcus aureus strains isolated from cows with mastitis in the eastern Poland. Pol J Microbiol, 61 ( 1): 65–9. [PubMed] [Google Scholar]
- 30. Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. (1999). The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun, 67 ( 10): 5427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]