Abstract
Background
Magnetic resonance imaging (MRI) has highlighted the effects of chronic cocaine exposure on cerebral structures and functions, and implicated the prefrontal cortices in deficits of cognitive control. Recent investigations suggest power spectrum scale invariance (PSSI) of cerebral blood oxygenation level dependent (BOLD) signals as a neural marker of cerebral activity. We examined here how PSSI is altered in association with cocaine misuse and impaired cognitive control.
Methods
Eighty-eight healthy (HC) and seventy-five age and gender matched cocaine dependent (CD) adults participated in functional MRI of a stop signal task (SST). BOLD images were preprocessed using standard procedures in SPM, including detrending, band-pass filtering (0.01–0.25 Hz), and correction for head motions. Voxel-wise PSSI measures were estimated by a linear fit of the power spectrum with a log-log scale. In group analyses, we examined differences in PSSI between HC and CD, and its association with clinical and behavioral variables using a multiple regression. A critical component of cognitive control is post-signal behavioral adjustment, which is compromised in cocaine dependence. Therefore, we examined the PSSI changes in association with post-signal slowing (PSS) in the SST.
Results
Compared to HC, CD showed decreased PSS and PSSI in multiple frontoparietal regions. PSSI was positively correlated with PSS in HC in multiple regions, including the left inferior frontal gyrus (IFG) and right supramarginal gyrus (SMG), which showed reduced PSSI in CD.
Conclusions
These findings suggest disrupted connectivity dynamics in the fronto-parietal areas in association with post-signal behavioral adjustment in cocaine addicts. These new findings support PSSI as a neural marker of impaired cognitive control in cocaine addiction.
Keywords: PSSI, stimulant, Cocaine addiction, Cognitive control, Conflict monitoring, Post-signal slowing, post-error slowing
Highlights
-
•
We examined power spectrum scale invariance (PSSI) in association with cocaine misuse.
-
•
Compared to controls, cocaine addicts showed decreased PSSI in frontoparietal regions.
-
•
Reduced PSSI in the IFG and SMG were associated with deficits in post-signal-slowing.
1. Introduction
Scale-free brain activity (He, 2014) has been examined and modeled on many levels, from neurotransmitter release (Lowen et al., 1997), neuronal spiking (Levina, A., et al., 2007, Rubinov, M., et al., 2011) and local field potentials (Bedard and Destexhe, 2009), slow cortical potentials (He and Raichle, 2009), and electroencephalography (Freyer et al., 2009), suggesting the importance in examining the power-law property (e.g., not restricted to a particular temporal frequency/scale) of neural signals across multiple scales. Recent research suggests complexity measures of cerebral blood oxygenation level dependent (BOLD) signals as an important neural marker of cerebral activity dynamics (Anderson, J.S., et al., 2014, Bassett, D.S. and Gazzaniga, M.S., 2011, Ciuciu, P., et al., 2014, He, B.J., et al., 2010). Deviations from the typical range of power-exponents in BOLD time series have been noted in neuropsychiatric disorders (Lai, M.C., et al., 2010, Maxim, V., et al., 2005, Mujica-Parodi, L.R., et al., 2014, Radulescu, A.R., et al., 2012, Tolkunov, D., et al., 2010). For instance, male adults with an autism spectrum condition showed a significant shift to randomness in endogenous brain oscillations, as compared to neurotypical individuals, in brain regions implicated in autism (Lai et al., 2010). Our previous studies of power spectrum scale invariance (PSSI) as a measure of nonlinear complexity demonstrated that as the neural circuits become increasingly dysregulated, signal complexity of affected nodes deviates from an equilibrium value, as observed in trait anxiety, epilepsy and schizophrenia (Mujica-Parodi, L.R., et al., 2014, Nedic, S., et al., 2015, Radulescu, A.R., et al., 2012, Tolkunov, D., et al., 2010).
This intriguing scale-free property of brain activity is noted for more than a decade for fMRI signals (Bullmore et al., 2001). Although initially it was treated as a fractal noise, recent studies have shown that scale-free brain activity is not structured noise but rather is associated with rich temporal structures and functional significance (Ciuciu, P., et al., 2014, El Boustani, S., et al., 2009, Fransson, P., et al., 2013, He, B.J., 2011, He, B.J., et al., 2010). For instance, in an fMRI study, the power-law exponent decreased during performance of a visual detection task as compared to resting state (He, 2011). The power-law exponents presented distinct variation across functional brain networks, being larger for default-mode, saliency and visual networks (Fransson, P., et al., 2013, He, B.J., 2011). Further, this increase in power-law exponents was correlated with glucose metabolism (He, 2011). These observations rule out the possibility that scale-free property of brain activity is simply noise. It was also reported that power-law exponents were correlated with fMRI signal variance across different brain regions, thus providing unique information complementing univariate and multivariate mean-based fMRI analyses (He, 2011). In fact, recent studies reported altered fMRI signal variance in task-induced activations (Bianciardi, M., et al., 2009, Fransson, P., 2006), highlighting the association between brain activity and second order statistics. Finally, it was also observed that complexity of low-frequency BOLD signals covaries with local connectivity (Anderson et al., 2014). This relationship persisted even after regressing out the gray matter density and its standard deviation of the BOLD signal, suggesting that local interconnectivity may play a key role in establishing the complexity of low-frequency fluctuations. Thus, PSSI as a neural measure may capture an additional dimension of brain activity and connectivity that is not available from conventional analyses.
Chronic cocaine exposure is known to influence cerebral structures and functions, as highlighted by magnetic resonance imaging (MRI). For instance, functional MRI described altered regional activations in chronic cocaine users and individuals with prenatal exposure to drugs of abuse during a variety of behavioral challenges (Crunelle, C.L., et al., 2012, Garavan, H. and Hester, R., 2007, Li, C.S. and Sinha, R., 2008, Moeller, S.J., et al., 2014, Morein-Zamir, S., et al., 2013, Morein-Zamir, S., et al., 2015, Roussotte, F., et al., 2010). In particular, frontal cortical regions including the dorsolateral prefrontal and anterior cingulate cortices have consistently been implicated in deficits of decision making in association with cocaine misuse (Connolly, C.G., et al., 2012, Hester, R., et al., 2013, Hester, R. and Garavan, H., 2004, Lundqvist, T., 2010). Furthermore, recent work showed altered functional and effective connectivity of prefrontal, cingulate and subcortical structures during cognitive challenges in cocaine abusers (Cisler, J.M., et al., 2013, Ma, L., et al., 2015, Zhang, S., et al., 2014). Together, these studies suggest the utility of fMRI to delineate the dysregulated neurocircuitry of cocaine addiction (Koob and Volkow, 2010). However, it remains to be established how different components/nodes of the dysregulated circuit can be localized and quantified in terms of their dynamics. Conventional mean-based activation analyses do not necessarily identify regions where power spectrum and complexity properties are altered as a consequence of changes in interregional dynamic interactions.
Cocaine addiction is known to involve deficits in cognitive control (Ersche, K.D., et al., 2011, Garavan, H. and Hester, R., 2007, Goldstein, R.Z. and Volkow, N.D., 2011). Dysfunctional error-related processes not only characterize cocaine addiction but also predict relapse in a longitudinal setting (Luo et al., 2013). In our previous work, we have employed a stop signal task (SST) to describe how participants respond trial by trial in anticipation of a stop signal (Hu et al., 2015, Ide et al., 2013). While healthy individuals respond to stop signals by slowing down, cocaine addicts tend to be deficient in post-signal slowing, suggesting an impairment in signal monitoring and cognitive control (Franken, I.H., et al., 2007, Ide, J.S., et al., 2015, Ide, J.S. and Li, C.S.R., 2011, Li, C.S., et al., 2006b, Li, C.S., et al., 2008a, Li, C.S., et al., 2008b, Li, C.S., et al., 2008c). Here, we hypothesize that dysfunctional cognitive control in cocaine abusers can be characterized by altered power-exponent property of the underlying cerebral hemodynamics.
Considering that PSSI, along with other power-exponent measures, is closely associated with brain activity (Ciuciu, P., et al., 2014, Ciuciu, P., et al., 2012, He, B.J., 2011, He, B.J., et al., 2010) and has been used to describe circuit-level changes in fMRI signals (Mujica-Parodi, L.R., et al., 2014, Nedic, S., et al., 2015, Radulescu, A. and Mujica-Parodi, L.R., 2014, Radulescu, A.R., et al., 2012, Tolkunov, D., et al., 2010), we took the logical step to examine differences in PSSI between cocaine dependent individuals and healthy controls and explore whether the differences in this dynamics marker are related to impaired post-signal slowing in cocaine addicts.
2. Material and methods
2.1. Subjects, informed consent, and assessment
Seventy-five patients (50 men) with cocaine dependence (CD) and eighty-eight age and gender matched healthy control (HC) subjects (49 men) participated in this study (Table 1). CD participants were recruited from the local, greater New Haven area via newspapers and flyers as part of a prospective study (Luo et al., 2013) and met criteria for current cocaine dependence, as diagnosed by the Structured Clinical Interview for DSM-IV (First et al., 1995). Of the 84 CD participants examined in our previous morphometry study (Ide et al., 2014), 9 oldest subjects were excluded in order to match HC in age and gender. Thus, the subjects represented a convenience sample as no power calculation was performed to predetermine the sample size. Recent cocaine use was confirmed by urine toxicology screens upon admission. They were drug-free while staying in an inpatient treatment unit at the Connecticut Mental Health Center during the study period. All subjects were physically healthy with no major medical illnesses or current use of prescription medications. None of them reported having a history of head injury or neurological illness. Other exclusion criteria included dependence on other psychoactive substances (except nicotine) and current or past history of psychotic disorders. Individuals with current depressive or anxiety symptoms requiring treatment or currently being treated for these symptoms were excluded as well. The Human Investigation committee at Yale University School of Medicine approved the study, and all subjects signed an informed consent prior to participation.
Table 1.
Demographics of cocaine dependent (CD) and healthy control (HC) subjects.
| Subject characteristic | CD (n = 75) | HC (n = 88) | p-Value |
|---|---|---|---|
| Age (years) | 39.9 ± 7.6 | 38.7 ± 10.9 | 0.43a |
| Gender (M/F) | 50/25 | 49/39 | 0.15b |
| Beck Depression Inventory (BDI) | 12.2 ± 9.2 | N/A | N/A |
| STAI State | 37.6 ± 11.0 | N/A | N/A |
| STAI Trait | 41.7 ± 11.7 | N/A | N/A |
| CCQ-Brief | 20.8 ± 8.3 | N/A | N/A |
| Current smoker (yes/no) | 59/16 | 31/57 | 1e − 12b |
| Years of alcohol use | 16.1 ± 9.3 | 14.9 ± 14.3 | 0.57a |
| Days of drinking (prior month) | 12.7 ± 7.5 | 3.4 ± 5.3 | 1e − 16a |
| Years of marijuana use | 10.0 ± 4.1 | N/A | N/A |
| Average monthly cocaine use (gm), prior year | 18.8 ± 27.3 | N/A | N/A |
| Days of cocaine use (prior month) | 15.3 ± 8.7 | N/A | N/A |
| Years of cocaine use | 18.0 ± 8.2 | N/A | N/A |
| Days abstinent prior to assessment | 18.0 ± 5.9 | N/A | N/A |
Note: values are mean ± S.D.
Two-tailed two-sample t-test.
χ2 test.
All CD participants were assessed with the Beck Depression Inventory (Beck et al., 1961) and the State-Trait Anxiety Inventory (Speilberger et al., 1970) at admission, both with scores within the range reported previously for individuals with cocaine dependence (Falck, R.S., et al., 2002, Karlsgodt, K.H., et al., 2003, Lopez, A. and Becona, E., 2007, Rubin, E., et al., 2007) (Table 1). Cocaine craving was assessed with the Cocaine Craving Questionnaire, brief version (CCQ-Brief), for all participants on the same day of the fMRI (Sussner et al., 2006). The CCQ-Brief is a 10-item questionnaire, abbreviated from the CCQ—Now (Tiffany et al., 1993). It is highly correlated with the CCQ—Now and other cocaine craving measures (Sussner et al., 2006). Each item was rated on a scale from 1 to 7, with a higher total score (ranging from 10 to 70) indicating greater craving (Table 1).
Healthy control participants (HC) were drawn from the local community, underwent a thorough interview by a psychiatrist (C.-S. R. Li) to rule out a DSM-IV diagnosis including abuse of or dependence on a substance other than nicotine, and all tested negative for illicit substances on the day of imaging. Smoking status and use of alcohol was documented. Previous use of any illicit substances and marijuana for longer than one year were exclusion criteria. As none of the HC reported depression or anxiety symptoms, HC were not assessed with the BDI or STAI. None of the HC were under any psychotropic medications during the year prior to the current study.
2.2. Behavioral task
We employed a simple reaction time task in this stop-signal paradigm (Farr, O.M., et al., 2012, Hendrick, O.M., et al., 2010, Hu, S., et al., 2014b, Ide, J.S. and Li, C.S.R., 2011, Li, C.S., et al., 2006b, Li, C.S., et al., 2010, Logan, G.D., et al., 1984, Winkler, A.D., et al., 2012). There are two trial types: “go” and “stop,” presented with an inter-trial interval of 2 s, and occurring on each trial with 0.75 probability of being a go trial (0.25 probability stop trial). A small dot appears on the screen to engage attention at the beginning of a go trial. After a randomized time interval (fore-period) between 1 and 5 s, drawn from a uniform distribution, the dot turns into a circle (the “go” signal), prompting the subjects to quickly press a button. The circle vanishes at a button press or after 1 s has elapsed, whichever coming first, and the trial terminates. A premature button press prior to the appearance of the circle also terminates the trial. On a stop trial, an additional “X,” the “stop” signal, appears after and replaces the go signal, and instructs participants to withhold their response. Similar to go trials, a stop trial terminates at button press or 1 s after the appearance of the stop signal. Failure to withhold the go response for the 1 s constitutes a stop error. The stop signal delay (SSD) – the time interval between go and stop signals – starts at 200 ms and is adjusted according to a staircase procedure, increasing and decreasing by 67 ms each for a successful and failed stop (Levitt, 1971). Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up occasionally. The staircase procedure ensures that subjects would succeed in withholding their response in approximately half of the stop trials.
2.3. Analyses of behavioral performance in the stop signal task
We computed a critical SSD that represents the time delay between go and stop signals that a subject would need to succeed in 50% of the stop trials (Levitt, 1971). Specifically, SSDs across trials were grouped into runs, with each run defined as a monotonically increasing or decreasing series. We derived a mid-run estimate by taking the middle SSD (or average of the two middle SSDs when there was an even number of SSDs) of every second run. The critical SSD was computed by taking the mean of all mid-run SSDs. It was reported that, except for experiments with a small number of trials (< 30), the mid-run estimate was close to the maximum likelihood estimate of X50 (50% positive response; i.e., 50% SS in the SST, (Wetherill et al., 1966)). The stop signal reaction time (SSRT) was computed by subtracting the critical SSD from the median go trial RT (Logan, 1994).
It is known that in the SST the RT of a correct response is prolonged following a stop signal, compared with other correct responses, and this prolonged RT is thought to reflect conflict monitoring. We thus computed the RT difference between the go trials that followed a stop trial and those that followed another go trial, and termed the effect size of this RT difference “post-signal slowing” (PSS) (Li et al., 2009).
2.4. Image acquisition, preprocessing and statistical tests
All imaging data were collected in the same 3T Siemens Trio scanner while subjects performed the SST, as described in detail in our previous work (Li, C.S., et al., 2006a, Li, C.S., et al., 2006c). Each scan comprised four 10-min runs of the SST. Smokers and caffeine-using subjects were allowed to smoke and drink coffee or other caffeinated beverages until 1 h before the fMRI studies. Functional blood oxygen level dependent (BOLD) signals were acquired with a single-shot gradient echo echo-planar imaging (EPI) sequence, with 32 axial slices parallel to the AC-PC line covering the whole brain, using our published parameters (Li, C.S., et al., 2006a, Li, C.S., et al., 2006c): TR = 2000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, FOV = 220 × 220 mm2, matrix = 64 × 64, slice thickness = 4 mm and no gap. A high-resolution 3D structural image (MPRAGE; 1 mm resolution) was also obtained for anatomical co-registration.
Functional MRI data was preprocessed with Statistical Parametric Mapping 12 (SPM12) (Wellcome Department of Imaging Neuroscience, University College London, U.K.). Images from the first five TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were first corrected for slice timing, realigned (motion-corrected) (Andersson, J.L., et al., 2001, Hutton, C., et al., 2002). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. The anatomical images (T1-weighted) were co-registered to the mean functional image, and normalized to an MNI (Montreal Neurological Institute) template with affine registration followed by nonlinear transformation using a unified segmentation and registration framework (Ashburner and Friston, 2005). The normalization parameters determined for the anatomical volume were then applied to the corresponding functional image volumes for each subject. In addition, the preprocessing procedures included detrending, and regression of global signal, cerebral spinal fluid, white matter, and six degrees of motion following our optimized pipeline for PSSI estimation (Rubin et al., 2013). Group analyses were performed also using SPM12 on the computed PSSI maps using two sample t-tests and multiple regressions, using age as covariate (Hu et al., 2012). In additional analyses, we examined whether clinical characteristics including alcohol use were associated with the findings on PSSI (Bednarski, S., et al., 2012, Yan, P. and Li, C.S., 2009).
2.5. PSSI
Using methods optimized for fMRI (Rubin et al., 2013), we estimated PSSI β from each FFT-transformed time series S(f) as per S(f) ∝ f− β. Power spectrum densities were computed from preprocessed BOLD images on a voxel-wise basis and plotted on a log-log scale. We computed the slope of the linear fit (β) within a frequency window of 0.01–0.25 Hz using least squares fitting; this range of frequency was adopted to exclude low fluctuations drifts (lower limit) and to avoid aliasing (upper limit) following previous experiments on PSSI computation on task data (Tolkunov et al., 2010). Following our previous work and others' on the PSSI, we used preprocessed time-series without taking the derivative and reported β to simplify interpretation of correlations and having PSSI represented by positive numbers. Thus, β = 0 represents a power spectrum with maximum entropy (white noise), and increasing β represents greater persistence (which can be due either to diminished excitatory inputs or tighter homeostatic constraint over the system via negative feedback (Radulescu and Mujica-Parodi, 2014)). PSSI β maps were smoothed with a Gaussian kernel of 6 mm at Full Width at Half Maximum, and were carried to second-level analyses.
3. Results
3.1. Behavioral performance
Behavioral performance is summarized in Table 2. CD showed a decrease in the percentage of successful go trials and in the extent (effect size) of post-signal slowing, as compared to HC individuals. CD showed longer SSRT as compared to HC, but the difference did not reach statistical significance. The latter likely resulted from an under-estimation of the SSRT because the “RT” of a larger number of go error trials could not be considered in the computation of SSRT for CD (Verbruggen et al., 2013).
Table 2.
Performance in the stop signal task.
| SSRT (ms) | Median go RT (ms) | %go | %stop | PSS (effect size) | |
|---|---|---|---|---|---|
| CD (n = 75) | 230 ± 50 | 594 ± 99 | 95.9 ± 1.4 | 52.4 ± 3.5 | 1.37 ± 2.10 |
| HC (n = 88) | 222 ± 45 | 624 ± 104 | 96.6 ± 1.9 | 52.8 ± 3.3 | 2.07 ± 1.98 |
| p-Valuea | 0.31 | 0.06 | 0.01 | 0.40 | 0.03 |
Note: All values are mean ± standard deviation; CD: individuals with cocaine dependence; HC: healthy controls; SSRT: stop signal reaction time; RT: reaction time; %go: percentage of go response trials; PSS: post-signal slowing.
p-Value based on 2-tailed 2-sample t-test.
3.2. The effects of cocaine misuse and post-signal behavioral adjustment
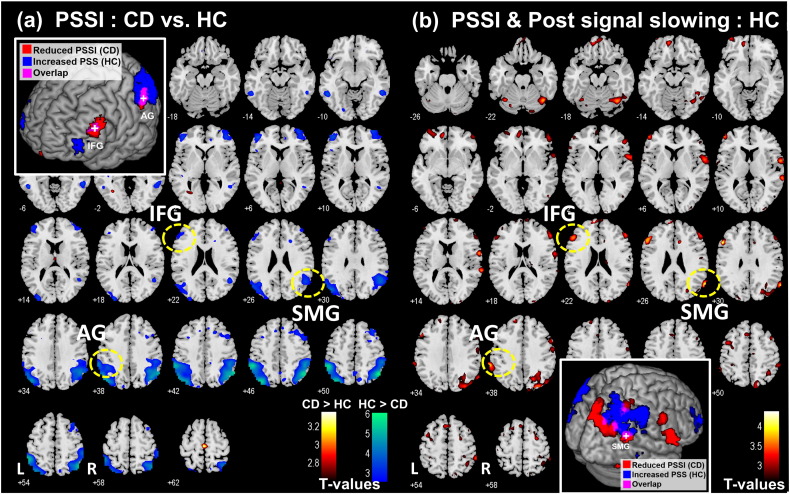
Compared to HC, CD presented a significant decrease of PSSI in several frontoparietal structures, including the angular and supramarginal gyri, as well as the inferior and middle frontal gyri (Fig. 1a and Table 3). We performed a linear regression of PSSI map against the effect size of post-signal slowing (PSS) each in HC and CD. In HC, multiple brain regions in the frontal and parietal cortex showed PSSI in positive correlation with PSS (Fig. 1b and Table 4). Regions were identified both using a published atlas (Duvernoy, 1999) and with reference to the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002). At the same threshold, CD did not show any significant regional association of PSSI to PSS. Notably, subregions of the left inferior frontal gyrus (IFG), left angular gyrus (G), and right supramarginal gyrus (SMG) with reduced PSSI in CD as compared to HC overlapped regions where the PSSI was in positive correlation with PSS in HC (Fig. 1, insets; Fig. 2b).
Fig. 1.
(a) Regions showing reduced PSSI in CD as compared to HC; two sample t-test with age as covariate. No brain regions showed increased PSSI in CD as compared to HC at the same threshold. Clusters that survived the corrected extent threshold are shown in Table 3. (b) Regions with PSSI in linear correlation with post-signal slowing (PSS) in the HC; multiple regression with PSS and age as covariates. p < 0.05, corrected AlphaSim threshold. Clusters that survived the corrected extent threshold are shown in Table 4. The inset each highlights the overlap in the left inferior frontal gyrus (IFG), left angular gyrus (AG) and right supramarginal gyrus (SMG) between these two group analyses.
Table 3.
Regions with reduced PSSI in CD (n = 75) as compared to HC (n = 88); t-test with age as covariate, p < 0.05, corrected AlphaSim threshold. Cluster k > 100. All peak voxels 8 mm apart are identified.
| Volume voxels (k) | Peak voxel |
MNI coordinate |
Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| Z | x | y | z | |||
| 951 | 5.76 | 48 | − 61 | 50 | R | Angular gyrusa |
| 5.20 | 57 | − 58 | 41 | R | Inferior parietal cortexa | |
| 4.56 | 48 | − 43 | 44 | R | Supramarginal gyrusa | |
| 947 | 5.47 | − 36 | − 76 | 47 | L | Middle occipital cortexa |
| 5.46 | − 54 | − 43 | 47 | L | Supramarginal gyrusa | |
| 4.76 | − 45 | − 73 | 38 | L | Middle occipital cortexa | |
| 114 | 3.70 | − 45 | 47 | 8 | L | Inferior frontal cortex |
| 2.95 | − 45 | 38 | 23 | L | Inferior frontal gyrus | |
| 2.91 | − 51 | 26 | 29 | L | Inferior frontal gyrus | |
| 136 | 3.54 | 42 | 50 | − 1 | R | Lateral orbitofrontal cortex |
| 3.49 | 36 | 59 | 8 | R | Lateral frontal cortex | |
Indicates clusters significant at p < 0.05, FWE corrected.
Table 4.
Regions with PSSI in positive correlation with the extent of post-signal slowing (PSS) in HC; multiple regression with age as a covariate, p < 0.05, corrected AlphaSim threshold. Cluster k > 100. All peak voxels 8 mm apart are identified. No clusters met the threshold in CD.
| Volume voxels (k) | Peak voxel |
MNI coordinate |
Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| Z | x | y | z | |||
| 109 | 4.16 | − 54 | 23 | 29 | L | Inferior frontal gyrus |
| 3.62 | − 48 | 32 | 23 | L | Inferior frontal gyrus | |
| 618 | 4.06 | 63 | − 61 | 26 | R | Supramarginal/superior temporal gyrus |
| 3.71 | 24 | − 85 | 41 | R | Superior occipital gyrus | |
| 111 | 3.73 | − 60 | − 46 | 44 | L | Angular gyrus |
| 3.71 | − 66 | − 40 | 38 | L | Angular gyrus | |
| 155 | 3.64 | 36 | − 64 | − 19 | R | Fusiform gyrus |
| 3.24 | 54 | − 58 | − 16 | R | Inferior temporal gyrus | |
| 3.17 | 45 | − 49 | − 13 | R | Inferior temporal gyrus | |
| 213 | 3.60 | 63 | 2 | 11 | R | Rolandic operculum |
| 3.05 | 60 | − 7 | 38 | R | Postcentral gyrus | |
| 2.72 | 54 | − 7 | 26 | R | Postcentral gyrus | |
| 127 | 3.42 | − 9 | 53 | − 16 | L | Rectus gyrus |
| 3.33 | − 9 | 53 | − 4 | L | Medial orbitofrontal cortex | |
| 3.12 | 3 | 59 | − 19 | R | Rectus gyrus | |
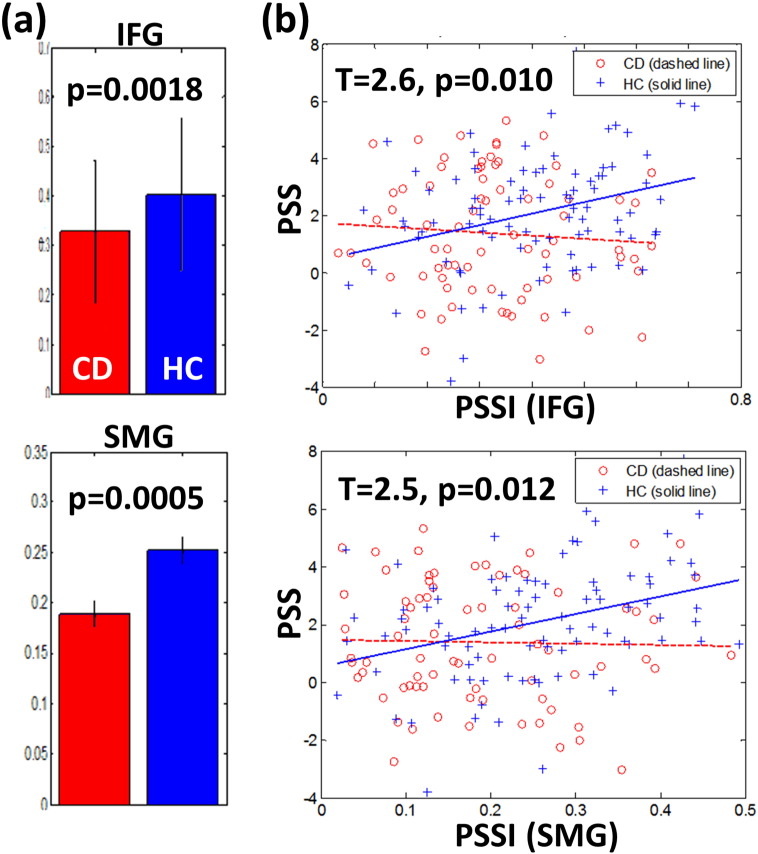
Fig. 2.
(a) Compared to HC, CD showed decreased PSSI in the left inferior frontal gyrus (IFG) and right supramarginal gyrus (SMG) (two sample t-test); (b) PSSI increased in association with post-signal slowing in the IFG (r = 0.31, p = 0.003) and SMG (r = 0.35, p = 0.001) in HC but not CD. A direct test shows a significant difference in slope of the linear regression in both cases (Zar, 1999).
We extracted the PSSI of the left IFG, left AG, and right SMG for all individual CD and HC subjects and confirmed that the PSSI of the left IFG and right SMG were significantly lower in CD than in HC (Fig. 2a). Further, the differences in slope in the linear regression of the PSS vs. PSSI were significant for both regions (Fig. 2b; Zar, 1999). The PSSI of the left AG was also significantly lower in CD than in HC (p < 1e − 05); however, the difference in slope in the linear regression of the PSS vs. PSSI was not significant between CD and HC (p > 0.13). The PSSI of none of these ROIs showed a significant correlation with the SSRT (all p's > 0.69), go success % (all p's > 0.02), stop success % (all p's > 0.18), or median go RT (all p's > 0.35) for CD or HC.
3.3. Correlation of PSSI with clinical characteristics and other SST performance measures
We examined whether the PPSI of the identified clusters was correlated with clinical characteristics of the CD subjects, including BDI score, STAI State/Trait score, CCQ-Brief score, years of alcohol use, days of drinking in the prior month, years of marijuana use, average monthly cocaine use in grams in the prior year, days of cocaine use in the prior month, years of cocaine use, and days abstinent prior to assessment. None of the correlations were significant with correction for multiple corrections (all p's > 0.01). The PSSI also did not differ with respect to their smoking status (p's > 0.13, main effects and interaction effect, two-way ANOVA).
We also examined whether the PSSI of the identified clusters (Table 3) was correlated with other performance measures of the SST in CD. No clusters showed a significant correlation with the SSRT (all p's > 0.37), the go success % (all p's > 0.12), the stop success % (all p's > 0.30), or the median go RT (all p's > 0.13).
3.4. Alcohol use as a potential confound
Cocaine addicts showed higher alcohol consumption as compared to controls. Although none of the cocaine dependent participants met the DSM-IV criteria for alcohol dependence — an exclusion condition for the current study, we examined whether alcohol use was related to PSSI. We performed a linear regression of PSSI against alcohol use measures for individual ROIs, each for CD and HC, and the results showed no significant correlations with years of alcohol use (all p's > 0.71 for CD, all p's > 0.19 for HC) or days of drinking in the prior month (all p's > 0.73 for CD, all p's > 0.18 for HC).
3.5. A comparison of GLM based findings and PSSI
We conducted an additional analysis using GLM and queried regional activations to stop versus go trials in correlation with PSS in a linear regression each for HC and CD. At the same statistical threshold, the results showed little overlap with the PSSI maps (Supplementary Fig. S1).
4. Discussion
We observed reduced post-signal slowing (PSS) in the CD as compared to HC groups (Table 1), confirming our previous report of impaired Bayesian learning for cognitive control in cocaine dependence (Ide et al., 2015). We also observed significant reduction of PSSI in the CD as compared to HC in the left inferior frontal gyrus (IFG) and right supramarginal gyrus (SMG). Compared to HC, CD showed decreased PSS, and the PSSI of the left IFG and right SMG correlated with the extent of PSS in HC but not CD. These findings suggest PSSI as a biomarker of impaired cognitive control in cocaine addiction.
The IFG is part of the ventral attention system, which activates to the detection of a salient, behaviorally relevant target (for review, see (Corbetta et al., 2008). Although the bulk of this literature has focused on the role of the right IFG in attention (Hampshire, A., et al., 2010, Konishi, S., et al., 1999) and its link to response inhibition (Chao, H.H., et al., 2009, Duann, J.R., et al., 2009, Erika-Florence, M., et al., 2014), studies have implicated the left IFG in attentional processes including responses to mismatch negativity (Hedge et al., 2015), as well as to repetition priming during word recognition (Pas, M., et al., 2015, Thiel, A., et al., 2005) and sound tone identification (Asaridou et al., 2015). The left IFG responds to the resolution of semantic conflicts between linguistic inputs (Ye and Zhou, 2009). In a perceptual decision task, activations of the left IFG varied with response bias to changing motivational context, suggesting a role in flexible behavioral control (Reckless et al., 2014). Studies with transcranial magnetic stimulation also demonstrated an interaction between the left IFG and pre-supplementary motor area to facilitate response inhibition (Obeso et al., 2013). The left IFG responds to inhibition of proactive interference (Feredoes, E., et al., 2006, Jimura, K., et al., 2009). Fatigue in interference control resulted in decreased activity in the left IFG and other cortical and subcortical structures (Persson et al., 2013). Further, patients with left IFC lesions were compromised in inhibitory control in the stop signal task (Swick et al., 2008). In studies of clinical populations, hypoactivation of the left IFG was noted in problem video gamers during Stroop interference (Luijten et al., 2015). Compared to non-smokers, smokers demonstrated decreased activity in the left IFG in link with impaired performance on the go/no-go task (Luijten et al., 2013). In reappraising evoked emotions, older participants demonstrated reduced activation of the left IFG, as compared to young adults (Winecoff et al., 2011). Together, these studies support a role of the left IFG in attention and cognitive control. The current findings demonstrated PSSI as a neural correlate of post-signal behavioral adjustment and diminished PSSI in the left IFG as an additional neural marker of impaired control in cocaine addicts.
As part of the inferior parietal lobule, the SMG also partakes in the ventral attentional network (Corbetta et al., 2008). The SMG is responsible for the attentional component of episodic memory (Cabeza et al., 2008) and through functional connectivity with the somatomotor cortex, supports attentional mechanisms that underlie visuomotor learning (Ma et al., 2011). The right but not left SMG responds to saccadic eye movements made to a peripheral visual target (Perry and Zeki, 2000) and to “inhibition of return”, a mechanism that prioritizes attention to new, yet-to-be-explored spatial locations (Lepsien, J. and Pollmann, S., 2002, Li, C.S., et al., 2003, Li, C.S. and Lin, S.C., 2002a, Li, C.S. and Lin, S.C., 2002b). In cognitive control, the right SMG responds to set shifting contingent on outcome evaluation (Hildebrandt et al., 2013). Proactive emotional control engages multiple brain regions including the pre-supplementary motor area and right SMG (Vanderhasselt et al., 2013). The supplementary eye field, anterior cingulate cortex, inferior frontal gyri and right SMG respond to inhibition of saccadic eye movements in an oculomotor go/nogo task (Brown, M.R., et al., 2006, Brown, M.R., et al., 2008, Ettinger, U., et al., 2008). The cortical thickness and white matter integrity of the right inferior parietal regions are linked to the capacity of attentional orienting in a visuospatial task (Yin et al., 2012). In clinical populations, voxel-based morphometry showed lower gray matter volumes in the striatum and right SMG in cocaine dependent patients, as compared to controls (Barros-Loscertales et al., 2011). Chronic methamphetamine users also demonstrated loss of gray matter volume in the right SMG (Hall et al., 2015). Frontoparietal functional connectivity involving the right SMG was decreased in adolescents at risk for dysfunctional control and depression (Clasen et al., 2014). Together, these findings support a role of the right SMG in orienting attention to external stimuli in support of motor decision making in a variety of behavioral contexts. These processes, including post-signal slowing in the SST, are likely compromised in chronic cocaine users and, as the current study shows, may be reflected in altered PSSI as a dynamics measure of cerebral cortical activity.
In addition to the IFG and SMG, we observed significant reduction of PSSI values in several other fronto-parietal regions in CD as compared to HC. This suggests that the fMRI signals overall are more random in these regions. Considering the equivalence between auto-correlation and PSSI measures (He, B.J., 2014, Nedic, S., et al., 2015), the reduction in PSSI indicates weaker autocorrelation/persistence in these regions. In line with previous reports of PSSI deviations from the neurotypical range in other neurological and psychiatric disorders (Lai, M.C., et al., 2010, Maxim, V., et al., 2005, Mujica-Parodi, L.R., et al., 2014, Nedic, S., et al., 2015, Radulescu, A.R., et al., 2012, Tolkunov, D., et al., 2010), the current study is the first to report reduced PSSI in cocaine addicts and its association with deficits in cognitive control.
By comparing findings from GLM and PSSI analyses, we observed little overlap between the maps (Supplementary Fig. S1). This finding may suggest that PSSI captures changes in circuit dynamics that elude GLM analyses, with the latter characterizing mean-based brain responses and PSSI describing altered second order statistics of fMRI signals (He, 2011). However, this observation also calls for a broader question regarding the relationship between various neural measures investigators have used to quantify cerebral structural integrity and highlight regional activations in health and illness. For instance, we examined whole-brain morphometry and fractional amplitude of low-frequency fluctuation (fALFF) of fMRI signals and noted similar changes in the prefrontal and frontal cortices in fALFF and gray matter density during healthy aging (Hu et al., 2014a) but not in cocaine addicts (Ide et al., 2014). Further, these findings do not mirror age-related changes identified for various GLM contrasts, including response inhibition, error processing, and risk taking, from the stop signal task (Hu et al., 2012) or age-related changes in PSSI (unpublished observations). More studies are clearly needed to provide insight to this important issue.
Finally, although we employed a previously optimized preprocessing pipeline for PSSI estimation in fMRI data (Rubin et al., 2013), aiming to remove measurement and physiological noises, we are not able to rule out the possibility of PSSI changes due to the effects of chronic cocaine use on the vascular system. Further studies to include imaging of cerebral perfusion are necessary to dissociate neural from vascular consequences of cocaine abuse.
5. Conclusions
Taken together, the current findings suggest altered PSSI as a useful neural marker for cocaine misuse and impaired cognitive control. Along with our previous work (Radulescu, A.R., et al., 2012, Rubin, D., et al., 2013, Tolkunov, D., et al., 2010), these new findings support the utility of PSSI in delineating the complex neural circuitry of various psychopathologies. Future work is needed to examine what is driving the decreased PPSI in the IFG and SMG by delineating the connectivity between regions in the frontoparietal network (Radulescu and Mujica-Parodi, 2014).
The following is the supplementary data related to this article.
(a) GLM contrast map (Stop > Go) correlated with PSS for HC. (b) PSSI map correlated with PSS for HC. (c) GLM contrast map (Stop > Go) correlated with PSS for CD. (d) PSSI map correlated with PSS for CD. Hot color: positive correlation, cold color: negative correlation. p < 0.05, corrected AlphaSim threshold.
Acknowledgements
This study is supported by NIH grants DA026990 (Li), DA023248 (Li), K25DA040032 (Zhang), and DA03846702 (Mujica-Parodi) as well as the Peter McManus Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Drug Abuse or the National Institutes of Health. We have disclosed all research support and do not have conflicts of interest in the current work.
Contributor Information
Jaime S. Ide, Email: jaime.ide@stonybrook.edu.
Chiang-shan R. Li, Email: chiang-shan.li@yale.edu.
References
- Anderson J.S., Zielinski B.A., Nielsen J.A., Ferguson M.A. Complexity of low-frequency blood oxygen level-dependent fluctuations covaries with local connectivity. Hum. Brain Mapp. 2014;35:1273–1283. doi: 10.1002/hbm.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Hutton C., Ashburner J., Turner R., Friston K. Modeling geometric deformations in EPI time series. NeuroImage. 2001;13:903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Asaridou S.S., Takashima A., Dediu D., Hagoort P., McQueen J.M. 2015. Repetition Suppression in the Left Inferior Frontal Gyrus Predicts Tone Learning Performance. Cerebral cortex. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barros-Loscertales A., Garavan H., Bustamante J.C., Ventura-Campos N., Llopis J.J., Belloch V., Parcet M.A., Avila C. Reduced striatal volume in cocaine-dependent patients. NeuroImage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Gazzaniga M.S. Understanding complexity in the human brain. Trends Cogn. Sci. 2011;15:200–209. doi: 10.1016/j.tics.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bedard C., Destexhe A. Macroscopic models of local field potentials and the apparent 1/f noise in brain activity. Biophys. J. 2009;96:2589–2603. doi: 10.1016/j.bpj.2008.12.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarski S., Zhang S., Luo X., Erdman E., Li C.-S. Neural correlates of an indirect analogue of risk taking in non-dependent heavy alcohol drinkers. Alcohol. Clin. Exp. Res. 2012;36:768–779. doi: 10.1111/j.1530-0277.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M., Fukunaga M., van Gelderen P., Horovitz S.G., de Zwart J.A., Duyn J.H. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. NeuroImage. 2009;45:160–168. doi: 10.1016/j.neuroimage.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.R., Goltz H.C., Vilis T., Ford K.A., Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. NeuroImage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown M.R., Vilis T., Everling S. Isolation of saccade inhibition processes: rapid event-related fMRI of saccades and nogo trials. NeuroImage. 2008;39:793–804. doi: 10.1016/j.neuroimage.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Long C., Suckling J., Fadili J., Calvert G., Zelaya F., Carpenter T.A., Brammer M. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum. Brain Mapp. 2001;12:61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H.H., Luo X., Chang J.L., Li C.S. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time — an intra-subject analysis. BMC Neurosci. 2009;10:75. doi: 10.1186/1471-2202-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Elton A., Kennedy A.P., Young J., Smitherman S., Andrew James G., Kilts C.D. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res. 2013;213:39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuciu P., Abry P., He B.J. Interplay between functional connectivity and scale-free dynamics in intrinsic fMRI networks. NeuroImage. 2014;95:248–263. doi: 10.1016/j.neuroimage.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuciu P., Varoquaux G., Abry P., Sadaghiani S., Kleinschmidt A. Scale-free and multifractal time dynamics of fMRI signals during rest and task. Front. Physiol. 2012;3:186. doi: 10.3389/fphys.2012.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen P.C., Beevers C.G., Mumford J.A., Schnyer D.M. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev. Cogn. Neurosci. 2014;7:13–22. doi: 10.1016/j.dcn.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C.G., Foxe J.J., Nierenberg J., Shpaner M., Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2012;121:45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelle C.L., Veltman D.J., Booij J., Emmerik-van Oortmerssen K., van den Brink W. Substrates of neuropsychological functioning in stimulant dependence: a review of functional neuroimaging research. Brain Behav. 2012;2:499–523. doi: 10.1002/brb3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann J.R., Ide J.S., Luo X., Li C.S. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H. Springer Verlag; New York, NY: 1999. The Human Brain: Surface, Blood Supply, and Three-Dimensional Sectional Anatomy. [Google Scholar]
- El Boustani S., Marre O., Behuret S., Baudot P., Yger P., Bal T., Destexhe A., Fregnac Y. Network-state modulation of power-law frequency-scaling in visual cortical neurons. PLoS Comput. Biol. 2009;5 doi: 10.1371/journal.pcbi.1000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erika-Florence M., Leech R., Hampshire A. A functional network perspective on response inhibition and attentional control. Nat. Commun. 2014;5:4073. doi: 10.1038/ncomms5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche K.D., Barnes A., Jones P.S., Morein-Zamir S., Robbins T.W., Bullmore E.T. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U., Ffytche D.H., Kumari V., Kathmann N., Reuter B., Zelaya F., Williams S.C. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cereb. Cortex. 2008;18:1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- Falck R.S., Wang J., Carlson R.G., Eddy M., Siegal H.A. The prevalence and correlates of depressive symptomatology among a community sample of crack-cocaine smokers. J. Psychoactive Drugs. 2002;34:281–288. doi: 10.1080/02791072.2002.10399964. [DOI] [PubMed] [Google Scholar]
- Farr O.M., Hu S., Zhang S., Li C.S. Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. NeuroImage. 2012;63:1070–1077. doi: 10.1016/j.neuroimage.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E., Tononi G., Postle B.R. Direct evidence for a prefrontal contribution to the control of proactive interference in verbal working memory. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19530–19534. doi: 10.1073/pnas.0604509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Williams J., Gibbon M. American Psychiatric Association; Washington DC: 1995. Structured Clinical Interview for DSM-IV (SCID) [Google Scholar]
- Franken I.H., van Strien J.W., Franzek E.J., van de Wetering B.J. Error-processing deficits in patients with cocaine dependence. Biol. Psychol. 2007;75:45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fransson P., Metsaranta M., Blennow M., Aden U., Lagercrantz H., Vanhatalo S. Early development of spatial patterns of power-law frequency scaling in FMRI resting-state and EEG data in the newborn brain. Cereb. Cortex. 2013;23:638–646. doi: 10.1093/cercor/bhs047. [DOI] [PubMed] [Google Scholar]
- Freyer F., Aquino K., Robinson P.A., Ritter P., Breakspear M. Bistability and non-Gaussian fluctuations in spontaneous cortical activity. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:8512–8524. doi: 10.1523/JNEUROSCI.0754-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Hester R. The role of cognitive control in cocaine dependence. Neuropsychol. Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M.G., Alhassoon O.M., Stern M.J., Wollman S.C., Kimmel C.L., Perez-Figueroa A., Radua J. Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am. J. Drug Alcohol Abuse. 2015;41:290–299. doi: 10.3109/00952990.2015.1044607. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Scale-free properties of the functional magnetic resonance imaging signal during rest and task. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:13786–13795. doi: 10.1523/JNEUROSCI.2111-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Scale-free brain activity: past, present, and future. Trends Cogn. Sci. 2014;18:480–487. doi: 10.1016/j.tics.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J., Raichle M.E. The fMRI signal, slow cortical potential and consciousness. Trends Cogn. Sci. 2009;13:302–309. doi: 10.1016/j.tics.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J., Zempel J.M., Snyder A.Z., Raichle M.E. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66:353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedge C., Stothart G., Todd Jones J., Rojas Frias P., Magee K.L., Brooks J.C. A frontal attention mechanism in the visual mismatch negativity. Behav. Brain Res. 2015;293:173–181. doi: 10.1016/j.bbr.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick O.M., Ide J.S., Luo X., Li C.S. Dissociable processes of cognitive control during error and non-error conflicts: a study of the stop signal task. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R., Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R., Bell R.P., Foxe J.J., Garavan H. The influence of monetary punishment on cognitive control in abstinent cocaine-users. Drug Alcohol Depend. 2013;133:86–93. doi: 10.1016/j.drugalcdep.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt H., Fink F., Eling P., Stuke H., Klein J., Lentschig M., Kastrup A., Thiel C., Breckel T. Neural correlates of stimulus response and stimulus outcome shifting in healthy participants and MS patients. Brain Cogn. 2013;81:57–66. doi: 10.1016/j.bandc.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Hu S., Chao H.H., Zhang S., Ide J.S., Li C.S. Changes in cerebral morphometry and amplitude of low-frequency fluctuations of BOLD signals during healthy aging: correlation with inhibitory control. Brain Struct. Funct. 2014 doi: 10.1007/s00429-013-0548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Tseng Y.C., Winkler A.D., Li C.S. Neural bases of individual variation in decision time. Hum. Brain Mapp. 2014;35:2531–2542. doi: 10.1002/hbm.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Chao H.H., Winkler A.D., Li C.S. The effects of age on cerebral activations: internally versus externally driven processes. Front. Aging Neurosci. 2012;4:4. doi: 10.3389/fnagi.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Ide J.S., Zhang S., Li C.S. Anticipating conflict: Neural correlates of a Bayesian belief and its motor consequence. Neuroimage. 2015;119:286–295. doi: 10.1016/j.neuroimage.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C., Bork A., Josephs O., Deichmann R., Ashburner J., Turner R. Image distortion correction in fMRI: a quantitative evaluation. NeuroImage. 2002;16:217–240. doi: 10.1006/nimg.2001.1054. [DOI] [PubMed] [Google Scholar]
- Ide J.S., Li C.S.R. Error-related functional connectivity of the habenula in humans. Front. Hum. Neurosci. 2011:5. doi: 10.3389/fnhum.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Shenoy P., Yu A.J., Li C.S. Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci. 2013;33(5):2039–2047. doi: 10.1523/JNEUROSCI.2201-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Hu S., Zhang S., Yu A.J., Li C.S. Impaired Bayesian learning for cognitive control in cocaine dependence. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide J.S., Zhang S., Hu S., Sinha R., Mazure C.M., Li C.S. Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend. 2014;134:51–62. doi: 10.1016/j.drugalcdep.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K., Yamashita K., Chikazoe J., Hirose S., Miyashita Y., Konishi S. A critical component that activates the left inferior prefrontal cortex during interference resolution. Eur. J. Neurosci. 2009;29:1915–1920. doi: 10.1111/j.1460-9568.2009.06731.x. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K.H., Lukas S.E., Elman I. Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. Am. J. Drug Alcohol Abuse. 2003;29:539–551. doi: 10.1081/ada-120023457. [DOI] [PubMed] [Google Scholar]
- Konishi S., Nakajima K., Uchida I., Kikyo H., Kameyama M., Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.C., Lombardo M.V., Chakrabarti B., Sadek S.A., Pasco G., Wheelwright S.J., Bullmore E.T., Baron-Cohen S., Suckling J. A shift to randomness of brain oscillations in people with autism. Biol. Psychiatry. 2010;68:1092–1099. doi: 10.1016/j.biopsych.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Lepsien J., Pollmann S. Covert reorienting and inhibition of return: an event-related fMRI study. J. Cogn. Neurosci. 2002;14:127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Levina A., Herrmann J.M., Geisel T. Dynamical synapses causing self-organized criticality in neural networks. Nat. Phys. 2007;3:857–860. [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 1971;49(Suppl. 2):467 +. [PubMed] [Google Scholar]
- Li C.S., Lin S.C. Inhibition of return in temporal order saccades. Vis. Res. 2002;42:2089–2093. doi: 10.1016/s0042-6989(02)00123-2. [DOI] [PubMed] [Google Scholar]
- Li C.S., Lin S.C. A perceptual level mechanism of the inhibition of return in oculomotor planning. Brain Res. Cogn. Brain Res. 2002;14:269–276. doi: 10.1016/s0926-6410(02)00129-5. [DOI] [PubMed] [Google Scholar]
- Li C.S., Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci. Biobehav. Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.S., Huang C., Yan P., Bhagwagar Z., Milivojevic V., Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.S., Huang C., Yan P., Paliwal P., Constable R.T., Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J. Cogn. Neurosci. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.S., Chang H.L., Lin S.C. Inhibition of return in children with attention deficit hyperactivity disorder. Exp. Brain Res. 2003;149:125–130. doi: 10.1007/s00221-002-1362-8. [DOI] [PubMed] [Google Scholar]
- Li C.S., Huang C., Constable R.T., Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. NeuroImage. 2006;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li C.S., Huang C., Constable R.T., Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J. Neurosci. Off. J. Soc. Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.S., Luo X., Sinha R., Rounsaville B.J., Carroll K.M., Malison R.T., Ding Y.S., Zhang S., Ide J.S. Increased error-related thalamic activity during early compared to late cocaine abstinence. Drug Alcohol Depend. 2010;109:181–189. doi: 10.1016/j.drugalcdep.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.S., Milivojevic V., Kemp K., Hong K., Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend. 2006;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Li C.S., Yan P., Chao H.H., Sinha R., Paliwal P., Constable R.T., Zhang S., Lee T.W. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience. 2008;155:1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.-S.R., Luo X., Yan P., Bergquist K., Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol. Clin. Exp. Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan G.D. On the Ability to Inhibit Thought and Action: A User's Guide to the Stop Signal Paradigm. In: Dagenbach D., Carr T.H., editors. Inhibitory Processes in Attention, Memory and Language. Academic Press; San Diego: 1994. pp. 189–239. [Google Scholar]
- Logan G.D., Cowan W.B., Davis K.A. On the ability to inhibit simple and choice reaction time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Lopez A., Becona E. Depression and cocaine dependence. Psychol. Rep. 2007;100:520–524. doi: 10.2466/pr0.100.2.520-524. [DOI] [PubMed] [Google Scholar]
- Lowen S.B., Cash S.S., Poo M., Teich M.C. Quantal neurotransmitter secretion rate exhibits fractal behavior. J. Neurosci. Off. J. Soc. Neurosci. 1997;17:5666–5677. doi: 10.1523/JNEUROSCI.17-15-05666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M., Meerkerk G.J., Franken I.H., van de Wetering B.J., Schoenmakers T.M. An fMRI study of cognitive control in problem gamers. Psychiatry Res. 2015;231:262–268. doi: 10.1016/j.pscychresns.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Luijten M., Veltman D.J., Hester R., Smits M., Nijs I.M., Pepplinkhuizen L., Franken I.H. The role of dopamine in inhibitory control in smokers and non-smokers: a pharmacological fMRI study. Eur. Neuropsychopharmacol. 2013;23:1247–1256. doi: 10.1016/j.euroneuro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Imaging cognitive deficits in drug abuse. Curr. Top. Behav. Neurosci. 2010;3:247–275. doi: 10.1007/7854_2009_26. [DOI] [PubMed] [Google Scholar]
- Luo X., Zhang S., Hu S., Bednarski S.R., Erdman E., Farr O.M., Hong K.I., Sinha R., Mazure C.M., Li C.S. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136:1231–1244. doi: 10.1093/brain/awt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Narayana S., Robin D.A., Fox P.T., Xiong J. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. NeuroImage. 2011;58:226–233. doi: 10.1016/j.neuroimage.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Steinberg J.L., Cunningham K.A., Lane S.D., Bjork J.M., Neelakantan H., Price A.E., Narayana P.A., Kosten T.R., Bechara A., Moeller F.G. Inhibitory behavioral control: a stochastic dynamic causal modeling study comparing cocaine dependent subjects and controls. NeuroImage Clin. 2015;7:837–847. doi: 10.1016/j.nicl.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxim V., Sendur L., Fadili J., Suckling J., Gould R., Howard R., Bullmore E. Fractional Gaussian noise, functional MRI and Alzheimer's disease. NeuroImage. 2005;25:141–158. doi: 10.1016/j.neuroimage.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Moeller S.J., Honorio J., Tomasi D., Parvaz M.A., Woicik P.A., Volkow N.D., Goldstein R.Z. Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cereb. Cortex. 2014;24:643–653. doi: 10.1093/cercor/bhs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein-Zamir S., Simon Jones P., Bullmore E.T., Robbins T.W., Ersche K.D. Prefrontal hypoactivity associated with impaired inhibition in stimulant-dependent individuals but evidence for hyperactivation in their unaffected siblings. Neuropsychopharmacology. 2013;38:1945–1953. doi: 10.1038/npp.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morein-Zamir S., Simon Jones P., Bullmore E.T., Robbins T.W., Ersche K.D. Take it or leave it: prefrontal control in recreational cocaine users. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujica-Parodi L.R., Carlson J.M., Cha J., Rubin D. The fine line between ‘brave’ and ‘reckless’: amygdala reactivity and regulation predict recognition of risk. NeuroImage. 2014;103C:1–9. doi: 10.1016/j.neuroimage.2014.08.038. [DOI] [PubMed] [Google Scholar]
- Nedic S., Stufflebeam S.M., Rondinoni C., Velasco T.R., Dos Santos A.C., Leite J.P., Gargaro A.C., Mujica-Parodi L.R., Ide J.S. Using network dynamic fMRI for detection of epileptogenic foci. BMC Neurol. 2015;15:262. doi: 10.1186/s12883-015-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I., Cho S.S., Antonelli F., Houle S., Jahanshahi M., Ko J.H., Strafella A.P. Stimulation of the pre-SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul. 2013;6:769–776. doi: 10.1016/j.brs.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Pas M., Nakamura K., Sawamoto N., Aso T., Fukuyama H. Stimulus-driven changes in the direction of neural priming during visual word recognition. NeuroImage. 2015;125:428–436. doi: 10.1016/j.neuroimage.2015.10.063. [DOI] [PubMed] [Google Scholar]
- Perry R.J., Zeki S. The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain. 2000;123(Pt 11):2273–2288. doi: 10.1093/brain/123.11.2273. [DOI] [PubMed] [Google Scholar]
- Persson J., Larsson A., Reuter-Lorenz P.A. Imaging fatigue of interference control reveals the neural basis of executive resource depletion. J. Cogn. Neurosci. 2013;25:338–351. doi: 10.1162/jocn_a_00321. [DOI] [PubMed] [Google Scholar]
- Radulescu A., Mujica-Parodi L.R. Network connectivity modulates power spectrum scale invariance. NeuroImage. 2014;90:436–448. doi: 10.1016/j.neuroimage.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Radulescu A.R., Rubin D., Strey H.H., Mujica-Parodi L.R. Power spectrum scale invariance identifies prefrontal dysregulation in paranoid schizophrenia. Hum. Brain Mapp. 2012;33:1582–1593. doi: 10.1002/hbm.21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckless G.E., Ousdal O.T., Server A., Walter H., Andreassen O.A., Jensen J. The left inferior frontal gyrus is involved in adjusting response bias during a perceptual decision-making task. Brain Behav. 2014;4:398–407. doi: 10.1002/brb3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte F., Soderberg L., Sowell E. Structural, metabolic, and functional brain abnormalities as a result of prenatal exposure to drugs of abuse: evidence from neuroimaging. Neuropsychol. Rev. 2010;20:376–397. doi: 10.1007/s11065-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D., Fekete T., Mujica-Parodi L.R. Optimizing complexity measures for FMRI data: algorithm, artifact, and sensitivity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E., Aharonovich E., Bisaga A., Levin F.R., Raby W.N., Nunes E.V. Early abstinence in cocaine dependence: influence of comorbid major depression. Am. J. Addict. 2007;16:283–290. doi: 10.1080/10550490701389880. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O., Thivierge J.P., Breakspear M. Neurobiologically realistic determinants of self-organized criticality in networks of spiking neurons. PLoS Comput. Biol. 2011;7 doi: 10.1371/journal.pcbi.1002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speilberger C., Gorsuch R., Lushene R. Consulting Psychologist Press; Palo Alto, CA: 1970. STAI Manual. [Google Scholar]
- Sussner B.D., Smelson D.A., Rodrigues S., Kline A., Losonczy M., Ziedonis D. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83:233–237. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken A.U. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel A., Haupt W.F., Habedank B., Winhuisen L., Herholz K., Kessler J., Markowitsch H.J., Heiss W.D. Neuroimaging-guided rTMS of the left inferior frontal gyrus interferes with repetition priming. NeuroImage. 2005;25:815–823. doi: 10.1016/j.neuroimage.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Tiffany S.T., Singleton E., Haertzen C.A., Henningfield J.E. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Tolkunov D., Rubin D., Mujica-Parodi L. Power spectrum scale invariance quantifies limbic dysregulation in trait anxious adults using fMRI: adapting methods optimized for characterizing autonomic dysregulation to neural dynamic time series. NeuroImage. 2010;50:72–80. doi: 10.1016/j.neuroimage.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M.A., Kuhn S., De Raedt R. ‘Put on your poker face’: neural systems supporting the anticipation for expressive suppression and cognitive reappraisal. Soc. Cogn. Affect. Neurosci. 2013;8:903–910. doi: 10.1093/scan/nss090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F., Chambers C.D., Logan G.D. Fictitious inhibitory differences: how skewness and slowing distort the estimation of stopping latencies. Psychol. Sci. 2013;24:352–362. doi: 10.1177/0956797612457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill G.B., Chen H., Vasudeva R.B. Sequential estimation of quantal response curves: a new method of estimation. Biometrika. 1966;53:439–454. [Google Scholar]
- Winecoff A., Labar K.S., Madden D.J., Cabeza R., Huettel S.A. Cognitive and neural contributors to emotion regulation in aging. Soc. Cogn. Affect. Neurosci. 2011;6:165–176. doi: 10.1093/scan/nsq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.D., Hu S., Li C.S. The influence of risky and conservative mental sets on cerebral activations of cognitive control. Int. J. Psychophysiol. 2012 doi: 10.1016/j.ijpsycho.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P., Li C.S. Decreased amygdala activation during risk taking in non-dependent habitual alcohol users: a preliminary fMRI study of the stop signal task. Am. J. Drug Alcohol Abuse. 2009;35:284–289. doi: 10.1080/00952990902968569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Zhou X. Conflict control during sentence comprehension: fMRI evidence. NeuroImage. 2009;48:280–290. doi: 10.1016/j.neuroimage.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yin X., Zhao L., Xu J., Evans A.C., Fan L., Ge H., Tang Y., Khundrakpam B., Wang J., Liu S. Anatomical substrates of the alerting, orienting and executive control components of attention: focus on the posterior parietal lobe. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J.H. Prentice-Hall, Inc.; New Jersey: 1999. Biostatistical Analysis. [Google Scholar]
- Zhang S., Hu S., Bednarski S.R., Erdman E., Li C.S. Error-related functional connectivity of the thalamus in cocaine dependence. NeuroImage Clin. 2014;4:585–592. doi: 10.1016/j.nicl.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) GLM contrast map (Stop > Go) correlated with PSS for HC. (b) PSSI map correlated with PSS for HC. (c) GLM contrast map (Stop > Go) correlated with PSS for CD. (d) PSSI map correlated with PSS for CD. Hot color: positive correlation, cold color: negative correlation. p < 0.05, corrected AlphaSim threshold.