Abstract
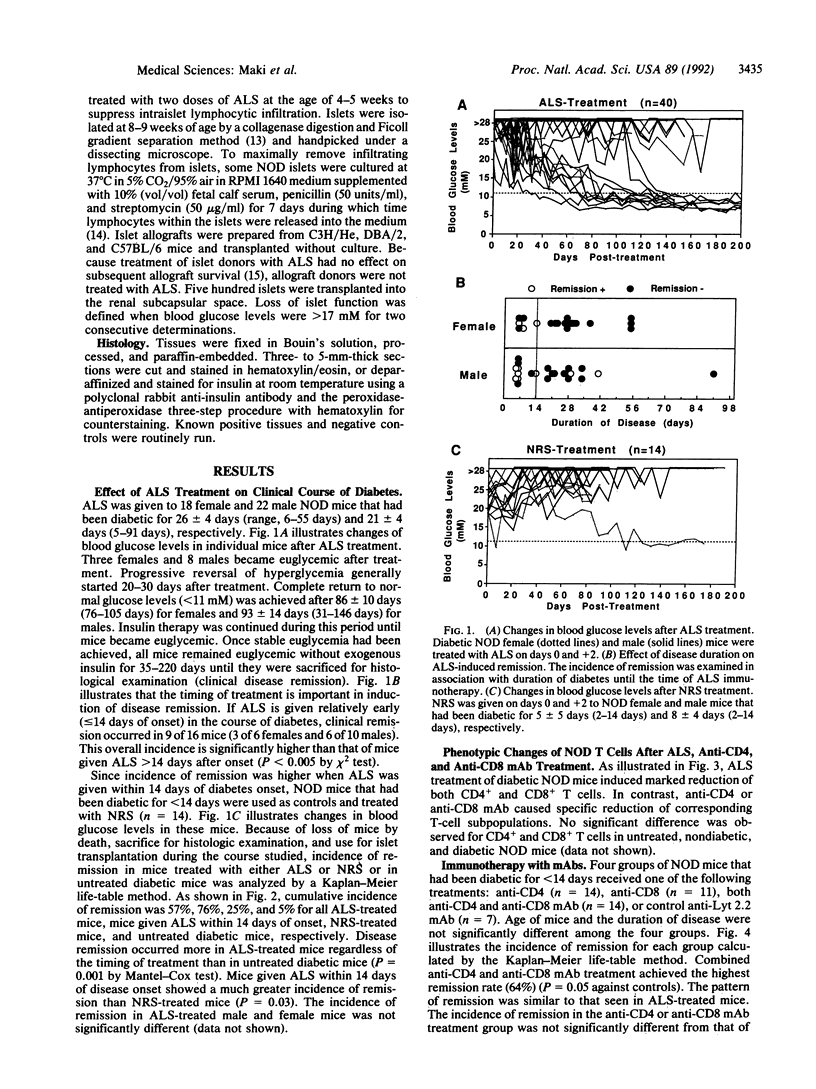
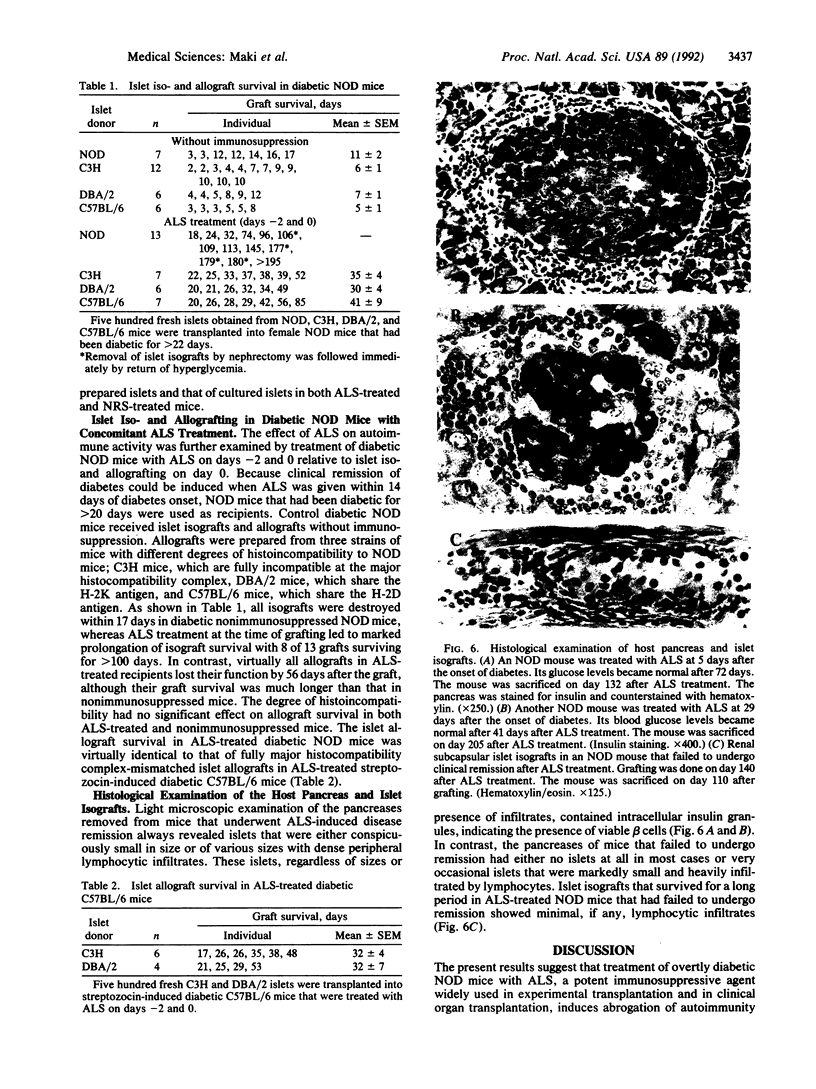
We investigated the therapeutic effect of anti-lymphocyte serum (ALS) on clinically overt diabetes by using a nonobese diabetic (NOD) mouse model of type I diabetes mellitus. ALS given within 14 days of disease onset gradually reversed hyperglycemia with a 76% cumulative incidence of remission. Combined use of anti-CD4 and anti-CD8 monoclonal antibodies, but not anti-CD4 or anti-CD8 antibody alone, was also effective with overall 64% remission. Diabetic NOD mice that failed to respond to ALS treatment accepted subsequent islet isografts for a prolonged period (mostly greater than 100 days), whereas islet isografts in diabetic NOD mice previously treated with normal rabbit serum were all destroyed as acutely as isografts in untreated diabetic NOD mice. These results suggest that persistence of diabetes was due to irreversible beta-cell destruction and that ALS has indeed abrogated autoimmunity. In addition, ALS treatment at the time of islet isografting achieved significant prolongation of graft survival with 8 of 13 mice maintaining euglycemia for greater than 100 days. Although ALS prolonged islet allograft survival in diabetic NOD mice, the degree of prolongation was much less for allografts than for isografts, suggesting that ALS is capable of suppressing autoimmunity more effectively than allograft responses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C., Yasunami R., Dardenne M., Bach J. F. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J Exp Med. 1989 May 1;169(5):1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockenbrough J. S., Weir G. C., Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes. 1988 Feb;37(2):232–236. doi: 10.2337/diab.37.2.232. [DOI] [PubMed] [Google Scholar]
- Charlton B., Bacelj A., Mandel T. E. Administration of silica particles or anti-Lyt2 antibody prevents beta-cell destruction in NOD mice given cyclophosphamide. Diabetes. 1988 Jul;37(7):930–935. doi: 10.2337/diab.37.7.930. [DOI] [PubMed] [Google Scholar]
- Gotoh M., Maki T., Satomi S., Porter J., Bonner-Weir S., O'Hara C. J., Monaco A. P. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987 May;43(5):725–730. doi: 10.1097/00007890-198705000-00024. [DOI] [PubMed] [Google Scholar]
- Gotoh M., Maki T., Satomi S., Porter J., Monaco A. P. Effect of antilymphocyte serum on crude pancreatic islet allograft survival. Transplant Proc. 1987 Feb;19(1 Pt 1):576–577. [PubMed] [Google Scholar]
- Hanafusa T., Sugihara S., Fujino-Kurihara H., Miyagawa J., Miyazaki A., Yoshioka T., Yamada K., Nakajima H., Asakawa H., Kono N. Induction of insulitis by adoptive transfer with L3T4+Lyt2- T-lymphocytes in T-lymphocyte-depleted NOD mice. Diabetes. 1988 Feb;37(2):204–208. doi: 10.2337/diab.37.2.204. [DOI] [PubMed] [Google Scholar]
- Harada M., Makino S. Suppression of overt diabetes in NOD mice by anti-thymocyte serum or anti-Thy 1, 2 antibody. Jikken Dobutsu. 1986 Oct;35(4):501–504. doi: 10.1538/expanim1978.35.4_501. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Shreiber M. Neonatal injection of CD3 antibody into nonobese diabetic mice reduces the incidence of insulitis and diabetes. J Immunol. 1989 Sep 1;143(5):1555–1559. [PubMed] [Google Scholar]
- Ichikawa T., Nakayama E., Uenaka A., Monden M., Mori T. Effector cells in allelic H-2 class I-incompatible skin graft rejection. J Exp Med. 1987 Oct 1;166(4):982–990. doi: 10.1084/jem.166.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T., Itoh Y., Ishii T., Ito I., Takabayashi K., Maruyama N., Tomioka H., Yoshida S. Preventive effect of monoclonal anti-L3T4 antibody on development of diabetes in NOD mice. Diabetes. 1987 Apr;36(4):539–541. doi: 10.2337/diab.36.4.539. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A., Guberski D. L., Appel M. C., Williams R. M. Spontaneous diabetes mellitus: reversal and prevention in the BB/W rat with antiserum to rat lymphocytes. Science. 1979 Dec 21;206(4425):1421–1423. doi: 10.1126/science.388619. [DOI] [PubMed] [Google Scholar]
- Maki T., Gottschalk R., Wood M. L., Monaco A. P. Specific unresponsiveness to skin allografts in anti-lymphocyte serum-treated, marrow-injected mice: participation of donor marrow-derived suppressor T cells. J Immunol. 1981 Oct;127(4):1433–1438. [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Monaco A. P., Wood M. L. Studies on heterologous antilymphocyte serum in mice. VII. Optimal cellular antigen for induction of immunologic tolerance with antilymphocyte serum. Transplant Proc. 1970 Dec;2(4):489–496. [PubMed] [Google Scholar]
- Mori Y., Suko M., Okudaira H., Matsuba I., Tsuruoka A., Sasaki A., Yokoyama H., Tanase T., Shida T., Nishimura M. Preventive effects of cyclosporin on diabetes in NOD mice. Diabetologia. 1986 Apr;29(4):244–247. doi: 10.1007/BF00454884. [DOI] [PubMed] [Google Scholar]
- Reich E. P., Scaringe D., Yagi J., Sherwin R. S., Janeway C. A., Jr Prevention of diabetes in NOD mice by injection of autoreactive T-lymphocytes. Diabetes. 1989 Dec;38(12):1647–1651. doi: 10.2337/diab.38.12.1647. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Mafune K., Maki T. Cloning of self-major histocompatibility complex antigen-specific suppressor cells from adult bone marrow. J Exp Med. 1990 Sep 1;172(3):901–909. doi: 10.1084/jem.172.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Maki T. Prolongation of mouse skin allograft survival by cloned veto suppressor cells. Transplant Proc. 1991 Feb;23(1 Pt 1):192–193. [PubMed] [Google Scholar]
- Wang Y., Hao L., Gill R. G., Lafferty K. J. Autoimmune diabetes in NOD mouse is L3T4 T-lymphocyte dependent. Diabetes. 1987 Apr;36(4):535–538. doi: 10.2337/diab.36.4.535. [DOI] [PubMed] [Google Scholar]




