Abstract
t(8;22)(p11;q11) is a rare but recurrent genetic alteration in various hematological disorders. Patients with t(8;22)(p11;q11) may be misdiagnosed with chronic myelogenous leukemia (CML), due to the similar clinical features. Thus, the current study presents a patient with t(8;22)(p11;q11) who was previously misdiagnosed with CML in the chronic phase. The current patient was a 26-year-old woman who was 4-weeks pregnant and in whom an increased white blood cell count (4.0×1010/l) was found upon physical examination. The patient had no history of hematological disease. Although cytogenetics showed a normal karyotype and no breakpoint cluster region/Abelson murine leukemia viral oncogene homolog 1 (BCR/ABL) fusion gene was detected by reverse transcription-polymerase chain reaction, a diagnosis of chronic myelogenous leukemia (CML) was initially made according to the clinical and morphological features. Another 6 weeks later, t(8;22)(p11;q11) rearrangement was present in 9 out of 10 analyzed metaphases. Fluorescence in situ hybridization and reverse transcription-polymerase chain reaction indicated a negative result for the BCR/ABL fusion, but gave a positive result for the BCR-fibroblast growth factor receptor 1 fusion. A hematological diagnosis of atypical CML was again formed.
Keywords: chronic myelogenous leukemia, BCR-FGFR1
Introduction
Chronic myelogenous leukemia (CML) is characterized by the presence of the Philadelphia chromosome, which is generated by the reciprocal translocation, t(9;22) (1). This abnormality is found in >90% of CML cases, in 15–20% of acute lymphoblastic leukemia cases, and a small number of acute myeloid leukemia cases (1). Recently, certain studies have reported that patients with t(8;22) exhibit similar morphological and clinical features to those observed in CML patients (2–4). Since the first case reported by Fioretos in 2001 (5), to the best of our knowledge, t(8;22)(p11;q11) has been reported in only 17 cases with hematological malignancies (2–17). Of these, 6 cases presented with atypical CML (2–4,6,7), 3 with myeloproliferative neoplasms (5,8,9), 3 with B-cell acute lymphoblastic leukemia (10–12) and 5 with other types of hematological neoplasms (13–17). t(8;22) results in an in-frame fusion of FGFR1 on 8p11 and BCR on 22q11, and causes constitutive activation of the tyrosine kinase of the breakpoint cluster region/fibroblast growth factor receptor 1 (BCR/FGFR1) chimera protein, similar to Abelson murine leukemia viral oncogene homolog 1 (ABL) kinase activity in the BCR/ABL chimera (2,18).
The present study reports the 18th case of a CML-like hematological tumor bearing t(8;22)(p11;q11) with no other cell lineages involved.
Case report
On February 23, 2013, a 26-year-old woman who was 4-weeks pregnant, with no history of hematological disease, was found to exhibit an increased white blood cell (WBC) count (4.0×1010/l; normal range, 0.37–0.92×1010/l) upon physical examination. Cytogenetic study on the patient's bone marrow (BM) cells showed a normal female karyotype in all metaphases. Tests for the BCR/ABL fusion gene and the Janus kinase 2 (JAK2)/Val617Phe mutation were negative. Based on the morphological examination of the BM, the patient was diagnosed with CML in Jiangsu Province People's Hospital (Nanjing, China).
On April 9, 2013, the patient was transferred to Shanghai First People's Hospital (Shanghai, China). Apart from general fatigue, no other clinical symptoms were apparent. Laboratory data were as follows: Hemoglobin (Hb), 120 g/l (normal range, 113–151 g/l); hematocrit, 33.4% (normal range, 35–47%); WBC count, 3.46×109/l (normal range, 0.37–0.92×109/l); and platelet (Plt) count, 233×109/l (normal range, 100–400×109/l). The BM differential count was as follows: 3% blasts (normal range, 0.3–2.0%), 2% promyelocytes (normal range, 1–8%), 18% neutrophilic myelocytes (normal range, 5–20%), 16% neutrophilic metamyelocytes (normal range, 9–18%), 9% banded neutrophils (normal range, 4–14%), 15% segmented neutrophils (normal range, 7–30%), 13% eosinophils (normal range, 0.5–4.0%), 2% basophils (normal range, 0–1%), 10% erythrocytes (normal range, 18.5–39.0%), 7% monocytes (normal range, 0.5–5.0%) and 5% lymphocytes (normal range, 3–20%) (Fig. 1). The myeloid and erythroid ratio was increased to 7.8:1 (normal range, 3–5:1), and the neutrophil alkaline phosphatase score was 4 (normal range, 35–70). No other cell lineages were proved to be involved, as determined by multicolor flow cytometry (FCM).
Figure 1.
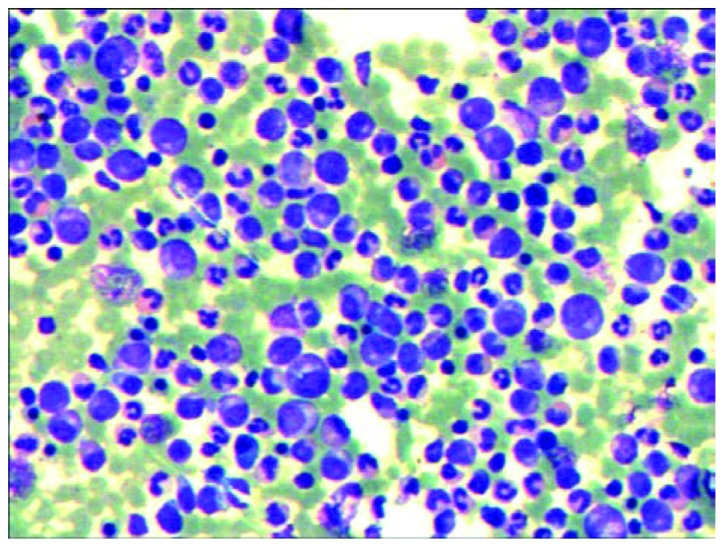
Bone marrow aspirate showing a chronic myelogenous leukemia-like image.
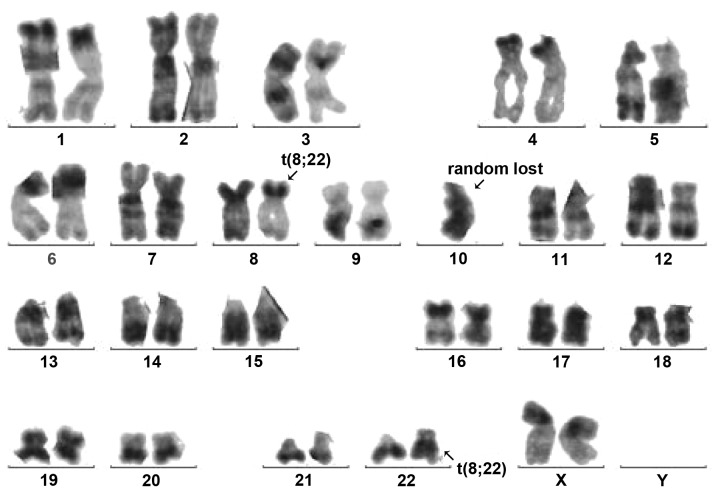
Conventional R-banding analysis of the BM cells showed 46,XX,t(8;22)(p11;q11) in 9 out of 10 metaphases (Fig. 2). Transcripts of BCR/ABL p210 and p190 were all negative. Using the LSI BCR/ABL Dual-Color Dual-Fusion Translocation Probe (Vysis, Downers Grove, IL, USA), fluorescence in situ hybridization indicated a negative result for the BCR-ABL fusion, but the BCR probe displayed a split signal, which suggested that the BCR gene was disrupted (Fig. 3). Reverse transcription-polymerase chain reaction using primers specific for BCR exon 4 and FGFR1 exon 9 [BCR-E1+ and FGFR9− (2)] confirmed the presence of BCR/FGFR1 transcripts at the molecular level. This result was confirmed by direct sequencing of the amplified fragment. The diagnosis of atypical CML was formed once again and hydroxyurea therapy (3 g, daily, for 3 weeks) was started. However, the patient underwent an abortion 2 weeks later and returned home to receive further therapy. The patient was experiencing progression-free survival with a partial hematological response at the time of publication.
Figure 2.
Karyotype depicting a t(8;22) translocation, as highlighted by the arrows.
Figure 3.
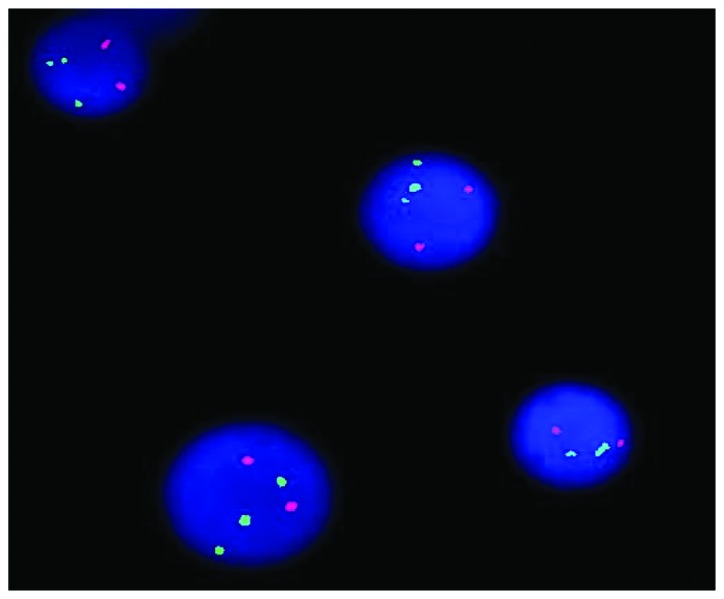
Fluorescence in situ hybridization demonstrating a split breakpoint cluster region(22q11) signal on chromosome 22 in 164/200 cells.
Discussion
To date, the recurrent t(8;22)(p11;q11) translocation, which results in a BCR/FGFR1 fusion, has been reported in 17 patients (2–17), including 16 adult patients (2–8,10–17) and 1 pediatric patient (9); 10 females and 7 males. Of these patients, 14 (82.4%) were >50 years old. Laboratory data were not available in 2 out of 17 cases (6,8). Of the 15 patients with laboratory data, 13 patients showed an increased WBC count (median, 5.56×1010/l; range, 1.84–19.8×1010/l), while the remaining 2 displayed a normal and a decreased WBC count of 5.1×109/l (13) and 1.5×109/l (16), respectively. Among the 15 cases, 10 displayed a decreased Hb level (median, 108 g/l; range, 72–131 g/l) (4,5,9–11,13–17), and the majority of these cases exhibited a marginal decrease in Hb levels. While 2 out of the 15 patients showed increased Plt levels (9,13), decreased levels were observed in 4 cases (11,14–16) and the remaining 9 displayed normal levels.
The clinical features reported in the 6 atypical CML cases with t(8;22) (2–4,6,7) included an older age, systemic symptoms (fatigue, night sweats and weight loss) and splenomegaly. In the present case, however, no clinical symptoms were observed upon physical examination and the patient had been in fair health. In agreement with the previously described 6 cases, the morphological picture of the present case was indistinguishable from typical CML. In the present case, and also in the previously reported 6 cases, no other additional chromosomal abnormalities were observed except for t(8;22). FCM also did not show any positive immunophenotype in the present and previous cases. These results highlight the importance of cytogenetic and molecular analysis in patients that present with features of atypical CML. Subsequently, detection of the genes involved in tyrosine kinase pathways may become an increasingly important feature of diagnosis.
The clinical features of the remaining 11 cases are heterogeneous. Among these cases, 9 exhibited a complex karyotype. The immunophenotypic data of 6 patients had not been described in the literature (2,3,6,8,9,13). A total of 10 cases exhibited aberrant proliferation of myeloid and B-lymphoid cells, and T-lymphoid cells were involved in 1 case (14). Additional chromosomal abnormalities may explain the observed heterogeneity with regard to the clinical features and affected lineages in these patients. These reported cases suggest that t(8;22) usually presents as CML-like disease, however, it may also present as AML, T or B lymphoblastic lymphoma/leukemia or a mixed phenotype acute leukemia. Furthermore, we hypothesize that the multiphenotypic nature of the disease indicates that the disease may originate from early progenitor cells, which retain the potential for both myeloid and lymphoid differentiation. Further identification and characterization are required to elucidate the possible molecular mechanisms underlying the disease.
In conclusion, the current study presented a case of a CML-like patient with t(8;22)(p11;q11) translocation. Detection of this translocation at diagnosis may become increasingly important, considering the recent promising development of tyrosine kinase inhibitory agents.
References
- 1.Wong S, Witte ON. The BCR-ABL story: Bench to bedside and back. Annu Rev Immunol. 2004;22:247–306. doi: 10.1146/annurev.immunol.22.012703.104753. [DOI] [PubMed] [Google Scholar]
- 2.Demiroglu A, Steer EJ, Heath C, Taylor K, Bentley M, Allen SL, Koduru P, Brody JP, Hawson G, Rodwell R, et al. The t(8;22) in chronic myeloid leukemia fuses BCR to FGFR1: Transforming activity and specific inhibition of FGFR1 fusion proteins. Blood. 2001;98:3778–3783. doi: 10.1182/blood.V98.13.3778. [DOI] [PubMed] [Google Scholar]
- 3.Pini M, Gottardi E, Scaravaglio P, Giugliano E, Libener R, Baraldi A, Muzio A, Cornaglia E, Saglio G, Levis A. A fourth case of BCR-FGFR1 positive CML-like disease with t(8;22) translocation showing an extensive deletion on the derivative chromosome 8p. Hematol J. 2002;3:315–316. doi: 10.1038/sj.thj.6200201. [DOI] [PubMed] [Google Scholar]
- 4.Murati A, Arnoulet C, Lafage-Pochitaloff M, Adélaide J, Derré M, Slama B, Delaval B, Popovici C, Vey N, Xerri L, et al. Dual lympho-myeloproliferative disorder in a patient with t(8;22) with BCR-FGFR1 gene fusion. Int J Oncol. 2005;26:1485–1492. [PubMed] [Google Scholar]
- 5.Fioretos T, Panagopoulos I, Lassen C, Swedin A, Billström R, Isaksson M, Strömbeck B, Olofsson T, Mitelman F, Johansson B. Fusion of the BCR and the fibroblast growth factor receptor-1 (FGFR1) genes as a result of t(8;22)(p11;q11) in a myeloproliferative disorder: The first fusion gene involving BCR but not ABL. Genes Chromosomes Cancer. 2001;32:302–310. doi: 10.1002/gcc.1195. [DOI] [PubMed] [Google Scholar]
- 6.Agerstam H, Lilljebjörn H, Lassen C, Swedin A, Richter J, Vandenberghe P, Johansson B, Fioretos T. Fusion gene-mediated truncation of RUNX1 as a potential mechanism underlying disease progression in the 8p11 myeloproliferative syndrome. Genes Chromosomes Cancer. 2007;46:635–643. doi: 10.1002/gcc.20442. [DOI] [PubMed] [Google Scholar]
- 7.Richebourg S, Theisen O, Plantier I, Parry A, Soenen-Cornu V, Lepelley P, Preudhomme C, Renneville A, Laï JL, Roche-Lestienne C. Chronic myeloproliferative disorder with t(8;22)(p11;q11) can mime clonal cytogenetic evolution of authentic chronic myelogeneous leukemia. Genes Chromosomes Cancer. 2008;47:915–918. doi: 10.1002/gcc.20588. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik MM, Gangat N, Knudson RA, Keefe JG, Hanson CA, Pardanani A, Ketterling RP, Tefferi A. Chromosome 8p11.2 translocations: Prevalence, FISH analysis for FGFR1 and MYST3 and clinicopathologic correlates in a consecutive cohort of 13 cases from a single institution. Am J Hematol. 2010;85:238–242. doi: 10.1002/ajh.21631. [DOI] [PubMed] [Google Scholar]
- 9.Dolan M, Cioc A, Cross NC, Neglia JP, Tolar J. Favorable outcome of allogeneic hematopoietic cell transplantation for 8p11 myeloproliferative syndrome associated with BCR-FGFR1 gene fusion. Pediatr Blood Cancer. 2012;59:194–196. doi: 10.1002/pbc.23404. [DOI] [PubMed] [Google Scholar]
- 10.Baldazzi C, Iacobucci I, Luatti S, Ottaviani E, Marzocchi G, Paolini S, Stacchini M, Papayannidis C, Gamberini C, Martinelli G, et al. B-cell acute lymphoblastic leukemia as evolution of a 8p11 myeloproliferative syndrome with t(8;22)(p11;q11) and BCRFGFR1 fusion gene. Leuk Res. 2010;34:e282–e285. doi: 10.1016/j.leukres.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Wakim JJ, Tirado CA, Chen W, Collins R. t(8;22)/BCR-FGFR1 myeloproliferative disorder presenting as B acute lymphoblastic leukemia: Report of a case treated with sorafenib and review of the literature. Leuk Res. 2011;35:e151–e153. doi: 10.1016/j.leukres.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Haslam K, Langabeer SE, Kelly J, Coen N, O'Connell NM, Conneally E. Allogeneic hematopoietic stem cell transplantation for a BCR-FGFR1 myeloproliferative neoplasm presenting as acute lymphoblastic leukemia. Case Rep Hematol. 2012;2012:620967. doi: 10.1155/2012/620967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SG, Park TS, Lee ST, Lee KA, Song J, Kim J, Suh B, Choi JR, Park R. Rare translocations involving chromosome band 8p11 in myeloid neoplasms. Cancer Genet Cytogenet. 2008;186:127–129. doi: 10.1016/j.cancergencyto.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Kim SY, Oh B, She CJ, Kim HK, Jeon YK, Shin MG, Yoon SS, Lee DS. 8p11 myeloproliferative syndrome with BCR-FGFR1 rearrangement resenting with T-lymphoblastic lymphoma and bone marrow stromal cell proliferation: A case report and review of the literature. Leuk Res. 2011;35:e30–e34. doi: 10.1016/j.leukres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Morishige S, Oku E, Takata Y, Kimura Y, Arakawa F, Seki R, Imamura R, Osaki K, Hashiguchi M, Yakushiji K, et al. A Case of 8p11 myeloproliferative syndrome with BCR-FGFR1 gene fusion presenting with trilineage acute leukemia/lymphoma, successfully treated by cord blood transplantation. Acta Haematol. 2013;129:83–89. doi: 10.1159/000341289. [DOI] [PubMed] [Google Scholar]
- 16.Shimanuki M, Sonoki T, Hosoi H, Watanuki J, Murata S, Mushino T, Kuriyama K, Tamura S, Hatanaka K, Hanaoka N, Nakakuma H. Acute leukemia showing t(8;22)(p11;q11), myelodysplasia, CD13/CD33/CD19 expression and immunoglobulin heavy chain gene rearrangement. Acta Haematol. 2013;129:238–242. doi: 10.1159/000345727. [DOI] [PubMed] [Google Scholar]
- 17.Matikas A, Tzannou I, Oikonomopoulou D, Bakiri M. A case of acute myelogenous leukaemia characterised by the BCR-FGFR1 translocation. BMJ Case Rep. 2013;2013:bcr2013008834. doi: 10.1136/bcr-2013-008834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roumiantsev S, Krause DS, Neumann CA, Dimitri CA, Asiedu F, Cross NC, Van Etten RA. Distinct stem cell myeloproliferative/T lymphoma syndromes induced by ZNF198-FGFR1 and BCR-FGFR1 fusion genes from 8p11 translocations. Cancer Cell. 2004;5:287–298. doi: 10.1016/S1535-6108(04)00053-4. [DOI] [PubMed] [Google Scholar]