Abstract
The concentrations required for curcumin to exert its anticancer activity (IC50, 20 µM) are difficult to achieve in the blood plasma of patients, due to the low bioavailability of the compound. Therefore, much effort has been devoted to the development of curcumin analogues that exhibit stronger anticancer activity and a lower IC50 than curcumin. The present study investigated twelve pyridine analogues of curcumin, labeled as groups AN, BN, EN and FN, to determine their effects in CWR-22Rv1 human prostate cancer cells. The inhibitory effects of these compounds on testosterone (TT)-induced androgen receptor (AR) activity was determined by performing an AR-linked luciferase assay and by TT-induced expression of prostate-specific antigen. The results of the current study suggested that the FN group of analogues had the strongest inhibitory effect of growth on CWR-22Rv1 cultured cells, and were the most potent inhibitor of AR activity compared with curcumin, and the AN, BN and EN analogues. Thus, the results of the present study indicate the inhibition of the AR pathways as a potential mechanism for the anticancer effect of curcumin analogues in human prostate cancer cells. Furthermore, curcumin analogues with pyridine as a distal ring and tetrahydrothiopyran-4-one as a linker may be good candidates for the development of novel drugs for the treatment of prostate cancer, by targeting the AR signaling pathway.
Keywords: prostate cancer, curcumin analogues, androgen receptor
Introduction
Prostate cancer is the second most common cause of cancer-related mortality in American men. The androgen receptor (AR) is a ligand-activated steroid hormone receptor that regulates normal prostate development and function (1). It is also critical in the development and progression of prostate cancer (2). Current therapeutic strategies for prostate cancer, such as androgen ablation therapy, inhibit AR function (3). A combination of androgen synthesis suppression and AR inhibition may be used as a more aggressive form therapy (4). Therefore, identification of the chemical agents and mechanisms that inhibit AR signaling warrant further investigation for the development of novel prostate cancer therapeutics.
Curcumin is a non-nutritive yellow pigment found in turmeric, a rhizome-derivative of the plant Curcuma longa Linn. Numerous studies have demonstrated the anticancer activity of curcumin and curcumin analogues in animal models (5–10), as well as the effects on cell growth and apoptosis in vitro (11–19). However, it should be noted that the clinical efficacy of curcumin is limited, possibly due to its low bioavailability (20–22).
In our previous study, we synthesized a series of 12 pyridine analogues of curcumin with cyclohexanone, cyclopentanone, tetrahydropyran-4-one or tetrahydrothiopyran-4-one linkers, and determined their anticancer activities in cultured human cancer cells (23). It was determined that these pyridine analogues exhibited stronger inhibitory effects than curcumin on the growth of a number of human cancer cell lines, including human prostate cancer PC-3 cells (23). Therefore, the aim of the present study was to investigate the effects and mechanisms of several pyridine curcumin analogues in CWR-22Rv1 human prostate cancer cells, as CWR-22Rv1 cells exhibit ARs and thus were considered suitable to investigate AR activity. Group FN showed a stronger inhibitory effect than groups AN, BN and EN on human prostate cancer cell growth. We also demonstrated that group FN was a greater inhibitor of AR activity and testosterone (TT)-induced prostate-specific antigen (PSA) expression than groups AN, BN, EN and curcumin in CWR-22Rv1 cells.
Materials and methods
Chemistry
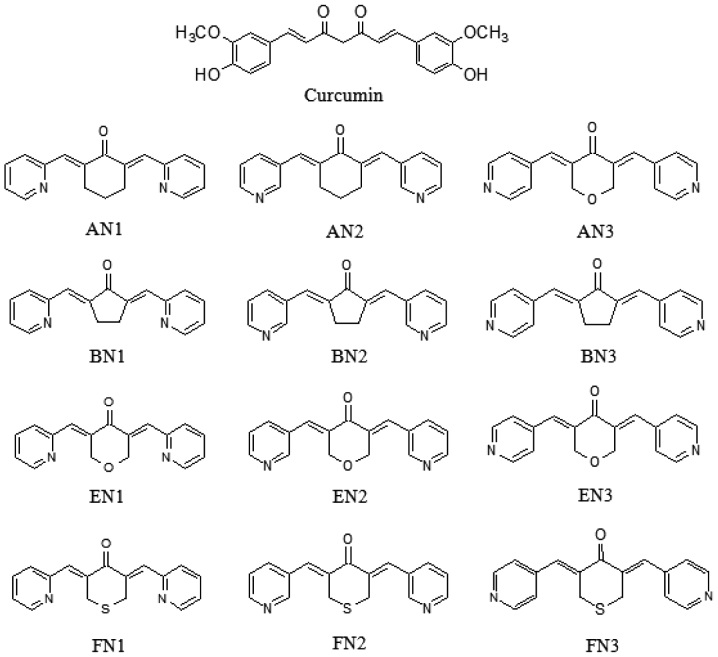
Twelve pyridine analogues of curcumin (Fig. 1) were synthesized by coupling the appropriate substituted benzaldehyde with cyclohexanone, cyclopentanone, tetrahydropyran-4-one or tetrahydrothiopyran-4-one, as previously described (23). Characterization of the compounds (2E,6E)-2,6-bis(pyridin-2/3/4-methylene)cyclohexanone (AN1/2/3), (2E,5E)-2,5-bis(pyridin-2/3/4-methylene)cyclopentanone (BN1/2/3), (3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydropyran-4-one (EN1/2/3) and (3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydrothiopyran-4-one (FN1/2/3) was previously described in detail (23).
Figure 1.
Structures of curcumin and its analogues. AN1/2/3, (2E,6E)-2,6-bis(pyridin-2/3/4-methylene)cyclohexanone; BN1/2/3, (2E,5E)-2,5-bis(pyridin-2/3/4-methylene)cyclopentanone; EN1/2/3, (3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydropyran-4-one; FN1/2/3, (3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydrothiopyran-4-one.
Cell culture and reagents
CWR-22Rv1 cells were acquired from the American Type Culture Collection (Rockville, MD, USA). RPMI-1640 tissue culture medium, penicillin-streptomycin, L-glutamine and fetal bovine serum (FBS) were from Gibco (Thermo Fisher Scientific, Waltham, MA, USA). CWR-22Rv1 cells were maintained in RPMI-1640 culture medium, and the medium was supplemented with 10% FBS, penicillin (100 U/ml)-streptomycin (100 µg/ml) and L-glutamine (300 µg/ml). Cells were grown at 37°C in a humidified atmosphere of 5% CO2 and were passaged two times per week. Analogues were dissolved in dimethylsulfoxide (DMSO; concentration, 100%; Sigma-Aldrich, St. Louis, MO, USA). A final concentration of 0.1% DMSO was used in all experiments.
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay
CWR-22Rv1 cells were seeded at a density of 2×104 cells/ml of medium in a 96-well plate (0.2 ml/well) and incubated for 24 h. The cells were then treated with various concentrations (0.5, 1, 2 and 5µM) of curcumin analogues for 72 h. After treatment, 5 mg/ml MTT (Sigma-Aldrich) was added to each well of the plate and incubated for 1 h. After careful removal of the medium, 0.1 ml DMSO was added to each well and absorbance was recorded on a microplate reader (Infinite® 200 PRO; Tecan, Männedorf, Switzerland) at 550 nm. The number of viable cells after each treatment was determined using a hemacytometer (Bright-line #1475; Thermo Fisher Scientific) under a light microscope (BH-2; Olympus Corporation, Tokyo, Japan). Cell viability was determined by performing a trypan blue exclusion assay, as follows: 80 µl of cell suspension was mixed with 20 µl of 0.4% trypan blue stain solution (Sigma-Aldrich) for 2 min. Blue cells were counted as dead cells and the cells that did not absorb dye were counted as live cells.
AR luciferase reporter assay
AR transcriptional activity was measured by performing an AR luciferase reporter gene expression assay. An AR luciferase construct was stably transfected into CWR-22Rv1 cells to generate a single stable clone, CWR-22Rv1/AR, which was used in the present study. Briefly, CWR22-Rv-1 cells cultured in 10% FBS RPMI-1640 medium were infected with lentivirus carrying Cignal Lenti AR reporter (catalog no. CLS-8019L; Qiagen, Inc., Valencia, CA, USA) in medium containing 8 µg/ml Polybrene (Sigma-Aldrich). Following incubation for 6 h, the culture medium was replaced with fresh 10% RPMI-1640 medium. Cells expressing stable AR luciferase reporter were selected using puromycin (5 µg/ml) three days after infection for 1 week. Selected cells (CWR-22Rv1/AR) were then used for the reporter assay for AR activity.
CWR-22Rv1/AR cells were seeded at a density of 0.1×105 cells/ml of medium for 24 h. Then the medium was changed to RPMI-1640 without FBS, and the cells were treated with DMSO solvent as the vehicle (control) or with TT (100 nM; Sigma-Aldrich) alone or in combination with curcumin and curcumin analogues (1 µM) for 24 h. The luciferase activities were measured using luciferase assay kits from Promega Corporation (Madison, WI, USA; catalog no., E1531). Briefly, the treated cells were washed with ice-cold phosphate-buffered saline (PBS; Gibco; Thermo Fisher Scientific, Inc.), harvested in reporter lysis buffer (Thermo Fisher Scientific, Inc.) and centrifuged for 5 min at 193 × g. Aliquots (10 µl) of the supernatants were measured for luciferase activity using a luminometer (Multiskan FC; Thermo Fisher Scientific). The luciferase activity was normalized against protein concentration and expressed as percent of luciferase activity in the control cells. The protein concentration levels were determined using protein assay reagents (Reagent B, catalog no., 500-0114; Reagent A, catalog no., 500-0113; Reagent S, catalog no., 500-0115; Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the manufacturer's protocol.
Western blot analysis
CWR-22Rv1 cells were seeded in 100-mm culture dishes (10 ml/dish) at a density of 1×105 cells/ml medium and incubated for 24 h. The medium was changed to RPMI-1640 without FBS, and the cells were then treated with vehicle, 100 nM TT alone or together with 1 µM curcumin, AN1, BN1, EN1 or FN1 for 24 h. Treated CWR-22Rv1 cells were washed with ice-cold PBS and lysed with 800 µl lysis buffer (10 mM Tris-HCl, pH 8.0, 10 mM EDTA, 150 mM sodium chloride, 1% NP-40, 0.5% SDS, in deionized water). The resulting homogenates were centrifuged at 193 × g for 15 min at 4°C. The protein concentration of whole cell lysates was determined using a protein assay kit (Bio-Rad Laboratories, Inc.). Equal amounts (50 µg) of protein were then resolved on a 10% Criterion precast gel (Bio-Rad Laboratories, Inc.) and transferred to a PVDF membrane using a semi-dry transfer system. The membrane was then probed with a mouse anti-human monoclonal PSA primary antibody (dilution, 1:10,000; catalog no., CBL252; EMD Millipore, Billerica, MA, USA). Following hybridization with primary antibody, the membrane was washed with Tris-buffered saline (Alfa Aesar, Haverhill, MA, USA) three times, then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (dilution, 1:5,000; catalog no., sc-2055; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and again washed with Tris-buffered saline three times. Final detection was performed with enhanced chemiluminescent reagents (Thermo Fisher Scientific, Inc.). The extent of protein loading was determined by blotting for β-actin (mouse anti-human monoclonal antibody; dilution, 1:1,000–5,000; catalog no., sc-8432; Santa Cruz Biotechnology, Inc.). The membrane was incubated in stripping buffer (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl, pH 6.7) at 50°C for 30 min with occasional agitation prior to incubation in blocking buffer and re-probing using anti-β-actin antibody (Santa Cruz Biotechnology, Inc.).
Statistical analysis
A comparative analysis of the TT-induced activation of curcumin and its analogues was based on a repeated measurement model. The effects of the treatments were assessed by comparing the rates of change over time between treatment groups (comparing the slopes between treatment groups). The analysis of variance (ANOVA) method with the Tukey-Kramer test was used to compare effects among the different treatment groups at study completion. All data analyses were performed using GraphPad InState (GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as mean ± standard error of the mean. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of curcumin analogues on CWR-22Rv1 cell growth
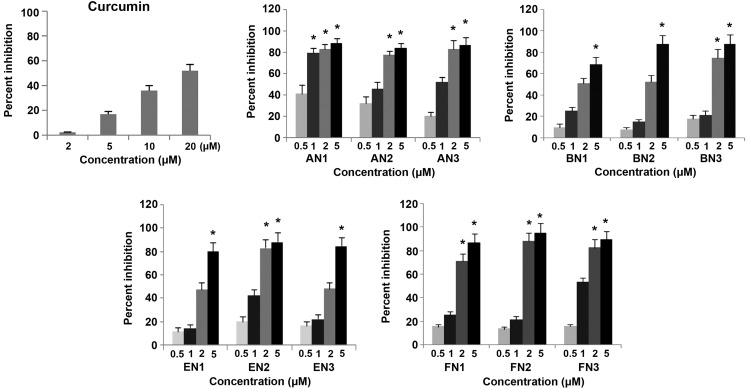
The inhibitory effects of curcumin analogues on the growth of cultured CWR-22Rv1 cells are presented in Fig. 2. All analogous compounds demonstrated a stronger inhibitory effect than curcumin (IC50=16.99 µM) (24) as determined by MTT assay. Among the series of four pyridine analogues of curcumin analyzed in the present study, the FN and AN groups exhibited the most potent inhibitory effects on the growth of cultured CWR-22Rv1 cells. The IC50 values for the FN and AN groups were <1 µM in CWR-22Rv1 cells, indicating that these compounds were ~20-fold more active than curcumin (IC50=16.99 µM). The IC50 values of the series of four pyridine analogues of curcumin ranged between 0.49 and 4.99 µM, as indicated in Table I. Statistical analysis using ANOVA demonstrated that the IC50 for the curcumin analogues were significantly lower than that of curcumin (P<0.001).
Figure 2.
Inhibitory effects of curcumin analogues on the growth of the CWR-22Rv1 prostate cancer cell line. CWR-22Rv1 cells were seeded at a density of 2×104 cells/ml medium in 96-well plates (0.2 ml/well) and incubated for 24 h. The cells were then treated with various concentrations (0.5, 1, 2 and 5µM) of different curcumin and its analogues for 72 h. Effects of the different compounds on the growth of CWR-22Rv1 cells were determined by performing a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay. Values are presented as the mean ± standard error of the mean from three separate experiments. *P<0.001 for 20 µM CUR (IC50) vs. AN, BN, EN and FN. AN1/2/3, (2E,6E)-2,6-bis(pyridin-2/3/4-methylene)cyclohexanone; BN1/2/3, (2E,5E)-2,5-bis(pyridin-2/3/4-methylene)cyclopentanone; EN1/2/3, (3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydropyran-4-one; FN1/2/3, (3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydrothiopyran-4-one.
Table I.
Inhibitory effects of curcumin and its analogues on the growth of CWR-22Rv1 cells.
| Compound | IC50, µM | P-value |
|---|---|---|
| Curcumin | 16.99±2.1 | 0.00092 |
| AN1 | 0.53±0.1 | 0.00065 |
| AN2 | 0.92±0.1 | 0.00071 |
| AN3 | 0.95±0.2 | 0.00065 |
| BN1 | 4.75±0.5 | 0.00095 |
| BN2 | 4.99±0.5 | 0.00096 |
| BN3 | 3.03±0.4 | 0.00083 |
| EN1 | 2.18±0.2 | 0.00079 |
| EN2 | 1.07±0.1 | 0.00080 |
| EN3 | 1.80±0.2 | 0.00082 |
| FN1 | 0.66±0.1 | 0.00072 |
| FN2 | 0.55±0.1 | 0.00073 |
| FN3 | 0.49±0.1 | 0.00061 |
CWR-22Rv1 prostate cancer cells were seeded at a density of 2×104 cells/ml of medium in 96-well plates (0.2 ml/well) and incubated for 24 h. The cells were then treated with various concentrations (0.5–30 µM) of curcumin and its analogues for 72 h. Effects of the different compounds on the growth of CWR-22Rv1 cells were determined by performing a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay. Values are presented as the mean ± standard error of the mean from three separate experiments. IC50, half maximal inhibitory concentration; AN1/2/3, (2E,6E)-2,6-bis(pyridin-2/3/4-methylene)cyclohexanone; BN1/2/3, (2E,5E)-2,5-bis(pyridin-2/3/4-methylene)cyclopentanone; EN1/2/3, (3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydropyran-4-one; FN1/2/3, (3E,5E)-3,5-bis(pyridin-2/3/4-methylene)-tetrahydrothiopyran-4-one.
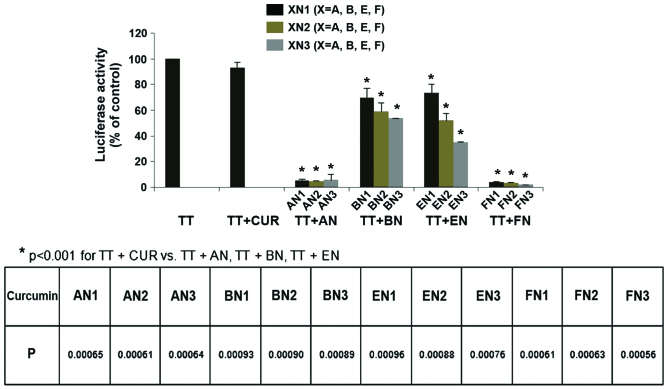
Effects of curcumin and its analogues on AR activity in CWR-22Rv1/AR cells
An AR-luciferase reporter gene expression assay was performed in CWR-22Rv1/AR cells to determine the effect of curcumin and its analogues on TT-induced activation of AR. Cultured CWR-22Rv1/AR cells were treated with a combination of TT and a specific curcumin analogue (1 µM) for 24 h. A marginal inhibitory effect on the TT-induced increase in AR activity was observed in cultured CWR-22Rv1/AR cells treated with 1 µM curcumin, or the BN or EN groups, while greater inhibitory effects were observed in CWR-22Rv1/AR cells treated with 1 µM of the AN or FN groups (Fig. 3). Statistical analysis using ANOVA with Tukey's multiple comparison tests demonstrated that AR activity was significantly lower in cells treated with AN and FN compounds than in cells treated with curcumin, BN and EN compounds (P<0.001 for: TT vs. TT + AN, TT + BN, TT + EN or TT + FN; TT + CUR vs. TT + AN, TT + BN, TT + EN or TT + FN; TT + AN vs. TT + BN, TT + EN or TT + FN; and TT + BN vs. TT + EN or TT + FN.
Figure 3.
Effect of CUR analogues on TT-induced increases in androgen receptor reporter activity in CWR-22Rv1/AR cells. CWR-22Rv1/AR cells were seeded at a density of 0.1×105 cells/ml of medium for 24 h. Then the medium was changed to RPMI-1640 without fetal bovine serum, and the cells were treated with vehicle (control) or TT (100 nM) alone or in combination with CUR and CUR analogues (1 µM) for 24 h. Luciferase activity and protein concentration were measured to determine androgen receptor reporter activity in the CWR-22Rv1/AR cells. Values are presented as the mean ± standard error of the mean from three separate experiments. TT, testosterone; CUR, curcumin; AN, (2E,6E)-2,6-bis(pyridin-n-methylene)cyclohexanone; BN, (2E,5E)-2,5-bis(pyridin-n-methylene)cyclopentanone; EN, (3E,5E)-3,5-bis(pyridin-n-methylene)-tetrahydropyran-4-one; FN, (3E,5E)-3,5-bis(pyridin-n-methylene)-tetrahydrothiopyran-4-one.
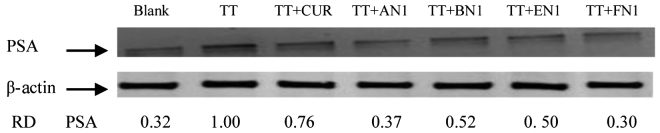
Effects of curcumin and its analogues on protein expression of PSA in CWR-22Rv1 cells
PSA protein levels were evaluated by western blot analysis using an anti-PSA antibody on cultured CWR-22Rv1 cells treated with TT, curcumin and curcumin analogues AN1, BN1, EN1 or FN1 for 24 h. Treatment of CWR-22Rv1 cells with AN1 and FN1 resulted in a strong decrease in the level of PSA compared with TT treatment alone, while the other compounds (curcumin, BN1 and EN1) were less active (Fig. 4). The current results indicate that the effects of AN1 and FN1 on CWR-22Rv1 cells are associated with a decrease in PSA protein expression in TT-induced cells.
Figure 4.
Effect of curcumin and its analogues on TT-induced increases in PSA formation in CWR-22Rv1 cells. CWR-22Rv1 cells were seeded at a density of 1×105 cells/ml medium in 100-mm culture dishes (10 ml/dish) and incubated for 24 h. The medium was changed to RPMI-1640 without fetal bovine serum, and the cells were then treated with vehicle, or 100 nM TT alone or in combination with 1 µM curcumin, AN1, BN1, EN1 or FN1 for 24 h. PSA expression was determined by western blot analysis with anti-PSA antibody. The extent of protein loading was determined by blotting for β-actin, and the levels of PSA in western blots were analyzed by optical density measurements and normalized for β-actin to obtain the RD for the various samples. RD values represent individual selected bands. Representative blots from three experiments are shown. PSA, prostate-specific antigen; TT, testosterone; AN, (2E,6E)-2,6-bis(pyridin-n-methylene)cyclohexanone; BN, (2E,5E)-2,5-bis(pyridin-n-methylene)cyclopentanone; EN, (3E,5E)-3,5-bis(pyridin-n-methylene)-tetrahydropyran-4-one; FN, (3E,5E)-3,5-bis(pyridin-n-methylene)-tetrahydrothiopyran-4-one; RD, relative optical density.
Discussion
Our previous study reported the synthesis and evaluation of five series of curcumin-related compounds (a total of 61 compounds) for their inhibitory effects on cultured prostate, pancreatic and colon cancer cells (23). The present study focused on pyridine analogues of curcumin, which are a subset of active compounds from our previous study. The present study provides the first evidence that pyridine analogues of curcumin inhibit AR activity in prostate cancer cells cultured in vitro.
The twelve pyridine analogues of curcumin (AN, BN, EN and FN groups) exhibited stronger anticancer activities than curcumin in cultured CWR-22Rv1 human prostate cancer cells in the current study. Among the curcumin analogues, the FN group demonstrated the strongest inhibitory effect CWR-22Rv1 cell growth compared with the other curcumin analogues. In addition, the present study observed that all curcumin analogues analyzed were more potent inhibitors of AR in CWR-22Rv1 cells than curcumin. Group FN was the most potent of the four curcumin analogues for inhibiting the activation of AR.
The natural product curcumin (diferuloylmethane) has been demonstrated to inhibit various targets in prostate epithelial cells, particularly in cancer formation and progression. Among these targets are transcription factors, receptors, intracellular kinases, cytokines and growth factors (25). The effect of curcumin on AR and its target PSA, using both endogenously expressed AR in LNCaP cells and ectopically expressed AR in PC-3 cells, has been demonstrated by several independent studies (26–27). However, in these reports, curcumin was used at relatively high concentrations, typically at >20 µM. It has previously been reported that curcumin has poor bioavailability in animal models and humans (28). This limitation has led researchers to generate a variety of synthetic analogues of curcumin, and investigate their capability to affect a number of molecular pathways implicated in tumorigenesis and cancer progression (29–32). Typical structure modifications include the introduction of substituents on the phenyl rings and modifications of the length of the linker between the phenyl rings. The addition of two bulky side chains on the phenyl rings of curcumin analogues has been shown to inhibit AR function (33), and diketone or enol-ketone modification of the linker also inhibits the expression of AR (34). The present study demonstrated that replacing the phenyl ring with pyridine significantly enhanced the inhibitory effect of curcumin analogues on the AR. Furthermore, curcumin analogues with pyridine rings and cyclohexanone or tetrahydrothiopyran-4-one linkers exhibited a more potent inhibitory effect on AR than analogues with cyclopentanone or tetrahydropyran-4-one linkers. These results indicate that both the distal rings and the linker are important for the inhibitory effect of curcumin analogues on AR activity.
During the past decade, numerous curcumin analogues have been synthesized to improve the stability and anticancer activity of curcumin. Our previous study and another previous study indicated that cyclohexanone-containing analogues of curcumin have more potent anticancer activities than curcumin (23,24). Further modification of cyclohexanone-containing curcumin analogues by replacing the ortho-hydroxylmethoxyl benzene with a pyridine ring strongly enhanced the anticancer activities of these curcumin analogues (23). In the present study, pyridine analogues of curcumin composed of a five-carbon linker with a cyclohexanone, cyclopentanone, tetrahydropyran-4-one or tetrahydrothiopyran-4-one moiety were synthesized, and evaluated for their effects on growth inhibition in CWR-22Rv1 cells. The present study identified that analogues with tetrahydrothiopyran-4-one (FN) as the linker were more potent inhibitors than those with tetrahydropyran-4-one (EN), cyclohexanone (AN) or cyclopentanone (BN). Earlier studies revealed that the six-membered cyclohexanone ring system is, in general, superior to the five-membered cyclopentanone system for inhibiting the growth of several cancer cell lines (23,24). The current results confirmed this structure activity association, as pyridine cyclohexanone curcumin analogues displayed stronger inhibitory activities than pyridine cyclopentanone curcumin analogues. The present study also demonstrated that the replacement of the cyclohexanone core with tetrahydrothiopyran-4-one further increased the anticancer activities, while replacement of cyclohexanone with tetrahydropyran-4-one did not. This indicates that the introduction of sulfur in the linker may enhance the anticancer activities of pyridine curcumin analogues. Additionally, compounds with a nitrogen heteroatom in the ortho position of the pyridine ring typically exhibited activities than compounds with a nitrogen heteroatom in the meta or para position of the pyridine ring.
In conclusion, the results of the present study demonstrated that pyridine analogues of curcumin with tetrahydrothiopyran-4-one as the linker (FN group compounds) possessed more potent inhibitory effects on cultured prostate cancer cells compared with those using cyclohexanone, cyclopentanone or tetrahydropyran-4-one as the linker. The strong effects of the FN group compounds on prostate cancer cells were associated with inhibition of AR activity. The present identified FN group compounds as leading compounds for the further development of novel anti-prostate cancer drugs that target AR signaling, which is important for the survival of prostate cancer cells. Thus, FN compounds warrant additional studies using suitable animal models.
Acknowledgements
The present study was supported by the China National Science Foundation Grants (grant no. 81272452), the PhD Start-up Fund of Natural Science Foundation of Guangdong Province (grant no. 2014A030310329) and the Medical Scientific Research Foundation of Guangdong Province (grant no. B2014072).
References
- 1.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105:3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L. Hormone therapy for patients with prostate carcinoma. Cancer. 2000;88(12 Suppl):S3009–S3014. doi: 10.1002/1097-0142(20000615)88:12+<3009::AID-CNCR17>3.3.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Simmons MN, Klein EA. Combined androgen blockade revisited: Emerging options for the treatment of castration-resistant prostate cancer. Urology. 2009;73:697–705. doi: 10.1016/j.urology.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- 6.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48:5941–5946. [PubMed] [Google Scholar]
- 7.Huang MT, Wang ZY, Georgiadis CA, Laskin JD, Conney AH. Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7, 12-dimethylbenz[a]anthracene. Carcinogenesis. 1992;13:2183–2186. doi: 10.1093/carcin/13.11.2183. [DOI] [PubMed] [Google Scholar]
- 8.Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–5847. [PubMed] [Google Scholar]
- 9.Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- 10.Leite KR, Chade DC, Sanudo A, Sakiyama BY, Batocchio G, Srougi M. Effects of curcumin in an orthotopic murine bladder tumor model. Int Braz J Urol. 2009;35:599–606. doi: 10.1590/S1677-55382009000500012. discussion 606–607. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal DK, Mishra PK. Curcumin and its analogues: Potential anticancer agents. Med Res Rev. 2010;30:818–860. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 12.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Teiten MH, Gaascht F, Eifes S, Dicato M, Diederich M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010;5:61–74. doi: 10.1007/s12263-009-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bill MA, Bakan C, Benson DM, Jr, Fuchs J, Young G, Lesinski GB. Curcumin induces proapoptotic effects against human melanoma cells and modulates the cellular response to immunotherapeutic cytokines. Mol Cancer Ther. 2009;8:2726–2735. doi: 10.1158/1535-7163.MCT-09-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson SM, Gulhati P, Arrieta I, Wang X, Uchida T, Gao T, Evers BM. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009;29:3185–3190. [PMC free article] [PubMed] [Google Scholar]
- 16.Piantino CB, Salvadori FA, Ayres PP, Kato RB, Srougi V, Leite KR, Srougi M. An evaluation of the anti-neoplastic activity of curcumin in prostate cancer cell lines. Int Braz J Urol. 2009;35:354–360. doi: 10.1590/S1677-55382009000300012. discussion 361. [DOI] [PubMed] [Google Scholar]
- 17.Kuo CT, Chen BC, Yu CC, Weng CM, Hsu MJ, Chen CC, Chen MC, Teng CM, Pan SL, Bien MY, et al. Apoptosis signal-regulating kinase 1 mediates denbinobin-induced apoptosis in human lung adenocarcinoma cells. J Biomed Sci. 2009;16:43. doi: 10.1186/1423-0127-16-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Cancer. 2009;100:1425–1433. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–E449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An ‘old-age’ disease with an ‘age-old’ solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 22.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 23.Wei X, Du ZY, Zheng X, Cui XX, Conney AH, Zhang K. Synthesis and evaluation of curcumin-related compounds for anticancer activity. Eur J Med Chem. 2012;53:235–245. doi: 10.1016/j.ejmech.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Zhou DY, Ding N, Van Doren J, Wei XC, Du ZY, Conney AH, Zhang K, Zheng X. Effects of curcumin analogues for inhibiting human prostate cancer cells and the growth of human PC-3 prostate xenografts in immunodeficient mice. Biol Pharm Bull. 2014;37:1029–1034. doi: 10.1248/bpb.b14-00044. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal BB. Prostate cancer and curcumin: Add spice to your life. Cancer Biol Ther. 2008;7:1436–1440. doi: 10.4161/cbt.7.9.6659. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S, Rhim JS. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002;21:825–830. [PubMed] [Google Scholar]
- 27.Tsui KH, Feng TH, Lin CM, Chang PL, Juang HH. Curcumin blocks the activation of androgen and interlukin-6 on prostate specific antigen expression in human prostatic carcinoma cells. J Androl. 2008;29:661–668. doi: 10.2164/jandrol.108.004911. [DOI] [PubMed] [Google Scholar]
- 28.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 29.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78:1305–1315. doi: 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- 31.Ishida J, Ohtsu H, Tachibana Y, Nakanishi Y, Bastow KF, Nagai M, Wang HK, Itokawa H, Lee KH. Antitumor agents. Part 214: Synthesis and evaluation of curcumin analogues as cytotoxic agents. Bioorg Med Chem. 2002;10:3481–3487. doi: 10.1016/S0968-0896(02)00249-3. [DOI] [PubMed] [Google Scholar]
- 32.Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C, et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5:2563–2571. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, Geng G, Shi Q, Sauriol F, Wu JH. Design and synthesis of androgen receptor antagonists with bulky side chains for overcoming antiandrogen resistance. J Med Chem. 2009;52:5546–5550. doi: 10.1021/jm801218k. [DOI] [PubMed] [Google Scholar]
- 34.Shi Q, Shih CC, Lee KH. Novel anti-prostate cancer curcumin analogues that enhance androgen receptor degradation activity. Anticancer Agents Med Chem. 2009;9:904–912. doi: 10.2174/187152009789124655. [DOI] [PubMed] [Google Scholar]