Abstract
Schwannomas rarely occur in the retroperitoneum, and are normally not aggressive. Preoperative diagnosis is difficult and the surgeon may confront blood vessels, nerves or organs damage, since the intra-operative findings are various. The diagnosis and treatment of malignant schwannomas in the retroperitoneum are even more challenging. In addition, the prognosis of malignant schwannomas is extremely poor. The present study reports the case of a 52-year-old woman who presented with a 6-month history of an abdominal mass in the left lower quadrant. The local doctor determined a preliminary diagnosis of abdominal giant tumor and referred the patient to the First Affiliated Hospital, Medical School of Xi'an Jiaotong University, (Xi'an, China). Following discussion, the patient underwent a surgical resection. Low-grade malignant schwannoma was diagnosed following histological examination. No evidence of recurrence or any other complication was observed at the 18-month follow-up examination. The present study reports a case of giant retroperitoneal schwannoma (RS), and includes a literature review in order to provide an overview of the diagnosis, treatment and prognosis of RS and discuss preoperative management strategies for the disease.
Keywords: retroperitoneal schwannoma, diagnosis, preoperative management, treatment
Introduction
Retroperitoneal schwannomas (RSs) are mostly benign and account for ~2% of all retroperitoneal tumors (1,2). RS is typically diagnosed in young and middle-aged patients (3). Furthermore, analysis of the literature found that women exhibit a slightly higher morbidity rate (4,5). The occurrence of malignant RS is even more rare (5–18% of RSs) (6) and has an even worse prognosis (7). The risk factors for benign RSs remain unknown, however, malignant RSs occur as a result of inherited autosomal-dominant neurocutaneous disorders (8). Despite the existence of advanced imaging modalities, such as ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI), only a few cases of RSs are diagnosed prior to resection (9). Diagnosis is usually made following excision and histological examination (10). Surgery is the primary treatment choice for RS; however, in malignant RS, a poor prognosis, such as neurofibromatosis type 1 (von Recklinghausen's disease), which is characterized by the occurrence of visceral neoplasms along with abnormal and numerous skin diseases (11,12), may occur (4). The aim of the present study was to present a case of RS and review the literature, in order to provide an overall understanding of the diagnosis, treatment and prognosis of RS, particularly by discussing the preoperative management of the disease, which reduces complications. Written informed consent was obtained from the patient.
Case report
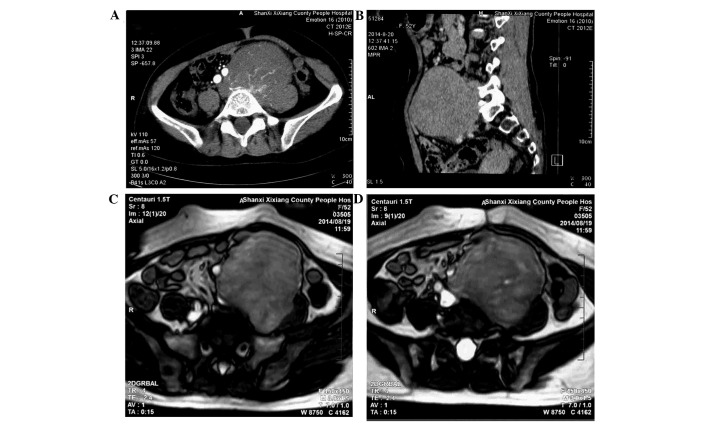
A 52-year-old woman presented to a local hospital (Shaanxi Xixiang County People's Hospital, Hanzhong, China) on August 19, 2014, with a 6-month history of an palpable abdominal mass in the left lower quadrant that was gradually increasing in size for 2 weeks. With the exception of the progressive enlargement of the mass, no other physical symptoms were observed. The patient had no family history of RS. Bowel and bladder function were normal. On physical examination, a large, firm, non-tender abdominal mass was palpable inferior to and to the left of the umbilicus. No lymphadenopathy was found. All vital signs, chest X-ray, hemogram, biochemical analysis and tumor marker results were normal. Computed tomography (CT; Siemens Somatom Emotion 16 CT scanner; Siemens AG, Munich, Germany) confirmed the presence of a 10×9×8-cm heterogeneous mass with a clear boundary and density lower than soft tissue in the left lower quadrant. The CT value was 36 Hu. During the arterial phase, multiple rete vasculums were observed in the lesion (Fig. 1A); these vasculums aggregated in the venous phase (Fig. 1B). Equal or low signal intensity was observed on T1-weighted MRI, and mixed high signal intensity was identified on T2-weighted MRI (Centauri 1.5T; AllTech Medical Systems, Chengdu, China). The vessels close to the tumor had been oppressed and shifted by the tumor. The surrounding fat gap of the tumor was clear (Fig. 1C and D). The patient was diagnosed with giant retroperitoneal tumor and referred to The First Affiliated Hospital, Medical School of Xi'an Jiaotong University on August 28, 2014.
Figure 1.
Abdominal CT scan revealing a 10×9×8-cm, low-density soft tissue heterogeneous mass with a clear boundary in the left lower quadrant. The CT value was 36 Hu. (A) During the arterial phase, multiple rete vasculums were observed in the lesion and (B) multiple rete vasculums aggregated uniformly in the venous phase. Magnetic resonance imaging scan showing (C) an equal or low signal on T1-weighted images and (D) a mixed high signal on T2-weighted images. (C and D) The vessels close to the tumor had been oppressed and shifted, and the surrounding fat gap of the tumor was clear. CT, computed tomography.
In case intestine resection was required, the intestinal tract was cleaned. An adequate volume of blood was prepared for the patient. An F6 double J stent (L260; Zhuzhou Reborn Medical Equipment Co., Ltd., Zhuzhou, China) was implanted into the ureter through a flexible cystoscope (Olympus, Tokyo, Japan), to protect the ureter. The anesthetist was prepared for the possibility of a major catecholamine surge.
Following preparation, the patient underwent surgery. Intraoperatively, the encapsulated mass, measuring 10 cm in diameter, was found to be located in the retroperitoneum. The mass was densely adherent to the psoas major and the left common iliac artery, and the nearest distance between the ureter and the mass was ~2.5 cm. The ureter was located left rear to the mass. The intestines had mostly been squeezed to the right side of the abdomen. Following the separation of the mass from all adjacent tissues, the mass was completely excised from the paravertebral region, using a harmonic scalpel (Ethicon Ultracision Harmonic Scalpel Generator 300 Gen 4; Johnson & Johnson GmbH, Neuss, Germany) and an electrotome (300 W Electrosurgical Generator Electrotome Digital; Beijing Dongfang Shenjian Medical Equipments Co., Ltd., Beijing, China). The blood loss during vessel dissection was estimated at ~300 ml.
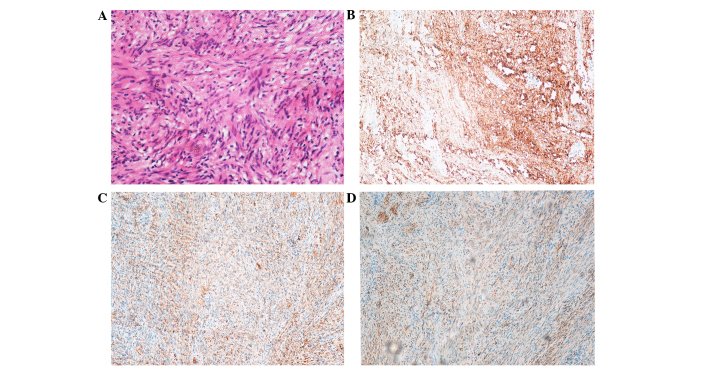
Macroscopically, the mass was oval and well-circumscribed, with a diameter of 10.5×8.5×6 cm (Figs. 2 and 3). All tissue specimens were fixed in 10% buffered-formalin, embedded in paraffin and cut into 4-mm sections. Hematoxylin and eosin-stained section were examined using an Olympus BX51 microscope (magnification, ×200), revealing thin and long bipolar spindle cells with a typical palisading pattern. Mitotic figures were rarely observed. Antoni B areas characterized by less cellular, loosely textured Schwann cells (Fig. 4A).
Figure 2.
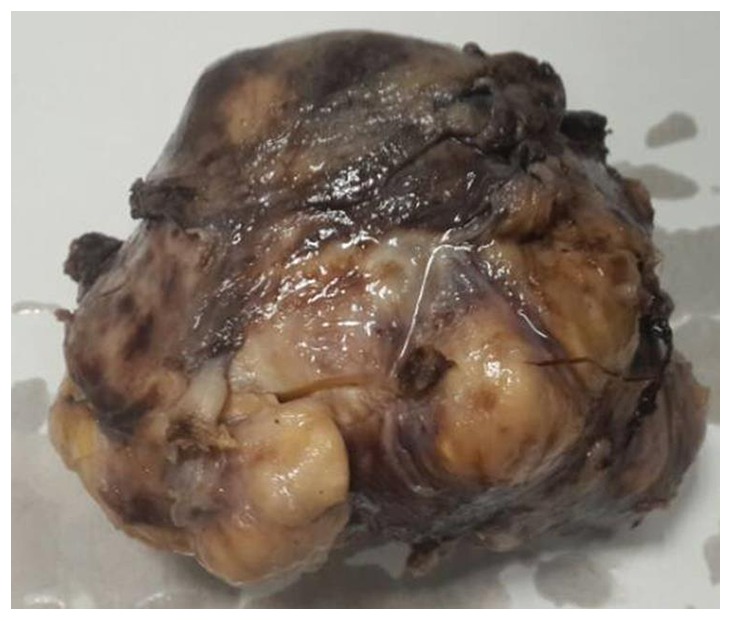
Macroscopically, the mass was oval and well-circumscribed, with a diameter of 10.5×8.5×6 cm.
Figure 3.

Macroscopically, the mass was cut in half, revealing calcified foci and numerous nerve fibers.
Figure 4.
(A) Hematoxylin and eosin staining revealed thin and long bipolar spindle cells with a typical palisading pattern. Hardly any mitotic figures were observed, and Antoni B areas characterized with less cellular, loosely textured Schwann cells were identified (original magnification, ×200). (B-D) Immumohistochemical staining showing positivity for (B) S100, (C) vimentin, (D) glial fibrillary acidic protein.
Immunohistochemical staining revealed positivity for S100 (monoclonal mouse anti-human antibody; cat. no. 5529; 1:500; Cell Signaling Technology, Inc., Danvers, MA, USA) (Fig. 4B), vimentin (monoclonal rabbit antibody; cat. no. 5741; 1:500; Cell Signaling Technology, Inc.) (Fig. 4C) and glial fibrillary acidic protein (monoclonal mouse anti-human antibody; cat. no. 3670; 1:500; Cell Signaling Technology, Inc.) (Fig. 4D). Histopathology was consistent with RS. The postoperative course of the patient was uneventful. No evidence of recurrence or any other operation-correlated complication was observed at the 3-month follow-up; however, 1 month later the patient developed a urinary system infection imputable to delayed removal of the double J stent.
Discussion
RS was first reported by Stallworthy in 1944 (13), and the majority of RSs are benign. Certain studies have reported that equal morbidity was observed in males and females (4,14,15), but Lin et al (16) and Cotran et al (5) found a slightly higher morbidity rate in women. Li et al (3) reviewed 82 cases of RS and the findings were similar to those of Cotran et al (5). RS usually occurs in patients aged 20–50 years old (4). The patient in the present study was a 52 year-old woman. It remains controversial whether these malignant tumors arise from benign or de novo schwannomas (10). It is likely that they have a benign origin, with mutations occurring during tumor growth, a common characteristic of the majority of tumors; however, additional research on gene mutations is required (17).
The symptoms of RS vary, due to the flexibility of the retroperitoneal cavity. The most common symptom is slowly-progressing abdominal pain. Pressure symptoms, also known as ‘symptoms due to organ displacement’ (18), such as urinary or fecal incontinence and neurological symptoms in the lower extremities, are rarely observed (4). Certain individuals, including the present patient, may not exhibit any abnormalities prior to routine examinations.
Clinically, RS tends to be misdiagnosed as appendicitis, adrenal adenoma (4), pancreatic tumor, mesenteric tumor (19), plexiform malignant peripheral nerve sheath tumor or sacral meningioma. Furthermore, RS must be distinguished from paraganglioma, pheochromocytoma, liposarcoma and malignant fibrous histiocytoma in histology (20). Imaging examinations, such as US, CT and MRI, may provide certain clues for the diagnosis of these tumors. On CT scans, they typically appear as well-defined, low- or mixed-attenuation masses with cystic necrotic central areas. On MRI scan, the ‘fascicular sign’, which stands for the presence of bundles, and the ‘target sign’, which stands for the presence of a hypointense center and hyperintense peripheries, are general properties of neurogenic tumors (20,21), which is why MRI is regarded as the diagnostic modality of choice in the evaluation of retroperitoneal tumors. In addition, MRI allows for improved elucidation of the origin, extent and internal composition of the tumors (18).
Differentiating between benign and malignant RS is extremely challenging, since laboratory tests and imaging examinations, such as US, CT and MRI scans, continue to identify few specific features that can distinguish malignant from benign RS (15). Numerous studies have attempted to identify an association between these specific features and malignant RS using large sample analysis (3,4,15). Song et al (4) hypothesized that an association exists between retroperitoneal tumors and adjacent neural structures, which, if identified, may act as evidence of malignancy. In addition, it is considered that malignant schwannomas have irregular contours and tend to demonstrate invasiveness into the adjacent structures.
There is no non-invasive diagnostic method for RS (19,21). CT- and ultrasound-guided biopsies have been shown to be effective invasive diagnostic methods. The present patient was recommended to undergo US-guided biopsy; however, the final diagnosis was not made using the aforementioned technique, as there was not enough tissue to perform the immunohistochemical analysis. In addition, hemorrhage, infection and tumor seeding are known risks of percutaneous biopsy, and due to cellular pleomorphism in degenerated areas, misdiagnosis as malignancy may occur (22). The present study is therefore consistent with the majority of studies in supporting that the final diagnosis should be made based on the findings of postoperative pathology.
Surgery is the primary treatment option for RS (18), due to the lack of sensitivity of these tumors to radiation and chemotherapy (23). Endoscopic surgery (24) and robotic laparoscopic resection (25) are promising surgical approaches, but preoperative management is crucial, since it is closely associated with prognosis.
For preoperative management, cleaning of the intestinal tract should be performed as a routine procedure and an adequate volume of blood or, if endoscopic surgery is selected, a suitable vascular clamp should be available for the patient. The organized structures around the tumor should also be protected during and prior to surgery. Subsequently, the implantation of a double J tube into the ureter through a cystoscope may help protect the ureter (19). In addition, preoperative arterial embolization may not only aid in reducing the risk of bleeding, but is also in favor of stripping the mass absolutely. The anesthesiologist should be prepared for the possibility of a major catecholamine surge (26).
The use of a double J tube was crucial, so the ureters left-rear corner to the mass was protected in advance; however, as Ueda et al (27) reported, the pedicle continuing to the vertebral nerve roots was not found, and the origin of the tumor was not confirmed intraoperatively.
A definitive diagnosis of RS is usually based on pathological, histological and immunohistochemical findings (22). Histologically, schwannomas can be distinguished by the presence of areas of high and low cellularity, termed Antoni A and B tissue patterns, respectively (18,21,22). Microscopically, schwannomas demonstrate Antoni A areas (densely cellular, arranged in short bundles or interlacing fascicles) and Antoni B areas (fewer cells, organized, with great myxoid component) (28). Histopathological staining results showing positivity for S100 (9,21,22) and vimentin (29), and negativity for CD34 (9) are the most accurate markers for a final diagnosis. The results of the present patient agreed with the aforementioned concepts.
The prognosis of benign RS is favorable following complete excision. Recurrence is the most frequent complication reported, mainly due to incomplete excision. In the present case, no evidence of recurrence or any other complication was observed at the 3-month follow-up; however, 1 month later the patient suffered urinary system infection imputable to a delayed removal of the double J stent.
In conclusion, the majority of RSs are benign. Imaging examinations are useful in the differential diagnosis of these tumors. The final diagnosis is based on postoperative histopathological findings and immunohistochemistry. Surgery is the primary treatment option. Although endoscopic surgery and robotic laparoscopic resection are promising surgical approaches, preoperative management, as aforementioned, is crucial for a favorable prognosis.
Acknowledgements
The present study was supported by grants from the National Natural Scientific Foundation of China (grant no. 81370069 to Dr Kang Li) and the Natural Science Basic Research Program of Shaanxi (grant no. 405053047011 to Professor Chengxue Dang).
References
- 1.Kececi Y, Gurler T, Gundogan H, Bilkay U, Cagdas A. Benign giant schwannoma located in the upper arm. Ann Plast Surg. 1997;39:100–102. doi: 10.1097/00000637-199707000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Takatera H, Takiuchi H, Namiki M, Takaha M, Ohnishi S, Sonoda T. Retroperitoneal schwannoma. Urology. 1986;28:529–531. doi: 10.1016/0090-4295(86)90161-5. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Gao C, Juzi JT, Hao X. Analysis of 82 cases of retroperitoneal schwannoma. ANZ J Surg. 2007;77:237–240. doi: 10.1111/j.1445-2197.2007.04025.x. [DOI] [PubMed] [Google Scholar]
- 4.Song JY, Kim SY, Park EG, Kim CJ, do Kim G, Lee HK, Park IY. Schwannoma in the retroperitoneum. J Obstet Gynaecol Res. 2007;33:371–375. doi: 10.1111/j.1447-0756.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- 5.Cotran RS, Kumar V, Robbins SL, editors. Robbins' Pathologic Basis of Disease. 4th. WB Saunders Co.; Philadelphia: 1989. pp. 897–900. [Google Scholar]
- 6.Daneshmand S, Youssefzadeh D, Chamie K, Boswell W, Wu N, Stein JP, Boyd S, Skinner DG. Benign retroperitoneal schwannoma: a case series and review of the literature. Urology. 2003;62:993–997. doi: 10.1016/S0090-4295(03)00792-1. [DOI] [PubMed] [Google Scholar]
- 7.Enzinger FM, Weiss SW. Benign tumours of peripheral nerves. In: Edzinger FM, Weiss SW, editors. Soft Tissue Tumours. 3rd. Mosby, St. Louis: 1995. pp. 821–888. [Google Scholar]
- 8.Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: A twenty first century perspective. Lancet Neurol. 2007;6:340–351. doi: 10.1016/S1474-4422(07)70075-3. [DOI] [PubMed] [Google Scholar]
- 9.Hijioka S, Sawaki A, Mizuno N, Hara K, Mekky MA, Bhatia V, Hosoda W, Yatabe Y, Shimizu Y, Tamada K, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of retroperitoneal schwannoma. Endoscopy. 2010;42(Suppl 2):E296. doi: 10.1055/s-0030-1255786. [DOI] [PubMed] [Google Scholar]
- 10.Shelat VG, Li K, Naik S, Ng CY, Rao N, Rao J, Koura A. Abdominal schwannomas: Case report with literature review. Int Surg. 2013;98:214–218. doi: 10.9738/INTSURG-D-13-00019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nirhale DS, Parasnis A, Bora C, Gupta R, Aulakh P. Retroperitoneal peripheral nerve sheath tumour of triton type-a case report. Indian J Surg. 2013;75(Suppl 1):S12–S14. doi: 10.1007/s12262-011-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avila Herrera P, González Domínguez M, Hernández Ordóñez OF, Gutiérrez Aceves J. Laparoscopic resection of a recurrent retroperitoneal schwannoma (case report and review of the literature) Actas Urol Esp. 2010;34:479–480. doi: 10.1016/j.acuro.2009.11.007. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 13.Stallworthy J. Brief clinical notes on two cases of retroperitoneal schwannoma. Proc R Soc Med. 1944;37:439–440. [PMC free article] [PubMed] [Google Scholar]
- 14.Cui H, Li P, Lu C, Huang X, Chen L, Liu N, She Y. Clinical diagnosis and treatment of primary retroperitoneal schwannoma: A report of 109 cases. Zhonghua Yi Xue Za Zhi. 2015;95:1755–1758. (In Chinese) [PubMed] [Google Scholar]
- 15.Ghosh BC, Luna G, Huvos AG, Fortner JG. Malignant schwannoma. A clinicopathologic study. Cancer. 1973;31:184–190. doi: 10.1002/1097-0142(197301)31:1<184::AID-CNCR2820310126>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Lin PP, Horenstein MG, Healey JH. Sacral mass in a 56-year-old woman. Clin Orthop Relat Res. 1997;333–337:341–343. [PubMed] [Google Scholar]
- 17.Jacoby LB, MacCollin M, Barone R, Ramesh V, Gusella JF. Frequency and distribution of NF2 mutations in schwannomas. Genes Chromosomes Cancer. 1996;17:45–55. doi: 10.1002/(SICI)1098-2264(199609)17:1<45::AID-GCC7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Mastoraki A, Toska F, Tsiverdis I, Kyriazi M, Tsagkas A, Danias N, Smyrniotis V, Arkadopoulos N. Retroperitoneal schwannomas: Dilemmas in diagnostic approach and therapeutic management. J Gastrointest Cancer. 2013;44:371–374. doi: 10.1007/s12029-013-9510-x. [DOI] [PubMed] [Google Scholar]
- 19.Okuyama T, Tagaya N, Saito K, Takahashi S, Shibusawa H, Oya M. Laparoscopic resection of a retroperitoneal pelvic schwannoma. J Surg Case Rep. 2014;2014 doi: 10.1093/jscr/rjt122. pii: rjt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalaycı M, Akyüz U, Demirağ A, Gürses B, Ozkan F, Gökçe O. Retroperitoneal schwannoma: A rare case. Case Rep Gastrointest Med. 2011;2011:465062. doi: 10.1155/2011/465062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theodosopoulos T, Stafyla VK, Tsiantoula P, Yiallourou A, Marinis A, Kondi-Pafitis A, Chatziioannou A, Boviatsis E, Voros D. Special problems encountering surgical management of large retroperitoneal schwannomas. World J Surg Oncol. 2008;6:107. doi: 10.1186/1477-7819-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshino T, Yoneda K. Laparoscopic resection of a retroperitoneal ancient schwannoma: A case report and review of the literature. Anticancer Res. 2008;28:2889–2891. [PubMed] [Google Scholar]
- 23.Kapan M, Onder A, Gümüş M, Gümüş H, Girgin S. Retroperitoneal schwannoma. J Surg Case Rep. 2011;2011:1. doi: 10.1093/jscr/2011.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki A, Suto T, Nitta H, Shimooki O, Obuchi T, Wakabayashi G. Laparoscopic excision of retroperitoneal tumors: Report of three cases. Surg Today. 2010;40:176–180. doi: 10.1007/s00595-008-4009-y. [DOI] [PubMed] [Google Scholar]
- 25.Deboudt C, Labat JJ, Riant T, Bouchot O, Robert R, Rigaud J. Pelvic schwannoma: Robotic laparoscopic resection. Neurosurgery. 2013;72(1 Suppl Operative):2–5. doi: 10.1227/NEU.0b013e31826e2d00. discussion 5. [DOI] [PubMed] [Google Scholar]
- 26.Misra MC, Bhattacharjee HK, Hemal AK, Bansal VK. Laparoscopic management of rare retroperitoneal tumors. Surg Laparosc Endosc Percutan Tech. 2010;20:e117–e122. doi: 10.1097/SLE.0b013e3181df2286. [DOI] [PubMed] [Google Scholar]
- 27.Ueda M, Okamoto Y, Ueki M. A pelvic retroperitoneal schwannoma arising in the right paracolpium. Gynecol Oncol. 1996;60:480–483. doi: 10.1006/gyno.1996.0077. [DOI] [PubMed] [Google Scholar]
- 28.Wong CS, Chu TY, Tam KF. Retroperitoneal schwannoma: A common tumour in an uncommon site. Hong Kong Med J. 2010;16:66–68. [PubMed] [Google Scholar]
- 29.Veliovits D, Fiska A, Zorbas G, Tentes AA. Retroperitoneal schwannomas. Am J Case Rep. 2012;13:244–246. doi: 10.12659/AJCR.883494. [DOI] [PMC free article] [PubMed] [Google Scholar]