Abstract
Boswellic acids (BAs) have long been considered as useful adjunct pharmacological agents for the treatment of patients with malignant brain tumors, notably glioblastoma. Two principal modes of action associated with BAs have been postulated: i) Anti-inflammatory properties, which are useful for containing edema formation, and ii) intrinsic antitumor cell properties, with a hitherto ill-defined mode of action. The present study assessed the effects of various BA derivatives on the viability and clonogenicity of a panel of nine long-term glioma cell lines and five glioma-initiating cell lines, studied cell cycle progression and the mode of cell death induction, and explored potential synergy with temozolomide (TMZ) or irradiation. BA induced the concentration-dependent loss of viability and clonogenicity that was independent of tumor protein 53 status and O6-methylguanine DNA methyltransferase expression. The treatment of glioma cells with BA resulted in cell death induction, prior to or upon S phase entry, and exhibited features of apoptotic cell death. Synergy with irradiation or TMZ was detected at certain concentrations; however, the inhibitory effects were mostly additive, and never antagonistic. While the intrinsic cytotoxic properties of BA at low micromolecular concentrations were confirmed and the potential synergy with irradiation and TMZ was identified, the proximate pharmacodynamic target of BA remains to be identified.
Keywords: boswellic acid, glioma, irradiation, temozolomide
Introduction
Boswellic acids (BAs) are gum resin extracts of Boswellia serrata (Indian frankincense) and other Boswellia species, which are mainly composed of volatile oils (5–15%), pure resin (55–66%) and mucus (12–23%) (1,2). The gum resin typically contains 30% BA (3); however, gum resins from various Boswellia species contrast in BA composition. For example, the gum resin of Boswellia serrata contains similar amounts of 11-keto-β-boswellic acid (KBA) and acetyl-KBA (AKBA) (3.0–4.7% and 2.2–2.9%, respectively); whereas, the gum resin of Boswellia carterii Birdw (African frankincense) contains decreased amounts of KBA (0.5%) compared with AKBA (3.3%). BAs are pentacyclic triterpenes, which may exist in an α- or β-configuration (2,4). Various pharmacological studies indicate that β-configurated derivatives exert stronger bioactivities compared with the respective α-isomers (5). Numerous Boswellia serrata products with varied compositions have been subject to clinical investigation.
BAs have long been considered useful adjunct pharmacological agents for the treatment of patients with malignant gliomas (6) and, more recently, brain metastasis (7). Two principal modes of action associated with BAs have been postulated. One theory suggests that BAs have anti-inflammatory properties, which are useful for containing edema formation in the context of growing brain tumors (6). The second theory proposes that BAs possess intrinsic antitumor cell properties, which may decrease the tumor burden. The latter assumption has been largely based on cell culture studies, which includes a previous study from the Laboratory of Molecular Neuro-Oncology, Department of Neurology, University Hospital Zurich (Zurich, Switzerland) (8) and one in vivo study on the C6 rat model, published over a decade ago (9). BA-induced glioma cell death was previously characterized as apoptotic by morphology, but appeared not to involve caspase activation or be rescued by B-cell lymphoma 2 family proteins (8).
The present study examined the effects of selected BA derivatives (α- and β-configuration), with a focus on human glioma stem-like cells in vitro, and examined whether these agents act in synergy with temozolomide (TMZ) or irradiation, which are the standard choice of care in glioblastoma (10).
Materials and methods
Materials and cell lines
AKBA and β-BA were isolated from the resin of Boswellia serrata and purified by the Alpinia Institute (Alpinia Laudanum Institute of Phytopharmaceutical Sciences AG, Walenstadt, Switzerland). α-BA was purchased from Fluka (Sigma-Aldrich International GmbH, St. Gallen, Switzerland). Stock solutions of AKBA, α-BA and β-BA were prepared in 100% ethanol (MSD Merck Sharp & Dohme AG, Lucerne, Switzerland). Batch-specific control of AKBA and β-BA was performed using high-pressure liquid chromatography (Alpinia Laudanum Institute of Phytopharmaceutical Sciences AG, Walenstadt, Switzerland). Aliquots were stored at 4°C. Schering-Plough TMZ was provided by Merck (Kenilworth, NJ, USA). A 200 mM stock solution of TMZ was prepared in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA) and stored at −20°C.
The human glioma U87MG and T-98 G cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). The other long-term cell lines (LTCs), including LN-18, LN-428, D-247MG, LN-319, A-172, LN-308 and LNT-229, were kindly provided by Professor N. de Tribolet (Lausanne, Switzerland). In addition to established cell lines, the primary patient-derived glioma initiating cells (GICs) T-269, T-325, S-24, ZH-161 and ZH-305 were also used in the present study, which were isolated in the Laboratory of Molecular Neuro-Oncology, Department of Neurology, University Hospital Zurich, as previously described (11). LTCs were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc.) and 2 mM glutamine (Gibco; Thermo Fisher Scientific, Inc.). GIC were maintained in Neurobasal Medium® (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 2% B-27 supplement (Gibco; Thermo Fisher Scientific, Inc.), 2 mM glutamine, 20 ng/ml epidermal growth factor (PeproTech EC Ltd., London, UK), 20 ng/ml fibroblast growth factor (PeproTech EC Ltd.) and 32 international units/ml heparin (Sigma-Aldrich). For irradiation experiments, cells were irradiated in a Co-radiation source (60-Co Thermal Energy Systems; Sulzer AG, Winterthur, Switzerland) at 1, 3 and 9 Gray (Gy) prior to the addition of BA derivatives for 72 h in serum-free medium (SFM; Gibco; Thermo Fisher Scientific, Inc.).
Acute viability assays
For LTCs, 10,000 cells/well were seeded in 96-well plates (TPP; Sigma-Aldrich) in full medium (Gibco; Thermo Fisher Scientific, Inc.) and allowed to attach for 24 h. The cells were then exposed to the BA derivatives at 1.95–220.00 µM for 72 h in SFM. GICs were seeded using the aforementioned method in neurobasal medium (Gibco; Thermo Fisher Scientific, Inc.), incubated for 24 h and then exposed to BA derivatives for 72 h. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine the metabolic activity of LTC and GIC. Briefly, MTT (Sigma-Aldrich) was dissolved in phosphate-buffered saline (PBS). Sodium dodecyl sulfate (10%; Fluka; Sigma-Aldrich) was used to dissolve the purple formazan. Absorbance was measured using the Infinite M200 spectrophotometer (Tecan Schweiz AG, Männedorf, Switzerland) at 540 nm. Solvent-treated cells (0.5% ethanol; Merck) were used as a reference for comparison. In addition, flow cytometry was used to assess the induction of apoptotic vs. necrotic cell death. The cells were harvested with accutase (Thermo Fisher Scientific, Inc.) and treated as indicated in Fig. 3 in SFM, washed in PBS, and resuspended in 10 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid/NaOH (pH 7.4), 140 mmol/l NaCl and 2.5 mmol/l CaCl2. Fluorescein isothiocyanate (dilution, 1:100; Becton Dickinson AG, Allschwil, Switzerland) or Pacific Blue-labelled Annexin V (AnxV; dilution, 1:100; BioLegend, Inc., San Diego, CA, USA) and propidium iodide (PI; 50 µg/ml; Sigma-Aldrich) were added, and the fluorescence of unfixed cells in a total of 10,000 events per condition was recorded in a BD FACSVerse™ flow cytometer (Becton Dickinson AG). AnxV- or PI-single or double-positive cells were quantified using FlowJo Software (version 10.0.8; FlowJo LLC, Ashland, OR, USA).
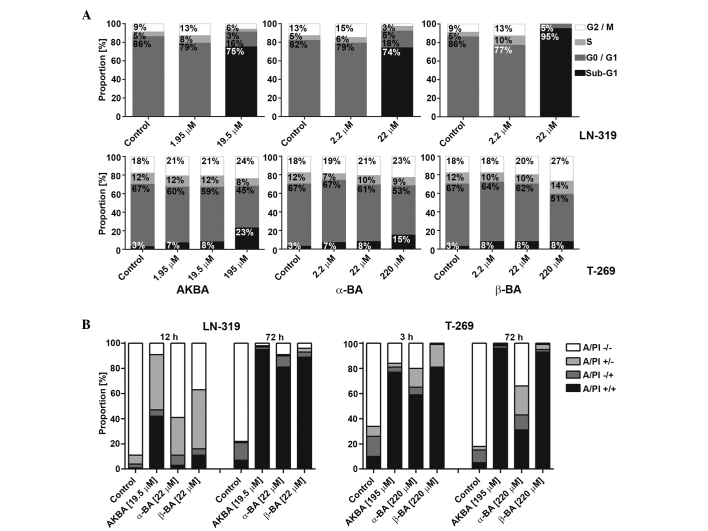
Figure 3.
Mode of glioma cell death induced by AKBA, α-BA or β-BA. (A) LTC (LN-319) or GIC (T-269) were exposed to AKBA at 1.95, 19.50 or 195.00 µM and α-BA or β-BA at 2.2, 22.0 or 220.0 µM for 72 h in serum-free (LTC) or neurobasal medium (GIC), prior to flow cytometric cell cycle analysis. Cell distributions are shown as bar graphs (black, sub-G1; dark grey, G0/G1; light grey, S; white, G2/M). (B) Viability of LN-319 or T-269 was determined by flow cytometry using A/PI staining following exposure to 19.5 µM (LN-319) or 195.0 µM (T-269) AKBA and 22 µM (LN-319) or 220 µM (T-269) α-BA or β-BA for 3 or 12 and 72 h. Frequency plots of double-negative (A/PI-/-), single-positive (A/PI-/+ or A/PI+/-), or double-positive cells (A/PI+/+) are shown as bar graphs. BA, boswellic acid; AKBA, acetyl-11-keto-β-boswellic acid; LTC, long-term cell line; GIC, glioma stem-like culture; A/PI, Annexin V/propidium iodide.
Cell cycle analysis
The cells were treated as indicated in Fig. 3 in SFM, harvested with accutase, fixed and permeabilized overnight in ice-cold 70% ethanol (Merck). The cells were washed twice with PBS. RNA was digested with RNase A (Gibco; Thermo Fisher Scientific, Inc.) and DNA was stained with PI (50 µg/ml; Sigma-Aldrich) containing 0.1% Triton X-100 (Sigma-Aldrich) to permeabilize the cells. Fluorescence was recorded in a BD FACSVerseTM flow cytometer (Becton Dickinson AG) and data was analyzed using FlowJo Software (version 10.0.8). Cells left of the G0/G1 peak were considered to have a DNA content of <2n, indicative of cell death. Aggregated cells were gated out.
Clonogenicity and spherogenicity assays
For LTC, clonogenicity assays were performed by seeding 200 cells per well in 96-well plates that were allowed to adhere overnight in full medium. The cells were then exposed to BA derivatives at increasing concentrations in SFM, as indicated in Fig. 1. Subsequent to 24 h exposure, FCS was added to each well to a final concentration of 10%, and the assay was observed for at least 7 days. GICs were seeded at 200–400 cells per well in neurobasal medium and treated consecutively in the aforementioned manner in neurobasal medium. The cell metabolism of LTC and GIC was assessed by MTT assay.
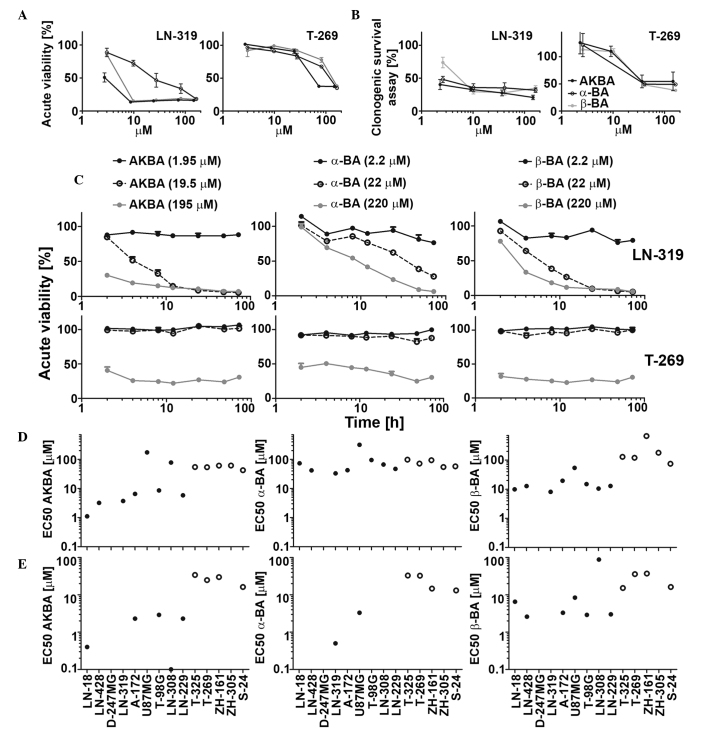
Figure 1.
Sensitivity of LTC and GIC to single BA derivatives (AKBA, α-BA and β-BA) in acute viability and in clonogenic survival assays. LN-319 LTCs or T-269 GICs were exposed to increasing drug concentrations and metabolic activity was assessed by MTT assay in (A) acute viability or (B) clonogenic survival assays. (C) Cells were exposed to drugs (AKBA, 1.95, 19.50 or 195.00 µM; α-BA and β-BA, 2.2, 22.0 or 220.0 µM) for 2, 4, 8, 12, 24, 48 or 72 h and then subjected to MTT assays. Data are expressed as mean and SEM (n=2). LTC (left, filled symbols) or GIC (right, open symbols) were exposed to BA in a concentration-dependent manner, and metabolic activity was assessed by MTT assay in (D) acute viability or (E) clonogenicity assays. EC50 values were calculated for each BA in every tested cell line. Representative results of two independent experiments are shown. The SEM was <15%. BA, boswellic acid; AKBA, acetyl-11-keto-β-boswellic acid; LTC, long-term cell line; GIC, glioma stem-like culture; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; EC50, half maximal effective concentration; SEM, standard error of the mean.
Data analysis
Data are representative of experiments performed three times and the results were similar in all three experiments. Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). The association between the sensitivity of cell lines to BA and p53 and MGMT expression was determined by Spearman's rank correlation coefficient. Synergy of irradiation or TMZ and BA derivatives was assessed by the fractional product method (12). Differences of 10% of observed vs. predicted (additive) effect were considered synergistic. P<0.05 was considered to indicate a statistically significant difference. The data are expressed as the mean ± standard error of the mean.
Results
Intrinsic BA activity against LTC and GIC glioma models
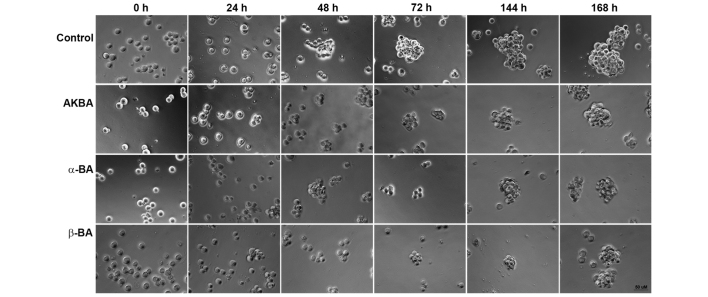
Three out of six major primary BA derivatives were tested for acute growth inhibitory and anti-clonogenic properties in a panel of nine LTC and five GIC models. Representative concentration and time response curves for selected BA derivatives are shown in Fig. 1A–C. The half maximal effective concentration (EC50) values in acute viability assays ranged between 1.1 and 314.4 µM in LTCs and between 42.1 and 668.2 µM in GICs. AKBA showed the highest activity in six out of nine LTCs and four out of five GICs (Fig. 1D). In clonogenic survival assays, the EC50 values ranged between 0.1 and 88 µM in LTCs and 13.1 and 34.1 µM in GICs (Fig. 1E). The EC50 values in acute viability and in clonogenic assays were correlated (r=0.80, P=0.0025). Therefore, the results of acute viability and clonogenic survival assays were similar overall, suggesting that acute cytotoxicity is largely responsible for the loss of clonogenicity or spherogenicity. Exposure to AKBA (19.5 µM), α-BA (22.0 µM) or β-BA (22.0 µM) inhibited sphere formation in ZH-161 (Fig. 2), T-269 or T-325 (data not shown).
Figure 2.
Inhibition of sphere formation by AKBA, α-BA or β-BA. ZH-161 (10,000 cells/well) were exposed to AKBA (19.5 µM), α-BA (22.0 µM) or β-BA (22.0 µM) and monitored for 168 h for sphere formation by phase contrast microscopy. Ethanol (1%) was included as solvent control. BA, boswellic acid; AKBA, acetyl-11-keto-β-boswellic acid.
In order to characterize the mode of growth inhibition in the short-term assays, the cell cycle progression of representative LTC (LN-319) or GIC (T-269) cells exposed to AKBA, α-BA and β-BA were examined using flow cytometry at 72 h. In LTC, within the concentration and time ranges of these experiments, AKBA, α-BA and β-BA induced an increase of the sub-G1 fraction associated with a strong decrease in G1, but only minor change in S or G2/M cells, indicating cell death induction prior to or upon S phase entry (Fig. 3A; upper panel). In GIC, neither a cell cycle arrest nor an induction of cell death prior or upon S phase entry was observed upon treatment with BA. However, at the greatest drug concentrations, a trend towards increased sub-G1 and G2/M fractions was observed (Fig. 3A; lower panel). In addition, flow cytometry using AnxV/PI labeling for the early signs of apoptosis and the assessment of primary or secondary necrosis confirmed the induction of cell death, which evolved with early AnxV positivity following 3 h (GIC) and 12 h (LTC) incubation and resulted in secondary apoptotic events following 72 h incubation, consistent with apoptotic cell death in LTC and GIC (Fig. 3B; left and right panels, respectively). A possible association between tumor protein 53 (p53) or O6-methylguanine DNA methyltransferase (MGMT) status and the sensitivity to BAs has also been identified (13,14). Of the LTCs, U87MG, LNT-229, A-172 and D247MG retain wild-type p53 function, determined by transcriptional activity (13), whereas LN-18 and T-98 G express MGMT (14). There was no association between the sensitivity of the cell lines to BA and p53 or MGMT status determined by the Spearman's rank correlation coefficient (p53, r=−0.056, P=0.88; MGMT, r=0.089, P=0.77) (13,14).
Synergistic activity of β-BA with irradiation or TMZ
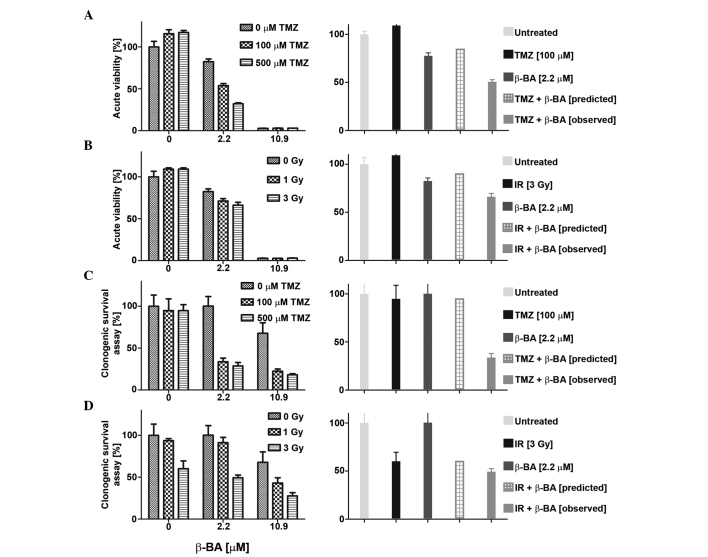
The sensitivity of glioma cell lines to combination treatment with BA (AKBA, a-BA or β-BA) and TMZ (0, 100 or 500 µM) or irradiation (1, 3 or 9 Gy) was also examined in acute viability and clonogenic survival assays. Overall, the effects of combination therapy were mostly additive and never antagonistic, as defined by the fractional product method (data not shown) (12). Certain combinations of BA and TMZ or irradiation met the criteria for synergistic inhibition, as representatively shown in Fig. 4A–D. For instance, co-treatment with β-BA and TMZ (Fig. 4A and C) or irradiation (Fig. 4B and D) revealed synergistic effects at low BA concentrations (<EC50) in LN-319 cells in the two experimental set-ups. No such synergistic effects were observed for T-269 with either combination (data not shown).
Figure 4.
Synergistic effects of BA and TMZ or irradiation. LN-319 cells were exposed for 72 h to β-BA (2.2 and 10.9 µM) (A) alone or (B) in combination with either TMZ (100 or 500 µM) or irradiation (1 or 3 Gray). Metabolic activity was assessed by MTT assay and the predicted vs. observed effect at 2.2 µM was compared using the fractional product method (12). Similar studies were performed as clonogenic survival assays, with β-BA (C) alone or (D) in combination with either TMZ (100 or 500 µM) or irradiation (1 or 3 Gray). Differences of 10% of observed vs. predicted (additive) effect were considered synergistic. Data are expressed as the mean ± standard error of the mean. BA, boswellic acid; TMZ, temozolomide; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Discussion
There is a great interest and requirement for novel therapeutic options for glioblastoma, including phytotherapeutic agents and agents targeting specifically tumor-associated edema (6). BA derivatives have been used in this indication for over a decade, mostly in central European countries (2).
The present study confirms that BAs can exert significant cytotoxic and antiproliferative activities on a panel of LTCs and GICs (Fig. 1). These results extend the similar findings of previous studies on LTCs (8) to GIC models that were not available at the time. Sphere formation by GICs was inhibited in a concentration-dependent manner (Fig. 2). The biochemical mode of cytotoxic action of these compounds and associated proximate molecular targets remains unclear. Furthermore, the profound induction of cell death in glioma cell cultures may require certain concentrations of BA; whether the concentrations can be achieved for prolonged time periods in an in vivo dosing setting remains uncertain. Several studies examining BA plasma and brain levels in rats following the oral administration of Boswellia serrate gum resin extracts (9,15,16) revealed that the brain demonstrates availability of the major BAs, with β-BA and α-BA exhibiting greatest and KBA and AKBA exhibiting the lowest levels (15). However, the achievable BA levels described by Gerbeth et al (15) were decreased compared with the EC50 concentrations determined to be cytotoxic and antiproliferative for a panel of LTCs and GICs in the present study. This inconsistency may be due to the lack of radiological partial or complete responses in glioblastoma patients exposed to these agents.
From the current knowledge of glioma cells, it is unlikely that the candidate biochemical targets of BAs, including 5-lipoxygenase, prostaglandin E synthase (PGES) or cathepsin G, are sufficiently essential for glioma cell viability to represent a lethal pharmacological target (2). However, to formally prove or disprove this hypothesis, the targets would require pharmacological or genetic deletion in order to assess whether the toxic effect of BAs may be reproduced.
Alternatively, in an in vivo setting, the targets of BA may be affected in glioma cells and in the infiltrating host cell population, which contributes to tumor growth and is now considered a promising co-target in the treatment of gliomas (17). For example, the inhibition of cyclooxygenase-2, which regulates the production of prostaglandin E2 by PGES-1, was indicated to delay glioma development in a murine glioma model by inhibiting the development of myeloid derived suppressor cells (MDSCs) and the accumulation of the cells in the tumor microenvironment (18).
Therefore, anti-glioma effects may be inhibited by interfering with the infiltration of the tumor by MDSCs, microglial cells and macrophages, and with proinflammatory or potentially protumorigenic activities. To corroborate this assumption would require the establishment of an in vivo paradigm of BA, the control of glioma growth and the careful characterization of the host cell composition and inflammatory and immunological activity. The present data confirm that BAs possess intrinsic cytotoxic properties at low micromolecular concentrations and may potentially obtain synergy with irradiation and temozolomide. Therefore, BAs should be considered for the treatment of glioblastoma, although the proximate pharmacodynamic targets remain to be identified.
Acknowledgements
The present study was supported by the Alpinia Institute (Walenstadt, Switzerland).
References
- 1.Kreck C, Saller R. Indischer Weihrauch und seine Zubereitungen einschliesslich H15 als traditionelles und modernes Therapeutikum. Internist Prax. 1998;38:857–872. [Google Scholar]
- 2.Abdel-Tawab M, Werz O, Schubert-Zsilavecz M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin Pharmacokinet. 2011;50:349–369. doi: 10.2165/11586800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Basch E, Boon H, Davies-Heerema T, Foppo I, Hashmi S, Hasskarl J, Sollars D, Ulbricht C. Boswellia: An evidence-based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 2004;4:63–83. doi: 10.1080/J157v04n03_06. [DOI] [PubMed] [Google Scholar]
- 4.Gerbeth K, Meins J, Kirste S, Momm F, Schubert-Zsilavecz M, Abdel-Tawab M. Determination of major boswellic acids in plasma by high-pressure liquid chromatography/mass spectrometry. J Pharm Biomed Anal. 2011;56:998–1005. doi: 10.1016/j.jpba.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Poeckel D, Werz O. Boswellic acids: Biological actions and molecular targets. Curr Med Chem. 2006;13:3359–3369. doi: 10.2174/092986706779010333. [DOI] [PubMed] [Google Scholar]
- 6.Streffer JR, Bitzer M, Schabet M, Dichgans J, Weller M. Response of radiochemotherapy-associated cerebral edema to a phytotherapeutic agent, H15. Neurology. 2001;56:1219–1221. doi: 10.1212/WNL.56.9.1219. [DOI] [PubMed] [Google Scholar]
- 7.Kirste S, Treier M, Wehrle SJ, Becker G, Abdel-Tawab M, Gerbeth K, Hug MJ, Lubrich B, Grosu AL, Momm F. Boswellia serrata acts on cerebral edema in patients irradiated for brain tumors: A prospective, randomized, placebo-controlled, double-blind pilot trial. Cancer. 2011;117:3788–3795. doi: 10.1002/cncr.25945. [DOI] [PubMed] [Google Scholar]
- 8.Glaser T, Winter S, Groscurth P, Safayhi H, Sailer ER, Ammon HP, Schabet M, Weller M. Boswellic acids and malignant glioma: Induction of apoptosis but no modulation of drug sensitivity. Br J Cancer. 1999;80:756–765. doi: 10.1038/sj.bjc.6690419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winking M, Sarikaya S, Rahmanian A, Jödicke A, Böker DK. Boswellic acids inhibit glioma growth: A new treatment option? J Neurooncol. 2000;46:97–103. doi: 10.1023/A:1006387010528. [DOI] [PubMed] [Google Scholar]
- 10.Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D, Henriksson R, Balana C, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 11.Günther HS, Schmidt NO, Phillips HS, Kemming D, Kharbanda S, Soriano R, Modrusan Z, Meissner H, Westphal M, Lamszus K. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene. 2008;27:2897–2909. doi: 10.1038/sj.onc.1210949. [DOI] [PubMed] [Google Scholar]
- 12.Webb JL. Enzyme and Metabolic Inhibitors. Vol. 1. New York, NY: Academic Press; 1963. pp. 55–79. and pp 488–512. [Google Scholar]
- 13.Wischhusen J, Naumann U, Ohgaki H, Rastinejad F, Weller M. CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene. 2003;22:8233–8245. doi: 10.1038/sj.onc.1207198. [DOI] [PubMed] [Google Scholar]
- 14.Hermisson M, Klumpp A, Wick W, Wischhusen J, Nagel G, Roos W, Kaina B, Weller M. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem. 2006;96:766–776. doi: 10.1111/j.1471-4159.2005.03583.x. [DOI] [PubMed] [Google Scholar]
- 15.Gerbeth K, Hüsch J, Fricker G, Werz O, Schubert-Zsilavecz M, Abdel-Tawab M. In vitro metabolism, permeation and brain availability of six major boswellic acids from Boswellia serrata gum resins. Fitoterapia. 2013;84:99–106. doi: 10.1016/j.fitote.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Reising K, Meins J, Bastian B, Eckert G, Mueller WE, Schubert-Zsilavecz M, Abdel-Tawab M. Determination of boswellic acids in brain and plasma by high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:6640–6645. doi: 10.1021/ac0506478. [DOI] [PubMed] [Google Scholar]
- 17.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59:1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 18.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]