Abstract
Background and Aims: To evaluate the outcome of patients with Guillain–Barre syndrome (GBS) having respiratory failure treated with modified intubation policy.
Design and Methods: Consecutive patients with GBS having single breath count below 12 and respiratory rate >30/min were included and their clinical details noted. The patients were intubated and mechanically ventilated (MV) if their PaO2 was <60 mmHg on venturi mask, PaCo2 > 50 mmHg or pH < 7.3. Their electrophysiological subtypes and complications were noted. The hospital mortality and 3 months outcome were compared in MV and those could be managed without MV even with respiratory compromise.
Results: Out of 369 patients, 102 (27.6%) patients had respiratory compromise who were included in this study. Of the patients with respiratory compromise, 44 (43.1%) were intubated and mechanically ventilated after a median of 4 days of hospitalization. The median duration of MV was 21 (range 1–88) days. The patients with autonomic dysfunction (56.8% vs. 19%), facial weakness (78% vs. 36.2%), bulbar weakness (81.8% vs. 31%), severe weakness (63.8% vs. 31%) and high transaminase level (47.7% vs. 25.9%) needed MV more frequently. In our study, 6.8% patients died and 26.6% had poor outcome which was similar between MV and non-MV patients. The MV patients had longer hospitalization and more complications compared with non-MV group.
Conclusion: In GBS patients with respiratory compromise, conservative intubation does not increase mortality and disability.
Introduction
Guillain–Barre syndrome (GBS) is rapidly progressive symmetrical weakness of upper and lower limbs with or without sensory or autonomic disturbances associated with hyporeflexia or areflexia in absence of cerebrospinal fluid (CSF) pleocytosis. GBS is the commonest cause of acute flaccid paraplegia and its worldwide incidence is 1.2–3/100 000 population per year.1 The incidence of GBS increases with age from 0.8 in those below 18 years to 3.2 in those 60 years or more.2 Respiratory paralysis is a dreaded complication of GBS and occurs in up to 40% patients.3–5 Severely affected GBS patients need close monitoring in intensive care unit (ICU) and artificial ventilation may be lifesaving.
In the GBS patients with impending respiratory failure and bulbar weakness, elective intubation and artificial ventilation have been suggested.6 Respiratory paralysis is more frequent in the patients with acute motor axonal neuropathy (AMAN) which has been reported commonly from South East Asia.7–9 In the developing countries, there is scarcity of ICU, moreover the available ICUs are beyond the reach of common patients. Mechanical ventilation (MV) has inherent complications such as ventilator associated pneumonia (VAP), sepsis, pneumothorax and line infection, which occur in 15–40% patients.10 These complications may result in prolonged ICU stay and mortality.10 Many patients with respiratory impairment may also recover without MV.
In our practice, we follow conservative policy for MV because of scarcity of ventilators.11 In the present study, we therefore compare the clinical, neurophysiological characteristics and outcome of the patients with respiratory impairment who were mechanically ventilated based on alteration of arterial blood gas (ABG) parameters with those who were not mechanically ventilated although they fulfilled the criteria for elective ventilation in GBS.6 The study may help in defining the characteristics of the patients with GBS with respiratory involvement in whom MV can be deferred without compromising the safety.
Subjects and methods
Study design and setting
Consecutive patients with GBS having respiratory involvement were prospectively evaluated. The study has been approved by Institute Ethics Committee.
Selection of patients
The diagnosis of GBS was based on National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) criteria.12 The patients with periodic paralysis, porphyria, viral myositis, polio and non-polio enteroviral diseases, diphtheria, botulism, rabies and toxic neuropathy were excluded.
Evaluation
Detailed medical history including preceding events such as flue like illness, rash, diarrhea, vaccination, trauma, pregnancy, childbirth and surgery were noted. The demographic variables, season of occurrence, duration of illness and onset to peak disability were noted. Presence of cranial nerve palsy was recorded. Muscle weakness was graded on a 0–5 MRC (Medical Research Council) scale. Muscle tone and tendon reflexes were categorized into decreased, absent or normal. Autonomic functions such as tachycardia or bradycardia, sinus arrhythmia, fluctuation of blood pressure, sweating abnormality and bowel and bladder dysfunction were noted. Postural hypotension was assessed in the patients who could sit or stand. Peak disability was assessed on a 0–10 scale.13
Blood counts, urinalysis, hemoglobin, ESR for the first hour, fasting blood sugar, serum creatinine, blood urea nitrogen, serum sodium, potassium, bilirubin, transaminase and creatinine kinase were measured. Urine porphobilinogen, HIV serology and chest radiograph were done in all the patients. CSF was examined for protein, cell and sugar. Motor nerve conduction of median, ulnar and peroneal and sensory nerve conduction study of median, ulnar and sural nerves were carried out bilaterally. Based on the motor nerve conduction parameters, i.e. distal motor latency, nerve conduction velocity, conduction block (>20%) and minimal F wave latency, the patients were categorized into different subgroups of GBS.14 On the basis of nerve conduction studies, the GBS were categorized in to (i) acute inflammatory demyelinating polyradiculoneuropathy (AIDP), (ii) AMAN, (iii) acute motor sensory axonal neuropathy (AMSAN) and (iv) equivocal.14,15
The patients were defined to have respiratory compromise by the clinical criteria; single breath count <12 and respiratory rate >30/min. These patients were closely monitored in ICU and those patients were intubated and mechanically ventilated who had the following features on ABG analysis (a + b or c).
(a) Inability to maintain PaO2 > 60 mmHg on ventimask.
(b) PaCO2 > 50 mmHg
(c) pH < 7.3
Those patients that not meet the criteria for intubation stated above were not intubated. The patients were closely followed up in ICU. Pulse, blood pressure, respiration and pulse oxymetry were continuously monitored in all. The ABG analysis was done daily or more frequently if needed. Blood counts, hemoglobin and serum electrolytes were done twice weekly and X-ray chest weekly or earlier if indicated. Blood culture, endotracheal or tracheostomy tube aspirate culture and urine culture were carried out as indicated. Pneumonia, pneumothorax, urinary infection, pressure sore, sepsis, deep vein thrombosis and pulmonary thromboembolism were closely monitored. Tracheostomy was done after 1–2 weeks of intubation based on the clinical condition.
The patients admitted within 14 days of illness were prescribed IVIg 400 mg/kg/day for 5 days if they could afford. Calories, fluid, electrolytes and nutrition were maintained by intravenous and/or nasogastric feeding. Heparin prophylaxis was given to the patients who had severe weakness (MRC grade <2). The patients weaned from ventilator when they could maintain normal saturation on continuous positive airway pressure (CPAP) or T pies and extubated when they maintained saturation for 24–48 hr on room air.
Outcome
Outcome was defined as hospital mortality, duration of hospital stay and disability at 3 months. Three months functional outcome was defined as complete (independent for activities of daily living), partial (dependent for activities of daily living) and poor (wheel chair bound or bedridden).16
Statistical analysis
The patients with respiratory impairment with or without MV were compared for demographic details, triggers, peak disability, onset to peak duration, duration of hospital stay, complications, death and 3 months disability using X2 for categorical and independent ‘t’ test or Mann–Whitney U-test for continuous variables. The predictors of MV were evaluated by logistic regression analysis. The variables having a P-values of <0.05 were considered significant. The statistical analysis was done by SPSS version 16 software and GraphPad prism 5.
Results
During last 14 years, 369 patients with GBS were admitted; 102 (27.6%) of them had respiratory compromise requiring ICU admission and they were included in the present study. Their median age was 25 (range 2–70) years and 27 were females. The patients were admitted after a median of 6 (1–25) days of illness. The triggering events were present in 65 (63.7%) patients and included diarrhea in 15 (23.1%), flu like illness in 42 (64.6%), surgery in 1 (1.5%), vaccination in 1 (1.5%) and others in 6 (9.2%) patients. The disability peaked after a median duration of 7 (range 2–24) days. The median peak disability grade was 8 (range 5–9). Facial weakness was present in 65 (63.7%), bulbar weakness in 57 (55.9%) and autonomic disturbances in 36 (35.3%) patients. After nerve conduction studies, the patients were categorized into AIDP in 65 (63.7%), AMAN in 19 (18.6%), AMSAN in 4 (3.9%), unclassifiable in 5 (4.9%) and inexcitable nerves in 5 (4.9%) patients. Intravenous immunoglobulin was given to 58 (56.9%) patients.
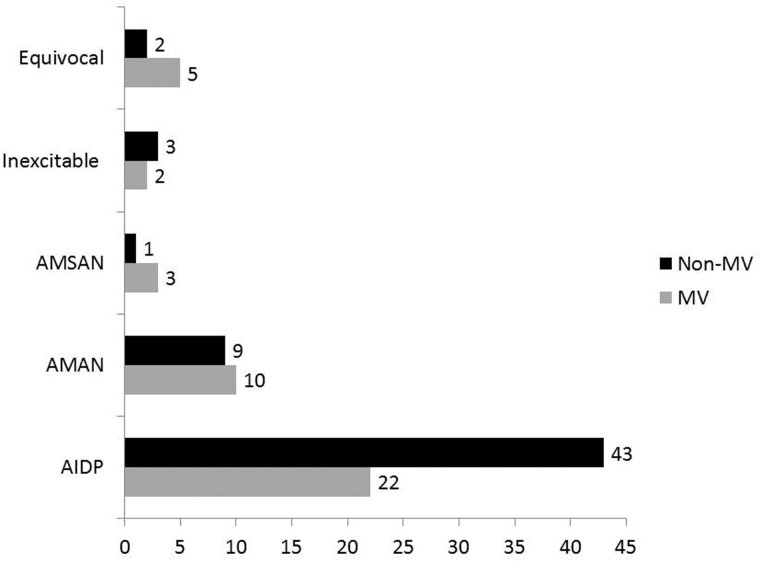
Out of 102 GBS patients with respiratory compromise, 44 (43.1%) needed mechanical ventilation based on ABG criteria after a median duration of 4 (1–20) days of hospitalization. There was no significant difference in the demographic variables in the MV and non-MV patients. Comparing the clinical data between the MV and non-MV patients, there was no significant difference in the day of admission from onset of illness (median 5 vs. 7 days; P = 0.18), the antecedent events (27 vs. 38; P = 0.40), frequency of transient bladder involvement (5 vs. 8; P = 0.71), radicular pain (29 vs. 45; P = 0.19), simultaneous involvement of upper and lower limbs (6 vs. 4; P = 0.25) and CSF protein (median 66 vs. 63.5 mg/dl; P = 0.35). The ventilated patients had insignificantly shorter median duration of onset to peak disability [5 (range 2–16) vs. 7 (range 3–14); P = 0.24]. The MV patients had more frequent dysautonomia (56.8% vs. 19%, P < 0.001), facial weakness (75% vs. 36.2%, P = 0.04), bulbar weakness (81.8% vs. 31.0%; P = 0.001), severe weakness [(MRC 0–2) 63.8% vs. 31% P = 0.001)] and high SGPT level (47.7% vs. 25.9%, P = 0.01). The details are summarized in Table 1. AMAN type of GBS although needed mechanical ventilation more frequently (9/19, 47.4%) compared with AIDP (22/65, 33.8%) but the difference was not statistically significant (P = 0.18). The details are shown in Figure 1. On multiple regression analysis, lower limb power MRC grade ≤2 (OR −6.8; 95% CI 0–0.29; P = 0.02) was independently associated with need of mechanical ventilation.
Table 1.
Comparison of admission parameters of the patients with Guillain–Barre syndrome having respiratory involvement who needed mechanical ventilation (MV) with those who did not based on arterial blood gas analysis
| Parameters | MV (n = 44) | Non-MV (n = 58) | P |
|---|---|---|---|
| Age (yrs) | 30.5 ± 15.9 | 28 ± 21.1 | 0.62 |
| Male | 34 (77.3%) | 41 (70.7%) | 0.30 |
| Day of admission (days) | 7.2 ± 6.1 | 8.2 ± 6.5 | 0.18 |
| Autonomic dysfunction | 25 (56.8%) | 11 (19%) | <0.001 |
| Antecedent illness | 27 (61.4%) | 38 (65.5%) | 0.40 |
| Radicular pain | 29 (65.9%) | 45 (77.6%) | 0.19 |
| Cranial nerve palsy | 27 (61.4%) | 34 (58.6%) | 0.75 |
| Facial palsy | 33 (75%) | 32 (36.2%) | 0.04 |
| Bulbar weakness | 36 (81.8%) | 21 (36.2%) | <0.001 |
| Power <3 UL | 31 (70.5%) | 18 (31%) | <0.001 |
| Wrist dorsiflexion Gr 0 | 16 (36.4%) | 5 (8.6%) | <0.001 |
| Lowe limb power <3 | 32 (72.7%) | 29 (50%) | <0.02 |
| Foot dorsiflexion Gr 0 | 18 (40.9%) | 10 (17.2%) | 0.005 |
| Power in all limbs <3 | 28 (63.6%) | 18 (31%) | 0.001 |
| Liver dysfunction | 21 (47.7%) | 15 (25.9) | 0.012 |
| CSF protein mg/dl | 66 (16–200) | 63.5 (18–422) | 0.35 |
| IVIg treatment | 32 (72.7%) | 26 (44.8%) | 0.001 |
CSF, cerebrospinal fluid; IVIg, intravenous immunoglobulin.
Figure 1.
Subtypes of Guillain–Barre syndrome with respiratory compromise who needed mechanical ventilation and those who did not.
Outcome
In this study, seven (6.8%) patients died during the hospital; five (11.4%) in the MV and two (3.4%) in the non-MV group. The relative risk of death in the ventilated group was 1.7 (95% CI 1.02–2.91; P = 0.23). The cause of death in the ventilated patients was pneumonia in two and dysautonomia in three patients. In the non-ventilated group, one died because of sudden cardiac arrest and the other due to dysautonomia. The median duration of MV was 21 (range 1–88) days. Prolonged ventilation (>15 days) was needed in 25 (56.8%) patients. The median duration of hospital stay of the MV patients was longer compared with those of non-MV patients [38 (range 3–105) vs. 12 (range 3–31) days; P < 0.001]. The ventilator-related complications were noted in 19 (43.2%) patients and included VAP in 16 (34.4%), lung collapse in 2 (4.5%), pneumothorax in 1 (2.3%) and sepsis in 1 (2.3%) patient. In the non-MV group, only three (5.2%) patients developed complications which included pneumonia in two (3.4%) and urinary tract infection in one (1.7%) patient (Table 2).
Table 2.
Complications during hospital stay between mechanically ventilated (MV) and non-ventilated Guillain–Barre patients
| Complications | MV N= 44 | Non-MV N= 58 |
|---|---|---|
| Pneumonia | 16 (34.4%) | 2 (3.4%) |
| Lung collapse | 2 (4.5%) | 0 (0%) |
| Pneumothorax | 1 (2.3%) | 0 (0%) |
| Fever without localization | 1 (2.3%) | 0 (0%) |
| Urinary tract infection | 1 (2.3%) | 1 (1.7%) |
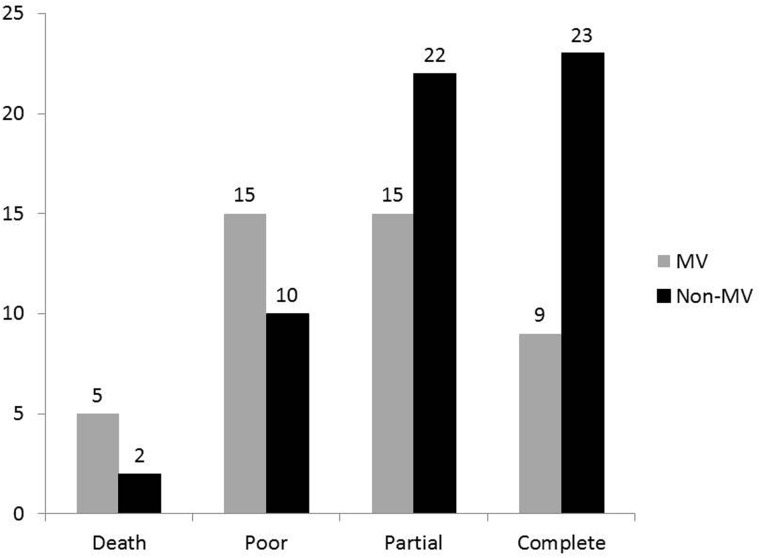
At 3 months follow up, 32 (43%) patients recovered completely, 37 (39.4%) partially and 25 (26.6%) had poor recovery. The GBS patients with respiratory compromise who did not require MV had insignificantly higher frequency of complete recovery (23/55; 41.8%) compared with those who were mechanically ventilated [9/39 (23%; P = 0.08)]. The details are shown in Figure 2.
Figure 2.
The mechanically ventilated (MV) Guillain–Barre patients had insignificantly higher mortality and poor outcome compared with the patients who did not require MV although had respiratory compromise.
Discussion
In our study, 102 (27.6%) patients with GBS had respiratory compromise, 43% of them needed MV on the basis of ABG criteria. Severity of weakness (MRC grade ≤2) was an independent predictor of mechanical ventilation. The patients with respiratory compromise who were mechanically ventilated had higher complications and more frequent deaths or disability compared with those who were not mechanically ventilated although fulfilled criteria of elective MV in GBS. We have followed conservative criteria for mechanical ventilation, i.e. we relied on ABG and have deferred intubation in those with respiratory impairment but maintained ABG and those with bulbar weakness.
In GB syndrome, elective intubation has been recommended based on 20, 30 and 40 rule (vital capacity <20 ml/kg, maximal inspiratory pressure <30 cm of water and maximum expiratory pressure <40 cm of water).5 Delaying ventilation by waiting for hypoxia and hypercarbia to occur may result in emergent intubation resulting in complications.6 Intubation and artificial ventilation have inherent complications of pneumonia, pneumothorax and sepsis resulting in higher mortality and morbidity. In MGH study, 83% of ventilated patients had pneumonia, 5% each had pulmonary embolism and tracheal stenosis but none died. In the Mayo clinic series out of 13 ventilated GBS patients; 38% had pneumonia, 5% pulmonary embolism, 7% tracheal stenosis and 15% patients died. Timely ventilation can reduce mortality by protecting airway and minimizing atelectasis.6 In this study, the criteria of ventilation were any of the following: ventilatory failure with reduced vital capacity of <12–15 ml/kg, pO2 below 70 mm of Hg on room air or severe oropharyngeal paresis with difficulty in clearing secretion or repeated coughing or aspiration after swallowing. In the study by Lawn et al.5, progression to respiratory failure is likely in the patients with rapid progression of weakness, bulbar dysfunction and dysautonomia. Progression to respiratory failure is likely in those patients having vital capacity <20 ml/kg, maximum inspiratory pressure <30 cm of water and maximum expiratory pressure <40 cm of water or reduction of >30% in vital capacity, maximum inspiratory pressure or expiratory pressure. The predictors of respiratory failure in GBS have been reported in a number of studies and include single breath count, neck weakness, bulbar weakness, limb power <3 (MRC grade) on admission, simultaneous upper and lower limb weakness and forced vital capacity.17,18
In our study, we have not intubated the patients with bulbar weakness unless they had hypoxia or ABG abnormality. Twenty-one out of 57 patients with bulbar weakness could be managed without artificial ventilation. There is no difference in mortality but ventilated patients had higher frequency of pneumonia and infection. In a study on mechanically ventilated GBS patients, death was related to old age, autonomic dysfunction, hypokalemia and bleeding from any site. The risk of death increased by 6.9 times with pneumonia, 2.55 times with hypokalemia and 3.14 times with autonomic dysfunction.19 In our study, hypokalemia was not an important predictor of mechanical ventilation or outcome. In our study, only seven patients died; five in MV and two in non-MV group. The results of our study regarding predictors of MV and outcome are different from the reported literature because we have included the patients with respiratory compromise and compared those requiring MV with those who did not based on ABG. Had we followed elective ventilation criteria, all these patients would have been mechanically ventilated. This may be the reason why the mortality and 3 month functional outcome were not significantly different between MV and non-MV patients in our study. The respiratory complications were significantly more in the MV patients compared with the non-MV patients (40.9% vs. 3.4%).
In this study, AIDP constituted 63.7% and AMAN in 18.6% patients. Half the patients with AMAN needed MV whereas ii was one-third in AIDP group. Higher frequency of AMAN although have been reported in the developing countries of Asia and America but on detailed nerve conduction studies, we have found AIDP as a predominant subtype of GBS.3,7,16,20,21
Limitations
The present study is limited by lack of spirometric assessment. We have relied on single breath count for defining respiratory compromise instead of spirometry because it is not easily available bedside and even many mildly affected patients had difficulty in performing spirometry test. Single breath count has been found to be a useful bedside pointer and correlated with peak expiratory flow rate and forced expiratory pressure in 1 s in the adults.22 Similar observation has also been reported in children.23 Our study is a retrospective analysis in a tertiary care teaching hospital and all the patients were examined by at least two of the authors. Our findings suggest that using ABG criteria, MV may be deferred in 55% patients with GBS who otherwise would have been ventilated. This protocol seems to be safe, reduce ventilator-related complications and may be applicable especially in resource poor countries. Further prospective study is needed to confirm these observations.
Acknowledgements
We acknowledge Mr Rakesh Kumar Nigam and Deepak Kumar Anand for secretarial help.
Ethical approval
The study PGI/BE/80/2014 has been approved by institutional ethics committee, SGPGIMS, Lucknow.
Conflict of interest: None declared.
References
- 1.Misra UK, Kalita J. Diagnosis and Management of Neurological Disorders, Vol. 18. New Delhi, Wolters Kluwer (India) Pvt. Ltd, 2011, 287. [Google Scholar]
- 2.Beghi E, Kurland LT, Mulder DW, Wiederholt WC. Guillain–Barré syndrome. Clinicoepidemiologic features and effect of influenza vaccine. Arch Neurol 1985; 42:1053–7. [DOI] [PubMed] [Google Scholar]
- 3.Kalita J, Misra UK, Goyal G, Das M. Guillain–Barré syndrome: subtypes and predictors of outcome from India. J Peripher Nerv Syst 2014; 19:36–43. [DOI] [PubMed] [Google Scholar]
- 4.Ropper AH. The Guillain–Barré syndrome. N Engl J Med 1992; 326:1130–6. [DOI] [PubMed] [Google Scholar]
- 5.Lawn ND, Fletcher DD, Henderson RD, Wolter TD, Wijdicks EF. Anticipating mechanical ventilation in Guillain–Barré syndrome. Arch Neurol 2001; 58:893–8. [DOI] [PubMed] [Google Scholar]
- 6.Ropper AH, Kehne SM. Guillain–Barré syndrome: management of respiratory failure. Neurology 1985; 35:1662–5. [DOI] [PubMed] [Google Scholar]
- 7.Islam Z, Jacobs BC, van Belkum A, Mohammad QD, Islam MB, Herbrink P, et al. Axonal variant of Guillain–Barre syndrome associated with Campylobacter infection in Bangladesh. Neurology 2010; 74:581–7. [DOI] [PubMed] [Google Scholar]
- 8.Ye Y, Wang K, Deng F, Xing Y. Electrophysiological subtypes and prognosis of Guillain–Barré syndrome in northeastern China. Muscle Nerve 2013; 47:68–71. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa K, Kuwabara S, Misawa S, Fujii K, Tanabe Y, Yuki N, et al. Electrophysiological subtypes and prognosis of childhood Guillain–Barré syndrome in Japan. Muscle Nerve 2006; 33:766–70. [DOI] [PubMed] [Google Scholar]
- 10.Ali MI, Fernández-Pérez ER, Pendem S, Brown DR, Wijdicks EF, Gajic O. Mechanical ventilation in patients with Guillain–Barré syndrome. Respir Care 2006; 51:1403–7. [PubMed] [Google Scholar]
- 11.Misra UK, Kalita J, Bhoi SK. Spectrum and outcome predictors of central nervous system infections in a neurological critical care unit in India: a retrospective review. Trans R Soc Trop Med Hyg 2014; 108:141–6. [DOI] [PubMed] [Google Scholar]
- 12.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain–Barré syndrome. Ann Neurol 1990; 27:S21–4. [DOI] [PubMed] [Google Scholar]
- 13.Hahn AF, Bolton CF, Pillay N, Chalk C, Benstead T, Bril V, et al. Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double-blind, sham controlled cross-over study. Brain 1996; 119:1055–66. [DOI] [PubMed] [Google Scholar]
- 14.Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, Toyka KV, et al. Electrophysiological classification of Guillain–Barré syndrome: clinical associations and outcome. Plasma exchange/Sandoglobulin Guillain–Barré syndrome trial group. Ann Neurol 1998; 44:780–8. [DOI] [PubMed] [Google Scholar]
- 15.Uncini A, Yuki N. Electrophysiologic and immunopathologic correlates in Guillain–Barré syndrome subtypes. Expert Rev Neurother 2009; 9:869–84. [DOI] [PubMed] [Google Scholar]
- 16.Kalita J, Misra UK, Das M. Neurophysiological criteria in the diagnosis of different clinical types of Guillain–Barre syndrome. J Neurol Neurosurg Psychiatry 2008; 79:289–93. [DOI] [PubMed] [Google Scholar]
- 17.Paul BS, Bhatia R, Prasad K, Padma MV, Tripathi M, Singh MB. Clinical predictors of mechanical ventilation in Guillain–Barré syndrome. Neurol India 2012; 60:150–3. [DOI] [PubMed] [Google Scholar]
- 18.Kannan Kanikannan MA, Durga P, Venigalla NK, Kandadai RM, Jabeen SA, Borgohain R. Simple bedside predictors of mechanical ventilation in patients with Guillain–Barre syndrome. J Crit Care 2014; 29:219–23. [DOI] [PubMed] [Google Scholar]
- 19.Netto AB, Taly AB, Kulkarni GB, Rao UG, Rao S. Mortality in mechanically ventilated patients of Guillain–Barré Syndrome. Ann Indian Acad Neurol 2011; 14:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachamkin I, Arzarte Barbosa P, Ung H, Lobato C, Gonzalez Rivera A, Rodriguez P, et al. Patterns of Guillain–Barre syndrome in children: results from a Mexican population. Neurology 2007; 69:1665–71. [DOI] [PubMed] [Google Scholar]
- 21.Misra UK, Kalita J. Patterns of Guillain–Barré syndrome in children: results from a Mexican population. Neurology 2008; 71:1203–4. [DOI] [PubMed] [Google Scholar]
- 22.Bartfield JM, Ushkow BS, Rosen JM, Dylong K. Single breath counting in the assessment of pulmonary function. Ann Emerg Med 1994; 24:256–9. [DOI] [PubMed] [Google Scholar]
- 23.Ali SS, O'Connell C, Kass L, Graff G. Single-breath counting: a pilot study of a novel technique for measuring pulmonary function in children. Am J Emerg Med 2011; 29:33–6. [DOI] [PubMed] [Google Scholar]