CXCR6 is required for homing of IL-17A+ T cells to atherosclerotic lesions
Key words: atherosclerosis, chemokine receptor, IL-17A, immune system, lymphocytes
Abstract
The adaptive immune response is involved in the development and progression of atherosclerosis and IL-17A+ cells play a role in this disease. Although elevated number of CD4+ IL-17A+ (Th17) and IL-17A+TCRγδ+ T cells are found within murine atherosclerotic aortas and human plaques, the mechanisms governing IL-17A+ T-cell migration to atherosclerotic lesions are unclear. The chemokine receptor CXCR6 is expressed on several T-cell subsets and plays a pro-atherogenic role in atherosclerosis. Here, we used CXCR6-deficient (Cxcr6 GFP/GFP) apolipoprotein E-deficient (Apoe −/−) mice to investigate the involvement of CXCR6 in the recruitment IL-17A+ T cells to atherosclerotic aortas. Flow cytometric analyses revealed reductions in Th17 and IL-17A+TCRγδ+ T cells within aged Cxcr6 GFP/GFP Apoe −/− aortas, in comparison with age-matched Cxcr6 GFP/+ Apoe −/− aortas. Although CXCR6-sufficient IL-17A+ T cells efficiently migrated toward CXCL16, the migration of CXCR6-deficient IL-17A+ T cells was abolished in transwell assays. Importantly, the recruitment of Cxcr6 GFP/GFP Apoe −/− IL-17A+ T cells into the aortas of Apoe −/− recipients was markedly reduced in short-term adoptive transfer experiments. Altogether these results demonstrate an important role of CXCR6 in the regulation of pathological Th17 and IL-17A+TCRγδ+ T-cell recruitment into atherosclerotic lesions.
Introduction
Atherosclerosis, the major etiological process responsible for 25% of global deaths, is the process through which inflamed arterial plaques form, persist and eventually rupture, resulting in myocardial infarctions and ischemic stroke. The etiology of atherosclerosis critically depends on the immune system, and recent work has demonstrated that many leukocytes are present within arterial lesions. Lesional monocytes, macrophages, dendritic cells and T cells, including Th1, Th2, Treg, Th17, and TCRγδ+ T-cell subsets, have been described (1, 2).
IL-17A, a major cytokine produced by Th17 and some TCRγδ+ T cells, is required to efficiently control bacterial and fungal infections at mucosal sites. However, IL-17A also actively participates in major autoimmune diseases (3–5). In the context of atherosclerosis, elevated levels of Th17 and IL-17A+TCRγδ+ T cells have been reported within atherosclerotic Apoe −/− and Ldlr −/− mice (6–9), coronary artery disease (CAD) and endarterectomy patients (10–12). Mechanistic studies in atherosclerotic mice have yielded at least two unifying hypotheses: that IL-17A plays a pro-atherogenic role by supporting aortic chemokine/cytokine production, myeloid cell recruitment (6, 7, 9, 10, 13–15) and activation; and an atheroprotective role, via the potential regulation of aortic Th1 or smooth-muscle-cell collagen deposition (8, 16, 17). Thus, while IL-17A may promote (8, 16, 17), not affect (9, 14, 18), or adversely affect (6, 12, 19) collagen synthesis and plaque stability; to date, the majority of evidence supports a pro-atherogenic role for IL-17A (6, 7, 9, 13–15, 18).
Although multiple T-cell subsets are present within the aortic wall, the mechanisms behind aortic and aortic adventitial T-cell homing are not completely understood. Several adhesion molecules and chemokines/chemokine receptors have been demonstrated to regulate aortic T-cell content. CCL5, CXCL10 and CXCL16 and their respective receptors CCR1, CXCR3 and CXCR6 support the migration of Th1 cells, and several studies have implicated CCL19/CCL21, CCL17 and the chemokine receptors CCR7 and CCR4 in the regulation of Treg homing (20, 21). In contrast, the mechanisms through which Th17 and IL-17A+TCRγδ+ T cells are recruited to atherosclerotic lesions are unknown; however, several candidates might be involved. The chemokine receptors CCR7 and CXCR5 generally support T-cell migration into secondary lymphoid tissues and the non-lymphoid homing receptors CCR4, CCR5, CCR6 and CXCR6 are expressed by Th17 cells (22). Interestingly, while CCR6 plays a central role in Th17-cell recruitment in experimental autoimmune encephalomyelitis (23), rheumatoid arthritis (24), and air pouch inflammation models (25) CCR6 did not affect the recruitment of aortic Th17 cells in atherosclerotic mice (26). Thus, the mechanisms through which Th17 and IL-17A+TCRγδ+ T cells are recruited to atherosclerotic lesions remains to be addressed.
In this study, we demonstrate that virtually all Th17 cells and IL-17A+TCRγδ+ T cells express high levels of the chemokine receptor CXCR6 in atherosclerotic aortas. In CXCR6-deficient Cxcr6 GFP/GFP Apoe −/− mice, CXCR6+ Th17 and IL-17A+TCRγδ+ T cells failed to accumulate within aortic atherosclerotic lesions. We assessed the role of CXCL16/CXCR6-dependent IL-17A+ T-cell chemotaxis in transwell assays and found that Th17 and IL-17A+TCRγδ+ T cells from Apoe −/− mice migrated towards CXCL16 in a dose-dependent manner. Lastly, in vivo competitive adoptive transfer experiments demonstrated that IL-17A+ T cells require CXCR6 to home to atherosclerotic lesions. Collectively, our data indicate that the chemokine receptor CXCR6 is required for efficient Th17 and IL-17A+TCRγδ+ T-cell recruitment to inflamed atherosclerotic lesions.
Methods
Mice
Cxcr6 GFP/+ and Cxcr6 GFP/GFP mice (27) (a kind gift of Dr Littman, Howard Hughes Medical Institute, New York University) were crossed with Apoe −/− mice (Jackson Laboratories, Bar Harbor, MN, USA) to obtain Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− mice. Mice were bred and maintained under specific pathogen-free conditions in the animal facilities of Eastern Virginia Medical School, Norfolk. Mice of 40–50 weeks old were used for the experiments described, in accordance with the EVMS Institutional Animal Care and Use Committee guidelines.
Flow cytometry
The preparation of aortic cell suspensions and intracellular flow cytometry staining protocols were conducted as previously described (14, 28, 29). Briefly, the mice were anesthetized and their vasculature was perfused with PBS containing 20U ml–1 sodium heparin via cardiac puncture. The aortas were subsequently dissected and digested for 1h at 37°C with 125U ml–1 Collagenase Type XI, 60U ml–1 Hyaluronidase Type 1-s, 60U ml–1 DNase 1 and 450U ml–1 Collagenase Type I in PBS (Sigma-Aldrich, St Louis, MO, USA). Single-cell suspensions were prepared from the spleens, peri-aortic lymph nodes (PALN) and digested aortas using 70 μm nylon cell strainers. To re-stimulate the cell suspensions for intracellular cytokine staining, the cells were cultured for 5h at 37°C with complete RPMI1640 (10% FBS, 2% penicillin/streptomycin) supplemented with 10ng ml–1 PMA, 500ng ml–1 Ionomycin C and 600ng ml–1 Brefeldin A (Sigma-Aldrich). To stain the re-stimulated cells, the single-cell suspensions were pre-incubated with anti-mouse CD16/32 antibodies (10min, room temperature), and stained with the following antibodies: CD45-Pacific Orange (Life Technologies), CXCR3-PerCP Cy5.5, CCR6-APC, CD3ε-APC Cy7, TCRβ-APC, TCRγδ-eF450 (all from eBioscience) or appropriate isotype controls. Intracellular staining for IL-17A-PE or IgG2a-PE (eBioscience) was performed using Fix and Perm kits (Invitrogen, Life Technologies) following the manufacturer’s instructions. Following the staining procedure, the samples were acquired using a CyanADPTM (Dako, CO, USA) or an upgraded CytekDevelopment 8DXP-upgraded FACS Calibur (BD Biosciences) and analyzed using FlowJo (Tree Star Inc.). For all flow cytometry experiments, the gates were set based on isotype or saline-recipient Apoe −/− organs.
Transwell migration experiments
Forty- to fifty-week-old Apoe −/− or Cxcr6 GFP/GFP Apoe −/− peripheral lymph nodes (cervical, axillary, brachial, inguinal lymph nodes) were sterilely collected and single-cell suspensions were prepared; 0.3–0.4×106 cells resuspended in 0.1ml RPMI1640 supplemented with 1% BSA and 10mM Hepes (‘migration media’ hereafter) were seeded onto the top well of the transwell. The bottom wells were loaded with 0.6ml migration media supplemented with the following: media alone (BSA control), 100ng ml–1 recombinant mouse CXCL16 (Peprotech, NJ, USA), 300ng ml–1 CXCL16 or 1000ng ml–1 CCL20 (Peprotech). The loaded cells were allowed to migrate for 3h at 37°C before being harvested for PMA/Ionomycin-based re-stimulation and intracellular cytokine staining. The migration index indicates the percentage of transmigrated IL-17A+ T cells normalized to the mean of the BSA controls for each genotype (30).
Short-term IL-17A+ T-cell homing experiments
Forty- to fifty-week-old Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− spleens were sterilely collected and the erythrocytes were lysed using ACK lysis buffer (5min, room temperature, 150mM NH4Cl, 10mM KHCO3, 0.1mM EDTA) and washed with PBS. Following the lysis procedure, Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− splenocytes were labeled with Cell Trace Violet or Cell Trace Far Red (Life Technologies) for 10min at 37°C and washed twice with 10% FBS/PBS, following the manufacturer’s instructions. The labeled cohorts were mixed in a 1:1 ratio and 100×106 cells (50×106 cells per donor population) were injected into each 40-week-old Apoe −/− recipient mouse. As a negative control, some age-matched Apoe −/− mice were injected with saline as dye-negative controls. At the time of the injection and 72h post-injection, the donor starting populations and the recipient Apoe −/− aortas, spleens, blood and PALN were collected for intracellular cytokine staining and assessed for IL-17A+ T cells. All gates were set based on isotype and non-recipient Apoe −/− controls. To control for differences in the starting percentage of IL-17A+ T cells and the migratory potential of Cxcr6 GFP/+ versus Cxcr6 GFP/GFP cells, the fold migration data were normalized to the starting percentage of IL-17A+ T cells and the percentage of donor IL-17A+ Cxcr6 GFP/+ T cells of each recipient tissue. Donor CD45+CD3+TCRαβ+GFP+IL-17A+ T cells were considered to be Th17 cells, whereas CD45+CD3+TCRαβ-GFP+IL-17A+ T cells were considered to be donor IL-17A+ γδ T cells as 75–80% of CD45+CD3+TCRαβ-IL-17A+ T cells are TCRγδ+.
Statistics
For comparisons between two groups, unpaired or paired (adoptive transfer experiments) two-tailed Student’s t-test were used. For comparisons of >2 conditions with a defined control group (transwell experiments), a one-way ANOVA with Dunnett post hoc tests (PASW Statistics, v18) were used. For all experiments, the means ± SEM are shown.
Results and discussion
Aortic Th17 and IL-17A+TCRγδ+ T cells express high levels of CXCR6 within atherosclerotic plaques
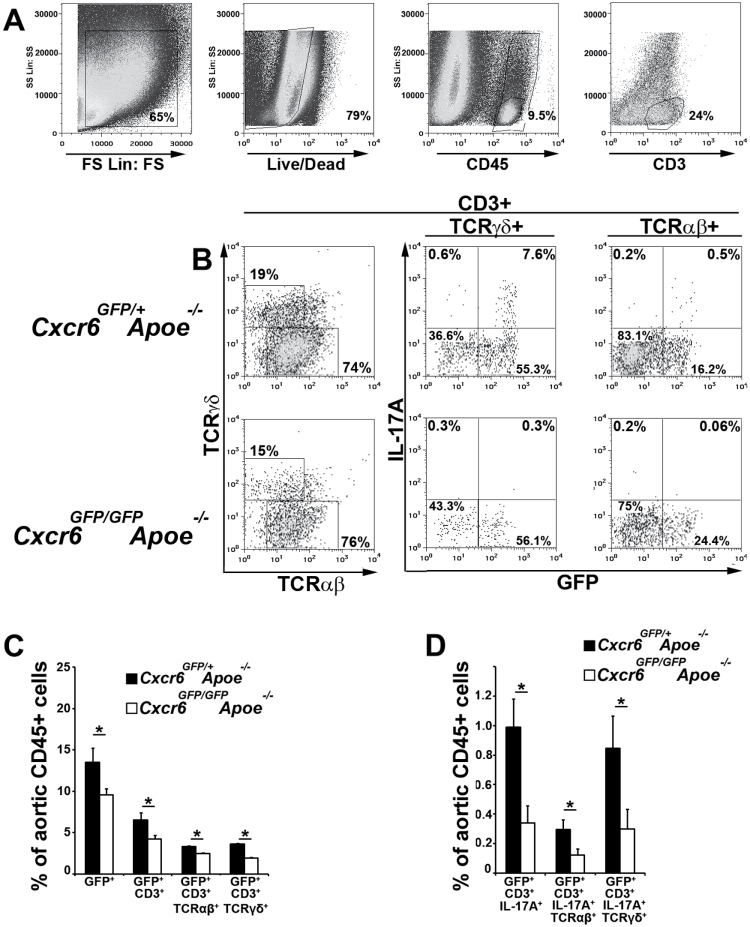
Th17 and IL-17A+TCRγδ+ T cells are present within atherosclerotic Apoe −/− aortas (7), and several lines of evidence suggest that they play a pro-atherogenic role (6, 7, 9, 14). However, the mechanisms behind their recruitment to the aortic wall are unclear. CXCL16 is one of the most abundant chemokines expressed in murine and human atherosclerotic plaques (21, 31) and its receptor, CXCR6, is known to participate in T-cell migration (32). Prior work has demonstrated a pro-atherogenic role for CXCR6, as CXCR6-GFP knockin Cxcr6 GFP/GFP Apoe −/− mice, which lack the functional CXCR6 receptor, displayed reductions in atherosclerotic lesions, aortic macrophage and Th1 accumulation (32). Thus, we hypothesized that CXCR6 might play a role in the recruitment of Th17 and IL-17A+TCRγδ+ T cells to atherosclerotic plaques. To test this hypothesis, we examined 40- to 50-week-old atherosclerotic Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− mice for aortic CXCR6-expressing Th17 and TCRγδ+ T cells (Fig. 1). CXCR6-GFP expression levels correlate with CXCR6 protein expression in Cxcr6 GFP/+ mice (33). As we previously reported, the percentage of CD45+GFP+ leukocytes was significantly decreased in Cxcr6 GFP/+ Apoe −/− versus Cxcr6 GFP/GFP Apoe −/− mice and approximately 25–35% of CD3+ cells expressed GFP within the aortas of Cxcr6 GFP/+ Apoe −/− mice [adapted from (32)]. In addition, we observed significantly less CXCR6-GFP-expressing CD3+ T cells, CD3+TCRαβ+ T cells and CD3+TCRγδ+ T cells in Cxcr6 GFP/GFP Apoe −/− aortas in comparison with Cxcr6 GFP/+ Apoe −/− controls (Fig. 1C). To determine if CXCR6 might be required for the accumulation of Th17 and IL-17A+TCRγδ+ T cells, we examined aged Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− aortas for IL-17A-expressing T cells. As previously described (7), we observed populations of aortic CD3+TCRαβ+ and CD3+TCRγδ+ T cells that expressed IL-17A (Fig. 1B). Interestingly, all aortic IL-17A+ T cells expressed high levels of CXCR6-GFP in Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− mice, and CXCR6GFP-expressing IL-17A+ T cells included both Th17 and TCRγδ+ T cells (Fig. 1B and D). Under steady-state conditions, CXCR6GFP-expressing Th17 and IL-17A+TCRγδ+ T cells were virtually absent in Cxcr6 GFP/GFP Apoe −/− aortas (Fig. 1B and D). Together, these results suggest that although there are many leukocytes that express CXCR6 within atherosclerotic plaques, aortic Th17 and IL-17A+TCRγδ+ T cells are defined by high expression of IL-17A and CXCR6.
Fig. 1.
Aortic Th17 and IL-17A+TCRγδ+ T cells express high levels of CXCR6 in atherosclerosis. Forty- to fifty-week-old Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− aortas were assessed for CD3ε, IL-17A, TCRαβ, TCRγδ and CXCR6-GFP expression by intracellular cytokine staining. (A) Representative flow cytometry gating scheme. For the analysis of CXCR6GFP and IL-17A expression by T cells, the aortic samples were gated on Forward/Side Scatter, Live events (Live/Dead Aqua low-negative), CD45+ cells and CD3+ T cells. (B) Representative TCRαβ and TCRγδ staining amongst aortic T cells, and CXCR6GFP expression and IL-17A staining within the CD3+TCRγδ+ and CD3+TCRαβ+ T-cell gates. (C) The average percentages of various CXCR6/GFP-expressing cells and (D) IL-17A+GFP+ populations among CD45+ aortic leukocytes. The mean ± SEM are shown. n = 13 Cxcr6 GFP/+ Apoe −/− mice, n = 7 Cxcr6 GFP/GFP Apoe −/− mice, three to six independent experiments. *P < 0.05.
Th17 cells rely on CXCL16/CXCR6-dependent chemotaxis to migrate to atherosclerotic plaques
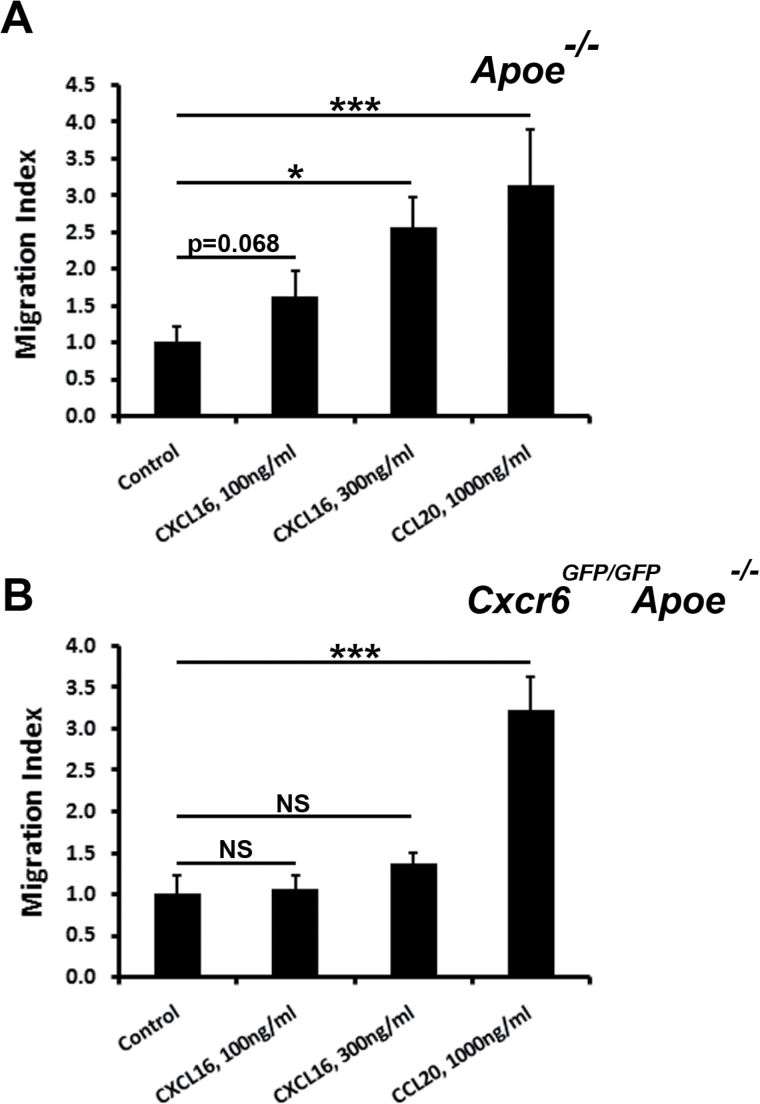
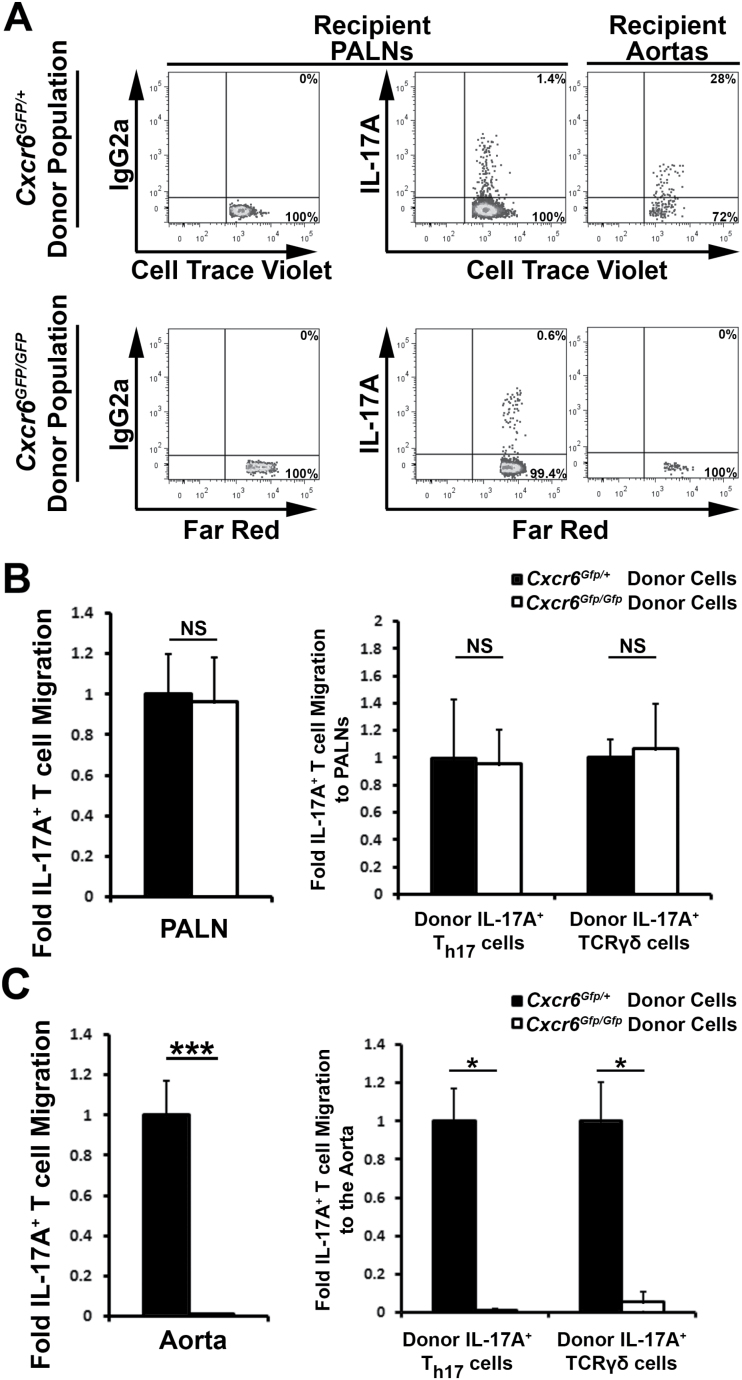
As all aortic IL-17A+ T cells express high levels of CXCR6, CXCR6 has been reported to be involved in the recruitment of IL-17A+TCRγδ+ T cells to the dermis (34) and Cxcr6 GFP/GFP Apoe −/− mice displayed a 60% reduction in aortic IL-17A+ T-cell content, we hypothesized that aortic Th17 and IL-17A+TCRγδ+ T-cell recruitment would partially rely on CXCL16/CXCR6-dependent chemotaxis. To test this hypothesis, we first examined the migratory potential of splenic Apoe −/− and Cxcr6 GFP/GFP Apoe −/− Th17 cells in vitro (Fig. 2). In transwell experiments, Apoe −/− splenic Th17 cells (Fig. 2A) migrated in a dose-dependent manner toward CXCL16, whereas CXCR6-deficient Th17 cells were unresponsive (Fig. 2B). In addition to CXCR6 and IL-17A, Th17 cells also express the chemokine receptor CCR6 (23, 24). Thus, as a positive control, we also determined the chemotactic potential of WT and CXCR6-deficient Th17 cells toward the CCR6 ligand, CCL20 (Fig. 2). As expected, Apoe −/− Th17 cells chemotactically migrated toward CCL20 in vitro. Thus, as CXCR6-dependent Th17 migration has not been demonstrated in atherosclerosis, we sought to formally test whether the CXCL16/CXCR6 axis is required for the recruitment of aortic Th17 cells in vivo. To test this, we adoptively transferred fluorescently labeled Cell Trace Violet+ Cxcr6 GFP/+ Apoe −/− and Far Red+ Cxcr6 GFP/GFP Apoe −/− splenocytes to 40-week-old atherosclerotic Apoe −/− recipients and tracked Th17 and IL-17A+TCRγδ+ T cell recruitment to the aorta and PALN 72h post-transfer (Fig. 3). As we demonstrated previously that Cxcr6 GFP/+ Apoe −/− and Apoe −/− mice display elevated atherogenesis (13.5±1.7% and 11.3±1.9% of aortic lesion area, respectively) in comparison with Cxcr6 GFP/GFP Apoe −/− mice (5.0±0.5% of lesion area) (32), Cxcr6 GFP/GFP and Cxcr6 GFP/+ splenocytes were used to specifically track the migration of CXCR6-GFP+ IL-17A+ T cells. In these experiments, Cxcr6 GFP/+ and Cxcr6 GFP/GFP donor T cells and IL-17A+ T cells accumulated to a similar extent in peripheral blood and lymphoid organs, including the PALN (Fig. 3A and B), spleens, peripheral lymph nodes (data not shown). In contrast, CXCR6-deficient T cells (1±0.2-fold Cxcr6 GFP/+ versus 0.26±0.15-fold Cxcr6 GFP/GFP T cells, P = 0.016), IL-17A+ T cells, Th17 cells and IL-17A+TCRγδ+ T cells failed to accumulate within atherosclerotic lesions, in comparison with CXCR6-sufficient T cells (Fig. 3A and C). Thus, CXCR6 is required for the efficient recruitment of IL-17A+ T cells to atherosclerotic lesions.
Fig. 2.
Th17 cells recovered from atherosclerotic mouse spleens chemotactically migrate toward CXCL16. Forty- to fifty-week-old Apoe −/− and Cxcr6 GFP/GFP Apoe −/− peripheral lymph nodes were collected and 0.3–0.4×106 cells were seeded into transwells containing complete RPMI1640 supplemented with BSA, 100ng ml–1 CXCL16, 300ng ml–1 CXCL16 or 1000ng ml–1 CCL20 for cell migration experiments. The cells were allowed to migrate for 3h before the cells in the bottom well were collected for re-stimulation and intracellular cytokine staining. The results depict IL-17A+ T cell migration normalized to the mean of the BSA controls (30). (A) Fold Cxcr6 GFP/+ Apoe −/− IL-17A+ T-cell transwell migration. (B) Fold Cxcr6 GFP/GFP Apoe −/− IL-17A+ T-cell transwell migration. The results are shown as the mean ± SEM and are representative of three technical replicates from three to four independent experiments. The data are expressed as the mean ± SD of triplicate wells. *P < 0.05, **P < 0.01, ***P < 0.001. NS, not significant.
Fig. 3.
CXCR6 is required for short-term IL-17A+ T-cell homing to atherosclerotic aortas; 40-week-old Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− splenocytes were labeled with Cell Trace Violet or Far Red fluorescent dyes, mixed in a 1:1 ratio, and adoptively transferred to 40-week-old atherosclerotic Apoe −/− recipients. Seventy-two hours post-transfer, the recipient spleens, PALN, and aortas were assessed for donor IL-17A+ T cells. (A) Representative flow cytometry plots from two pooled recipient Apoe −/− mice. All flow cytometry plots are gated on CD45+ dye+ donor leukocytes. Recipient Apoe −/− PALN isotype control staining (left), and IL-17A staining in the PALNs (middle) and aortas (right) within the gated Cxcr6 GFP/+ and Cxcr6 GFP/GFP donor populations. (B and C) Quantification of the IL-17A+ T-cell homing results. The percentage of IL-17A+ Cxcr6 GFP/+ and IL-17A+ Cxcr6 GFP/GFP T cells that successfully migrated to the recipient Apoe −/− PALNs, and aortas, normalized to the starting percentage of IL-17A+ T cells and IL-17A+ Cxcr6 GFP/+ T cells within the recipient organs. The mean ± SEM are shown. n = 9 independent transfer experiments. *P < 0.05, ***P < 0.001. NS, not significant.
Interestingly, peri-aortic and splenic IL-17A+ T cells, Th17, and IL-17A+TCRγδ+ T cells, express high levels of CCR6 (data not shown). Within the aorta, Th17 and IL-17A+TCRγδ+ T cells are similarly 98.96±0.84% and 95.1±2.62% positive for CCR6, respectively. However, as the deficiency of CXCR6 almost completely abolished the accumulation of IL-17A+ T cells into aorta, CCR6 may not compensate for the defective homing of CXCR6-deficient IL-17A+T cells to the aorta. This observation is in line with recent studies that demonstrated that CCR6 is dispensable for the recruitment of aortic Th17 cells in Ccr6 −/− Apoe −/− and Ccr6 −/− Ldlr −/− mice (26, 35). Thus, despite high expression of the chemokine receptor CCR6, Th17 cells might instead rely majorly on CXCR6-depedent chemotaxis to migrate toward atherosclerotic lesions.
Importantly, the recruitment of Cxcr6 GFP/+ IL-17A+ T cells to the aorta was disproportionately lower (1±0.2-fold Cxcr6 GFP/+ versus 0.052±0.03-fold Cxcr6 GFP/GFP T-cell recruitment, P = 0.039) than the proportion of recruited total Cxcr6 GFP/+ T cells (1±0.19-fold Cxcr6 GFP/+ versus 0.26±0.15 Cxcr6 GFP/GFP T-cell recruitment, P = 0.016). We therefore determined whether Cxcr6 GFP/+ IL-17A- T cells might express other chemokine receptors in addition to CXCR6. Aortic Cxcr6 GFP/+IL-17A−TCRαβ+ T cells, including IFNγ+ Th1 cells, majorly express the Th1-related chemokine receptor CXCR3 (80±2.5% of Cxcr6 GFP/+ IL-17A-IFNγ+ Th1 cells), whereas Cxcr6 GFP/+ Th17 and IL-17A+TCRγδ+ T cells are mostly negative (18±4% and 20±1.6%, respectively). Thus, Cxcr6 GFP/+ IL-17A− T cells likely compensate for CXCR6-deficient conditions via CXCR3, whereas CXCR6 is required for the recruitment of Th17 and IL-17A+TCRγδ+ T cells to atherosclerotic lesions.
Altogether, these data demonstrate that IL-17A+ T cells, including Th17 and IL-17A+ TCRγδ+ T cells, require CXCR6 in order to efficiently migrate to atherosclerotic lesions in Apoe −/− mice. These observations are important as it is currently unclear which chemokine receptors are required for the migration of IL-17A-producing cells in atherosclerosis, and aortic T-cell homing experiments are technically challenging. Prior work on the functions of Th17 and IL-17A+TCRγδ+ T cells have demonstrated that both subsets accumulate in atherosclerotic lesions and likely promote atherogenesis by supporting further monocyte recruitment and macrophage maturation (6, 7, 9, 14, 15, 18, 19, 36). Th17 cells are known to express the chemokine receptors CCR6 (23, 24) and CXCR6 (22) but the precise combination of chemokine receptors required for Th17 migration to atherosclerotic lesions was unclear. Several studies have reported the presence of IL-17A-producing T cells in sites of chronic inflammation (23–25) and have implicated the chemokine receptor CCR6 in their recruitment. However, the potential roles of other complementary Th17 chemokine receptors were not assessed. Prior work involving CCR6-deficient Apoe −/− and Ldlr −/− mice demonstrated a decidedly pro-atherogenic role for CCR6; however, IL-17A expression was unaltered between CCR6-deficient and CCR6-sufficient mice (26, 35). Here, we expand on these observations by reporting that CCR6 does not compensate for a genetic CXCR6-deficiency in short-term aortic IL-17A+ T-cell recruitment experiments, within atherosclerotic Apoe −/− mice.
Additionally, work from our group investigated the effects of CXCR6 on atherosclerosis using Cxcr6 GFP/+ Apoe −/− and Cxcr6 GFP/GFP Apoe −/− mice (32). In that study, atherosclerotic lesions—particularly in the aortic arch, macrophage accumulation, T-cell homing and IFNγ production—were significantly decreased in CXCR6-deficient mice. Thus, we hypothesized in the present study that CXCR6 might affect Th17 and IL-17A+TCRγδ+ T-cell recruitment to the aorta. Our results here demonstrated that all IL-17A+ T cells within the aorta express CXCR6 and that CXCR6-deficient Th17 and IL-17A+TCRγδ+ T cells were unable to effectively migrate in transwell and short-term homing experiments. CXCL16 is expressed by endothelial and smooth muscle cells, macrophages and dendritic cells (37). CXCR6 is the only known chemokine receptor for CXCL16, which can serve as a chemoattractant or mediate cell adhesion for T cells (37). It is possible that this dual function of CXCL16 provides a unique combination that successfully supports IL-17A+ T-cell migration into atherosclerotic lesions. Additionally, CXCL16-expressing cells are detected within human and murine atherosclerotic vessels, and circulating CXCL16 levels are elevated in patients with acute coronary syndromes (21). CXCL16 levels also positively correlate with the clinical outcomes of stroke and CAD. Thus, elevated CXCL16 production might accelerate the recruitment of Th17 and IL-17A+TCRγδ+ T cells in these conditions, thereby supporting further inflammation and atherogenesis. In summary, our results demonstrate that Th17 and IL-17A+TCRγδ+ T cells use CXCL16/CXCR6 in order to efficiently migrate toward atherosclerotic lesions.
Funding
National Heart, Lung, and Blood Institute (HL107522 to E.V.G.).
Acknowledgements
We thank T. Phillips for her technical assistance.
Conflict of interest statement: The authors declared no conflict of interests.
References
- 1. Galkina E. and Ley K. 2009. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 27:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Libby P. Lichtman A. H. and Hansson G. K. 2013. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity 38:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaffen S. L. 2009. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolls J. K. and Lindén A. 2004. Interleukin-17 family members and inflammation. Immunity 21:467. [DOI] [PubMed] [Google Scholar]
- 5. Reynolds J. M. Angkasekwinai P. and Dong C. 2010. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 21:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erbel C., Chen L., Bea F., et al. 2009. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 183:8167. [DOI] [PubMed] [Google Scholar]
- 7. Smith E. Prasad K. M. Butcher M. Dobrian A. Kolls J. K. Ley K. and Galkina E. 2010. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121:1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taleb S., Romain M., Ramkhelawon B., et al. 2009. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 206:2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Es T., van Puijvelde G. H., Ramos O. H., et al. 2009. Attenuated atherosclerosis upon IL-17R signaling disruption in LDLr deficient mice. Biochem. Biophys. Res. Commun. 388:261. [DOI] [PubMed] [Google Scholar]
- 10. Butcher M. and Galkina E. 2011. Current views on the functions of interleukin-17A-producing cells in atherosclerosis. Thromb. Haemost. 106:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eid R. E., Rao D. A., Zhou J., et al. 2009. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 119:1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erbel C., Dengler T. J., Wangler S., et al. 2011. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res. Cardiol. 106:125. [DOI] [PubMed] [Google Scholar]
- 13. Gao Q., Jiang Y., Ma T., et al. 2010. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J. Immunol. 185:5820. [DOI] [PubMed] [Google Scholar]
- 14. Butcher M. J. Gjurich B. N. Phillips T. and Galkina E. V. 2012. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ. Res. 110:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Usui F., Kimura H., Ohshiro T., et al. 2012. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem. Biophys. Res. Commun. 420:72. [DOI] [PubMed] [Google Scholar]
- 16. Danzaki K., Matsui Y., Ikesue M., et al. 2012. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 32:273. [DOI] [PubMed] [Google Scholar]
- 17. Gistera A., Robertson A. K., Andersson J., et al. 2013. Transforming growth factor-b signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci. Transl. Med. 5:196ra100. [DOI] [PubMed] [Google Scholar]
- 18. Madhur M. S., Funt S. A., Vinh A., et al. 2011. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 31:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erbel C., Akhavanpoor M., Okuyucu D., et al. 2014. IL-17A influences essential funcitons of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J. Immunol. 193:4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galkina E. and Ley K. 2007. Leukocyte influx in atherosclerosis. Curr. Drug Targets 8:1239. [DOI] [PubMed] [Google Scholar]
- 21. Zernecke A. and Weber C. 2014. Chemokines in atherosclerosis: proceedings resumed. Arterioscler. Thromb. Vasc. Biol. 34:742. [DOI] [PubMed] [Google Scholar]
- 22. Lim H. W. Lee J. Hillsamer P. and Kim C. H. 2008. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J. Immunol. 180:122. [DOI] [PubMed] [Google Scholar]
- 23. Yamazaki T., Yang X. O., Chung Y., et al. 2008. CCR6 Regulates the Migration of Inflammatory and Regulatory T Cells. J. Immunol. 181:8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hirota K., Yoshitomi H., Hashimoto M., et al. 2007. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 204:2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alcaide P., Maganto-Garcia E., Newton G., et al. 2012. Difference in Th1 and Th17 lymphocyte adhesion to endothelium. J. Immunol. 188:1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manthey H. D., Cochain C., Barnsteiner S., et al. 2013. CCR6 selectively promotes monocyte mediated inflammation and atherogenesis in mice. Thromb. Haemost. 110:1267. [DOI] [PubMed] [Google Scholar]
- 27. Unutmaz D. Xiang W. Sunshine M. J. Campbell J. Butcher E. and Littman D. R. 2000. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J. Immunol. 165:3284. [DOI] [PubMed] [Google Scholar]
- 28. Butcher M. J., Herre M., Ley K., Galkina E. 2011. Flow cytometry analysis of immune cells within murine aortas. J. Vis. Exp. 53. pii: 2848. doi:10.3791/2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galkina E. Kadl A. Sanders J. Varughese D. Sarembock I. J. and Ley K. 2006. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J. Exp. Med. 203:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valcu M. and Valcu C. M. 2011. Data transformation practices in biomedical sciences. Nat. Methods 8:104. [DOI] [PubMed] [Google Scholar]
- 31. Aslanian A. M. and Charo I. F. 2006. Targeted disruption of the scavenger receptor and chemokine CXCL16 accelerates atherosclerosis. Circulation 114:583. [DOI] [PubMed] [Google Scholar]
- 32. Galkina E., Harry B. L., Ludwig A., et al. 2007. CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation 116:1801. [DOI] [PubMed] [Google Scholar]
- 33. Geissmann F., Cameron T. O., Sidobre S., et al. 2005. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gray E. E. Suzuki K. and Cyster J. G. 2011. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J. Immunol. 186:6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wan W., Lim J. K., Lionakis M. S., et al. 2011. Genetic deletion of chemokine receptor Ccr6 decreases atherogenesis in ApoE-deficient mice. Circ. Res. 109:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lim H., Kim Y. U., Sun H., et al. 2014. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity 40:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matloubian M. David A. Engel S. Ryan J. E. and Cyster J. G. 2000. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 1:298. [DOI] [PubMed] [Google Scholar]