LPS-induced acute lung injury requires IL-23 and proceeds via IL-22 from memory Th17 cells
Keywords: acute lung injury, antibiotics, commensal bacteria, memory Th17
Abstract
Lipopolysaccharide (LPS)-induced acute lung injury (ALI) is known as a mouse model of acute respiratory distress syndrome; however, the function of T-cell-derived cytokines in ALI has not yet been established. We found that LPS challenge in one lung resulted in a rapid induction of innate-type pro-inflammatory cytokines such as IL-6 and TNF-α, followed by the expression of T-cell-type cytokines, including IL-17, IL-22 and IFN-γ. We discovered that IL-23 is important for ALI, since blockage of IL-23 by gene disruption or anti-IL-12/23p40 antibody treatment reduced neutrophil infiltration and inflammatory cytokine secretion into the bronchoalveolar lavage fluid (BALF). IL-23 was mostly produced from F4/80+CD11c+ alveolar macrophages, and IL-23 expression was markedly reduced by the pre-treatment of mice with antibiotics, suggesting that the development of IL-23-producing macrophages required commensal bacteria. Unexpectedly, among T-cell-derived cytokines, IL-22 rather than IL-17 or IFN-γ played a major role in LPS-induced ALI. IL-22 protein levels were higher than IL-17 in the BALF after LPS instillation, and the major source of IL-22 was memory Th17 cells. Lung memory CD4+ T cells had a potential to produce IL-22 at higher levels than IL-17 in response to IL-1β plus IL-23 without TCR stimulation. Our study revealed an innate-like function of the lung memory Th17 cells that produce IL-22 in response to IL-23 and are involved in exaggeration of ALI.
Introduction
Acute respiratory distress syndrome (ARDS) is a complicated syndrome that presents with progressive hypoxemia and dyspnea. Several disorders, including sepsis, pneumonia, pancreatitis and major trauma, are associated with the development of ARDS (1). In spite of advances in respiratory care, the mortality rate remains over 40% (2). Acute lung injury (ALI) is defined as mild ARDS (3). Endotoxin, also known as lipopolysaccharide (LPS), is located in the outer membrane of gram-negative bacteria and has been shown to induce ALI and ARDS-like symptoms (4). LPS activates innate immune responses and stimulates the production of pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6, and chemokines from macrophages, which have been known to induce neutrophil accumulation, leakage of proteins into the bronchoalveolar lavage fluid (BALF), edema, and blood vessel and lung tissue damage. The role of T-cell-derived cytokines such as IFN-γ and IL-17 has not been well characterized in ALI. Several clinical studies have demonstrated a relationship between ARDS and Th17 cells. One study suggested that the IL-17-secreting CD4+ T cells in ALI/ARDS are associated with a high level of T-cell activation and proliferation (5, 6). However, the involvement of Th17 cells in ALI using gene-disrupted mice has not yet been demonstrated.
Th17 cells produce IL-17F and IL-22 in addition to IL-17A, and function primarily in host defense against fungal and bacterial pathogens. Th17 cells have emerged as crucial mediators of various inflammatory diseases, such as inflammatory bowel disease, multiple sclerosis, psoriasis and rheumatoid arthritis (RA). The differentiation of Th17 cells from naive T cells requires TGF-β and IL-6, both in vitro and in vivo, which induces the nuclear orphan receptors RORγt and RORα.
IL-22 is a member of the IL-10 family of cytokines (7). IL-22 exerts a biological effect via a heterodimeric receptor consisting of IL-22R1 and IL-10R2 (8, 9). IL-22 plays an important role in host defense against extracellular pathogens through enhancing the epithelial barrier function in the lung (10, 11). Although IL-22 is produced from pathogenic Th17 cells, the significance of IL-22 in inflammation remains controversial. In many cases, IL-22 functions against tissue damage associated with inflammation. We have shown that IL-22 from type 3 innate lymphoid cells (ILC3s) is protective against ConA-induced hepatitis (12). In the lung, IL-22 has been reported to reduce inflammation during influenza A virus infection (13) and to be protective against secondary bacterial infection (14). In addition, IL-22 has been shown to be essential for lung repair after influenza A infection (15). Lung fibrosis correlates with outcome in ARDS (16). IL-22 is protective against Bacillus subtillis-exposure-induced lung fibrosis (17). In contrast, IL-22 has been shown to play a pro-inflammatory role and to worsen bleomycin-induced airway inflammation (18).
IL-22 has been shown to be produced from various types of cells in acute and chronic inflammation in mice. Th17 cells are an important source of IL-22 and are involved in mucosal host defense, chronic inflammation and autoimmunity (19). In the lung, Th17 cells have been reported to produce IL-22 in gram-negative bacterial pneumonia (11) and bleomycin-induced airway inflammation (18). In humans, IL-22 was produced from a subset of CD4+ T cells referred to as Th22 cells, which produce IL-22 in the absence of IL-17 (20). A role for Th22 cells has also been proposed in a murine infection model against Citrobacter rodentium (21). However, the allegiance of IL-22 to a specific CD4+ T-cell subset is currently under debate (22). NK cells produce IL-22 and promote host defense in Klebsiella pneumoniae pneumonia (23) and influenza A infection (13). Alveolar macrophages produce IL-22 after LPS intranasal administration (24). γδT cells, which are key innate immune cells, also produce IL-22 (17). IL-22-producing ILCs play an important role in mucosal immunity, especially in the gut (25). Although ILCs in the lung also produce IL-22 in Streptococcus pneumoniae infection (26), the role and cellular source of IL-22 in the LPS-induced ALI model remains to be clarified.
In this study, we clarified the role of Th17-type cytokines in the LPS-induced ALI model. We found that LPS-induced ALI was dependent on IL-23 and that IL-22 rather than IL-17 played an important role in the acute phase of ALI. Furthermore, we demonstrated that the major source of IL-22 in the lung was CD4+ T cells. We also found that lung memory CD4+ T cells had a potential to produce IL-22 at higher levels than IL-17 in response to IL-1β plus IL-23 without TCR stimulation, and ALI and IL-22 expression in the lung required commensal bacteria. Our results suggest that the IL-23-memory Th17-IL-22 axis could be a novel mechanism of ALI in ARDS.
Methods
Animals
C57BL/6 [wild type (WT)] mice, 8–12 weeks of age, were purchased from CLEA (Tokyo, Japan). IL-22−/− mice were described previously (10) and were kindly provided by Dr Yoshimoto (Tokyo Medical School). IL-23p19−/−, CD3ε−/−, IFN-γ−/− and IL-17−/− mice were described previously (27). All mice were on a C57BL/6 background. Mice were kept in specific pathogen-free facilities at Keio University. All experiments using mice were carried out in accordance with the guidelines for animal care and use approved by Keio University.
Acute lung injury model
Mice were anesthetized using halothane and N2O. LPS from Escherichia coli serotype 0111: B4 (Sigma-Aldrich, St Louis, MO, USA) or PBS was intratracheally instilled into one of the lung lobes as described previously (28). The trachea was exposed, and a 24-gauge angiocatheter was inserted into it. After insertion, the catheter was advanced into the left lung, and 0.3mg kg–1 of LPS or PBS was instilled through the catheter. LPS was challenged into one lobe because challenge into two lobes was highly lethal.
BALF was collected 12, 24 and 72h after intratracheal instillation. After euthanasia by injection of a lethal dose of pentobarbital, the trachea was exposed and a 22-gauge angiocatheter was inserted into the trachea. The whole lungs were washed by two aliquots of 0.7ml PBS. Cells in the BALF were counted using trypan blue dye exclusion. Neutrophils in the BALF were distinguished by cell size and expression of CD11b, Gr-1, F4/80 and Siglec-F. Supernatant of BALF was collected following centrifugation at 400 × g for 10min at 4°C and stored at −80°C.
mRNA preparation and quantitative real-time polymerase chain reaction
RNA was extracted from sorted cells using the ReliaPrepTM RNA cell Miniprep System (Promega) and subjected to reverse transcription using a High Capacity cDNA Synthesis Kit (Applied Biosystems). Quantitative real-time polymerase chain reaction (RT–PCR) was performed using SsoFAst EvaGreen Supermix (Bio-Rad) with a CFX384 real-time PCR detection system (Bio-Rad). The results were normalized to HPRT levels. The primers used were as follows: 5′-TGAAGAGCTACTGTAATGATCAGTC-3′ and 5′-AGCAAGCTTGCAACCTTAACCA for HPRT; 5′-AGCGG GACATATGAATCTACTAAGAGA-3′ and 5′-GTCCTAGTAGGG AGGTGTGAAGTTG-3′ for IL-23p19; 5′-TCCGAGGAGTCA GTGCTAAA-3′ and 5′-AGAACGTCTTCCAGGGTGAA-3′ for IL-22; 5′-GCTCCAGAAGGCCCTCAG-3′ and 5′-CTTTCCC TCGCATTGACA-3′ for IL-17A; and 5′-CCTCCACTGCC AGCTGTGTGCTGTC-3′ for RORγt.
Histologic analysis
Lung tissue was fixed by inflation with 4% paraformaldehyde in PBS at a pressure of 25cm H2O. The isolated lungs were immersed in 4% paraformaldehyde for 24h. Fixed tissues were embedded in paraffin and then cut into 5 µm sections and stained with hematoxylin and eosin (HE).
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was performed with an ELISA kit (eBioscience, San Diego, CA, USA) in accordance with the manufacturer’s instructions.
Cell isolation
The mice were perfused with PBS transcardially. The left lungs were removed and excised. Excised lungs were digested with collagenase D (1mg ml–1, Sigma-Aldrich) and DNase I (1mg ml–1, Roche) at 37°C for 40min. After filtration with a cell strainer, the extract was centrifugated at 1300rpm for 5min at 4°C and re-suspended in magnetic cell sorting buffer (PBS, 0.5% bovine serum albumin, and 2mM EDTA) buffer.
Flow cytometry and cell sorting
For intracellular cytokine staining, cells were stimulated for 4h in complete medium with PMA (50ng ml–1) and ionomycin (500ng ml–1; both from Sigma-Aldrich) in the presence of brefeldin A (eBioscience). Surface staining was performed in the presence of Fc-blocking antibody (2.4G2), followed by intracellular staining for anti-IL-17 with a fixation and permeabilization kit (eBioscience) according to the manufacturer’s instructions. A single suspension was stained with CD3-FITC, CD4-PE, CD44-APC, CD62L-PerCP, NK1.1-APC, Sca1-PE, Thy1.2-APC-Cy7, Gr-1-FITC, F4/80-APC, Siglec-F-PE, CD11b-PerCP, CD11c-PE and IL-17-APC. All antibodies were obtained from eBioscience. Data were acquired using BD FACSCanto II (BD Biosciences) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA). Isolated cells from lung tissue were sorted using SH800 (SONY, Tokyo, Japan). Memory T cells derived from the lung were sorted as CD3+CD4+CD44high cells.
Antibody treatment
Mice were intraperitoneally injected with IL-12/23p40 antibody (200 µg) or PBS immediately before LPS or PBS intratracheal instillation, as previously described (29).
Antibiotics treatment
Mice that received antibiotics treatment were fed water with ampicillin (0.5mg ml–1), gentamicin (0.5mg ml–1), metronidazole (0.5mg ml–1), neomycin (0.5mg ml–1) and vancomycin (0.25mg ml–1) via their water bottle for 10 days (30, 31).
Statistical analysis
Statistical analysis was performed by an unpaired Student’s t-test. A P value <0.05 was judged as significant. All error bars represent the standard deviation (SD).
Results
LPS instillation in one lung induced both innate and T-cell cytokines
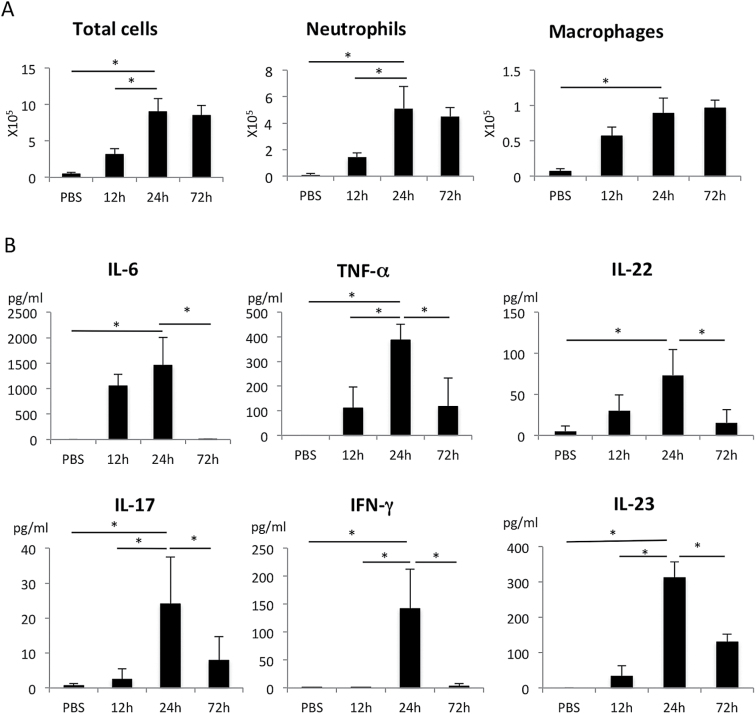
First, we established a model of ALI. Mice were anesthetized, and then LPS or PBS was intratracheally instilled in one lung. The LPS-administered lung became reddish due to bleeding after 24h, and the infiltration of inflammatory cells, which were mostly neutrophils and macrophages, was evident 24–72h after instillation (Fig. 1A). Innate inflammatory cytokines (IL-6, TNF-α and IL-23) were detected in the BALF 12–24h after instillation, which was preceded by the infiltration of immune cells. T-cell cytokines, IL-22, IL-17 and IFN-γ were highly expressed 24h after LPS instillation and were slightly delayed compared to innate inflammatory cytokine expression (Fig. 1B). IL-22 protein levels were 70–100 pg ml–1 in the BALF at 24h, which were always higher than those of IL-17 (10–40 pg ml–1) (Fig. 1B).
Fig. 1.
Time course of total cells, protein concentration and cytokines in BAL fluid after LPS challenge. The number of total cells, neutrophils and macrophages (A) and cytokine concentration (B) in BAL fluid 12, 24 and 72h after PBS or LPS instillation in WT mice. Neutrophils and macrophages were determined to be CD11b+Gr-1+ and F4/80+ using flow cytometry, respectively. Cytokine concentration was determined by ELISA. *P < 0.05 versus the value at 24h. n = 4 mice per group. Values represent the mean ± SD.
IL-23 is critical for the LPS-induced ALI model
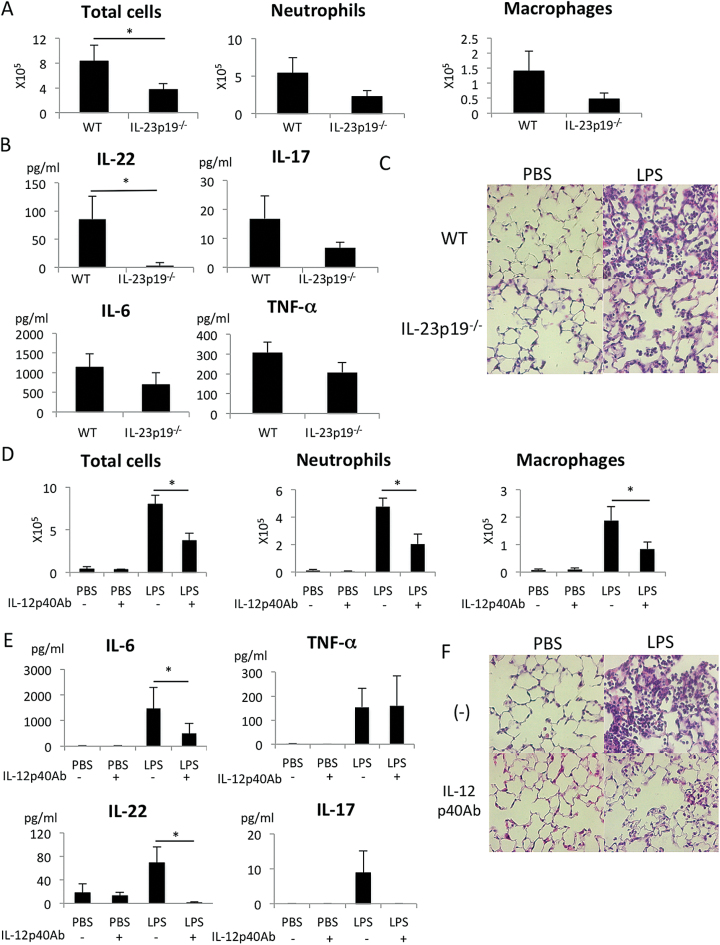
Since it has been shown that IL-23 is essential for the secretion of IL-17 and IL-22 from Th17, γδT cells and ILC3 cells, we first investigated the role of IL-23 in the LPS-induced ALI model. As shown in Fig. 2A, the infiltration of inflammatory cells into the BALF was strongly reduced in IL-23p19−/− mice compared with WT mice. IL-17 and IL-22 levels in the BALF were severely reduced in IL-23p19−/− mice (Fig. 2B). The expression of inflammatory cytokines, IL-6 and TNF-α, was also inhibited in IL-23p19−/− mice (Fig. 2B). Histological examination confirmed the much weaker inflammation in the LPS-induced ALI model in IL-23p19−/− mice compared with WT mice (Fig. 2C).
Fig. 2.
IL-23-dependent inflammation in the lung after LPS instillation. (A–C) The number of cells (A) and cytokine concentration (B) in the BAL fluid 24h after PBS or LPS instillation in WT or IL-23p19−/− (KO) mice. n = 4 per group. *P < 0.05. (C) Representative lung tissue sections stained with HE 24h after PBS or LPS instillation. Magnification: ×400. (D–F) The number of cells (D) and cytokine concentration (E) in the BAL fluid 24h after LPS instillation in mice treated with or without IL-12/23p40-neutralizing antibody. n = 5 per group. *P < 0.05. Values represent the mean ± SD. (F) Representative lung tissue sections stained with HE 24h after PBS or LPS instillation. Magnification: ×400.
IL-23 is a heterodimeric cytokine composed of IL-12/23p40 and IL-23p19 subunits (32). The pre-administration of IL-12p40-neutralizing antibody reduced the infiltration of inflammatory cells in the lung (Fig. 2D). The expression of IL-6, IL-22 and IL-23 was also inhibited in the group pre-administered with IL-12/23p40-neutralizing antibody (Fig. 2E). Histological examination also confirmed suppression of inflammation by the anti-p40 antibody (Fig. 2F). These data suggest that IL-23 is essential for IL-22 expression and is pro-inflammatory in the LPS-induced ALI model.
Commensal bacteria-dependent residential memory Th17 cells express IL-22 in response to IL-23
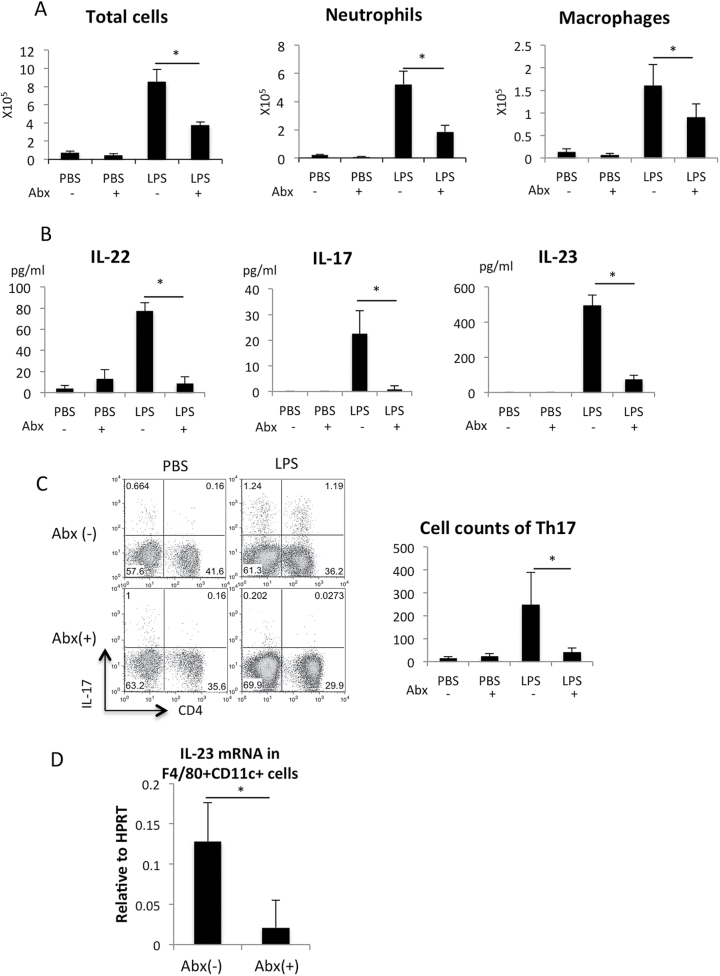
A previous report showed that Th17 differentiation in the lamina propria in the small intestine is dependent on commensal bacteria and reduced by treatment with selective antibiotics (33). Therefore, we suspected that commensal bacteria are also involved in Th17 cell development in the lung and LPS-induced ALI. As expected, the lung inflammation was suppressed in mice pre-treated with antibiotics (Fig. 3A ). The expression of IL-22, IL-17 and IL-23 was severely reduced in mice pre-treated with antibiotics (Fig. 3B). Although we failed to stain T cells with anti-IL-22 antibody, flow cytometric analysis of cells isolated from the lungs revealed that the IL-17+CD4+ (Th17) cell population was reduced in mice pre-treated with antibiotics (Fig. 3C). Alveolar and infiltrated macrophages have been shown to be distinguished by CD11b and CD11c expression (34). In the F4/80+ cells, about 70% were CD11blowCD11c+ alveolar macrophages and 30% were CD11bhigh infiltrated macrophages 24h after LPS challenge (data not shown). The F4/80− fraction was mostly Gr-1+ neutrophils. IL-23 has been shown to be produced from F4/80+CD11c+ alveolar macrophages in the lung (34). We confirmed that IL-23 expression in F4/80+CD11c+ alveolar macrophage fraction was markedly reduced by the pre-treatment of mice with antibiotics (Fig. 3D). These results indicate that the commensal bacteria in the lung are required for the production of IL-23, which is necessary for Th17 cell development and lung inflammation.
Fig. 3.
IL-23 expression and Th17 development were dependent on commensal bacteria. (A) LPS instillation was performed in mice treated with or without antibiotics (Abx) for 10 days. The number of cells in the BAL fluid 24h after PBS or LPS instillation in mice treated with antibiotics. n = 4–5 per group. *P < 0.05. (B) IL-22, IL-17 and IL-23 protein levels in the BAL fluid 24h after PBS or LPS instillation in mice treated with antibiotics. n = 4–5 per group. *P < 0.05. (C) IL-17 was detected by the intracellular staining of cells from the lungs 24h after instillation. Quantitative data of absolute number of Th17 cells are shown in the right. n = 4 per group. *P < 0.05. (D) F4/80+CD11c+ fraction in the BAL fluid were separated by FACS and the IL-23 mRNA levels were measured with quantitative RT–PCR. n = 4 per group. *P < 0.05.
IL-22 rather than IL-17 was critical for LPS-induced ALI
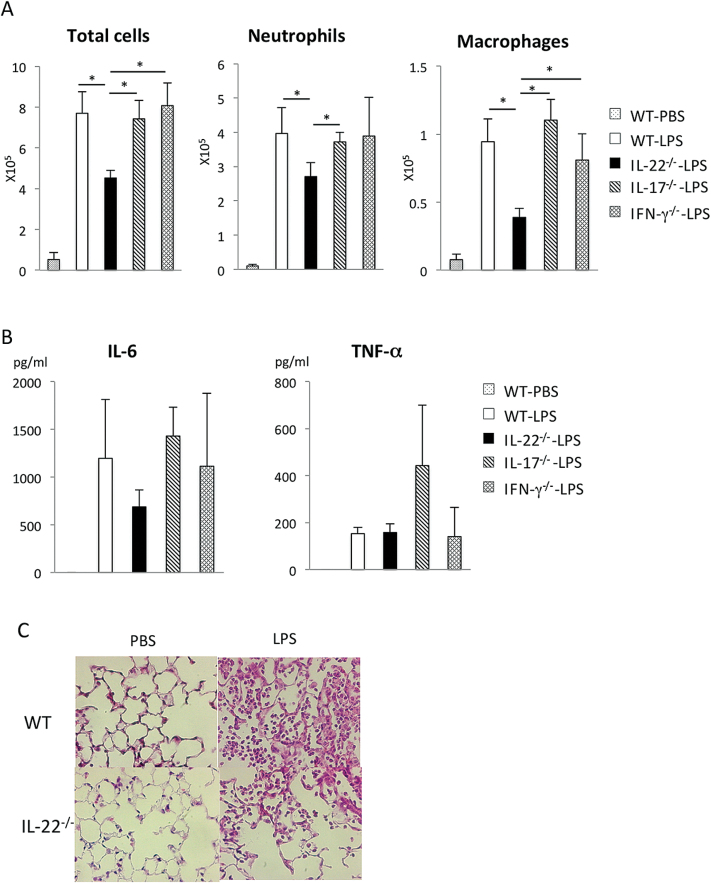
Next, we investigated the influence of IL-22, IL-17 and IFN-γ in LPS-induced ALI. The inflammatory cell infiltration and expression of inflammatory cytokines in the BALF were compared among WT, IL-22−/−, IL-17−/− and IFN-γ−/− mice. As shown in Fig. 4A, among these mice, the infiltration of inflammatory cells in the lung was significantly reduced in IL-22−/− mice, but not in other knockout (KO) mice. The expression of IL-6, but not TNF-α, was also reduced in IL-22−/− mice (Fig. 4B). In contrast, the expression of IL-6 in IL-17−/− and IFN-γ−/− mice was comparable to that in WT mice. Histological examination confirmed the much weaker inflammation in IL-22−/− mice compared with WT mice (Fig. 4C). Taken together, these results suggest that, among the T-cell-type cytokines we examined, IL-22 plays the most important role in promoting lung inflammation in the acute phase of LPS-induced ALI.
Fig. 4.
Role of cytokines in LPS-induced lung inflammation using cytokine knockout mice. (A and B) The number of cells (A) and cytokine concentration (B) in the BAL fluid from cytokine KO mice 24h after LPS instillation. n = 4 per group. *P < 0.05. (C) Representative HE staining of the lungs derived from WT or IL-22−/− mice 24h after PBS or LPS instillation. *P < 0.05. Values represent the mean ± SD. Magnification: ×400.
Memory CD4+ T cells were a major source of IL-22
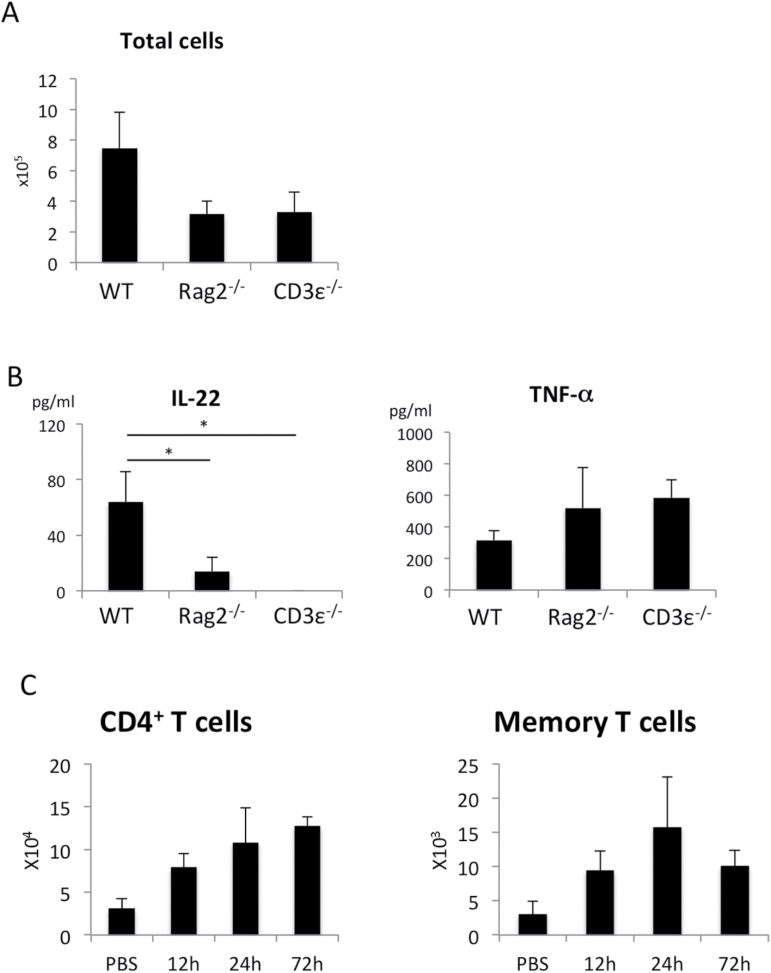
To investigate the major cellular source of IL-22, we utilized the ALI model with Rag2−/− and CD3ε−/− mice. Rag2−/− mice lack B cells and T cells, but possess ILCs. In contrast, CD3ε−/− mice lack only T cells. The infiltration of inflammatory cells in the LPS-treated lung was reduced in both Rag2−/− and CD3ε−/− mice (Fig. 5A). As shown in Fig. 5B, the expression of IL-22 was markedly reduced in Rag2−/− and CD3ε −/− mice, whereas the expression of TNF-α was comparable among WT, Rag2−/− and CD3ε−/− mice. These data indicate that T cells play an important role in the augmentation of inflammation and IL-22 production in the acute phase of LPS-induced ALI. As shown in Fig. 5C, LPS challenge resulted in an increase of total CD4+ T cells, as well as CD4+CD44highCD62Llow T cells in the lung (Fig. 5C), suggesting that helper T cells infiltrated and were activated after LPS challenge.
Fig. 5.
LPS-induced ALI was dependent on T cells. The number of total cells (A) and cytokine concentration (B) in the BAL fluid from WT, Rag2−/−, or CD3ε−/− mice 24h after LPS instillation. n = 3 per group. *P < 0.05. Values represent the mean ± SD. (C) The number of CD4+T cells and CD4+CD44highCD62Llow memory or effector T cells in the lung after LPS challenge. n = 4 for each point.
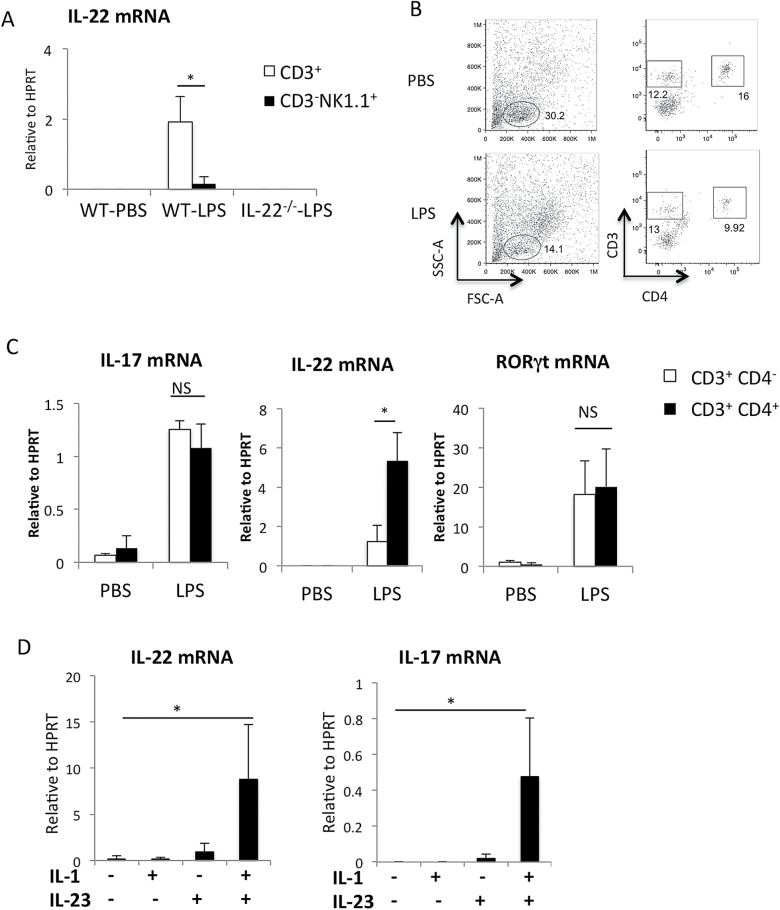
Next, we investigated IL-22 expression in T cells and other lymphoid cells after sorting. We could not detect IL-22 mRNA in the CD3− cell fraction or NK cell fraction in the LPS-treated lung (Fig. 5B and Fig. 6A). Since IL-22 levels were extremely low in Rag2−/− and CD3ε−/− mice (Fig. 5B), IL-22 was mostly produced from T cells. IL-22 mRNA expression was detected in sorted CD3+ T cells isolated from lungs instilled with LPS (Fig. 6A). Then we separated T cells with CD4 expression (Fig. 6B). The CD3+CD4− cell fraction contained mostly CD8+ T cells and low levels of γδT cells (data not shown). Although IL-17 mRNA and RORγt mRNA levels were comparable between CD4+ cells and CD4− cells, IL-22 mRNA expression was significantly higher in CD4+ cells compared with CD4− cells (Fig. 6C). In CD4+ T cells, IL-22 mRNA levels normalized to HPRT were about five times higher than those of IL-17 (Fig. 6C). These results indicate that memory CD4+ T cells were the main cellular source of IL-22 in LPS-induced ALI.
Fig. 6.
Detection of IL-22 in CD4+ T cells after LPS instillation in the lung and IL-22 production form memory CD4+ T cells in vitro. (A) Mononuclear cells isolated from the lungs 24h after PBS or LPS instillation were labeled with anti-CD3, anti-NK1.1 and anti-CD4 antibodies. Il22 mRNA expression in CD3+ T cells and NK cells (CD3-NK1.1+) FACS sorted from the lungs 24h after PBS or LPS instillation. n = 3 per group. *P < 0.05. (B) Representative data of flow cytometric analysis of cells isolated from the lungs 24h after PBS or LPS instillation. n > 5. (C) IL17, IL22 and RORγt mRNA expression in CD3+ CD4+ cells (Th cells) and CD3+ CD4- cells sorted from the lungs 24h after PBS or LPS instillation. mRNA levels were determined by quantitative RT–PCR. n = 3 per group. *P < 0.05. Values represent the mean ± SD. (D) Lung residential memory CD4+ T cells (CD4+CD44highCD62Llow) were purified by cell sorter and stimulated with IL-23 and/or IL-1β for 6h. mRNA levels of IL-22 and IL-17 were determined by quantitative RT–PCR.
Since IL-22 was detected within 24h after LPS stimulation, we speculated that memory-type helper T cells are involved in the acute response without TCR recognition. It has been shown that memory Th17 cells can produce IL-17 in response to IL-23 without TCR stimulation (35). It has also been shown that IL-1β augments IL-17 expression in IL-23-stimulated γδT cells (36). Thus, we examined whether IL-22 can be produced from lung memory helper T cells in response to IL-1β and IL-23 without TCR stimulation. As shown in Fig. 6D, both IL-17 and IL-22 mRNA levels were markedly elevated by IL-23 plus IL-1β treatment in CD4+CD44highCD62Llow T cells freshly isolated from the lung. Again, we found that IL-22 mRNA levels determined by quantitative RT–PCR were much higher than those of IL-17 (Fig. 6D). Similarly, splenic memory T cells produced IL-22 in response to IL-23 plus IL-1β (data not shown). Since IL-22 production from Th22 cells has been reported to be independent of IL-23 (21), we categorize IL-22-producing helper T cells as Th17 cells.
Discussion
In this study, we utilized a lung model for ALI and explored the underlying pathogenesis. The obtained data suggest the importance of the IL-23-memory Th17-IL-22 axis in the pathology of ALI.
Unlike many other immune-mediated diseases, our data suggest that IL-22, but not IL-17, is the major effector cytokine in ALI. IL-22 has been implicated in both pro- and anti-inflammatory properties in vivo. In a skin inflammation model, IL-22 was shown to be produced from memory T cells in response to IL-23 and to promote inflammation (37). Similarly, in our LPS-induced ALI, IL-22 acted mainly as a pro-inflammatory factor. One report using a bleomycin-induced airway inflammation model suggested that IL-22 was pathological only in the presence of IL-17A (18). In that study, IL-22 was shown to be protective against inflammation in the absence of IL-17A. In contrast to their model, the symptoms of LPS-induced ALI in the IL-22−/−, but not IL-17−/−, mice were ameliorated compared with WT mice. The exact mechanism of how IL-22 works as a pro-inflammatory cytokine in an LPS-induced ALI model remains to be determined. Interestingly, a previous report suggested that the systemic injection of IL-22 alone can cause most of the acute phase responses (38). In a psoriasis model, IL-22 KO mice showed less neutrophil accumulation (37). In these models, IL-22 has been shown to induce chemokines (CXCL1 and CXCL5), as well as inflammatory gene expression, from target tissue (i.e. lung or skin) (17, 38, 39). It was also proposed that serum amyloid A, induced by IL-22, may promote the downstream pathway of inflammation (38). These IL-22-mediated chemokines and inflammatory factors may be involved in LPS-induced ALI.
The production of effector cytokines after LPS instillation was rapid and happened only in T-cell-replete animals. This is suggestive of the memory Th response rather than the de novo priming of naive T cells against LPS. A pre-treatment of mice by a cocktail of antibiotics, which is known to reduce the commensal bacterium load (22), strongly inhibited the development of ALI and IL-22 expression. Pre-treatment with antibiotics has been shown to inhibit the differentiation of Th17 cells in the intestine (33, 40). Similarly, we observed severe reduction of Th17 cells in the lung by the antibiotic treatment (Fig. 3C). Since IL-23 expression was also reduced in antibiotic-treated mice, there is a possibility of the reduction of IL-23-expressing macrophages and dendritic cells. In any case, it is likely that IL-22-producing Th17 cells are also involved in the pathogenesis of lung disease. In support of this, the neutralization/genetic ablation of IL-23 dramatically ameliorated the ALI in our model. Although IL-23 was initially thought to mediate the Th17 response, it was later found that it regulated the terminal differentiation of committed Th17 cells and their expansion, migration, survival and maintenance (41). Also, IL-23 was required for the recruitment of Th17 cells and mediated lung inflammation, as shown in an experimental silicosis (42). Therefore, it is likely that both the neutralization and deficiency of IL-23 ameliorate the disease by affecting the Th17 memory/effector response.
Since LPS challenge increased the number of memory CD4+ T cells in the BALF (Fig. 5C), it is not clear whether IL-22 was produced from residential memory Th17 cells or infiltrated activated Th17 cells. However, we have shown that residential memory Th17 cells have a potential to produce IL-17 and IL-22 in response to inflammatory cytokines (Fig. 6D). Thus, residential memory Th17 cells could be involved in IL-22 production at least in part. We found that the levels of IL-22 were much higher than IL-17 in the lung memory CD4+ T cells, as well as splenic memory CD4+ T cells (Fig. 6D and data not shown). We have shown that IL-17 levels were higher than IL-22 in Th17 cells induced from naive T cells in vitro (A. Yoshimura, unpublished data). Thus, higher expression of IL-22 than IL-17 could be characteristic of memory Th17 cells. The molecular basis for this phenomenon remains to be clarified. IL-22-producing lung CD4+ T cells could be Th22, a population of T cells that primarily produce IL-22 rather than Th17. The technical difficulty of detecting IL-22 by intracellular cytokine staining precluded us from the simultaneous intracellular detection of IL-22 and IL-17 at the protein level. Thus, we have not succeeded in determining whether memory CD4+ T cells produced both IL-17 and IL-22 simultaneously or a mixture of Th17 and Th22. In humans, Th22 cells are negative for the IL-23 receptor (43). In mice, the Th22 function has been proposed to be much less dependent on IL-23 compared with ILC3 or Th17 cells (21). Since our ALI model was strictly dependent on IL-23, these reports suggest that the LPS-induced ALI is largely dependent on Th17 rather than Th22.
Even though we showed that IL-17 and IL-22 were produced from lung memory CD4+ T cells in response to IL-23 plus IL-1β in the absence of TCR stimulation, it remains unclear whether IL-22 was produced from memory Th17 cells without antigen stimulation in vivo. It has also not been determined that Th17 cells were residential in the lung or infiltrated from other organs. Since antibiotics reduced the IL-22 secretion, it is possible that the rapid Th17 response was caused by the re-stimulation of memory Th17, specific for bacterial lipids derived from commensals. Alternatively, chronically stimulated memory Th17 by commensals may acquire the innate-like properties that are seen in γδ T cells and ILC3s (44, 45) and produce IL-22 in response to cytokines without TCR signaling. There is another possibility that the commensal bacteria are necessary for the development of myeloid cells that produce IL-23. Further study is necessary to clarify how IL-22-producing memory Th17 cells develop in the lung.
In conclusion, we showed for the first time that IL-22 and IL-23 play crucial roles in the development of ALI. The memory Th17 cell response is likely to mediate the ALI by causing inflammation. The IL-23 pathway may be a future target for the treatment of ALI. Further study is needed to clarify the mechanism of Th17 differentiation in ALI.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (S) (2522105) from JSPS, Advanced Research & Development Programs for Medical Innovation (AMED-CREST), an Intramural Research Grant (22–4) for Neurological and Psychiatric Disorders of NCNP, the Uehara Memorial Foundation to A.Y.; and by PRESTO (Precursory Research for Embryonic Science and Technology) for Takashi Shichita; the SENSHIN Research Foundation, the Kanae Foundation for the Promotion of Medical Science and the Takeda Science Foundation to T.S., S.C., T.S. and H.M.
Disclosures
The authors have no conflicting financial interests.
Acknowledgements
We thank K. Fujita for lung tissue fixation. We thank M. Asakawa and Y. Noguchi for their technical assistance.
References
- 1. Suresh R. Kupfer Y. and Tessler S. 2000. Acute respiratory distress syndrome. N. Engl. J. Med. 343:660. [DOI] [PubMed] [Google Scholar]
- 2. Rubenfeld G. D., Caldwell E., Peabody E., et al. 2005. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 353:1685. [DOI] [PubMed] [Google Scholar]
- 3. Bernard G. R., Artigas A., Brigham K. L., et al. 1994. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 149(3 Pt 1):818. [DOI] [PubMed] [Google Scholar]
- 4. Brigham K. L. and Meyrick B. 1986. Endotoxin and lung injury. Am. Rev. Respir. Dis. 133:913. [PubMed] [Google Scholar]
- 5. Risso K., Kumar G., Ticchioni M., et al. 2015. Early infectious acute respiratory distress syndrome is characterized by activation and proliferation of alveolar T-cells. Eur. J. Clin. Microbiol. Infect. Dis. 34:1111. [DOI] [PubMed] [Google Scholar]
- 6. Yu Z. X., Ji M. S., Yan J., et al. 2015. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit. Care 19:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ouyang W. Rutz S. Crellin N. K. Valdez P. A. and Hymowitz S. G. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 29:71. [DOI] [PubMed] [Google Scholar]
- 8. Dumoutier L. Lejeune D. Colau D. and Renauld J. C. 2001. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 166:7090. [DOI] [PubMed] [Google Scholar]
- 9. Kotenko S. V. Izotova L. S. Mirochnitchenko O. V. Esterova E. Dickensheets H. Donnelly R. P. and Pestka S. 2001. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem. 276:2725. [DOI] [PubMed] [Google Scholar]
- 10. Zheng Y., Valdez P. A., Danilenko D. M., et al. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282. [DOI] [PubMed] [Google Scholar]
- 11. Aujla S. J., Chan Y. R., Zheng M., et al. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abe H., Kimura A., Tsuruta S., et al. 2014. Aryl hydrocarbon receptor plays protective roles in ConA-induced hepatic injury by both suppressing IFN-gamma expression and inducing IL-22. Int. Immunol. 26:129. [DOI] [PubMed] [Google Scholar]
- 13. Paget C., Ivanov S., Fontaine J., et al. 2012. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J. Biol. Chem. 287:8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivanov S., Renneson J., Fontaine J., et al. 2013. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J. Virol. 87:6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pociask D. A., Scheller E. V., Mandalapu S., et al. 2013. IL-22 is essential for lung epithelial repair following influenza infection. Am. J. Pathol. 182:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin C. Papazian L. Payan M. J. Saux P. and Gouin F. 1995. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest 107:196. [DOI] [PubMed] [Google Scholar]
- 17. Simonian P. L. Wehrmann F. Roark C. L. Born W. K. O’Brien R. L. and Fontenot A. P. 2010. γδ T cells protect against lung fibrosis via IL-22. J. Exp. Med. 207:2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sonnenberg G. F. Nair M. G. Kirn T. J. Zaph C. Fouser L. A. and Artis D. 2010. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 207:1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aujla S. J. Dubin P. J. and Kolls J. K. 2007. Interleukin-17 in pulmonary host defense. Exp. Lung Res. 33:507. [DOI] [PubMed] [Google Scholar]
- 20. Duhen T. Geiger R. Jarrossay D. Lanzavecchia A. and Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10:857. [DOI] [PubMed] [Google Scholar]
- 21. Basu R., O’Quinn D. B., Silberger D. J., et al. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Basu R. Hatton R. D. and Weaver C. T. 2013. The Th17 family: flexibility follows function. Immunol. Rev. 252:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu X. Weiss I. D. Zhang H. H. Singh S. P. Wynn T. A. Wilson M. S. and Farber J. M. 2014. Conventional NK cells can produce IL-22 and promote host defense in Klebsiella pneumoniae pneumonia. J. Immunol. 192:1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansson M. Silverpil E. Lindén A. and Glader P. 2013. Interleukin-22 produced by alveolar macrophages during activation of the innate immune response. Inflamm. Res. 62:561. [DOI] [PubMed] [Google Scholar]
- 25. Vivier E. Spits H. and Cupedo T. 2009. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat. Rev. Immunol. 9:229. [DOI] [PubMed] [Google Scholar]
- 26. Van Maele L., Carnoy C., Cayet D., et al. 2014. Activation of type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during streptococcus pneumoniae infection. J. Infect. Dis. 210:493. [DOI] [PubMed] [Google Scholar]
- 27. Shichita T., Sugiyama Y., Ooboshi H., et al. 2009. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat. Med. 15:946. [DOI] [PubMed] [Google Scholar]
- 28. Seki H., Tasaka S., Fukunaga K., et al. 2010. Effect of Toll-like receptor 4 inhibitor on LPS-induced lung injury. Inflamm. Res. 59:837. [DOI] [PubMed] [Google Scholar]
- 29. Konoeda F., Shichita T., Yoshida H., et al. 2010. Therapeutic effect of IL-12/23 and their signaling pathway blockade on brain ischemia model. Biochem. Biophys. Res. Commun. 402:500. [DOI] [PubMed] [Google Scholar]
- 30. Hill D. A., Siracusa M. C., Abt M. C., et al. 2012. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat. Med. 18:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chinen T., Komai K., Muto G., et al. 2011. Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nat. Commun. 2:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oppmann B., Lesley R., Blom B., et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715. [DOI] [PubMed] [Google Scholar]
- 33. Ivanov I. I., Frutos R. de L., Manel N., et al. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bosmann M. Grailer J. J. Russkamp N. F. Ruemmler R. Zetoune F. S. Sarma J. V. and Ward P. A. 2013. CD11c+ alveolar macrophages are a source of IL-23 during lipopolysaccharide-induced acute lung injury. Shock 39:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aggarwal S. Ghilardi N. Xie M. H. de Sauvage F. J. and Gurney A. L. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910. [DOI] [PubMed] [Google Scholar]
- 36. Hasegawa E., Sonoda K. H., Shichita T., et al. 2013. IL-23-independent induction of IL-17 from γδT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J. Immunol. 190:1778. [DOI] [PubMed] [Google Scholar]
- 37. Zheng Y. Danilenko D. M. Valdez P. Kasman I. Eastham-Anderson J. Wu J. and Ouyang W. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445:648. [DOI] [PubMed] [Google Scholar]
- 38. Liang S. C., Nickerson-Nutter C., Pittman D. D., et al. 2010. IL-22 induces an acute-phase response. J. Immunol. 185:5531. [DOI] [PubMed] [Google Scholar]
- 39. Boniface K. Bernard F. X. Garcia M. Gurney A. L. Lecron J. C. and Morel F. 2005. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 174:3695. [DOI] [PubMed] [Google Scholar]
- 40. Nemoto Y., Kanai T., Takahara M., et al. 2013. Th1/Th17-mediated interstitial pneumonia in chronic colitis mice independent of intestinal microbiota. J. Immunol. 190:6616. [DOI] [PubMed] [Google Scholar]
- 41. McGeachy M. J., Chen Y., Tato C. M., et al. 2009. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lo Re S., Dumoutier L., Couillin I., et al. 2010. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. 184:6367. [DOI] [PubMed] [Google Scholar]
- 43. Eyerich S., Eyerich K., Pennino D., et al. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 119:3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sutton C. E. Lalor S. J. Sweeney C. M. Brereton C. F. Lavelle E. C. and Mills K. H. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331. [DOI] [PubMed] [Google Scholar]
- 45. Crellin N. K. Trifari S. Kaplan C. D. Satoh-Takayama N. Di Santo J. P. and Spits H. 2010. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity 33:752. [DOI] [PubMed] [Google Scholar]