Abstract
Major urinary proteins (MUPs) are the most abundant protein species in mouse urine, accounting for more than 90% of total protein content. Twenty-one Mup genes and 21 pseudogenes are clustered in a region of around 2 megabase pairs (Mbp) on chromosome 4. A Mup-knockout mouse model would greatly facilitate researches in the field of proteomic analysis of mouse urine. Here, we report the successful knockout of the Mup gene cluster of 2.2 Mbp using the CRISPR/Cas9 system. Homozygous Mup-knockout mice survived to adulthood and exhibited no obvious defects. The patterns of the proteomes of non-MUP urinary proteins in homozygous Mup-knockout mice were similar to those of wild-type mice judged by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The sensitivity of enzyme-linked immunosorbent assay to detect non-MUP urinary protein was significantly enhanced in Mup-knockout mice. In short, we have developed a Mup-knockout mouse model. This mouse model will be useful for the research of urinary biomarker testing that may have relevance for humans.
Keywords: major urinary protein, CRISPR/Cas9, knockout, genetically engineered mouse model, urine
Introduction
Disease-related biomarkers give an indication of a disease development in an individual case. An ideal biomarker for measuring pathological conditions, such as tumors, should be present in peripheral body fluids which can be collected through a noninvasive way, and capable of indicating the corresponding in vivo conditions faithfully. Current biomarker research focuses on metabonomics and biomacromolecule such as non-coding RNAs or proteins in serum and urine. Urine is protein-rich, containing ∼3000 detectable protein species [1,2]. In comparison to blood where proteins tend to be degraded by proteases like thrombin during coagulation, urine proteins are stable because these proteins are secreted mature. Moreover, owing to the availability, ease of collection and frequent correlation with the pathophysiology of diseases [3], it is not at all surprising that urinary proteins have been explored for use as biomarkers for a variety of diseases [4–6].
Genetically engineered mice have been extensively used to recapitulate the human disease conditions, and preclinical research on mouse models has provided many important clues for subsequent application in clinical settings. The association of urinary protein patterns with a disease, as revealed with a mouse model, could potentially shed light on the corresponding disease in humans. Unfortunately, the major urinary proteins (MUPs) account for more than 90% of the protein content in abundance in mouse urine [7]. The unusually high abundance of MUPs severely interferes with the successful analysis of the proteomic patterns of mouse urine. Therefore, the knockout of the entire Mup gene locus would greatly facilitate urine proteomic research in mouse.
MUPs are members of a large family of low-molecular weight (19–21 kDa) proteins known as lipocalins [8]. These proteins are the most abundant protein species in mouse urine [7]. MUPs, which provide persistent and highly diverse signals in mouse urinary scent marks, are responsible for the robust, innate, male–male territorial aggression social behavior observed in mice [9].
Sequence analysis revealed that the reference mouse genome contains at least 21 distinct Mup genes. In addition, 21 Mup pseudogenes, the reading frames of which are disrupted by a nonsense mutation or an incomplete gene duplication, also occur in the genome. The Mup genes and pseudogenes cluster in a region occupying around 2 megabase pairs (Mbp) of DNA on chromosome 4. Due to lack of a Mup-knockout mouse mode, the functions of MUPs have been studied through the use of purified proteins from mouse urine [10] or recombinant MUPs expressed in bacteria [9]. Thus, a Mup-knockout mouse model is urgently needed in fields of urinary biomarker study and mouse social behavioral study.
A conventional knockout mouse model was generated by deleting a DNA sequence through homologous recombination between an exogenously delivered targeting construct and endogenous gene locus in embryonic stem (ES) cells and subsequent derivation of mouse from the manipulated ES cell clone. This technique has been successful in deleting DNA fragment up to dozens of kilobase pairs (kbp). Owing to its unusually large size, the Mup-knockout mouse model has not been successful. Deletion of large DNA segments has been achieved via recombination of targeted loxP sites in mouse chromosomes. However, it can be time-consuming to knock-in two separate LoxP sites at both ends of the targeted region through successive targeting in ES cells. Moreover, the efficiency of deletion using Cre decreases with the increase in size of the target DNA fragment [11].
Here, we report the successful deletion of a 2.2-Mbp fragment encompassing the Mup gene cluster in mouse. We showed that homozygous Mup-knockout mice survived to adulthood and exhibited no obvious defects. We observed that the non-MUP urinary proteins of the Mup-knockout mice exhibited a similar pattern to those of wild-type (WT) mice in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). We were able to detect non-MUP proteins more sensitively in urine samples from Mup-knockout mice than in urine samples from WT mice by enzyme-linked immunosorbent assay (ELISA). These results indicate that our Mup-knockout mice can potentially serve as a model animal for studying the correlation between proteomic changes in urine and diseases.
Materials and Methods
Animals
All mice were housed in a specific pathogen-free environment (GB14925-2010, Laboratory Animal—Requirements of Environment and Housing Facilities) at the National Institute of Biological Sciences, Beijing (NIBS). All experimental protocols were approved by the Institutional Committee for Animal Care and Use at NIBS. All animal work was performed in accordance with the approved protocol.
Two single guide RNA (sgRNA) sequences targeting sites upstream (sgRNA1) and downstream (sgRNA2) of the Mup gene cluster, respectively, were transcribed in vitro using MEGAshortscript™ Kit (AM1345; Ambion Life Technologies, Carlsbad, USA). A mixture of plasmids encoding hCas9 [12], sgRNA1 and sgRNA2 was microinjected into the fertilized C57BL/6 eggs at a ratio of 100:35:35 (ng/μl), and the transgenic embryos were planted into pseudopregnant ICR recipients. The founder was genotyped with primers spanning the Cas9 cleavage sites. Founder lines of successful deletion of the Mup gene cluster were identified through PCR genotyping of tail DNA. PCR products were further verified through Sanger sequencing. The genotyping primers were detailed below. MupdelF1 (5′-GGGAGGCAAAATCCTGAAACT-3′) anneals to sequence upstream of Mup gene cluster. MupwtF1 (5′-AAACCAGAGGTCCCCAAACTT-3′) anneals to sequence within Mup gene cluster. MupR1 (5′-TTTGAAAAGGGCAGGAACAGT-3′) anneals to sequence downstream of Mup gene cluster. MupdelF1 and MupR1 amplify a fragment of ∼137 bp corresponding to the knockout mutant. MupwtF1 and MupR1 amplify a fragment of ∼485 bp corresponding to the WT allele. The Mup-knockout mice are available upon request.
Urine collection
Mouse urine was collected with metabolic cages (3700M022; TECNIPLAST, Buguggiate, Italy) and immediately processed via centrifugation at 12,550 g for 5 min at 4°C to remove fecal debris. Samples were treated with protease inhibitors (4693132001; Roche, Basel, Switzerland) to avoid further degradation, aliquoted into sterile centrifuge tubes, and stored at −80°C.
SDS-PAGE
Mouse urine was precipitated using TCA–acetone (1:8). The protein pellet was then washed three times with ice-cold acetone and dissolved in 100 mM Tris (pH 8.5)/8 M urea. The protein concentration was determined with a Bradford protein assay kit (500-0006; Bio-Rad, Hercules, USA). A total of 20 µg of urinary proteins were subject to 10% SDS-PAGE and Coomassie Brilliant Blue staining.
Pathology analysis
The liver, spleen, kidney, lung, and heart tissues were collected from WT and Mup-knockout mice, fixed with 10% phosphate buffered saline neutralized formalin and then embedded in paraffin. Five-micrometer slices were sectioned and stained with hematoxylin and eosin for pathological examination.
ELISA
Microalbuminuria (MAU) and uromodulin (UMOD) were quantified with a Mouse MAU ELISA Kit (E-EL-M0792C; Elabscience, Wuhan, China) and a Mouse UMOD ELISA Kit (xy-0916; X-Y Biotechnology, Hangzhou, China), following the manufacturer's instructions. Each experiment was performed in triplicate. Briefly, 10 µl of each urine sample was applied to plates pre-coated with primary monoclonal antibody for 30 min at 37°C. After washing five times, an enzyme-linked polyclonal antibody was added and incubated for an additional 30 min. After additional three washes, 100 µl of substrate was added to each well and incubated in a light-free environment at room temperature for 10 min, followed by the addition of 50 µl of stop solution to terminate the enzymatic reaction. OD450 values were measured, and the protein concentration was determined according to standard curves calculated from reads of sample wells with known concentration loaded side-by-side with urine samples.
Data analysis
Student's t-test was performed for comparison of two groups. P-values <0.05 were considered to be of statistically significant difference.
Results
CRISPR/Cas9 efficiently deleted a 2.2-Mbp region containing the Mup gene cluster
Given that the Mup genes and pseudogenes are clustered in a region spanning ∼2.2 Mbp on chromosome 4, conventional gene targeting in ES cells through homologous recombination is not a valid choice to delete the entire Mup cluster. CRISPR/Cas9 is now widely used for targeted DNA cutting and the ensuing repair and can be harnessed for introducing predictable, desired mutations. We therefore tested the possibility of deleting the entire Mup gene cluster through the use of CRISPR RNA molecules, such that the guided Cas9 would simultaneously generate double-strand DNA breaks at both ends of the region containing the cluster. The repair of these DNA breaks would ostensibly result in the deletion of the entire region in a portion of cells.
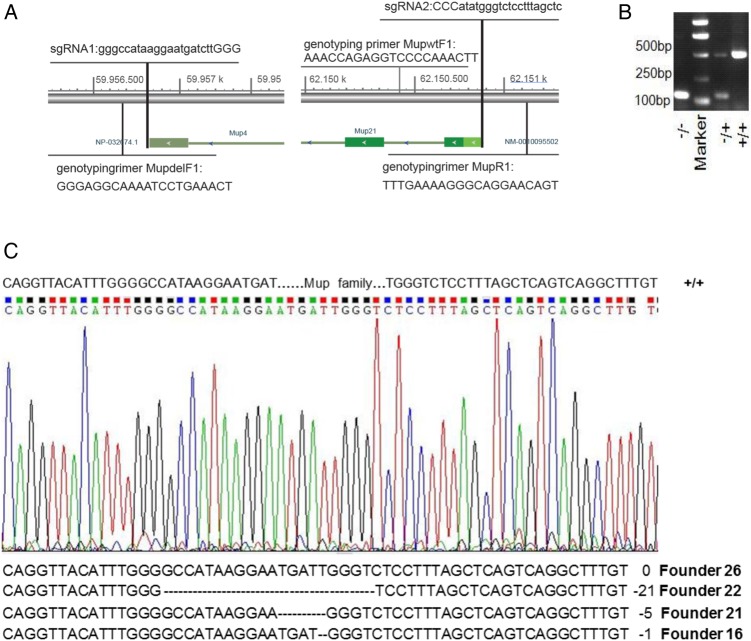
sgRNA1 at chr4: 59956711-59956730 and sgRNA2 at chr4: 62150877-62150899 were designed for the deletion of a 2.2-Mbp region of Mup gene cluster (Fig. 1A). We did the thorough blast on both sgRNAs against mouse genome and listed the detailed information of blast result in Supplementary Tables S1 and S2. Then, the Cas9 and sgRNA1 or sgRNA2 constructs were electroporated into mouse fibroblast NIH-3T3 cells. And 72 h after eletroporation, T7E1 assay [13] was carried out to evaluate the issue of off-target. Among the 10 most likely off-targeting sites for both sgRNAs (Supplementary Table S3), T7E1 assay revealed no off-target digestion by Cas9 (Supplementary Fig. S1). Therefore, both sgRNAs and Cas9 were injected into the pronuclei of fertilized eggs of C57BL/6 mice. A total of 311 embryos were injected, and 217 embryos survived the microinjection and were transferred to pseudopregnant females. Finally, 28 pups were born (Supplementary Table S4). These founders were genotyped with primers annealing to genomic DNA sequences upstream of sgRNA1 (MupdelF1) and downstream of sgRNA2 (MupR1), resulting in an expected 137 bp amplification product for pup with the deletion. The expected 485 bp WT amplification product was amplified with the MupR1 primer and a primer annealing to a region downstream of the Mup21 gene locus (MupwtF1). Furthermore, 5 out of 28 founders were identified to harbor knockout allele based on the size of the PCR product (Fig. 1B and Supplementary Fig. S2). Sequencing of the PCR product amplified from four of these positive founders confirmed the deletion of Mup gene cluster in these pups (Fig. 1C). Interestingly, the deletion pattern was not identical among these founders. Our results are consistent with the low accuracy of non-homologous end joining (NHEJ) repair patterns on the cut DNA reported for the Cas9 system [14].
Figure 1.
Generation of Mup-knockout mouse (A) Scheme of CRISPR/Cas9-mediated knockout of Mup; (B) representative genotyping PCR results of WT (Mup+/+), heterozygote knockout (Mup+/−) and homozygote knockout (Mup−/−) mouse; and (C) PCR-sequencing chromatograph of the knockout (Mup−/−) founders.
Mup-knockout mice developed to adulthood with no obvious defects
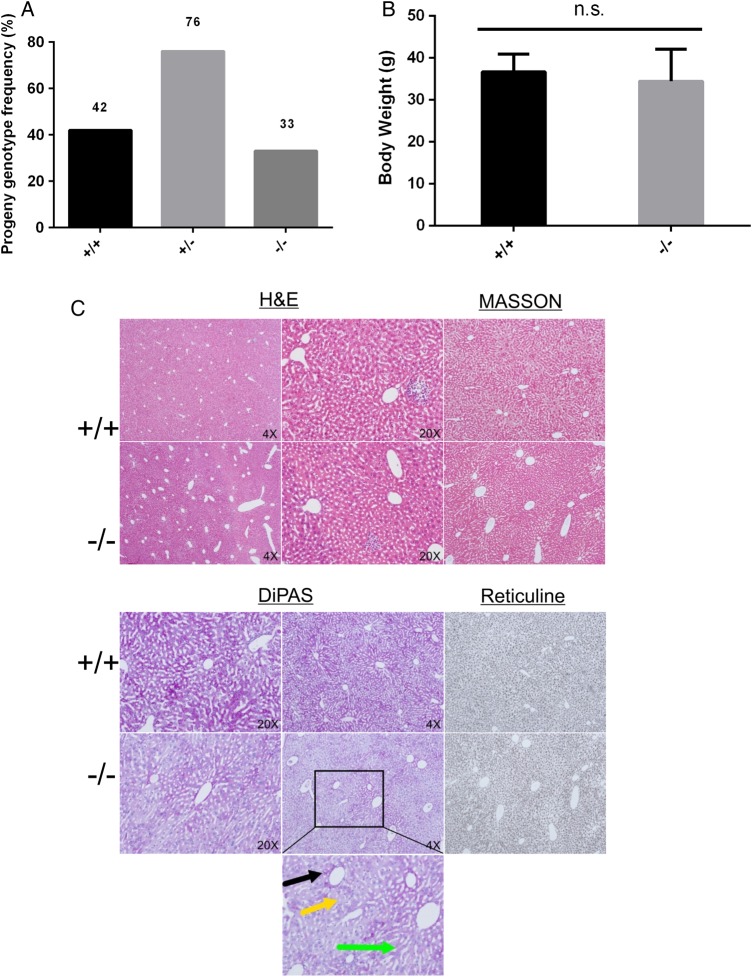
MUPs function as pheromones. While it is known that these proteins are important for social behaviors, they are most likely dispensable for the development and survival of individuals. We expected that Mup-knockout mice would likely survive to adulthood. We therefore established a large cohort of breeding pairs between heterozygous knockout mice. Interestingly, it was found that WT, heterozygous, and homozygous pups were born at the typical Mendelian ratio (Fig. 2A). The pups of different genotypes were not distinguishable from each other. Indeed, these mice were healthy for the 2-year period of observation. The body weights of these mice were also checked and no significant difference was found among 4-month-old WT, heterozygous, and homozygous mice (Fig. 2B).
Figure 2.
Mup-knockout mice are healthy (A) Mendelian ratio distribution of WT (Mup+/+), heterozygous (Mup+/−) and homozygous (Mup−/−) progenies; (B) body weight comparison between Mup+/+ and Mup−/− mice; and (C) pathological analysis of liver of Mup−/− mice in comparison with WT mice. n.s., not significant.
We also checked the pathology of important organs of the homozygous deletion mice. No obvious abnormalities were detected in kidney, spleen, lung, and heart in the 4-month-old homozygous mice (Supplementary Fig. S3 for heart, liver, spleen, lung, and kidney). As MUPs were produced in liver [15,16], the pathology of liver of Mup-knockout mice was thoroughly checked. Livers of knockout mice were processed with routine procedures in the Department of Pathology in Beijing Ditan Hospital and then stained with H&E, PAS, Masson, and reticulin stains, respectively (Fig. 2C). The architecture of lobes was well preserved, and hepatocytes were well-lined in cords without predominant necrosis or even balloon degeneration (Fig. 2C). The peri-portal areas were clear and had minimal numbers of inflammation cells infiltrated. Some vesicles were observed at a frequency of one out of six mice, which were suggestive of fatty vesicles in hepatocytes (Fig. 2C, the lower panel for high magnifications images; black arrow head for peri-portal, yellow for fatty vesicles and green for cords). Therefore, livers of homozygous knockout mice are normal.
Our results therefore indicated that the complete knockout of the Mup gene cluster did not cause obvious deleterious effects on important organs or development of mice.
Mup deletion resulted in enhanced ability to detect non-MUP proteins in urine
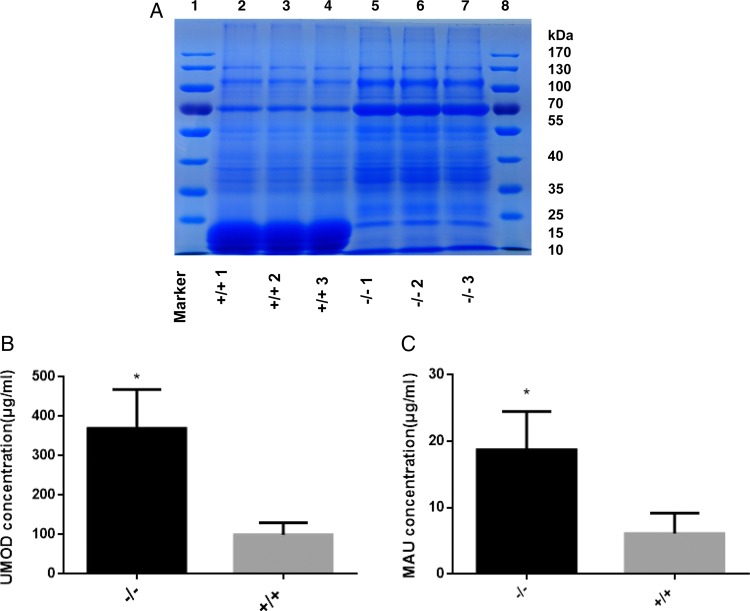
MUPs are the most abundant proteins in mouse urine. The extremely high abundance of MUPs severely interferes with analysis of non-MUP proteins in mouse urine samples. We evaluated the use of our Mup-knockout mice for the characterization of urine samples. We first checked whether MUPs were absent in the urine of Mup-knockout mice. Proteins were purified from urine and separated by SDS-PAGE. Coomassie Brilliant Blue staining revealed that MUPs were the predominant protein species in WT mouse urine (Fig. 3A, lanes 2–4). In striking contrast, no MUPs were detected in urine samples from Mup-knockout mice (Fig. 3A, lanes 5–7). Interestingly, it was found that the protein band distribution pattern of non-MUP proteins were similar between WT and Mup-knockout mice (Fig. 3A).
Figure 3.
Mup-knockout mouse model enables analyzing non-MUP urinary proteins with enhanced sensitivity (A) Coomassie Brilliant Blue staining of an SDS-PAGE showing the absence of MUPs in Mup−/− mice and similar patterns of non-MUP proteins between WT and Mup−/− mice. (B) The sensitivity of ELISA toward UMOD was increased in Mup−/− mice. (C) The sensitivity of ELISA toward MAU was increased in Mup−/− mice. *P<0.05.
As MUPs are the predominant protein species in mouse urine, low abundance non-MUP proteins are blocked by MUPs for detection. Urine samples of Mup-knockout mice contain no MUPs, thus rendering the non-MUP proteins relatively higher in abundance, which will result in detection of these non-MUP proteins at higher sensitivity. We then performed ELISA analysis targeting some non-MUP proteins.
UMOD protein, also known as Tamm–Horsfall protein (THP), is primarily synthesized and secreted into the urine by thick ascending limb (TAL) of kidney tubules medullary loop and epithelial cells in the initial segment of the distal convoluted tubule. This protein has been detected in mouse urine in earlier reports [17]. As expected, for the detection of the UMOD protein, OD reading was significantly higher in urine samples of Mup-knockout mice than that in WT samples in the ELISA assay (Fig. 3B).
Human urine contains low level of micro albumin (also called urine albumin). Abnormally high level of micro albumin in urine is called MAU, resulting from abnormally high permeability for albumin in the glomerulus of the kidney. In clinic, MAU serves as an independent marker of vascular endothelial dysfunction and an important prognostic marker for kidney disease [18]. Therefore, the levels of MAU were measured in the urine samples of WT and Mup-knockout mice. Similarly, significantly higher OD reading was found in urine samples from knockout mice than in those from WT mice (Fig. 3C).
Discussion
Owing to its unusually large size, Mup-knockout mouse has not been generated to date. Here, we report the use of the CRISPR/Cas9 system to successfully delete a 2.2-Mbp DNA fragment harboring the Mup gene cluster. We showed that this mouse model is useful to increase the sensitivity of analytical methods for exploring the proteomes of the urine of these mice.
Traditionally, gene targeting in ES cells has been the choice to delete a DNA fragment for generating gene-knockout mice. However, this technique is not a suitable choice for the deletion of large DNA fragments. In earlier studies, the deletion of large DNA segments has been obtained via recombination of targeted loxP sites in mouse chromosomes. However, the deleting efficiency decreases with the increase of the size of DNA fragment. Very recently, CRISPR/Cas9 has been used to delete a 1.5-Mbp fragment in ES cells to generate gene-knockout mouse [19]. In our study, we deleted a 2.2-Mbp fragment through pronuclear injection of sgRNAs and Cas9. Use of CRISPR/Cas9 greatly shortens the time needed to generate F1 mice by circumventing germline transmission. Our work therefore clearly demonstrated the advantage of pronuclear injection with CRISPR RNA and Cas9 for generating gene-knockout mice.
Major bioactive excreta reported in urine include metabolites, non-coding RNA molecules, and proteins. Metabolomics analyses have revealed more than 2000 metabolites in mouse urine [20]. Small volatile organic compounds have been studied as potential biomarkers of lung cancer in a recent study [21]. Urinary miRNA molecules have also been evaluated as biomarkers [22]. Urine of mammals is protein-rich. Human urinary proteins have been used to successfully diagnose disease conditions in clinical settings. Human urine has advantages over serum for use in testing proteins in that: (i) urine collection is absolutely noninvasive; (ii) no predominant proteins exist in urine, unlike the predominance of albumin in serum and (iii) urinary proteins are secreted as mature globulins of low-molecular weight suitable for biochemical analysis [3]. Correlation of changes in the urine proteome with the pathophysiology of diseases has attracted intensive research seeking to identify urinary protein markers of disease.
Genetically engineered mice have been extensively used to recapitulate the human disease conditions. However, MUPs severely hinder the successful identification of protein biomarkers in the studies with mouse model because MUPs account for up to 90% of total urinary proteins in mouse. This unusually high abundance interferes with biochemical and biophysical analyses of mouse urinary proteins. Our Mup-knockout mouse model may therefore represent an important new experimental tool for research seeking to identify disease biomarkers in urine. We found that knockout of the Mup gene cluster resulted in disappearance of MUPs in urine, thus significantly enhanced the ability to analyze mouse urinary proteins. Importantly, we found that the proteomic patterns of non-MUP urine proteins were similar between Mup-knockout and WT mice.
MUPs exert important biological functions. However, previous studies relied on WT mice, rather than gene-knockout mice. The Mup-knockout mice generated in this work will be useful to confirm several important conclusions from earlier studies that explored MUPs.
Supplementary Data
Funding
This work was supported by the grants from the Major State Basic Research Development Program of China (No. 2011CB812401 to L.C. and No. 2012CB837403 to F.W.).
Supplementary Material
Acknowledgement
The authors thank Dr Yanbin Liu (National Institute of Biological Sciences, Beijing, China) for his technical help in genotyping mice and preparing Figure 1.
References
- 1.Husi H, Barr JB, Skipworth RJ, Stephens NA, Greig CA, Wackerhage H, Barron R et al. The human urinary proteome fingerprint database UPdb. Int J Proteomics 2013, 2013: 760208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siwy J, Mullen W, Golovko I, Franke J, Zurbig P. Human urinary peptide database for multiple disease biomarker discovery. Proteom Clin Appl 2011, 5: 367–374. [DOI] [PubMed] [Google Scholar]
- 3.Husi H, Fearon KC, Ross JA. Can a simple proteomics urine test assist in the early diagnosis of early-stage cancer? Expert Rev Proteomics 2011, 8: 555–557. [DOI] [PubMed] [Google Scholar]
- 4.Pedroza-Diaz J, Rothlisberger S. Advances in urinary protein biomarkers for urogenital and non-urogenital pathologies. Biochem Med 2015, 25: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson DL, Broadbent JA, Parker AW, Upton Z, Parker TJ. Urinary biomarkers of physical activity: candidates and clinical utility. Expert Rev Proteomics 2014, 11: 91–106. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Cao J, Li L, Liu Y, Zhao H, Li N, Li B et al. Identification of urine protein biomarkers with the potential for early detection of lung cancer. Sci Rep 2015, 5: 11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beynon RJ, Hurst JL. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides 2004, 25: 1553–1563. [DOI] [PubMed] [Google Scholar]
- 8.Flower DR. The lipocalin protein family: structure and function. Biochem J 1996, 318: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF et al. Identification of protein pheromones that promote aggressive behaviour. Nature 2007, 450: 899–902. [DOI] [PubMed] [Google Scholar]
- 10.Hurst JL, Robertson DHL, Tolladay U, Beynon RJ. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim Behav 1998, 55: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 11.Coppoolse ER, de Vroomen MJ, van Gennip F, Hersmus BJ, van Haaren MJ. Size does matter: cre-mediated somatic deletion efficiency depends on the distance between the target lox-sites. Plant Mol Biol 2005, 58: 687–698. [DOI] [PubMed] [Google Scholar]
- 12.Bernascone I, Janas S, Ikehata M, Trudu M, Corbelli A, Schaeffer C, Rastaldi MP et al. A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Hum Mol Genet 2010, 19: 2998–3010. [DOI] [PubMed] [Google Scholar]
- 13.Xie S, Shen B, Zhang C, Huang X, Zhang Y. sgRNAcas9: a software package for designing CRISPR sgRNA and evaluating potential off-target cleavage sites. PLoS One 2014, 9: e100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canver MC, Bauer DE, Dass A, Yien YY, Chung J, Masuda T, Maeda T et al. Characterization of genomic deletion efficiency mediated by clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 nuclease system in mammalian cells. J Biol Chem 2014, 289: 21312–21324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlayson JS, Asofsky R, Potter M, Runner CC. Major urinary protein complex of normal mice: origin. Science 1965, 149: 981–982. [DOI] [PubMed] [Google Scholar]
- 16.Knopf JL, Gallagher JF, Held WA. Differential, multihormonal regulation of the mouse major urinary protein gene family in the liver. Mol Cell Biol 1983, 3: 2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun MC, Li L, Ke B, Dubinsky WP, Pickering MC, Chang JY. Proteomic profiling of urinary protein excretion in the factor H-deficient mouse. Am J Nephrol 2006, 26: 127–135. [DOI] [PubMed] [Google Scholar]
- 18.Yoon YE, Lee KS, Choi KH, Kim KH, Yang SC, Han WK. Prospective measurement of urinary microalbumin in living kidney donor nephrectomy: toward understanding the renal functional recovery period. J Urol 2014, 192: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 19.Kraft K, Geuer S, Will AJ, Chan WL, Paliou C, Borschiwer M, Harabula I et al. Deletions, inversions, duplications: engineering of structural variants using CRISPR/Cas in mice. Cell Rep 2015, 10: 833–839. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Watson DG. A short review of applications of liquid chromatography mass spectrometry based metabolomics techniques to the analysis of human urine. Analyst 2015, 140: 2907–2915. [DOI] [PubMed] [Google Scholar]
- 21.Mathe EA, Patterson AD, Haznadar M, Manna SK, Krausz KW, Bowman ED, Shields PG et al. Noninvasive urinary metabolomic profiling identifies diagnostic and prognostic markers in lung cancer. Cancer Res 2014, 74: 3259–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulos T, Belliere J, Bascands JL, Neau E, Klein J, Schanstra JP. miRNAs in urine: a mirror image of kidney disease? Expert Rev Mol Diagn 2015, 15: 361–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.