Abstract
Akt/PKB plays a pivotal role in cell proliferation and survival. However, the isotype-specific roles of Akt in mitochondrial function have not been fully addressed. In this study, we explored the role of Akt in mitochondrial function after stable knockdown of the Akt isoforms in EJ human bladder cancer cells. We found that the mitochondrial mass was significantly increased in the Akt1- and Akt3-knockdown cells, and this increase was accompanied by an increase in TFAM and NRF1. Akt2 knockdown did not cause a similar effect. Interestingly, Akt3 knockdown also led to severe structural defects in the mitochondria, an increase in doxorubicin-induced senescence, and impairment of cell proliferation in galactose medium. Consistent with these observations, the mitochondrial oxygen consumption rate was significantly reduced in the Akt3-knockdown cells. An Akt3 deficiency-induced decrease in mitochondrial respiration was also observed in A549 lung cancer cells. Collectively, these results suggest that the Akt isoforms play distinct roles in mitochondrial function and that Akt3 is critical for proper mitochondrial respiration in human cancer cells.
Keywords: Akt, isoform-specific function, Akt3, mitochondria
Introduction
Mitochondria are centers of energy production and cellular metabolism. Previous studies revealed that mitochondria were also involved in various other important cellular processes, including apoptosis and cellular senescence [1,2]. Normal mitochondrial activity is required for cell proliferation and other metabolic processes, whereas defective mitochondrial function has been associated with various human diseases, including diabetes mellitus and age-related disorders [1–3]. Mitochondrial dysfunction is often associated with structural alterations, such as the loss of matrix density and the disorganization of the inner membrane cristae. Previous studies also suggested that mitochondrial dysfunction contributed to the growth and progression of human cancers [3]. Thus, identifying proteins that regulate mitochondrial function is critical to elucidate how mitochondrial dysfunction leads to the development of human diseases and to aid in the development of efficient treatments for mitochondria-related diseases.
Akt is a Ser/Thr kinase that plays an essential role in cell proliferation and survival [4,5]. Akt is activated in a phosphatidylinositol 3-kinase (PI3-K)-dependent manner and then stimulates cell growth and proliferation by regulating various target proteins through phosphorylation [4]. Previous studies demonstrated that Akt was also activated in response to various types of stresses. Activated Akt enhances cell survival by modulating a variety of important regulatory proteins, including GSK3β, FOXO, MDM2, and ASK1 [6,7]. The studies discussed above showed that Akt regulated numerous cellular processes, including apoptosis, ROS generation, and energy metabolism, to protect cells from stress and enhance their proliferation.
Mammalian cells express three isoforms of the Akt protein: Akt1, Akt2, and Akt3 [5]. Although these isoforms share more than 80% sequence homology [6], previous studies demonstrated that they played distinct physiological roles in addition to their redundant and overlapping functions. For example, studies using genetically engineered mice deficient in either Akt1, Akt2, or Akt3 confirmed that the different Akt isoforms had different physiological functions. Akt1-knockout mice exhibited growth retardation and increased apoptosis [8]. The removal of Akt2 induced insulin resistance and diabetes [9,10]. Finally, Akt3-knockout mice exhibited reductions in brain size [11]. In addition to the above studies, we previously showed that Akt2 played a critical role in cell survival after UV irradiation and that Akt1 regulated cell migration and cell invasion activity, suggesting that the Akt isoforms played distinct roles during the stress response and cell migration [12,13]. However, the roles of the Akt isoforms in mitochondrial function have not been fully addressed.
In this study, we explored the role of Akt in mitochondrial function via isoform-specific knockdown of Akt in EJ human bladder carcinoma and A549 lung cancer cells. We found that knockdown of both Akt1 and Akt3 induced a significant increase in the mitochondrial mass in EJ cells. Akt3 knockdown led to the most serious mitochondrial dysfunctions, which suggested that the different Akt protein isoforms played distinct roles in the maintenance of proper mitochondrial function in human cancer cells.
Materials and Methods
Cell culture, plasmids, and creation of Akt-knockdown cell lines
EJ human bladder carcinoma cells and A549 human lung cancer cells were maintained in DMEM containing 10% FBS. To create cell lines harboring knockdowns of each of the Akt protein isoforms, lentivirus constructs in the pLKO.1 plasmid [13] encoding shRNAs against Akt1 (shAkt1), Akt2 (shAkt2), or Akt3 (shAkt3) were transfected into 293FT packaging cells, and the cell-free viral supernatants were used to infect EJ cells. After puromycin selection, the resistant cells were pooled and used for the remaining experiments.
Western blot analysis and antibodies
The cells were lysed in RIPA buffer and subject to western blot analysis as previously described [14]. Antibodies against Akt1 and Akt3 were purchased from Upstate Biotechnology (Lake Placid, USA). Antibodies against Akt2 were obtained from Merck Millipore (Billerica, USA). The actin levels were monitored as an internal loading control using an anti-actin (Sigma-Aldrich, St Louis, USA) antibody.
Measurement of mitochondrial mass
To determine the mitochondrial mass, 1 × 106 cells were trypsinized and fixed with cold 70% ethanol. The cells were subsequently stained with 0.5 µM 10-N-nonyl-acridine orange (NAO) for 15 min and then analyzed by flow cytometry using an FC500 cytometer and the CXP software (Beckman Coulter, Inc., Brea, USA). The experiment was repeated at least three times, and the results were presented as mean values with standard deviations.
Quantitative RT-PCR
To assess the mRNA levels, total RNA isolation and RT-PCR were performed as previously described [15]. β-Actin was amplified in the same reactions for all samples as an internal control, and the mRNA levels of other genes were normalized to the β-actin mRNA level. The expression level of each mRNA was determined using the 2−ΔCT-threshold cycle method. The primers used were as follows: TFAM forward primer 5′-CGTTGGAGGGAACTTCCTGAT-3′, TFAM reverse primer 5′-CCTGCCACTCCGCCCTATA-3′, NRF1 forward primer, 5′-GGGAGCTACAGTCACTATGGCG-3′, and NRF1 reverse primer 5′-ACAAGACGATCTGTCCCCCA-3′.
Electron microscopy
To analyze the mitochondrial structure, the cells were harvested and fixed with 2.5% glutaraldehyde (Sigma-Aldrich) in phosphate buffer (pH 7.4). After washing with phosphate buffer, the cells were post-fixed with 1% osmium tetroxide (Sigma-Aldrich) for 1 h. The samples were embedded in Epon812 (Sigma-Aldrich), and ultrathin sections were obtained with a Reichert Ultracut E microtome. The ultrathin sections were stained with uranyl acetate and lead citrate and observed using an H7650 transmission electron microscope (Hitachi, Tokyo, Japan).
Cell proliferation in galactose medium
To examine mitochondrial function, cell growth in galactose medium was measured as previously described [16]. Briefly, 1 × 104 cells were seeded into 6-well plates and cultured in DMEM-galactose medium (DMEM glucose-free medium supplemented with 1 g/l galactose, 1 mM sodium pyruvate, 10% fetal bovine serum, and 1% penicillin/streptomycin). The cells were counted every 2 days by trypan blue exclusion using a hemocytometer. To measure their colony-forming abilities in galactose medium, 500 cells were seeded into 60 mm dishes and incubated in DMEM-galactose medium. The medium was changed every 3 days. After 2 weeks, the resulting colonies were fixed, stained with 2% methylene blue and counted. The experiments were repeated independently at least twice, and the results were presented as mean values with standard deviations.
Senescence-associated (SA)-β-galactosidase staining
The cells were fixed with 0.25% glutaraldehyde, and SA β-galactosidase staining was performed at pH 6.0 as previously described [17]. After staining, at least 300 cells were examined in several different fields, and the SA β-gal-positive cells were counted. These experiments were repeated three times, and the results were presented as mean values with standard deviations.
Measurement of the respiration rate
The cellular respiration rates were measured using an XF24 flux analyzer (Seahorse Bioscience, Inc., North Billerica, USA) according to the manufacturer's instructions as previously described [18]. Briefly, the cells were plated at a density of 10,000 cells/well in an XF24 24-well plate. Their oxygen consumption rates were measured under basal conditions in the presence of 2,4-dinitrophenol (DNP, 100 μM) and rotenone (1 μM) to assess their maximal oxidative capacities. The measurements of oxygen consumption were normalized to the number of cells.
Results
Knockdown of Akt isoforms increases the mitochondrial mass
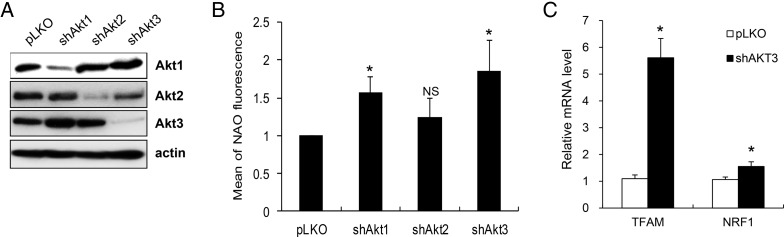
To examine the roles of the Akt protein isoforms in mitochondrial functions, we generated stable EJ cell lines that lacked Akt1, Akt2, or Akt3 using lentivirus-encoded shRNAs against each of the relevant genes. Protein knockdown was confirmed by western blot analysis (Fig. 1A), which indicated that each of the Akt protein isoforms could be individually knocked down in our EJ cell line. Additionally, quantitative analysis of three repeated western blots indicated that knockdown of the Akt isoform did not induce changes in the expression levels of the remaining isoforms, which was consistent with our and other groups' previous reports [13,19,20].
Figure 1.
Increases in the mitochondrial mass following knockdown of Akt protein isoforms in EJ cells (A) EJ cells expressing shAkt1, shAkt2, shAkt3, or pLKO vectors (pLKO) were lysed and subjected to western blotting using the indicated antibodies. The data shown are representative of three independent experiments. (B) The mitochondrial mass was determined by flow cytometry after staining with NAO. Relative NAO fluorescence was measured in three independent experiments, and the results are presented as mean values with standard deviations. (C) The TFAM and NRF1 mRNA levels were measured by quantitative RT-PCR. The relative TFAM and NRF1 mRNA levels obtained in two independent experiments are presented as mean values with standard deviations. *P< 0.05 compared with pLKO vector control cells according to Student's t-test. NS, not significant.
To address the isoform-specific roles of Akt in mitochondrial function, we measured the mitochondrial mass by staining with NAO. NAO enables quantitative measurement of the mitochondrial mass because it binds to cardiolipin in the inner mitochondrial membrane [21]. The analysis of the NAO signal revealed that the average mitochondrial masses were increased in both the Akt1-knockdown cells (shAkt1) and Akt3-knockdown cells (shAkt3) to ∼1.6 and 1.9 folds, respectively (Fig. 1B). The average mitochondrial mass in the Akt2-knockdown cells (shAkt2) was also slightly increased; however, this increase was not significant (P> 0.05). These results suggest that knockdown of the different Akt protein isoforms differentially affects the regulation of the mitochondrial mass in EJ cells.
The increased mitochondrial mass in the Akt3-knockdown cells suggested that the absence of Akt3 might induce mitochondrial biogenesis in EJ cells. To test whether the elevated mitochondrial mass in the Akt3-knockdown cells was associated with an increase in mitochondrial biogenesis, we examined the expression levels of TFAM and NRF1, which were master regulators of mitochondrial biogenesis [22]. The quantitative analysis of repeated experiments indicated that the TFAM and NRF1 levels were increased in the Akt3-knockdown cells to ∼5.5 and 1.5 folds, respectively (Fig. 1C). These results suggested that the absence of Akt3 induced an increase in mitochondrial biogenesis in the EJ cells.
Akt3 knockdown induced severe structural disorganization in the mitochondria
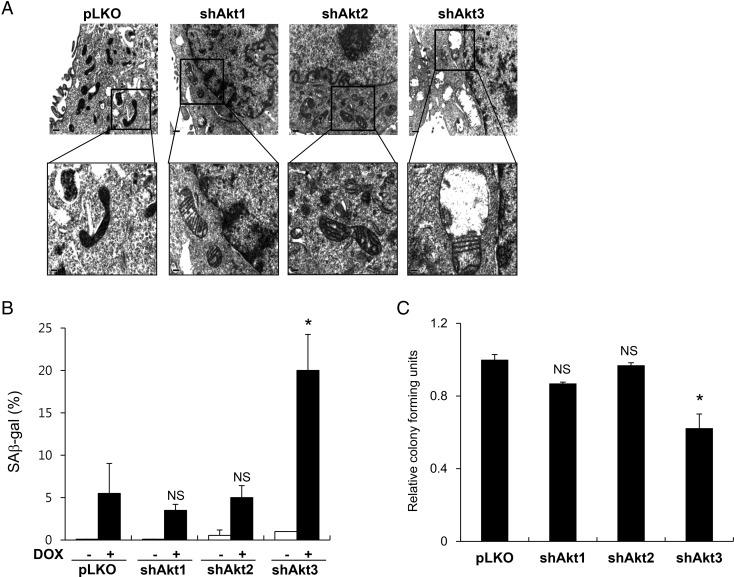
The dysfunction of important mitochondrial regulators often induces functional and structural changes in the mitochondria. To examine whether knockdown of the Akt isoforms affected the mitochondrial structural organization, we examined the detailed structures of the mitochondria using transmission electron microscopy. As shown in Fig. 2A, we found that the mitochondria were slightly larger in each of the Akt isoform-knockdown cell lines than those in the control EJ cells infected with the control lentivirus (pLKO). Additionally, while the mitochondria in the pLKO cells exhibited regular cristae arrangements and dense matrix staining, the mitochondria in cells harboring the knocked down Akt isoforms had disarrayed cristae arrangements and a considerably reduced matrix density. The mitochondria in the Akt3-knockdown cells (shAkt3) exhibited the most severely dysregulated cristae and a remarkable loss of matrix density. These results suggest that the Akt protein isoforms are involved in maintaining the proper infrastructure of the mitochondria and that Akt3 is particularly important to the structural integrity of the mitochondria in the EJ cells.
Figure 2.
Mitochondrial structural and functional defects following knockdown of Akt protein isoforms in EJ cells (A) Representative electron microscopy images of mitochondria in control EJ cells (pLKO) and in EJ cells expressing shAkt1, shAkt2, or shAkt3. The upper panels show ×20,000 magnification, and the indicated regions are enlarged in the bottom panel (×40,000). (B) Cells were treated with 25 nM DOX for 16 h. After 6 days of culture in DOX-free media, the cells were stained with freshly prepared SA β-gal staining solution. SA β-gal-positive cells counted in three independent experiments were presented as mean values with standard deviations. (C) A total of 500 cells were seeded per well in 6-well culture plates and cultured in DMEM-galactose media. After 2 weeks, the resulting colonies were fixed, stained with methylene blue, and counted. Each experiment was independently repeated in triplicate, and the results were presented as the mean values with standard deviations. *P< 0.05 compared with pLKO vector control cells using Student's t-test. NS, not significant.
Knockdown of Akt3 induces defects in mitochondrial functions in EJ cells
To examine whether the structural defects that result from the knockdown of the Akt protein isoforms correlated with mitochondrial dysfunction, we checked cellular responses to the anticancer drug doxorubicin (DOX). Treatment with sublethal doses of DOX induces premature cellular senescence in various cancer cells, including EJ cells [18,23]. Furthermore, both our group and others have previously shown that cellular responses to DOX treatment are closely correlated with functional defects in the mitochondria [18,24,25]. Interestingly, the Akt3-knockdown cells showed ∼3-fold higher levels of senescence following DOX treatment than the control cells (pLKO) (P< 0.05), whereas no significant differences were detected in the Akt1- and Akt2-knockdown cells (P> 0.05) (Fig. 2B).
To examine the effect of the knockdown of the Akt protein isoforms on mitochondrial function, we measured cell proliferation in DMEM glucose-free medium supplemented with galactose. Previous studies have demonstrated that cells with defects in the mitochondrial respiratory chain exhibit growth impairments in galactose medium because they rely primarily on oxidative phosphorylation to produce ATP [26,27]. As shown in Fig. 2C, Akt3-knockdown cells exhibited impaired growth in galactose medium (P< 0.05), whereas Akt1- and Akt2-knockdown cells exhibited growth similar to the control knockdown cells (pLKO) (P> 0.05). These results suggest that Akt3 knockdown in EJ cells results in mitochondrial dysfunction as well as structural defects.
Knockdown of Akt3 leads to decreased mitochondrial respiration
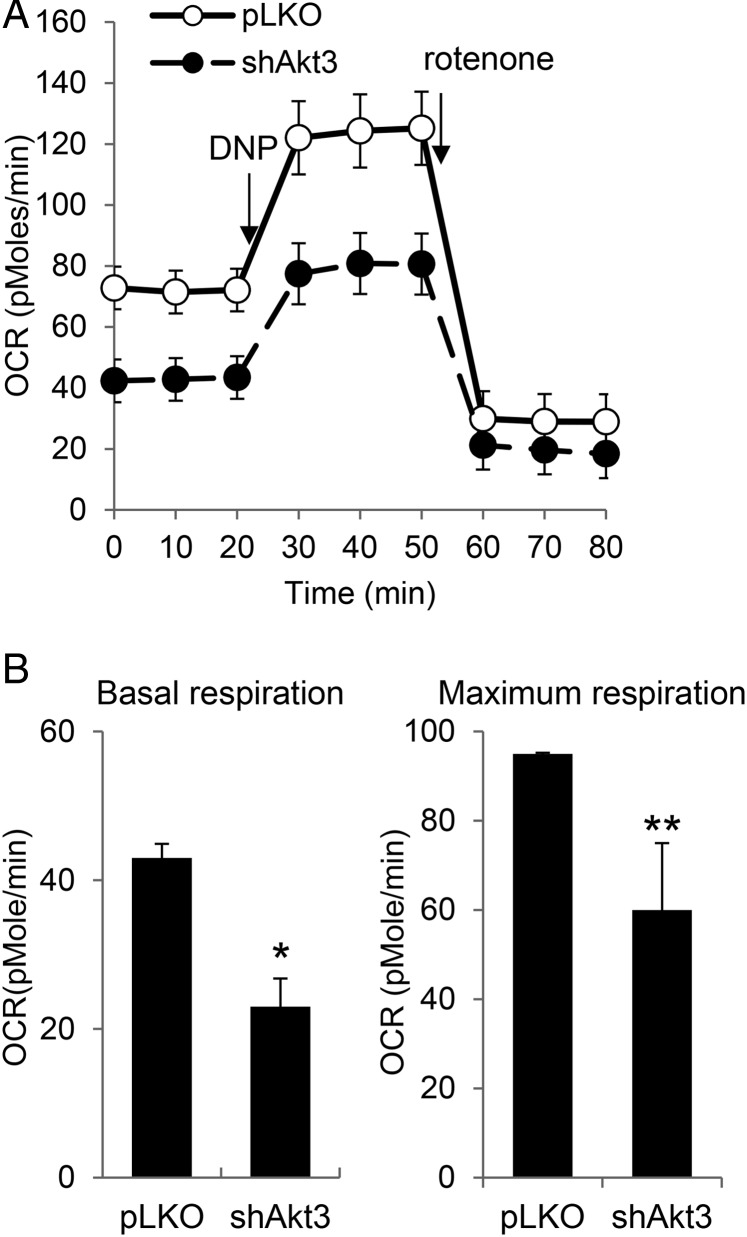
The results from the cell proliferation assays in galactose medium and the DOX-induced senescence assay suggested that Akt3 knockdown induced mitochondrial dysfunction in EJ cells. To confirm the effect of Akt3 knockdown on mitochondrial function, we measured the oxygen consumption rate, which is an informative indicator of mitochondrial respiration. We found that Akt3 knockdown significantly reduced the basal respiration rate of the EJ cells (Fig. 3A). Additionally, the maximal mitochondrial oxidative capacity was remarkably decreased following Akt3 knockdown based on the level of 2,4-dinitrophenol (DNP)-stimulated respiration. Quantitative analysis of three independent experiments revealed that Akt3 knockdown resulted in a 47% decrease in the basal respiration rate (P< 0.05) and a 37% decrease in the maximal mitochondrial oxidative capacity (P< 0.01) (Fig. 3B). These results confirmed that the mitochondria in the Akt3-knockdown cells had functional defects that included an impaired respiration capacity.
Figure 3.
Decreased oxygen consumption rates following Akt3 knockdown in EJ cells (A) The respiration rates of EJ cells expressing either the pLKO vector (pLKO) or shAkt3 were measured using the XF24 flux analyzer as described in the Materials and Methods section. Representative analyses of oxygen consumption performed in triplicate under basal conditions following the addition of either 2,4-dinitrophenol (DNP; 40 μM) or rotenone (1 μM) are shown. (B) The averaged metabolic profile of three repeated experiments. The results are presented as mean values with standard deviations. *P< 0.05, **P< 0.01 compared with pLKO vector control cells using Student's t-test.
Knockdown of Akt3 induced mitochondrial dysfunction in A549 cells
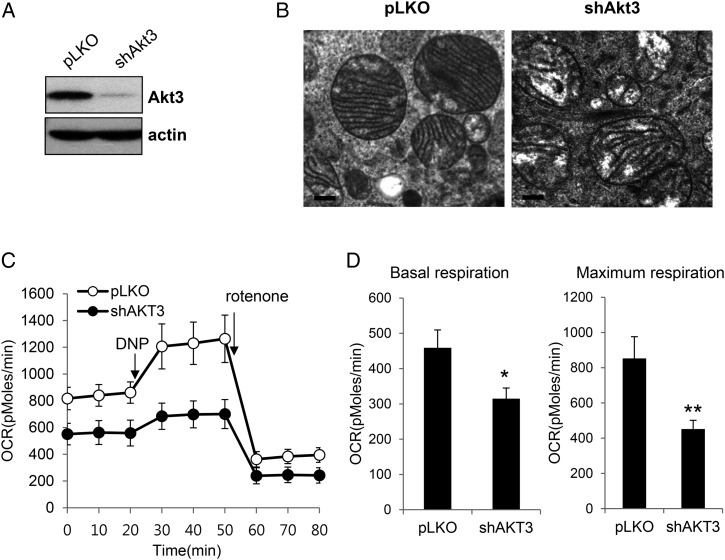
To confirm the role of Akt3 in mitochondrial function, we examined the effect of Akt3 knockdown in A549 human lung cancer cells. After confirmation of Akt3 knockdown by western blotting (Fig. 4A), we examined mitochondria structural organization using transmission electron microscopy. Consistent with the results of Akt3 knockdown in EJ cells, Akt3 knockdown also induced severe structural defects in mitochondria in A549 cells (Fig. 4B). Furthermore, oxygen consumption rate measured by using the XF24 flux analyzer was also remarkably reduced by Akt3 knockdown in A549 cells (Fig. 4C,D), suggesting that the mitochondrial respiration of A549 cells was impaired by Akt3 knockdown. These data further confirmed that Akt3 function is important for maintaining normal mitochondria function in human cancer cells.
Figure 4.
Effect of Akt3 knockdown on the mitochondrial mass and respiration rate in A549 cells (A) A549 cells expressing either the shAkt3 or pLKO vector were lysed and subject to western blotting using the indicated antibodies. The data shown are representative of three independent experiments. (B) Representative electron microscopy images of mitochondria in control A549 cells (pLKO) and in A549 cells expressing shAkt3. The images show ×40,000 magnification. (C) The respiration rates were measured using the XF24 flux analyzer as described in Fig. 3. Representative analyses of oxygen consumption performed in triplicate are shown. (D) The averaged metabolic profiles of three repeated experiments. The results are presented as mean values with standard deviations. *P< 0.05, **P< 0.01 compared with pLKO vector control cells using Student's t-test.
Discussion
In this report, we describe findings that indicate that the different Akt protein isoforms play distinct roles in mitochondrial functions in human cancer cells. First, we found that knockdown of either Akt1 or Akt3 increased the mitochondrial mass, whereas knockdown of Akt2 produced no significant changes in EJ human bladder carcinoma cells (Fig. 1B). Additionally, Akt3 deficiency induced remarkable structural disorganization in the mitochondria, whereas knockdown of either Akt1 or Akt2 resulted in only a moderate level of structural alteration (Fig. 2A). These results suggest that each Akt isoform plays a distinct role in maintaining mitochondrial abundance and structural integrity in EJ human bladder carcinoma cells.
Interestingly, the significant alterations in both the mitochondrial mass and mitochondrial structure that occurred after Akt3 knockdown suggested that Akt3 was particularly important for proper mitochondrial functions in EJ cells. Consistent with this hypothesis, Akt3 knockdown significantly increased DOX-induced premature senescence, whereas no differences were observed following Akt1 and Akt2 knockdown (Fig. 2B). Additionally, cell growth in galactose medium was significantly impaired following Akt3 knockdown, whereas Akt1- or Akt2-knockdown cells exhibited levels of growth similar to the control cells (Fig. 2C). These results suggest that the loss of Akt3 leads to defects in mitochondrial respiratory function. Indeed, Akt3-deficient EJ cells had significantly reduced oxygen consumption rates (Fig. 3A,B), indicating that the loss of Akt3 function induced mitochondrial dysfunction. A remarkable reduction in the oxygen consumption rates upon Akt3 knockdown was also observed in A549 human lung cancer cells (Fig. 4B,C) Therefore, these results confirm that Akt3 is required for the maintenance of proper respiratory functions in mitochondria in human cancer cells.
Akt has previously been implicated in mitochondrial functions. Akt is involved in the mitochondrial intrinsic apoptosis pathway by either directly or indirectly regulating various apoptotic factors, such as Bcl-2, JNK, GSK3β, and FOXO (reviewed in [28]). In addition to regulating this apoptotic pathway, the activation of Akt leads to changes in mitochondrial metabolism through its effects on the VDAC and hexokinase [29]. More directly, the accumulation of Akt within the mitochondria has also been reported. Bijur et al. demonstrated that Akt localized to the mitochondria in response to a number of stimuli, including cellular exposure to insulin-like growth factor-1, insulin, or heat shock stress [30]. This translocation of Akt was mediated by HSP90, and mitochondrial import occurred independent of Akt activation [31]. Miyamoto et al. indicated that the translocation of Akt into the mitochondria was important for the protection of cardiomyocytes against ischemic injury [32]. These studies indicate that Akt functionally cooperates with mitochondria when executing its protective role against various stressors. However, the involvement of Akt in basal mitochondrial functions (i.e. maintaining mitochondrial structural integrity and respiration) has not been fully addressed. In this study, we demonstrated that depletion of Akt expression resulted in defects in the mitochondrial structure and respiratory functions, suggesting that Akt was required for the maintenance of basal mitochondrial functions in addition to the stress responses.
Notably, our results highlight the importance of Akt3 in maintaining proper mitochondrial structure and respiration. Thus, it is possible that Akt3 is also directly involved in maintaining the integrity of the mitochondrial inner membrane structure. For example, Akt3 localized to the mitochondria could modulate critical regulators of mitochondrial integrity. Alternatively, Akt3 could indirectly participate in mitochondrial quality control by modulating either mitochondrial biogenesis or mitochondrial turnover through autophagic degradation (i.e. mitophagy). Alterations in mitochondrial biogenesis or degradation could lead to defects in mitochondrial quality control that would ultimately result in the accumulation of defective mitochondria [33]. Interestingly, Akt3 was recently shown to control both mitochondrial biogenesis and mitochondrial autophagy in human endothelial cells [34]. In this study, we demonstrated that Akt3 was required for mitochondrial biogenesis because of its ability to regulate the subcellular localization of PGC-1α (the master regulator of mitochondrial biogenesis) and to inhibit CRM-1-dependent autophagy. Interestingly, we found that Akt3 knockdown resulted in an increase in the mitochondrial mass and the induction of TFAM and NRF1 expression in EJ cells (Fig. 1B,C). These results suggest that the role of Akt3 in mitochondrial biogenesis could be cell type-dependent. The exact mechanism underlying how Akt3 is involved in mitochondrial biogenesis and whether Akt3 can regulate autophagy in EJ cells have not been determined.
In conclusion, this study revealed that Akt3 played an important role in maintaining the mitochondrial structure and respiratory activity in EJ and A549 cells. In conjunction with our previous studies, the present study provides evidence that different Akt protein isoforms play distinct roles within cells. Although the exact mechanisms that drive the contribution of Akt3 to normal mitochondrial function are unknown, future studies may provide critical information for the development of efficient strategies to treat cancer by targeting Akt family proteins.
Funding
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future (No. 2013R1A1A2A10008141).
References
- 1.Lightowlers RN, Taylor RW, Turnbull DM. Mutations causing mitochondrial disease: what is new and what challenges remain? Science 2015, 349: 1494–1499. [DOI] [PubMed] [Google Scholar]
- 2.Zeviani M, Carelli V. Mitochondrial disorders. Curr Opin Neurol 2003, 16: 585–594. [DOI] [PubMed] [Google Scholar]
- 3.Kang D, Hamasaki N. Alterations of mitochondrial DNA in common diseases and disease states: aging, neurodegeneration, heart failure, diabetes, and cancer. Curr Med Chem 2005, 12: 429–441. [DOI] [PubMed] [Google Scholar]
- 4.Franke TF. PI3K/Akt: getting it right matters. Oncogene 2008, 27: 6473–6488. [DOI] [PubMed] [Google Scholar]
- 5.Toker A, Marmiroli S. Signaling specificity in the Akt pathway in biology and disease. Adv Biol Regul 2014, 55: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci 2004, 29: 233–242. [DOI] [PubMed] [Google Scholar]
- 7.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 2005, 9: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 2001, 276: 38349–38352. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH et al. . Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 2001, 292: 1728–1731. [DOI] [PubMed] [Google Scholar]
- 10.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA et al. . A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 2004, 304: 1325–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM et al. . Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol 2005, 25: 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MA, Kim HJ, Jee HJ, Kim AJ, Bae YS, Bae SS, Yun J. Akt2, but not Akt1, is required for cell survival by inhibiting activation of JNK and p38 after UV irradiation. Oncogene 2009, 28: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 13.Kim EK, Yun SJ, Ha JM, Kim YW, Jin IH, Yun J, Shin HK et al. . Selective activation of Akt1 by mammalian target of rapamycin complex 2 regulates cancer cell migration, invasion, and metastasis. Oncogene 2011, 30: 2954–2963. [DOI] [PubMed] [Google Scholar]
- 14.Kim MA, Kim HJ, Brown AL, Lee MY, Bae YS, Park JI, Kwak JY et al. . Identification of novel substrates for human checkpoint kinase Chk1 and Chk2 through genome-wide screening using a consensus Chk phosphorylation motif. Exp Mol Med 2007, 39: 205–212. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Roh MS, Son CH, Kim AJ, Jee HJ, Song N, Kim M et al. . Loss of Med1/TRAP220 promotes the invasion and metastasis of human non-small-cell lung cancer cells by modulating the expression of metastasis-related genes. Cancer Lett 2012, 321: 195–202. [DOI] [PubMed] [Google Scholar]
- 16.Kim AJ, Jee HJ, Song N, Kim M, Jeong SY, Yun J. p21(WAF1/CIP1) deficiency induces mitochondrial dysfunction in HCT116 colon cancer cells. Biochem Biophys Res Commun 2013, 430: 653–658. [DOI] [PubMed] [Google Scholar]
- 17.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol 2007, 371: 21–31. [DOI] [PubMed] [Google Scholar]
- 18.Song N, Kim AJ, Kim HJ, Jee HJ, Kim M, Yoo YH, Yun J. Melatonin suppresses doxorubicin-induced premature senescence of A549 lung cancer cells by ameliorating mitochondrial dysfunction. J Pineal Res 2012, 53: 335–343. [DOI] [PubMed] [Google Scholar]
- 19.Grabinski N, Bartkowiak K, Grupp K, Brandt B, Pantel K, Jucker M. Distinct functional roles of Akt isoforms for proliferation, survival, migration and EGF-mediated signalling in lung cancer derived disseminated tumor cells. Cell Signal 2011, 23: 1952–1960. [DOI] [PubMed] [Google Scholar]
- 20.Lee MW, Kim DS, Lee JH, Lee BS, Lee SH, Jung HL, Sung KW et al. . Roles of AKT1 and AKT2 in non-small cell lung cancer cell survival, growth, and migration. Cancer Sci 2011, 102: 1822–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia Fernandez MI, Ceccarelli D, Muscatello U. Use of the fluorescent dye 10-N-nonyl acridine orange in quantitative and location assays of cardiolipin: a study on different experimental models. Anal Biochem 2004, 328: 174–180. [DOI] [PubMed] [Google Scholar]
- 22.Kanki T, Ohgaki K, Gaspari M, Gustafsson CM, Fukuoh A, Sasaki N, Hamasaki N et al. . Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol Cell Biol 2004, 24: 9823–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jee HJ, Kim HJ, Kim AJ, Song N, Kim M, Yun J. Nek6 suppresses the premature senescence of human cancer cells induced by camptothecin and doxorubicin treatment. Biochem Biophys Res Commun 2011, 408: 669–673. [DOI] [PubMed] [Google Scholar]
- 24.Green PS, Leeuwenburgh C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta 2002, 1588: 94–101. [DOI] [PubMed] [Google Scholar]
- 25.Huigsloot M, Tijdens IB, Mulder GJ, van de Water B. Differential regulation of doxorubicin-induced mitochondrial dysfunction and apoptosis by Bcl-2 in mammary adenocarcinoma (MTLn3) cells. J Biol Chem 2002, 277: 35869–35879. [DOI] [PubMed] [Google Scholar]
- 26.Aguer C, Gambarotta D, Mailloux RJ, Moffat C, Dent R, McPherson R, Harper ME. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One 2011, 6: e28536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci 2007, 97: 539–547. [DOI] [PubMed] [Google Scholar]
- 28.Stiles BL. PI-3-K and AKT: onto the mitochondria. Adv Drug Deliv Rev 2009, 61: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 29.Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 2001, 15: 1406–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem 2003, 87: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barksdale KA, Bijur GN. The basal flux of Akt in the mitochondria is mediated by heat shock protein 90. J Neurochem 2009, 108: 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ 2008, 15: 521–529. [DOI] [PubMed] [Google Scholar]
- 33.Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci 2010, 123: 2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corum DG, Tsichlis PN, Muise-Helmericks RC. AKT3 controls mitochondrial biogenesis and autophagy via regulation of the major nuclear export protein CRM-1. FASEB J 2014, 28: 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]