Abstract
Carcinoma-associated fibroblasts (CAFs) play central roles in facilitating tumor progression and metastasis in breast cancer. Galectin-1 (Gal-1), a marker of CAFs, was previously reported to be associated with tumorigenesis and metastasis of various types of tumors. The aim of this study is to investigate the role of Gal-1 in CAF-mediated breast cancer metastasis and its underlying molecular mechanisms. Our results showed that CAFs isolated from human breast tumor tissues expressed higher level of Gal-1 compared with paired normal fibroblasts, and the conditioned medium (CM) of CAFs significantly induced the migration and invasion of human MDA-MB-231 breast cancer cells. Knockdown of Gal-1 in CAFs dramatically inhibited CAF-CM-induced cell migration and invasion, probably by inhibiting the expression of matrix metalloprotein 9 (MMP-9). Our findings demonstrate that Gal-1-regulated CAFs activation promotes breast cancer cell metastasis by upregulating MMP-9 expression, which indicated that Gal-1 in CAFs might be a potential novel target for breast cancer therapy.
Keywords: carcinoma-associated fibroblasts, galectin-1, metastasis, matrix metalloprotein 9
Introduction
Breast tumor is a highly complex tissue composed of carcinoma cells and the surrounding stroma, and the stroma comprises of various different cells and extracellular matrix (ECM) [1]. Carcinoma-associated fibroblasts (CAFs, commonly referred to as myofibroblasts), the most abundant cell type in breast cancer stroma, are becoming increasingly apparent due to its effect on promoting tumor cell growth, migration, invasion, and drug resistance [2]. Increasing evidence indicated that CAFs could induce breast epithelial cell migration and invasion by producing a plethora of chemokines, growth factors, and ECM proteins [3], and a dynamic bidirectional signaling is detected between CAFs and breast cancer cells [4,5]. However, the precise molecular mechanisms of CAFs' effect on the migration and invasion of breast cancer are still far from clear.
Galectin-1 (Gal-1), a homodimer of 14 kDa subunits, is classified as a prototype member of carbohydrate-binding proteins, which shares the common affinity for β-galactoside. It is differentially expressed in normal and pathological tissues with a wide range of biological activity [6]. Gal-1 has become a molecule of interest in cancer research due to its role in apoptosis, cell transformation, angiogenesis, and cell invasiveness [7]. Gal-1 expression in tumor cells as well as in tumor stromal cells can be considered as a sign of the malignant tumor progression, but its overexpression in CAFs has been identified as a poor prognosis marker in breast cancer [3,8,9].
Metastatic breast cancer is a tumor with the highest mortality in women, which is even worse in triple-negative breast cancer patients [10]. In the present study, we investigated the effect of CAFs on the migration and invasion of MDA-MB-231 (ER-, PR-, and Her2/neu-negative) breast carcinoma cell line and explored the contribution of Gal-1 in this process.
Materials and Methods
Materials
Cell culture medium components were purchased from Gibco (New York, USA). Dimethyl sulfoxide, sodium bicarbonate, penicillin–streptomycin, trypsin, ribonuclease A (RNase A), polyvinylidene difluoridepolyvinylidenefluoride (PVDF) membrane, and enhanced chemiluminescence (ECL) assay kit were purchased from Beyotime (Nantong, China). Recombinant human TGF-β1 was purchased from PeproTech (Rocky Hill, USA). The antibodies used in this study include: anti-pan-cytokeratin (anti-Pan-CK) antibody (CK, 1 : 500; Santa Cruz, Santa Cruz, USA), anti-Vimentin antibody (1 : 1000; Santa Cruz), anti-α-SMA antibody (1 : 3000; Abcam, Cambridge, USA), anti-Gal-1 antibody (1 : 2000; Abcam), anti-fibroblast activation protein (FAP) antibody (1 : 1000; Abcam), anti-Desmin antibody (1 : 2000; Abcam), anti-fibronectin (FN) antibody (1 : 3000; Abcam), anti-CD31 antibody (1 : 1000; Abcam), and anti-β-actin antibody (1 : 2000; Abcam). Horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG; 1 : 2000) and goat anti-rabbit IgG (1 : 2000) secondary antibodies were from Santa Cruz.
Isolation of primary fibroblasts and culture of cells
All procedures performed in this study involving human participants were in accordance with the ethical standards of the research committee (Institutional and National) and with the Helsinki declaration and its later amendments or comparable ethical standards. Samples of this study were obtained from Department of Pathology, the Affiliated Maternity and Child Health Hospital of Nanjing Medical University (Wuxi, China), and informed consents were obtained from all subjects.
CAFs and normal fibroblasts (NFs) were isolated from breast tumor tissues and their normal counterparts as described previously [1,11]. The normal counterparts were defined in the noncancerous region of breast tissue at least 2 cm away from the outer tumor margin. Briefly, tumor tissue and paired normal mammary tissues were washed three times with sterile phosphate buffered saline (PBS), containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamycin. After being minced with sterile scissors, the tissues were digested with 0.1% collagenase type I at 37°C for 12 h. Then the tissues were carefully resuspended in Dulbecco's modified Eagle medium (DMEM)–low glucose culture medium. The mixtures were centrifuged and washed with DMEM to remove the fat and tissue debris. The mammary tissues were cultured in DMEM supplemented with 10% FBS for 3 days. After the suspending cells or tissues were removed, the most adherent cells were fibroblasts. The primary fibroblasts isolated from tumor tissues were CAFs, and from tumor paired normal tissues were NFs. The isolated CAFs and NFs were further identified by immunoblotting with antibodies against pan-CK, desmin, FN, vimentin, CD31, and α-SMA. Cells were routinely cultured in DMEM medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human breast carcinoma cell line MDA-MB-231 was obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China) and routinely cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Cell transfection
To knockdown the expression of Gal-1, CAFs and NFs were transfected with small interfering RNA (siRNA) in serum-free medium using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, USA) as described previously [12]. The predesigned siRNA for Gal-1 was obtained from Santa Cruz. The depletion of target gene was determined by real-time-polymerase chain reaction (qRT-PCR) and western blot analysis 24 h after transfection.
To overexpress Gal-1, the NFs were transfected with a plasmid that encodes Gal-1 cDNA under the control of the CMV promoter (pcDNA3.1; Invitrogen) using Attractene Transfection Reagent (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The expression of target gene was determined by qRT-PCR and western blot analysis 24 h after transfection.
Quantitative real-time-polymerase chain reaction
Total RNA was isolated using TRIzol reagent, and first-strand cDNA was synthesized using RT reagent kit (Takara, Shiga, Japan) according to the manufacturer's protocol. qRT-PCR was performed with the cDNA using SYBR green PCR Master Mix (Takara). The primer pairs were as follows—Gal-1: forward, 5′-CTGTGCCTGCACTTCAACC-3′ and reverse, 5′-CATCTGGCAGCTTGACGGT-3′; α-SMA: forward, 5′-CTATGAGGGCTATGCCTTGCC-3′ and reverse, 5′-GCTCAGCAGTAGTAACGAAGGA-3′; β-actin: forward, 5′-GGGACCTGACTGACTACCTCA-3′ and reverse, 5′-GACTCGTCATACTCCTGCTTG-3′. PCR was performed in an ABI7500 sequence detection system (Life Technologies, Carlsbad, USA) using the following procedure: denaturation at 95°C for 30 s, amplification for 30 cycles at 95°C for 5 s, and annealing and extension at 60°C for 20 s. The amount of target was normalized to β-actin and expressed relative to the control using the comparative ΔΔCt method [13].
Western blot analysis
For western blot analysis, cells (1 × 106) were collected and lysed in ice-cold RIPA buffer (50 mM Tris–HCl, 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid, 1 mM ethylenediaminetetraacetic acid, 20 mM NaF, 100 mM Na3VO4, 1% Nonidet P-40, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mg/ml aprotinin, and 10 mg/ml leupeptin) for 30 min. Protein concentration was determined with the Bradford method [14]. Samples (50 μg) were separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis gel (12%) and electrophoretically transferred onto PVDF membranes. The membranes were blocked with 5% bovine serum albumin in Tris-buffer saline (TBST) at 37°C for 1 h, and then incubated with the primary antibodies overnight, followed by incubation with HRP-conjugated secondary antibody for 1 h at room temperature. The protein bands were visualized by the enhanced chemiluminescence (ECL) detection kit (Beyotime).The density of each band was normalized to β-actin. HeLa whole cell lysate (Santa Cruz) was used as the positive control for pan-CK antibody. HSM (human skeletal muscle tissue lysate) (Abcam) was used as the positive control for desmin antibody, and HUVEC (human umbilical vein endothelial cell) whole cell lysate was used as the positive control for CD31 antibody.
Cell invasion and migration assay
Cell migration and invasion assays were performed in 24-well Transwell polycarbonate filters with 8-μm pore size (Corning Co., Corning, USA) as previously described [15]. For the invasion assay, MDA-MB-231 cells were starved overnight in serum-free medium. Then, 30 μl of Matrigel was added into culture inserts (BD Biosciences, San Diego, USA) and MDA-MB-231 cells were added in the upper chamber, and the conditioned media of NFs (NF/Con), NFs transfected with Gal-1 (NF/OE), CAFs (CAF/Con), NFs and CAFs with Gal-1 knockdown (NF/KD and CAF/KD) were added to the lower chamber, respectively. After 4 h of culture, non-penetrated cells were removed from the upper surface of the filter with a cotton swab.
The migration assay was performed using a transwell culture system without a Matrigel coating. The migrated and invaded cells were incubated for another 24 and 8 h, respectively. After being cultured for indicated time, cells on the lower surface of membrane were washed and fixed with methanol for 5 min. The numbers of invaded and migrated cells were estimated by staining of membranes with 0.1% crystal violet in PBS. The membranes were washed three times with PBS, and the dye was eluted with 500 ml of 10% acetic acid. The absorbance at 600 nm was measured. Background value was obtained from wells without cells.
Enzyme-linked immunosorbent assay
Levels of secreted matrix metalloprotein 9 (MMP-9) by the cells were measured using the Human MMP-9 Quantikine ELISA Kit (R&D Systems, Minneapolis, USA) according to the manufacturer's instructions. The supernatant of the cultured cells (6 × 105 cells per well in a 24-well plate) were collected by centrifugation at 1000 g for 20 min. The samples and standard substance were incubated in 96-well plates at 37°C for 30 min. Then the plates were washed three times with wash buffer. After reacting with chromogenic substrates, absorbance was measured at 450 nm with a microplate reader. The expression of MMP-9 was calculated from the standard curve.
Statistical analysis
All experiments were done in triplicates and the results were from three independent studies. Results were expressed as mean ± standard deviation (SD). Biostatistical analyses were conducted with the SPSS 19.0 software packages (IBM, Armonk, USA). Statistical comparisons were made by one-way analysis of variance followed by Tukey's post hoc test, and P<0.05 was considered as statistically significant.
Results
Gal-1 overexpression promotes fibroblast activation
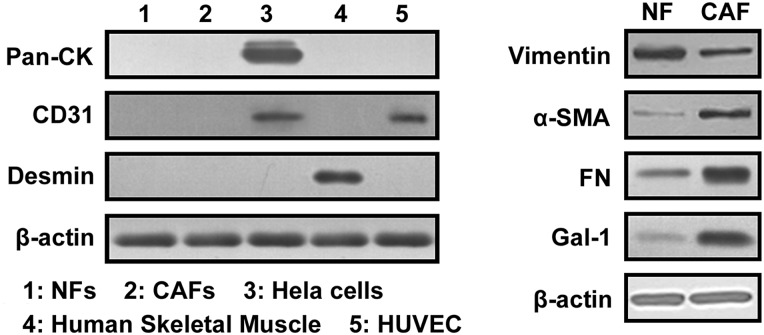
NFs and CAFs were isolated from clinical breast cancer tissue, and characterized as fibroblast with the absence of Pan-CK (an epithelial cell marker), Desmin (a muscle cell marker), and CD31 (an endothelial cell marker), but high expression of vimentin and FN (fibroblast markers). CAFs were further identified with strong expression of α-SMA (a CAF marker). As shown in Fig. 1, the absence of Pan-CK, Desmin, and CD31 expression in NFs and CAFs indicated that the samples were not contaminated by epithelial cells, smooth muscle cells, or endothelial cells. Vimentin and FN were strongly expressed in NFs and CAFs. Both Gal-1 and α-SMA expressions were higher in CAFs than in NFs, which suggested that Gal-1 expression might closely correlated with CAF activation.
Figure 1.
Gal-1 expression is correlated with α-SMA expression Paired NF and CAF cells were isolated from clinical breast cancer tissue. Western blot analysis was used to analyze the expression of Pan-CK (an epithelial cell marker), Desmin (a muscle cell marker), FN (a fibroblast marker), Vimentin (a fibroblast marker), and α-SMA (a CAF marker) and Gal-1. Each experiment included triplicate repeats.
Gal-1 stimulates the transdifferentiation of fibroblasts into myofibroblasts
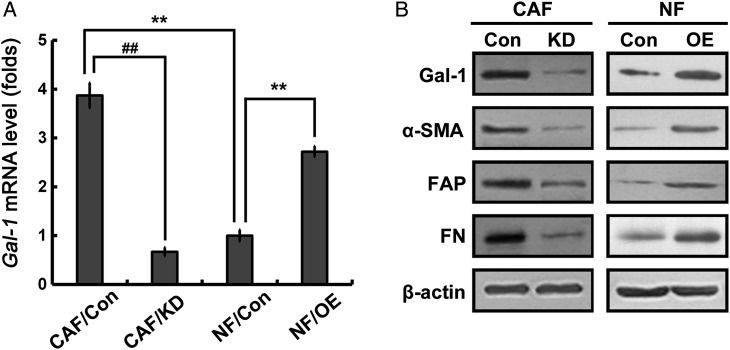
It is well known that fibroblasts to myofibroblasts differentiation is a key event in the physiological reconstruction [16]. Due to the association of Gal-1 expression and CAF activation [8], whether Gal-1 regulates the transdifferentiation of fibroblasts into myofibroblasts was examined. RNA interference was used to knockdown Gal-1 expression in CAFs (Fig. 2A). Strikingly, Gal-1 knockdown in CAFs significantly reduced the expression of α-SMA. FAP, a CAF marker, was also reduced upon knockdown of Gal-1 in CAFs (Fig. 2B). The expression of ECM protein FN was also suppressed (Fig. 2B). However, after transfection with Gal-1, NFs dramatically increased the expression of α-SMA, FAP, and FN (Fig. 2B). These data strongly support that Gal-1 modulates fibroblasts transdifferentiation into myofibroblasts.
Figure 2.
Gal-1 regulates fibroblast activation CAFs and NFs were transfected with Gal-1 siRNA and Gal-1 cDNA, respectively. The depletion and expression of Gal-1 were determined by qRT-PCR (A). Relative expression levels were normalized using β-actin. (B) Western blot analysis was used to analyze protein expressions of Gal-1, α-SMA, FAP, and FN. All data were expressed as mean ± SD of three experiments. **P< 0.01 vs. NF/Con, ##P< 0.01 vs. CAF/Con. Con, controls; OE, overexpressions; KD, knockdowns.
Targeting Gal-1 suppresses CAF-augmented breast cancer cell migration and invasion
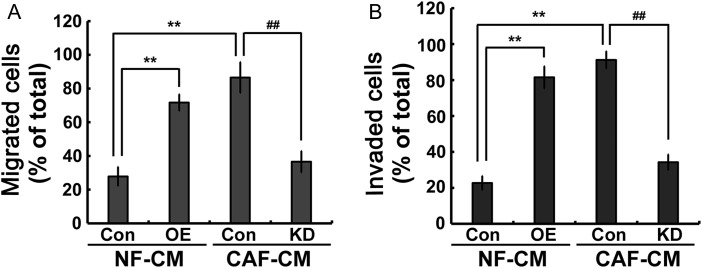
CAFs play an important role in promoting tumor invasion and migration; therefore, whether Gal-1 regulates CAF-augmented breast cancer cell invasion and migration was investigated. NFs and CAFs were transfected with Gal-1 cDNA and Gal-1 siRNA, respectively. The conditioned media (CM) were collected as chemoattractants for the invasion and migration chamber assays. CAF/Con-conditioned medium (CAF/Con-CM) and NF/OE-conditioned medium (NF/OE-CM) induced more cancer cell migration and invasion than NF/Con-conditioned medium (NF/Con-CM) (Fig. 3A,B); however, Gal-1 knockdown significantly attenuated the migratory and invasive abilities of MDA-MB-231 cells (Fig. 3A,B). These data suggested that Gal-1 might play an important role in regulating CAF-augmented cancer cell migration and invasion.
Figure 3.
Gal-1 regulates CAF-CM-augmented MDA-MB-231 cell migration and invasion NFs and CAFs were transfected with Gal-1 cDNA and Gal-1 siRNA, respectively. Cell migration (A) and invasion (B) were measured using a transwell culture system. The CM was collected as a chemoattractant. All data were expressed as mean ± SD of three experiments. **P< 0.01 vs. NF/Con-CM; ##P< 0.01 vs. CAF/Con-CM. Con, controls; OE, overexpressions; KD, knockdowns.
Gal-1 regulates CAF-augmented cancer cell migration and invasion via MMP-9 signaling pathway
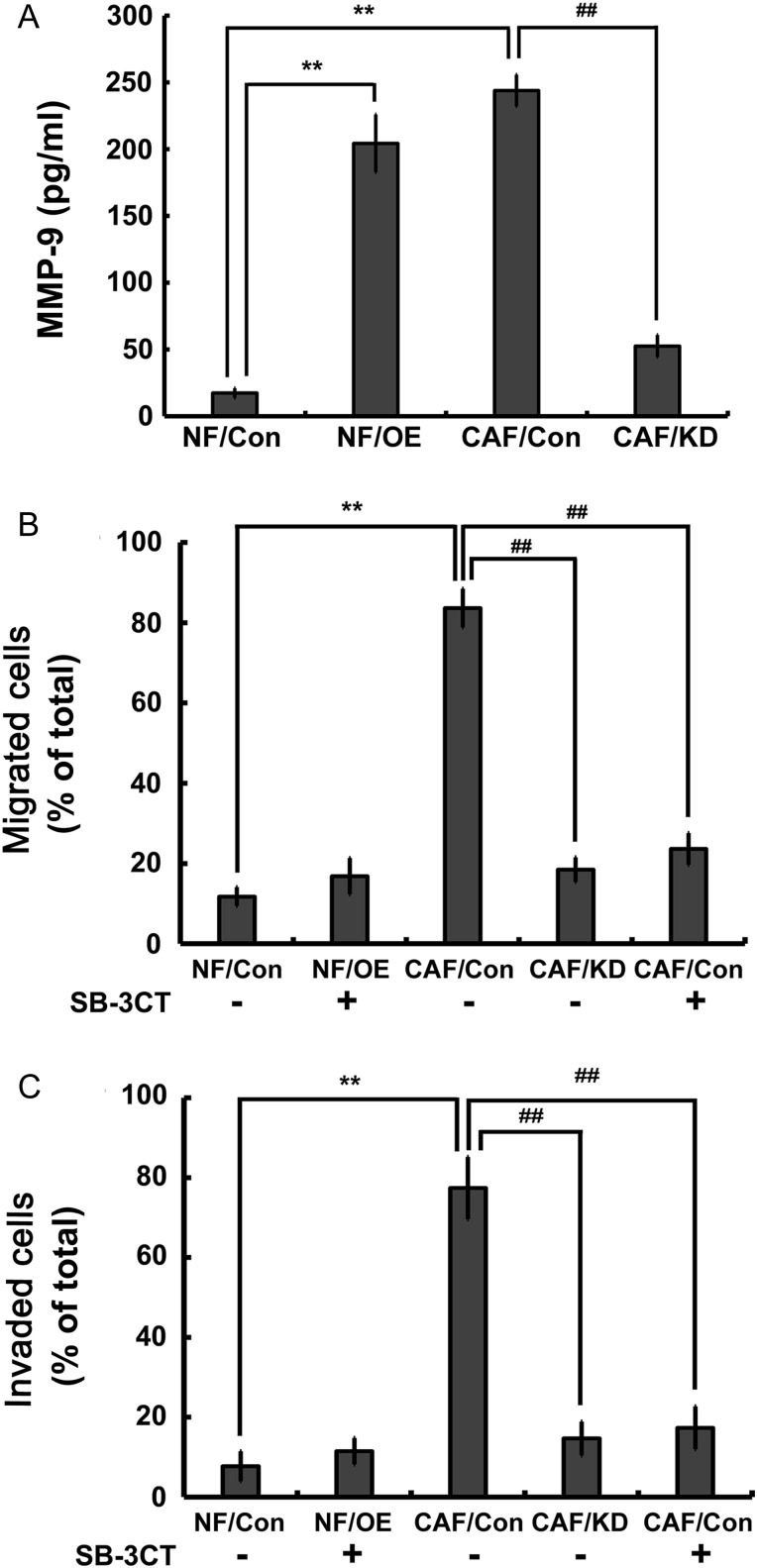
Gal-1-mediated MMP-9 secretion in CAFs is a prerequisite for tumor growth, angiogenesis, invasion, and metastasis [7,17]. First, we investigated whether MMP-9 expression was mediated by Gal-1 in breast cancer-associated fibroblasts. As shown in Fig. 4A, the level of MMP-9 in CAFs was significantly higher than that in NFs, and it was reduced upon suppression of Gal-1 expression. NFs transfected with Gal-1 cDNA also showed high level of MMP-9 expression. Whether MMP-9 is a critical regulator in CAF-augmented cancer cell migration and invasion was further evaluated. Addition of SB-3CT, an MMP-9 inhibitor, into CAF/Con-CM and NF/OE-CM inhibited the migration and invasion MDA-MB-231 cells (Fig. 4B,C). These data indicated that Gal-1 promoted cancer cell migration and invasion by modulating MMP-9 expression in CAFs.
Figure 4.
Gal-1 modulates CAF-augmented MDA-MB-231 cell migration and invasion by upregulating MMP-9 expression (A) The levels of secreted MMP-9 protein in the medium were determined by human MMP-9 ELISA kit. (B,C) Inhibition of MMP-9 activity significantly reduced CAF/Con-CM and NF/OE-CM-induced cell migration and invasion. All data were expressed as mean ± SD of three experiments. **P< 0.01 vs. NF/Con-CM; ##P< 0.01 vs. CAF/Con-CM. Con, controls; OE, overexpressions; KD, knockdowns.
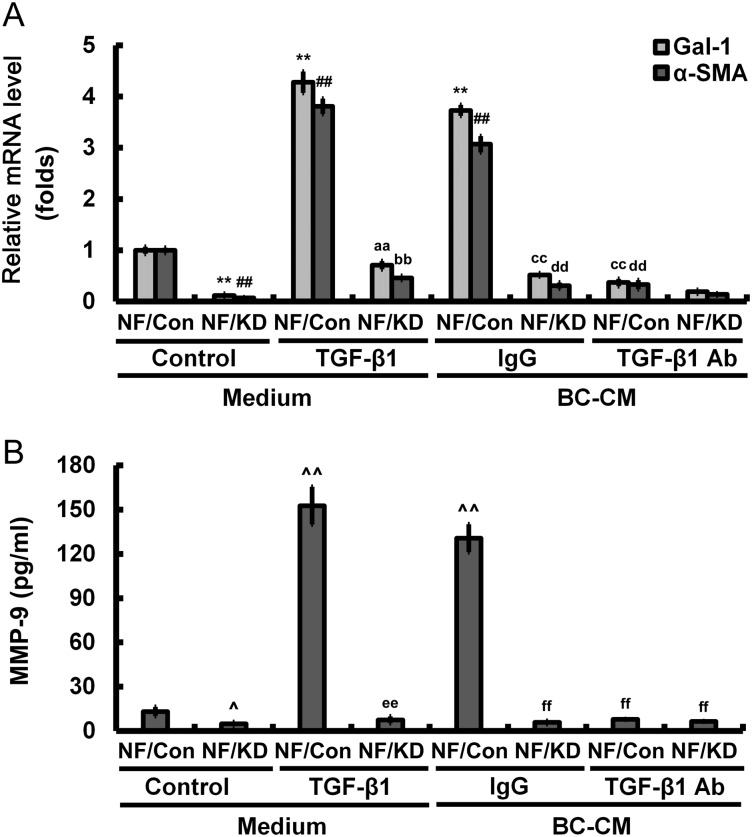
TGF-β1 promotes fibroblasts Gal-1 expression, transdifferentiation, and MMP-9 secretion
In breast cancer, TGF-β-signaling was shown to promote metastasis [18]. Therefore, we investigated whether TGF-β1 is a critical regulator in Gal-1-mediated tumor invasion and migration. With treatment of TGF-β1 (2 ng/ml), NFs induced Gal-1 and α-SMA expression; however, this effect was inhibited in NFs with Gal-1 knockdown. Treatment of NFs with breast cancer cell (MDA-MB-231)-conditioned medium (BC-CM) had the similar results. The addition of anti-TGF-β1 antibody in BC-CM almost abolished the expressions of Gal-1 and α-SMA, indicating that TGF-β1 regulated Gal-1 expression and NF transdifferentiation (Fig. 5A). Moreover, treatment with BC-CM containing TGF-β1 induced MMP-9 secretion in NFs, which could be blocked by cleanup of TGF-β1 (Fig. 5B). TGF-β1 treatment-induced MMP-9 secretion in NFs could also be inhibited by Gal-1 suppression (Fig. 5B). Gal-1 knockdown in NFs efficiently cuts off the cross talk between cancer cells and cancer-associated stroma.
Figure 5.
TGF-β1 expressed by breast cancer cells promotes Gal-1 expression, fibroblast transdifferentiation, and MMP-9 secretion (A) The relative expression of Gal-1 and α-SAM was analyzed by qRT-PCR. (B) The levels of secreted MMP-9 protein in the medium were determined by human MMP-9 ELISA kit. All data were expressed as mean ± SD of three experiments. **P< 0.01, ##P< 0.01, ^P< 0.05, ^^P< 0.05 vs. NF/Con treated with medium; aaP< 0.01, bbP< 0.01, eeP< 0.01 vs. NF/Con treated with TGF-β1; ccP< 0.01, ddP< 0.01, ffP< 0.01 vs. NF/Con treated with BC-CM. Con, controls; KD, knockdowns.
Discussion
Cancer cells exist within a complex ecosystem consisting of CAFs, endothelial cells, and immune cells, and actively interact with the adjacent tumor microenvironment to promote cancer growth, survival, and metastasis [19]. Increasing histopathological evidence indicates that tumor-associated stroma, especially CAFs, may refine the prognostic assessment of breast cancer [2]. Cross talk between CAFs and breast cancer cells promotes tumor growth, metastasis, and invasion, which raises an important question on the mechanism of their regulation. In this study, we first isolated CAFs and NFs from clinical breast cancer tissue and characterized these two different fibroblast phenotypes. Then, we investigated the regulatory mechanism of CAFs on breast cancer cell line MDA-MB-231. Our results showed that CAFs expressed a higher level of α-SMA compared with the paired NFs, and the CM of CAFs could induce the migration and invasion in MDA-MB-231 cells.
Gal-1 is a highly expressed marker of cancer stromal fibroblasts, which is associated with metastasis [3]. There is strong evidence supporting the involvement of Gal-1 in various biological processes linked to cell adhesion, motility, migration, and invasion [7]. However, the role of Gal-1 in cross talk between CAFs and breast cancer cells is far from clear. In the present, Gal-1 was confirmed to be highly expressed in CAFs from clinical breast cancer tissue than that in NFs. Our data also showed that Gal-1 could modulate fibroblasts transdifferentiation into myofibroblasts. Moreover, knockdown of Gal-1 in CAFs significantly suppressed CAF-augmented MDA-MB-231 cell migration and invasion. These results indicate that targeting Gal-1 in CAFs may provide a novel therapeutic strategy for breast cancer metastasis.
As the most common cellular population found in the tumor microenvironment, CAFs are responsible for the secretion of proteins and cytokines that regulate ECM remodeling, tumor cell proliferation, and metastasis [16]. The possible mechanism of how Gal-1 contributes to cancer progression and metastasis is that it mediates cell–cell or cell–ECM adhesion [20]. Wu et al. [21] reported that Gal-1-induced tumor invasion was related to the upregulation of matrix metalloproteinases expression, such as MMP-9 and MMP-2, in oral squamous cell carcinoma and lung adenocarcinoma. Compared with cancer cells, cancer stromal fibroblasts is known to secrete MMP-9, which is involved in ECM degradation and cancer invasion [22]. In the present study, we provided evidence that Gal-1 could induce MMP-9 expression in CAFs, and inhibition of MMP-9 could significantly abolish CAF/Con-CM-induced cell migration and invasion. These data indicated that Gal-1 regulated CAF-augmented breast cancer cell metastasis probably by upregulating MMP-9 expression. Moreover, the cross talk between tumor cells and stroma is not a one-way street. Our results indicated that TGF-β1, which is expressed by MDA-MB-231 cells, could also induce Gal-1 expression, promote fibroblast transdifferentiation and MMP-9 secretion. TGF-β1, as a key fibrogenic cytokine, strongly activates the differentiation of fibroblasts into myofibroblasts in vitro [23]. Both TGF-β1 and Gal-1 are involved in tumor progression and tumor-immune escape. TGF-β1 could upregulate the expression of Gal-1 in human metastatic mammary adenocarcinoma LM3 cells and MCF-3 cells [24]. TGF-β1-upregulated Gal-1 expression is likely to be mediated through binding with TβRII, activating TβRI, and triggering a Smad-dependent signaling pathway [24]. Our results indicated that TGF-β1-induced upregulation of Gal-1 expression occurs not only in cancer cells but also between the stroma and cancer cells. Knockdown of Gal-1 in CAFs could reverse the activation of fibroblasts, and may inhibit cancer metastasis.
Taken together, our findings may significantly contribute to a better understanding of the importance of Gal-1 in breast tumorigenesis. Gal-1 may be used not only as a prognosis marker but also as a therapeutic target.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 81300787), the Natural Science Foundation of Jiangsu Province (Nos. BK2012105 and BK20141103), the Project of Jiangsu Provincial Commission of Health and Family Planning (No. H201546), the Major Project of Wuxi Municipal Health Bureau (Nos. ZS201401, Z201508, and MS201404), and the Project of Wuxi Municipal Science and Technology Bureau (No. CSE31N1520).
References
- 1.Zhao L, Sun Y, Hou Y, Peng Q, Wang L, Luo H, Tang X et al. . MiRNA expression analysis of cancer-associated fibroblasts and normal fibroblasts in breast cancer. Int J Biochem Cell Biol 2012, 44: 2051–2059. [DOI] [PubMed] [Google Scholar]
- 2.Aboussekhra A. Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int J Dev Biol 2011, 55: 841–849. [DOI] [PubMed] [Google Scholar]
- 3.Folgueira MA, Maistro S, Katayama ML, Roela RA, Mundim FG, Nanogaki S, de Bock GH et al. . Markers of breast cancer stromal fibroblasts in the primary tumour site associated with lymph node metastasis: a systematic review including our case series. Biosci Rep 2013, 33 doi:10.1042/BSR20130060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos RP, Benvenuti TT, Honda ST, Del Valle PR, Katayama ML, Brentani HP, Carraro DM et al. . Influence of the interaction between nodal fibroblast and breast cancer cells on gene expression. Tumor Biol 2011, 32: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozenchan PB, Carraro DM, Brentani H, de Carvalho Mota LD, Bastos EP, e Ferreira EN, Torres CH et al. . Reciprocal changes in gene expression profiles of cocultured breast epithelial cells and primary fibroblasts. Int J Cancer 2009, 125: 2767–2777. [DOI] [PubMed] [Google Scholar]
- 6.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology 2006, 16: 137R–157R. [DOI] [PubMed] [Google Scholar]
- 7.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, Serova M, Neuzillet C, Albert S, Raymond E et al. . Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat Rev 2014, 40: 307–319. [DOI] [PubMed] [Google Scholar]
- 8.Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ, Hong SC et al. . Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int J Cancer 2007, 120: 2331–2338. [DOI] [PubMed] [Google Scholar]
- 9.Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Méndez-Huergo SP, Stupirski JC et al. . Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res 2013, 73: 1107–1117. [DOI] [PubMed] [Google Scholar]
- 10.Weide R, Feiten S, Friesenhahn V, Heymanns J, Kleboth K, Thomalla J, van Roye C et al. . Metastatic breast cancer: prolongation of survival in routine care is restricted to hormone-receptor- and Her2-positive tumors. SpringerPlus 2014, 3: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Q, Zhao L, Hou Y, Sun Y, Wang L, Luo H, Peng H et al. . Biological characteristics and genetic heterogeneity between carcinoma-associated fibroblasts and their paired normal fibroblasts in human breast cancer. PLoS One 2013, 8: e60321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu MH, Hong HC, Hong TM, Chiang WF, Jin YT, Chen YL. Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression. Clin Cancer Res 2011, 17: 1306–1316. [DOI] [PubMed] [Google Scholar]
- 13.Jung C, Lee M, Kang Y, Lee Y, Yoon H, Kang SW, Lee W et al. . Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Diabetol 2014, 13: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Casimiro MC, Wang C, Shirley LA, Jiao X, Katiyar S, Ju X et al. . p21CIP1 attenuates Ras- and c-Myc-dependent breast tumor epithelial mesenchymal transition and cancer stem cell-like gene expression in vivo. Proc Natl Acad Sci USA 2009, 106: 19035–19039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 2010, 21: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi A, Kawana K, Tomio K, Yamashita A, Isobe Y, Nagasaka K, Koga K et al. . Matrix metalloproteinase (MMP)-9 in cancer-associated fibroblasts (CAFs) is suppressed by omega-3 polyunsaturated fatty acids in vitro and in vivo. PLoS One 2014, 9: e89605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuelten CH, Byfield SD, Arany PR, Karpova TS, Stetler-Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-α and TGF-β. J Cell Sci 2005, 118: 2143–2153. [DOI] [PubMed] [Google Scholar]
- 19.De Wever O, Van Bockstal M, Mareel M, Hendrix A, Bracke M. Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Semin Cancer Biol 2014, 25: 33–46. [DOI] [PubMed] [Google Scholar]
- 20.van den Brule F, Califice S, Garnier F, Fernandez PL, Berchuck A, Castronovo V. Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Lab Invest 2003, 83: 377–386. [DOI] [PubMed] [Google Scholar]
- 21.Wu MH, Hong TM, Cheng HW, Pan SH, Liang YR, Hong HC, Chiang WF et al. . Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol Cancer Res 2009, 7: 311–318. [DOI] [PubMed] [Google Scholar]
- 22.Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int 2002, 52: 255–264. [DOI] [PubMed] [Google Scholar]
- 23.Lim MJ, Ahn J, Yi JY, Kim MH, Son AR, Lee SL, Lim DS et al. . Induction of galectin-1 by TGF-beta1 accelerates fibrosis through enhancing nuclear retention of Smad2. Exp Cell Res 2014, 326: 125–135. [DOI] [PubMed] [Google Scholar]
- 24.Daroqui CM, Ilarregui JM, Rubinstein N, Salatino M, Toscano MA, Vazquez P, Bakin A et al. . Regulation of galectin-1 expression by transforming growth factor beta1 in metastatic mammary adenocarcinoma cells: implications for tumor-immune escape. Cancer Immunol Immun 2007, 56: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]