Abstract
Inflammation is a response of body tissues to injury and infection. Compounds that can inhibit inflammation have been shown to have potential therapeutic clinical application. Gambogenic acid (GEA) has potent antitumor and anti-inflammatory activities. Herein, the molecular mechanisms of GEA's anti-inflammatory effect were investigated in lipopolysaccharide (LPS)-stimulated macrophage cells. The results showed that pretreatment with GEA could markedly inhibit interleukin (IL)-1α, IL-1β, tumor necrosis factor-α, IFN-β, IL-12b, and IL-23a production in a dose-dependent manner in LPS-induced model. Furthermore, this drug significantly reduced the release of nitric oxide (NO), and impaired the protein level of inducible NO synthase and the cyclooxygenase 2. The finding also showed that the effect of GEA may be related to the suppression of the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathway. These results indicate that GEA could suppress LPS-simulated inflammatory response partially by attenuating NO synthesis and NF-κB and MAPK activation, suggesting that it may become a potent therapeutic agent for the treatment of inflammatory diseases.
Keywords: inflammation, gambogenic acid, NO, NF-κB pathway, MAPK pathway
Introduction
Inflammation is an innate immune response for elimination of harmful particles, cell, and tissue injury. It acts as a key regulator in the pathogenesis of many diseases, such as infection, arteriosclerosis, metabolic disorders, and cancer [1]. The inflammation process is controlled by several different immune cells such as macrophage, neutrophils, and lymphocyte [2]. Macrophage plays a crucial role during inflammatory response by producing a large quantity of inflammatory mediators such as reactive oxygen species, nitric oxide (NO), and prostaglandin E2 (PGE2), as well as proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1α, IL-1β, and monocyte chemotactic protein-1 [3,4]. Previous studies have demonstrated that these genes or factors might mediate the initiation and persistence of inflammation, suggesting that proinflammatory is a promising target for the treatment of inflammatory disorders [5,6].
TLR4 is a member of the TLRs (Toll-like receptors) family, and mainly interacts with and is activated by pathogen-associated molecular pattern [7]. Lipopolysaccharide (LPS), a major component of gram-negative bacteria cell membrane, interacts with TLR4, leads to the initiation of downstream signaling pathways such as phosphatidylinositol 3-kinase (PI3K)/AKT, nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs), and stimulates the secretion of inflammation mediators [8]. Therefore, the inflammatory response in LPS-induced macrophages is a useful model to screen the pharmacological effect of agents on inflammation.
Epidemiological studies have indicated that Chinese herbal traditional medicines or their derivative products have anti-inflammatory properties in disease prevention and treatment [9,10]. Parthenolide, purified from the shoots of feverfew, attenuates the LPS-induced production of cytokines such as NO, PGE2, IL-1β, and TNF-α, and inhibits the activation of NF-κB and MAPKs in human and mouse macrophages [11]. Epigallocatechin-3-gallate is the major polyphenol component of green tea and has been shown to have anti-inflammatory effect via inactivating the NF-κB and MAPKs pathways [12]. In addition, antitumor agent curcumin significantly inhibits the release of NO, PGE2, and proinflammatory cytokines in LPS-stimulated immune cells [13]. It is urgent to develop new drugs from natural plants to attenuate the production of inflammatory mediators and combat various inflammatory diseases.
The genus Garcinia is known for its rich variety of oxygenated and prenylated phenol derivatives. Gambogic acid is a major active component of gamboge, which has been reported to have potent anticancer and anti-inflammatory activity [14,15]. It is authorized to be tested in Phase I in 2004 and now in Phase II clinical trials [16]. Gambogenic acid (GEA) is another important active ingredient isolated from the resin of Garcinia hanburyi. The structure of GEA is shown in Fig. 1A. It has potent anticancer activity via targeting various intracellular signaling pathways associated with G1/S arrest, cell apoptosis, and autophagy [17–21]. However, the anti-inflammatory activity of GEA has not yet been clearly defined. In the present study, we investigated the effects of GEA on LPS-induced inflammatory response and further explored molecular mechanisms involved in its anti-inflammatory activity in macrophages.
Figure 1.
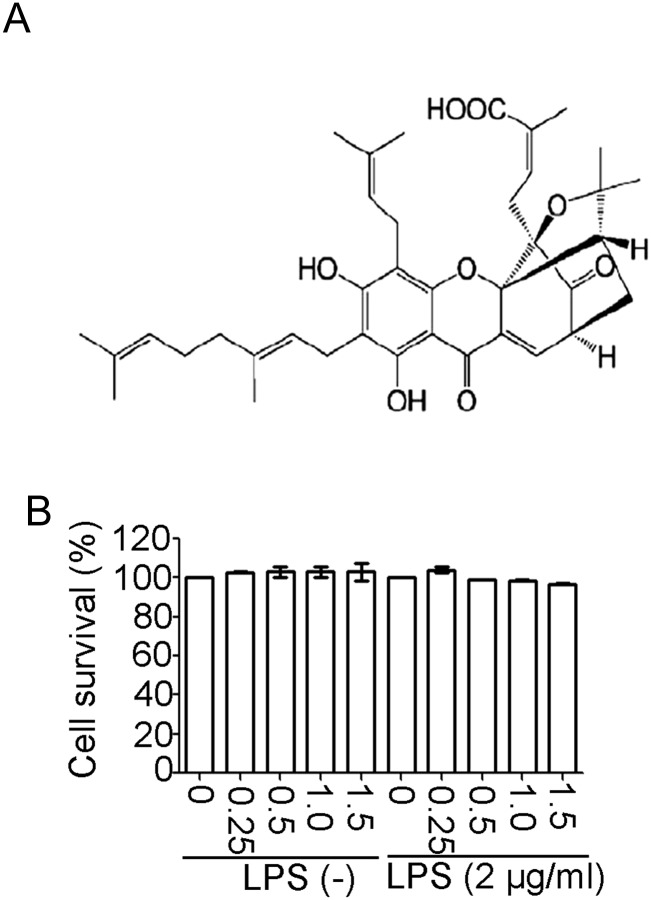
Effect of GEA on the viability of J774A.1 cells (A) The chemical structure of GEA. (B) J774A.1 cells were treated with GEA (0, 0.25, 0.5, 1.0, and 1.5 μM) for 24 h. Cell viability was assessed by cell titer assay. Data are from three independent experiments.
Materials and Methods
Chemicals and reagents
GEA was extracted from gamboges as previously described [18] and dissolved in dimethyl sulfoxide (Sigma, St Louis, USA) to make a stock solution (20 mM) and stored at −20°C. LPS, Poly I:C, and anti-β-actin antibody were purchased from Sigma. The primary antibodies including anti-cyclooxygenase 2 (COX-2), anti-phosphor-IκBα (p-IκBα), anti-IκBα, anti-phosphor-65 (p-p65), anti-p65, anti-extracellular signal regulated kinase 1/2 (ERK1/2), anti-phosphor-ERK1/2 (p-ERK1/2), anti-phosphor-JNK, anti-JNK, anti-phosphor-p38 (p-p38), anti-p38, anti-phosphor-AKT (p-AKT), and anti-AKT antibodies were purchased from Cell Signaling Technology (Beverly, USA). Rabbit monoclonal antibody against inducible NO synthase (iNOS) was purchased from Santa Cruz (Santa Cruz, USA).
Cell culture and treatment
J774A.1 cells were obtained from Shanghai Institutes for Biological Sciences (Shanghai, China) and maintained in Dulbecco's modified Eagle's medium (Gibco BRL, Gaithersburg, USA) supplemented with 10% fetal bovine serum (FBS; Biological Industries, Beit Ahemeq, Israel), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine (Invitrogen, Carlsbad, USA). Human THP-1 cells were grown in RPMI 1640 medium supplemented with 10% FBS and 50 μM 2-mercaptoethanol. THP-1 cells were differentiated for 3 h with 100 nM phorbol-12-myristate-13-acetate (PMA). Murine primary peritoneal macrophages were isolated from the mouse peritoneal cavity [22]. The cells were then incubated for 6 h at 37°C and removed nonadherent cells. The remaining cells were used as the peritoneal macrophages and cultured in RPMI 1640 medium supplemented with 10% FBS. All cells were cultured in a humidified incubator at 37°C under 5% CO2.
Cell viability assay
J774A.1 cells were seeded at a density of 1 × 105 cells/well in 96-well plates and treated with various concentrations of GEA (0, 0.25, 0.5, 1.0, and 1.5 µM) for 1 h, subsequently stimulated with LPS (2 μg/ml) for another 16 h. After incubation for the indicated time, cell viability assay was conducted by using a Cell Titer 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, USA). Three duplicate studies were performed for each experimental condition.
Enzyme-linked immunosorbent assay
Cells were seeded in 12-well plates and pretreated with GEA (0, 0.25, 0.5, 1.0, and 1.5 µM) for 1 h before treatment with LPS (2 μg/ml) for 24 h. Supernatants were collected, and the concentration of the proinflammatory cytokines was measured using enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (eBioscience, San Diego, USA). Each sample was measured and analyzed in triplicate.
NO assay
J774A.1 and murine primary peritoneal macrophage cells were seeded in 12-well plates and pretreated with GEA (0, 0.25, 0.5, 1.0, and 1.5 µM) for 1 h before treatment with LPS (2 μg/ml) for 24 h. The levels of nitrite, as an indicator of NO production, were measured in the supernatants with Griess reaction (Beyotime Institute of Biotechnology, Shanghai, China). The concentrations of were determined by a standard curve using NaNO2 as standard.
Real-time reverse transcription polymerase chain reaction
Total RNA was isolated from cells by using the TRIzol reagent (Invitrogen) according to the manufacturer's protocol. The first-strand complementary DNA was synthesized using PrimeScript™ RT reagent kit with gDNA Eraser (TaKaRa, Dalian, China). And real-time polymerase chain reaction (qRT-PCR) was performed using the SYBR Premix Ex Taq (TaKaRa). The primers are listed in Table 1. L32 was used as the internal control. The amplifications were performed as follows: 94°C for 10 min and then 40 cycles of 94°C for 15 s, 60°C for 30 s, and 72°C for 30 s.
Table 1.
Sequences of primers used in the study
| Gene | Sequence |
|---|---|
| IL12b | Forward: 5′-TGGTTTGCCATCGTTTTGCTG-3′ |
| Reverse: 5′-ACAGGTGAGGTTCACTGTTTCT-3′ | |
| IL23a | Forward: 5′-ATGCTGGATTGCAGAGCAGTA-3′ |
| Reverse: 5′-ACGGGGCACATTATTTTTAGTCT-3′ | |
| IL10 | Forward: 5′-GCTCTTACTGACTGGCATGAG-3′ |
| Reverse: 5′-CGCAGCTCTAGGAGCATGTG-3′ | |
| IFN-β | Forward: 5′-CAGCTCCAAGAAAGGACGAAC-3′, |
| Reverse: 5′-GGCAGTGTAACTCTTCTGCAT-3′ | |
| L32 | Forward: 5′- TTAAGCGAAACTGGCGGAAAC-3′ |
| Reverse: 5′-TTGTTGCTCCCATAACCGATG-3′ |
Western blot analysis
Cells were seeded in 6-well plates and pretreated with GEA (0, 0.25, 0.5, 1.0, and 1.5 µM) for 1 h and then treated with LPS (2 μg/ml) for 1 h. After LPS stimulation, the cells were collected and washed twice with PBS. The cell pellets were resuspended in lysis buffer containing 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 1 mM Na3VO4, 1 mM NaF, a cocktail of 1 mM phenylmethanesulfonyl fluoride, and 1 mM protease inhibitors. The lysates were centrifuged at 12,000 g for 10 min at 4°C. Total protein concentrations were determined by bicinchoninic acid (Thermo Fisher Scientific, USA). Samples were boiled at 100°C for 10 min and chilled on ice for 10 s. Proteins (20 μg) were separated on 10% SDS-polyacrylamide gel electrophoresis gel and transferred onto the polyvinylidene fluoride membrane. The membranes were incubated overnight with specific primary antibodies at 4°C after being blocked with 5% nonfat milk. After being washed three times with PBS containing 0.05% Tween 20 (PBST), the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology) for 1 h at room temperature, followed by three times wash with 0.05% Tween-20/PBST and then detected by using a chemiluminescent substrate (Thermo Scientific, Rockford, USA).
Statistical analysis
All experiments were performed three times. Data were presented as mean ± standard deviation (SD). Comparison between groups was performed by analysis of variance, and P values of 0.05 or less were considered statistically significant.
Results
Effect of GEA on J774A.1 cell viability
To assess the cytotoxic effect of GEA, cells were pretreated with various concentrations of the drug (0, 0.25, 0.5, 1.0, and 1.5 μM) for 24 h in the presence or absence of LPS (2 μg/ml), and the cell viability was determined by cell titer assay. Data showed that GEA, at a concentration of <1.5 μM, did not affect the viability of J774A.1 cells (Fig. 1B). Therefore, the concentrations under 1.5 μM (0, 0.25, 0.5, 1.0, and 1.5 μM) were selected for subsequent experiments.
Effect of GEA on LPS-induced NO production and the expression of iNOS and COX-2 protein
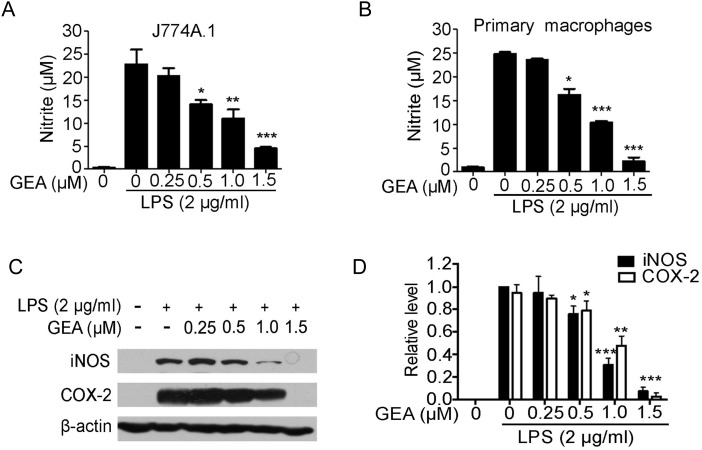
To assess the effect of GEA on LPS-induced NO production in J774A.1 cells, culture supernatant was harvested and nitrite levels were measured. As shown in Fig. 2A, the concentration of nitrite in culture media of unstimulated cells was very low; however, the NO production was markedly increased by 20 folds compared with control after LPS stimulation for 24 h. Compared with the LPS group, pretreatment of J774A.1 cells with GEA significantly inhibited LPS-induced NO production in a dose-dependent manner (Fig. 2A). The observed inhibitory effect of GEA on NO production was also confirmed in murine primary peritoneal macrophages (Fig. 2B).
Figure 2.
Effect of GEA on NO production and LPS-induced iNOS and COX-2 expression J774A.1 cells (A) and murine primary peritoneal macrophages (B) were treated with indicated concentrations of GEA for 1 h before LPS (2 μg/ml) treatment. After 24 h of treatment, the amounts of NO in the culture supernatants were measured. (C) The levels of iNOS and COX-2 protein were assessed by western blot analysis. β-Actin was used as an internal control. (D) The expression levels of iNOS and COX-2 protein were normalized to β-actin. Data are representative of three independent experiments. *P < 0.05, **P < 0.01, and ***P< 0.001 vs. LPS alone group.
GEA has been shown to have inhibitory effect on NO production, and NO was catalyzed by iNOS. Therefore, we speculated that the anti-inflammatory effect of GEA might be related to the production of NO. Western blot analysis showed that iNOS and COX-2 protein were hardly detectable in resting J774A.1 cells, but the level of these proteins was markedly induced in response to LPS stimulation, and GEA strongly suppressed the expression of these proteins in a concentration-dependent manner (Fig. 2C,D).
Effect of GEA on LPS-induced release of proinflammatory cytokines
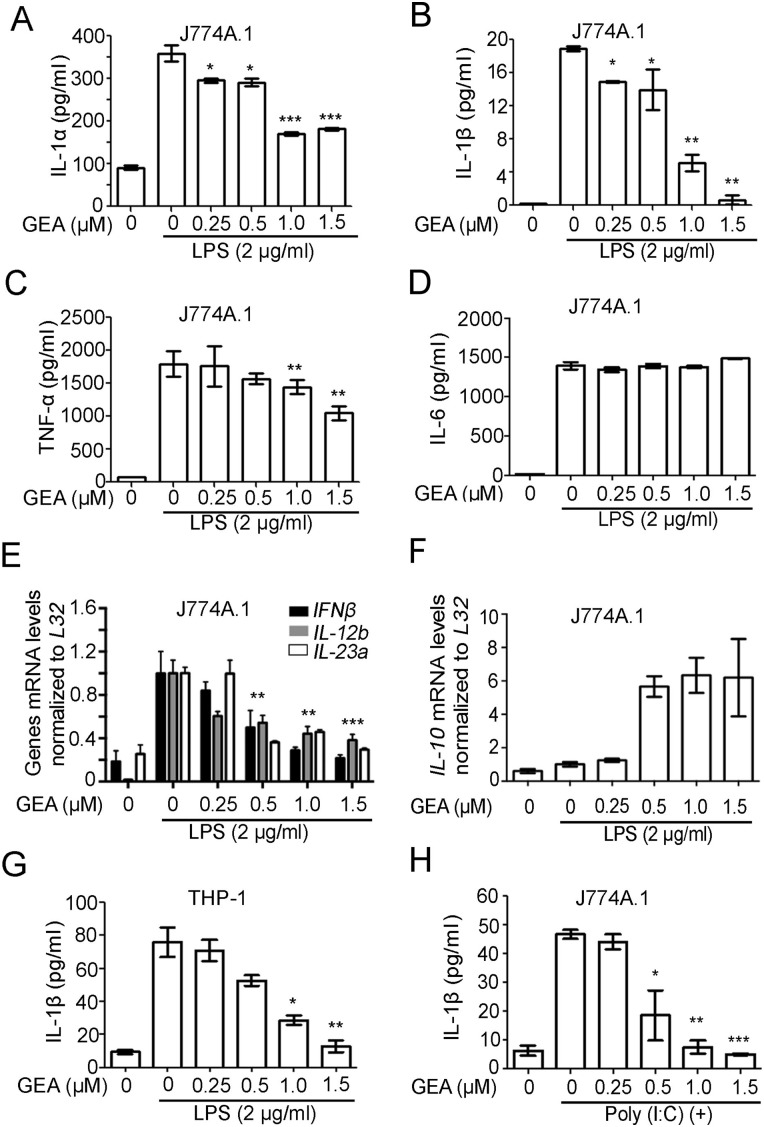
The release of proinflammatory cytokines, such as IL-1α, IL-1β, IL-6, and TNF-α, plays an important role in human inflammatory responses and inflammatory diseases. To evaluate the anti-inflammatory effect of GEA in LPS-stimulated J774A.1 cells, the levels of IL-1α, IL-1β, TNF-α, and IL-6 were determined by ELISA. The results showed that LPS markedly increased the expressions of these cytokines compared with nonstimulation group. However, treatment with GEA significantly impaired the LPS-induced production of proinflammatory cytokines in a dose-dependent manner, but no effect was found on IL-6 secretion (Fig. 3A–D). The effect of GEA on the expression of other pro- and anti-inflammatory mediators including IFN-β, IL-12b, IL-23a, and IL-10 was also examined using qRT-PCR (Fig. 3E,F). The data showed that LPS treatment led to an increase of IFN-β, IL-12b, and IL-23a at mRNA levels; however, GEA impaired the upregulations in a dose-dependent manner (Fig. 3E). Results also showed that GEA enhanced the expression of anti-inflammatory cytokine IL-10 in LPS-stimulated macrophage at mRNA level (Fig. 3F).
Figure 3.
Effect of GEA on LPS-induced proinflammatory cytokines (A–D) The J774A.1 cells were pretreated with GEA (0, 0.25, 0.5, 1.0, and 1.5 μM) for 1 h, followed by treatment with or without LPS (2 μg/ml) for 24 h. The levels of IL-1α, IL-1β, TNF-α, and IL-6 were determined by ELISA. (E,F) The J774A.1 cells were treated with 2 μg/ml LPS in the absence or presence of GEA for 12 h. The levels of IFN-β, IL-12b, IL-23a, and IL-10 were measured by qRT-PCR. (G) PMA-differentiated THP-1 cells were treated with GEA and then stimulated with LPS for 24 h. The levels of released human IL-1β were analyzed by ELISA. (H) The J774A.1 cells were pretreated with different doses of GEA for 1 h followed by treatment with or without 100 μg/ml of Poly (I:C) for 24 h. The levels of IL-1β were determined by ELISA. Data are from three independent experiments. *P < 0.05, **P < 0.01, and ***P< 0.001 vs. LPS alone group or Poly (I:C) alone group.
To investigate whether GEA exerts the same anti-inflammatory effect in human cell as in mouse macrophage cells, PMA-differentiated human THP-1 cells were primed and simulated with GEA and LPS. The data showed that GEA effectively diminished IL-1β secretion in the THP-1 cells (Fig. 3G). Poly (I:C) is a synthetic dsRNA that can induce a series of proinflammatory cytokines through binding to Toll-like receptor-3. When Poly (I:C) was used to simulate the macrophages, the secretion of IL-1β was significantly reduced after cells were treated with GEA (Fig. 3H).
Effect of GEA on the NF-κB signaling pathway in J774A.1 cells
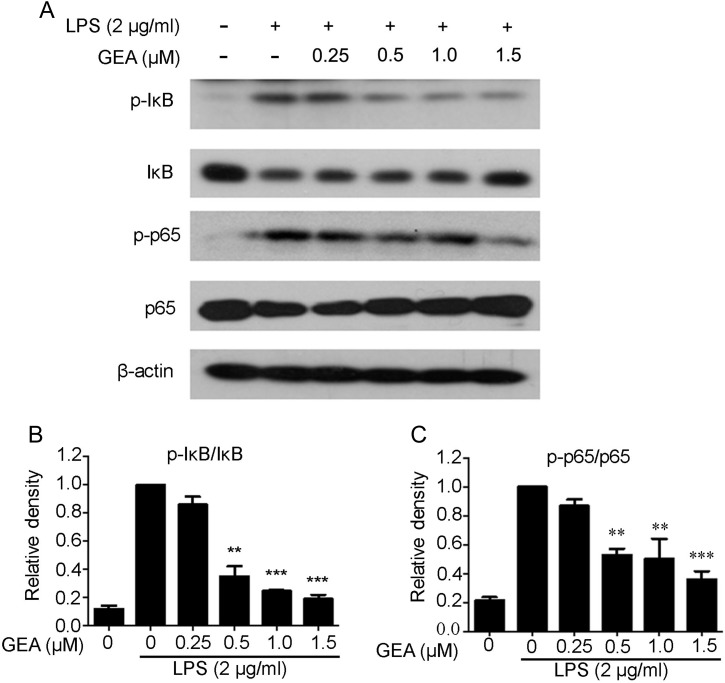
It is well known that NF-κB plays an important role in inflammation and innate immunity. Therefore, the expressions of p-IκBα, IκBα, p-p65, and p65 were detected to confirm whether GEA exerted anti-inflammatory response via the NF-κB pathway. As shown in Fig. 4, compared with the control group, LPS treatment significantly increased the expressions of p-IκBα and p-p65; however, GEA pretreatment significantly suppressed the levels of p-IκBα and p-p65 in a dose-dependent manner.
Figure 4.
Effect of GEA on LPS-induced NF-κB signaling in J774A.1 cells (A) Cells were pretreated with various concentrations of GEA for 1 h and then treated with 2 μg/ml LPS for another 1 h. The phosphorylation of p65 and IκBα was determined by western blot analysis using specific primary antibodies. (B) The band was quantified and expressed as the ratio of total p65 and IκBα intensities. Data are from three independent experiments. **P< 0.01 and ***P< 0.001 vs. LPS alone group.
Effect of GEA on the MAPK signaling pathway in J774A.1 cells
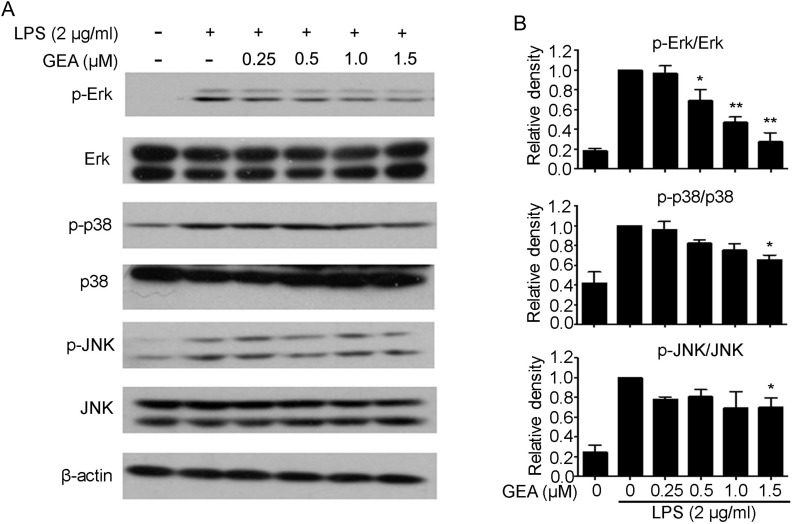
MAPKs play a critical role in the initiation of proinflammatory gene expression. To confirm whether the suppression of inflammatory response by GEA is mediated through the MAPK pathway, activation of JNK, ERK, and P38 MAPKs was examined by western blot analysis. As shown in Fig. 5A,B, stimulation with LPS dramatically triggered the activation of MAPK kinases, including p38, ERK, and JNK. Compared with group treated with LPS alone, GEA pretreatment significantly inhibited the level of p-ERK1/2, but had a minor decreasing effect on p-p38 and p-JNK.
Figure 5.
Effect of GEA on LPS-induced activation of MAPK signaling in J774A.1 cells (A) Cells were pretreated with different concentrations of GEA for 1 h and then treated with 2 μg/ml LPS for another 1 h. Subsequently, cells were harvested and the protein levels of p-Erk1/2, Erk1/2, p-JNK, JNK, p-p38, and p38 were detected by western blot analysis. (B) The relative level was quantified and expressed as the ratio of p-Erk1/2/Erk1/2, p-JNK/JNK, and p-p38/p38. Data are from three independent experiments. *P< 0.05 and **P< 0.01 vs. LPS alone group.
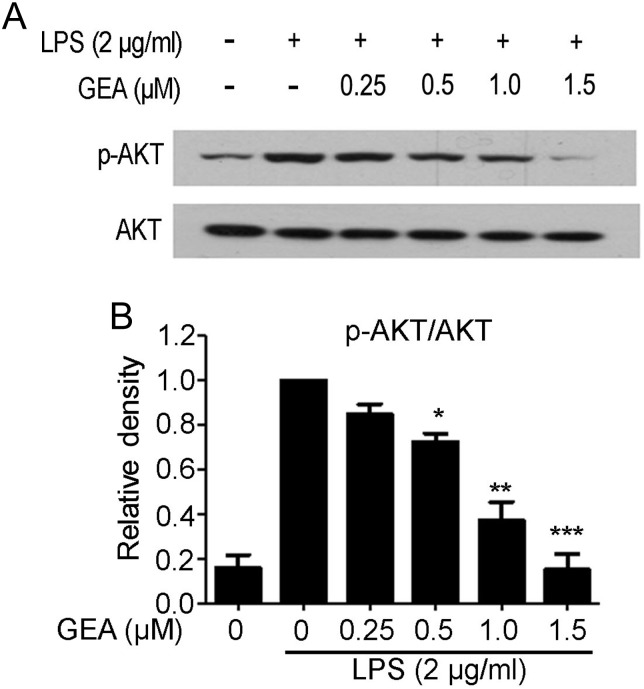
To assess the role of AKT in LPS-stimulated J774A.1 cells, the phosphorylation of AKT was examined by western blot analysis. As shown in Fig. 6A,B, pretreatment of GEA reduced the expression of p-AKT compared with the group treated with LPS alone, but did not influence the total protein of AKT.
Figure 6.
Effect of GEA on LPS-induced activation of AKT signaling in J774A.1 cells (A) The cells were preincubated with GEA at the indicated concentrations for 1 h, then treated with 2 μg/ml LPS for another 1 h. Cells were harvested and the protein levels of p-AKT and AKT were detected by western blot analysis. (B) The relative level was quantified and expressed as the ratio of p-AKT/AKT. Data are from three independent experiments. *P< 0.05, **P< 0.01, and ***P< 0.001 vs. LPS alone group.
Discussion
Recently, the interests in using Chinese herbal products to treat inflammatory diseases by blocking inflammatory mediators and critical signaling pathway are increasing [23]. GEA is an active ingredient isolated from G. hanburyi. The antitumor activities of GEA have been demonstrated in our and other studies [17–19,21]. Herein, we showed that this compound exhibits remarkable inhibitory activity against inflammation. Furthermore, the mechanisms underlying this anti-inflammatory effect were explored in LPS-stimulated experimental models.
It has been found that the excessive production of pro- and anti-inflammatory cytokines play pivotal roles in various inflammatory response [24]. The releases of these cytokines were obviously increased after LPS and Poly (I:C) treatment in macrophages; however, these increases were significantly reversed in a dose-dependent manner by pretreatment with GEA (Fig. 3). Accumulating evidence indicates that NO and PGE2 are important mediators of inflammation, such as autoimmune diseases and neurodegenerative disease [25–30]. The generation of excessive NO and PGE2 in response to various inflammatory stimuli contributes to the development of chronic inflammatory diseases [31–34]. For these reasons, the suppression of NO production may possibly be a promising strategy against inflammatory diseases. In this study, we assessed the anti-inflammatory effect of GEA via the decreasing of NO production in LPS-induced macrophage cells (Fig. 2A,B). Moreover, the levels of iNOS and COX-2 proteins were decreased by GEA compared with stimulation with LPS alone (Fig. 2C,D). Therefore, GEA may inhibit inflammation through reducing NO production and decreasing iNOS and COX-2 expression.
According to previous studies, NF-κB is a main transcription factor and plays a critical regulatory role in cellular response to numerous stimuli [35–39]. NF-κB exists in the cytosol in an inactive form and binds to its inhibitor protein IκB. Activated NF-κB is involved in immune regulation, inflammatory response, cell growth, and anti-apoptosis via phosphorylating IκB and shifting into the nucleus [40–42]. Notably, previous studies have shown that the suppression of NF-κB pathway is associated with anti-inflammatory responses [43–46]. Therefore, we explored whether GEA could regulate NF-κB signaling pathways. Our results confirmed that GEA inhibited NF-κB activation via inhibiting the phosphorylation of p65 and IκBα (Fig. 4). MAPK signaling pathway, including ERKs, JNKs, and p38 MAPKs, is critical for the regulation of proinflammatory mediator genes and inflammatory responses [47]. Thus, we investigated whether GEA exerts its anti-inflammatory effect through the MAPKs signaling. Our data showed that the effect of GEA on anti-inflammatory response may involve the inhibition of ERK signaling pathway in LPS-stimulated J774A.1 cells (Fig. 5). Our previous work indicated that GEA exerted its anticancer effect via inhibiting AKT/mTOR/p70S6K pathway [18], but others studies had reported that PI3K/AKT was involved in inflammatory response [48–50]. We also found that the GEA reduced the phosphorylation of AKT upon LPS stimulation in J774A.1 cells, which indicated that GEA partially suppressed LPS-induced inflammatory response via AKT pathway (Fig. 6).
In conclusion, in the current study, we demonstrated that GEA might significantly inhibit the production of NO and selectively inhibit IL-1α, IL-1β, TNF-α, IFN-β, IL-12b, and IL-23a in LPS-stimulated macrophages. Meanwhile, cell titer assay results exclude the possibility that the effect of GEA might be due to its cell cytotoxicity. Furthermore, data also showed that the inhibitory effect of GEA on inflammatory mediators is related to the prevention of NF-κB, MAPK, and PI3K/AKT pathways. Our findings provide a molecular basis and evidence to understand the therapeutic effect of GEA on various inflammatory diseases.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81502548 and 31571426), the Natural Science Foundation of Hubei Province of China (No. 2015CFB210), the Natural Science Foundation of Hubei Provincial Department of Education (No. Q20152101), the Scientific and Technological Project of Shiyan City of Hubei Province (No. 15Y08), the Initial Project for Post-Graduates of Hubei University of Medicine (No. 2014QDJZR09), the Foundation for Innovative Research Team of Hubei University of Medicine (No. 2014 CXX02), and Thousand Young Talents Program of the Chinese government.
References
- 1.Grange JM, Krone B, Mastrangelo G. Infection, inflammation and cancer. Int J Cancer 2011, 128: 2240–2241. [DOI] [PubMed] [Google Scholar]
- 2.Nowarski R, Gagliani N, Huber S, Flavell RA. Innate immune cells in inflammation and cancer. Cancer Immunol Res 2013, 1: 77–84. [DOI] [PubMed] [Google Scholar]
- 3.Rich AM, Hussaini HM, Parachuru VP, Seymour GJ. Toll-like receptors and cancer, particularly oral squamous cell carcinoma. Front Immunol 2014, 5: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risitano R, Curro M, Cirmi S, Ferlazzo N, Campiglia P, Caccamo D, Ientile R et al. . Flavonoid fraction of Bergamot juice reduces LPS-induced inflammatory response through SIRT1-mediated NF-kappaB inhibition in THP-1 monocytes. PLoS One 2014, 9: e107431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes 2005, 54(Suppl. 2): S73–S78. [DOI] [PubMed] [Google Scholar]
- 6.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005, 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plociennikowska A, Hromada-Judycka A, Borzecka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci 2015, 72: 557–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harada K, Ohira S, Isse K, Ozaki S, Zen Y, Sato Y, Nakanuma Y. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab Invest 2003, 83: 1657–1667. [DOI] [PubMed] [Google Scholar]
- 9.Deng S, Hu B, Shen KP, Xu L. Inflammation, macrophage in cancer progression and Chinese herbal treatment. J Basic Clin Pharm 2012, 3: 269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Astry B, Venkatesha SH, Laurence A, Christensen-Quick A, Garzino-Demo A, Frieman MB, O'Shea JJ et al. . Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin Immunol 2015, 157: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Gao X, Wu X, Wu Z, Cheng L, Zhu L, Shen D et al. . Parthenolide inhibits LPS-induced inflammatory cytokines through the toll-like receptor 4 signal pathway in THP-1 cells. Acta Biochim Biophys Sin 2015, 47: 368–375. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Qian Y, Chen F, Chen X, Chen Z, Zheng M. EGCG attenuates pro-inflammatory cytokines and chemokines production in LPS-stimulated L02 hepatocyte. Acta Biochim Biophys Sin 2014, 46: 31–39. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Peng X, Du W, Wu Y, Huang B, Xue L, Wu Q et al. . Curcumin attenuates cardiomyocyte hypertrophy induced by high glucose and insulin via the PPARgamma/Akt/NO signaling pathway. Diabetes Res Clin Pract 2015, 108: 235–242. [DOI] [PubMed] [Google Scholar]
- 14.Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood 2007, 110: 3517–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JY, Lee BH, Lee JY. Gambogic acid disrupts toll-like receptor4 activation by blocking lipopolysaccharides binding to myeloid differentiation factor 2. Toxicol Res 2015, 31: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi Y, Zhan XK, Yu H, Xie GR, Wang ZZ, Xiao W, Wang YG et al. . An open-labeled, randomized, multicenter phase IIa study of gambogic acid injection for advanced malignant tumors. Chin Med J 2013, 126: 1642–1646. [PubMed] [Google Scholar]
- 17.Mei W, Dong C, Hui C, Bin L, Fenggen Y, Jingjing S, Cheng P et al. . Gambogenic acid kills lung cancer cells through aberrant autophagy. PLoS One 2014, 9: e83604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu XJ, Han QB, Wen ZS, Ma L, Gao J, Zhou GB. Gambogenic acid induces G1 arrest via GSK3beta-dependent cyclin D1 degradation and triggers autophagy in lung cancer cells. Cancer Lett 2012, 322: 185–194. [DOI] [PubMed] [Google Scholar]
- 19.Yan F, Wang M, Li J, Cheng H, Su J, Wang X, Wu H et al. . Gambogenic acid induced mitochondrial-dependent apoptosis and referred to phospho-Erk1/2 and phospho-p38 MAPK in human hepatoma HepG2 cells. Environ Toxicol Pharmacol 2012, 33: 181–190. [DOI] [PubMed] [Google Scholar]
- 20.Yan F, Wang M, Chen H, Su J, Wang X, Wang F, Xia L et al. . Gambogenic acid mediated apoptosis through the mitochondrial oxidative stress and inactivation of Akt signaling pathway in human nasopharyngeal carcinoma CNE-1 cells. Eur J Pharmacol 2011, 652: 23–32. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Cheng H, Zhu G, Yang L, Zhou A, Wang X, Fang N et al. . Gambogenic acid inhibits proliferation of A549 cells through apoptosis-inducing and cell cycle arresting. Biol Pharm Bull 2010, 33: 415–420. [DOI] [PubMed] [Google Scholar]
- 22.Mao K, Chen S, Chen M, Ma Y, Wang Y, Huang B, He Z et al. . Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res 2013, 23: 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheu ML, Chiang CK, Tsai KS, Ho FM, Weng TI, Wu HY, Liu SH. Inhibition of NADPH oxidase-related oxidative stress-triggered signaling by honokiol suppresses high glucose-induced human endothelial cell apoptosis. Free Radic Biol Med 2008, 44: 2043–2050. [DOI] [PubMed] [Google Scholar]
- 24.Netea MG, van der Meer JW, van Deuren M, Kullberg BJ. Proinflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing? Trends Immunol 2003, 24: 254–258. [DOI] [PubMed] [Google Scholar]
- 25.Rahat MA, Hemmerlein B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Front Physiol 2013, 4: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H et al. . Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med 2013, 210: 855–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosca L, Zeini M, Traves PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology 2005, 208: 249–258. [DOI] [PubMed] [Google Scholar]
- 28.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol 1997, 15: 323–350. [DOI] [PubMed] [Google Scholar]
- 29.Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflam Allergy 2005, 4: 471–479. [DOI] [PubMed] [Google Scholar]
- 30.Gomez PF, Pillinger MH, Attur M, Marjanovic N, Dave M, Park J, Bingham CO III et al. . Resolution of inflammation: prostaglandin E2 dissociates nuclear trafficking of individual NF-kappaB subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J Immunol 2005, 175: 6924–6930. [DOI] [PubMed] [Google Scholar]
- 31.Yu C, Mei XT, Zheng YP, Xu DH. Gastroprotective effect of taurine zinc solid dispersions against absolute ethanol-induced gastric lesions is mediated by enhancement of antioxidant activity and endogenous PGE2 production and attenuation of NO production. Eur J Pharmacol 2014, 740: 329–336. [DOI] [PubMed] [Google Scholar]
- 32.Urdanibia I, Michelangeli F, Ruiz MC, Milano B, Taylor P. Anti-inflammatory and antitumoural effects of Uncaria guianensis bark. J Ethnopharmacol 2013, 150: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 33.Kwon DJ, Ju SM, Youn GS, Choi SY, Park J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-kappaB and AP-1 activation in RAW 264.7 macrophages. Food Chem Toxicol 2013, 58: 479–486. [DOI] [PubMed] [Google Scholar]
- 34.Tunctan B, Korkmaz B, Sari AN, Kacan M, Unsal D, Serin MS, Buharalioglu CK et al. . Contribution of iNOS/sGC/PKG pathway, COX-2, CYP4A1, and gp91(phox) to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against vasodilation, hypotension, tachycardia, and inflammation in a rat model of septic shock. Nitric Oxide-Biol Chem 2013, 33: 18–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J et al. . NF-kappaB in renal inflammation. J Am Soc Nephrol 2010, 21: 1254–1262. [DOI] [PubMed] [Google Scholar]
- 36.Munhoz CD, Garcia-Bueno B, Madrigal JL, Lepsch LB, Scavone C, Leza JC. Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res 2008, 41: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 37.Ear T, McDonald PP. Cytokine generation, promoter activation, and oxidant-independent NF-kappaB activation in a transfectable human neutrophilic cellular model. BMC Immunol 2008, 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal 2005, 7: 395–403. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol 2002, 30: 285–296. [DOI] [PubMed] [Google Scholar]
- 40.Jayandharan GR, Aslanidi G, Martino AT, Jahn SC, Perrin GQ, Herzog RW, Srivastava A. Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy. Proc Natl Acad Sci USA 2011, 108: 3743–3748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Qu Y, Zhang X, Wu R. Knockdown of NF-kappaB p65 subunit expression suppresses growth of nude mouse lung tumor cell xenografts by activation of Bax apoptotic pathway. Neoplasma 2015, 62: 34–40. [DOI] [PubMed] [Google Scholar]
- 42.Park J, Bae EK, Lee C, Choi JH, Jung WJ, Ahn KS, Yoon SS. Establishment and characterization of bortezomib-resistant U266 cell line: constitutive activation of NF-kappaB-mediated cell signals and/or alterations of ubiquitylation-related genes reduce bortezomib-induced apoptosis. BMB Rep 2014, 47: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim ME, Jung YC, Jung I, Lee HW, Youn HY, Lee JS. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C. Agardh on lipopolysaccharide-stimulated macrophage activation via NF-kappaB pathway regulation. Immunol Invest 2015, 44: 137–146. [DOI] [PubMed] [Google Scholar]
- 44.Koo HJ, Yoon WJ, Sohn EH, Ham YM, Jang SA, Kwon JE, Jeong YJ et al. . The analgesic and anti-inflammatory effects of Litsea japonica fruit are mediated via suppression of NF-kappaB and JNK/p38 MAPK activation. Int Immunopharmacol 2014, 22: 84–97. [DOI] [PubMed] [Google Scholar]
- 45.Choi YY, Kim MH, Han JM, Hong J, Lee TH, Kim SH, Yang WM. The anti-inflammatory potential of Cortex Phellodendron in vivo and in vitro: down-regulation of NO and iNOS through suppression of NF-kappaB and MAPK activation. Int Immunopharmacol 2014, 19: 214–220. [DOI] [PubMed] [Google Scholar]
- 46.Montoya-Rodriguez A, de Mejia EG, Dia VP, Reyes-Moreno C, Milan-Carrillo J. Extrusion improved the anti-inflammatory effect of amaranth (Amaranthus hypochondriacus) hydrolysates in LPS-induced human THP-1 macrophage-like and mouse RAW 264.7 macrophages by preventing activation of NF-kappaB signaling. Mol Nutr Food Res 2014, 58: 1028–1041. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases—regulating the immune response. Nat Rev Immunol 2007, 7: 202–212. [DOI] [PubMed] [Google Scholar]
- 48.Kao TC, Shyu MH, Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. J Agric Food Chem 2010, 58: 8623–8629. [DOI] [PubMed] [Google Scholar]
- 49.Choi JH, Kim NH, Kim SJ, Lee HJ, Kim S. Fucoxanthin inhibits the inflammation response in Paw Edema Model through suppressing MAPKs, Akt, and NFkappaB. J Biochem Mol Toxicol 2015. doi:10.1002/jbt.21769. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Zhang Q, Xi J, Xiao B, Li Y, Ma C. Neuroprotective effect of fasudil on inflammation through PI3K/Akt and Wnt/beta-catenin dependent pathways in a mice model of Parkinson's disease. Int J Clin Exp Pathol 2015, 8: 2354–2364. [PMC free article] [PubMed] [Google Scholar]