Abstract
Flavonoids have been shown to improve cognitive function and delay the dementia progression. However, the underlying mechanisms remain elusive. In the present study, we examined the effect of Scutellaria baicalensis stem-leaf total flavonoids (SSTFs) extracted from S. baicalensis Georgi on spatial learning and memory in a vascular dementia (VaD) rat model and explored its molecular mechanisms. The VaD rats were developed by permanent bilateral occlusion of the common carotid artery. Seven days after recovery, the VaD rats were treated with either 50 or 100 mg/kg of SSTF for 60 days. The spatial learning and memory was evaluated in the Morris water maze (MWM) test. The tau hyperphosphorylation and the levels of the related protein kinases or phosphatases were examined by western blot analysis. In VaD rats, SSTF treatment at 100 mg/kg significantly reduced the escape latency in training trial in MWM test. In the probe trial, SSTF treatment increased the searching time and travel distance in the target quadrant. SSTF treatment inhibited the tau phosphorylation in both cortex and hippocampus in VaD rats. Meanwhile, SSTF reduced the activity of glycogen synthase kinase 3β and cyclin-dependent kinase 5 in VaD rats. In contrast, SSTF treatment increased the level of the protein phosphatase 2A subunit B in VaD rats. SSTF treatment significantly improved the spatial cognition in VaD rats. Our results suggest that SSTF may alleviate tau-hyperphosphorylation-induced neurotoxicity through coordinating the activity of kinases and phosphatase after a stroke. SSTF may be developed into promising novel therapeutics for VaD.
Keywords: flavonoid, vascular dementia, deficits of spatial learning and memory, hyperphosphorylated tau
Introduction
Chronic hypoperfusion and thromboembolic events induce cognitive deficit that is typical in vascular dementia (VaD) mainly through encouraging brain damage [1]. Pathologically, both focal and global brain ischemia produce a stereotyped pattern of selective neuronal degeneration, which is just the same as in Alzheimer's type dementia [2]. Results from VaD animal models and clinical studies of ischemic stroke demonstrated increased expression and processing of amyloid precursor protein to oligomeric β-amyloid (Aβ) peptide as well as hyperphosphorylation of tau protein [2], indicating that angiopathy, Aβ peptides aggregates and tau hyperphosphorylation might share common features and convergent pathogenic mechanisms of Alzheimer's disease (AD) and VaD [3]. Current treatments for dementia are palliative rather than curative, as the mechanisms of cognitive deficit remain poorly characterized.
Flavonoid compounds have been found to attenuate learning and memory impairment and improve cognitive function in AD model [4–11]. In addition to the direct interference with the generation and assembly of Aβ peptides into neurotoxic oligomeric aggregates or regulation of prosurvival transcription factors in the brain [12–15], flavonoids encourage beneficial effects on the vascular system [16]. It has been noticed for decades that cerebral amyloid angiopathy in AD induces a severe form of cerebrovascular dysfunction, leading to intra- and postischemic cerebral blood flow deficits that ultimately exacerbate cerebral infarction [17]. Brain infarcts by themselves had little effect on cognitive status [18]; however, infarcts as well as vascular risk factors and cerebrovascular disease may accelerate Aβ production/aggregation/deposition and contribute to the pathology and symptomatology of AD [19]. For these reasons, flavonoid compounds may introduce changes in cerebrovascular blood flow and therefore promote angiogenesis, neurogenesis, and alterations in neuronal morphology, which may finally be beneficial for the improvement of cognitive function in patients with VaD.
Scutellaria baicalensis Georgi is a traditional Chinese herb that has been extensively used in traditional medicine for the treatment of cancers and inflammatory diseases. Baicalin, baicalein, and wogonin are the most commonly isolated medicinal constituents in the root of the herb, the main part used in Chinese medicine [20]. While these flavonoids have been shown to protect neuronal cells from oxidative damage [21], reverse aging-related cognitive impairment [22], reduce Aβ, and promote nonamyloidogenic amyloid precursor protein processing [23] in AD or VaD animal models, their effect on tau phosphorylation has not been systematically investigated [24]. In AD, the tau hyperphosphorylation makes Aβ more toxic and eventually causes neurons to die. In VaD, the underlying reasons for the degeneration and death of neurons are the deprivation of oxygen and other nutrients from ischemic stroke. However, the increased tau phosphorylation after mild repetitive brain injury may imply a role of the tau hyperphosphorylation in VaD [25]. In the present study, using VaD animals induced by permanent internal carotid ligation, we evaluated the effects of flavonoids extracted from aerial part of S. baicalensis Georgi (S. baicalensis stem-leaf total flavonoids: SSTFs) on spatial learning and memory improvement. The possible mechanisms of action of SSTF were also investigated.
Materials and Methods
Drugs and reagents
The SSTF was provided by the Institute of Chinese Traditional Medicine, Chengde Medical College (Chengde, China). The SSTF powder (Lot No. 010608) was prepared as described previously [26] and dissolved in 0.9% normal saline (pH 7.4) for intraperitoneal injection. Eleven flavonoid compounds have been identified in SSTF including (i) chrysin, (ii) 5,7,4′-trihydroxy-6-methoxyflavone, (iii) wogonin, (iv) 5,4′-dihydroxy-6,7,3′,5′-tetramethoxyflavone, (v) apigenin, (vi) isoscutellarein, (vii) baicalein-7-O-β-d-glucopyranoside, (viii) baicalin, (ix) apigenin-7-O-β-d-glucopyranoside, (x) chrysin-7-O-β-d-glucuronopyranoside, and (xi) oroxylin-7-O-β-d-glucopyranoside [27]. The purity of SSTF was ≥62%.
Animal and experimental procedures
All protocols were in accordance with the Guidelines for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China and approved by the Society for Neuroscience Research in Zhejiang Chinese Medical University. Chronic cerebral ischemia (CCI) was induced in adult male Sprague-Dawley rats (480–600 g, 19 months; Experimental Animal Center of Zhejiang Chinese Medical University) through bilateral common carotid artery occlusion (BCCAo) as described previously [28]. Briefly, the rat was anesthetized with halothane, then the common carotid was exposed and occluded with 4-0 silk suture with a 3-day interval between interventions, the left common carotid being the first to be manipulated and the right one being ligated permanently 3 days later. All rats were maintained in a temperature-controlled room (20–23°C), on a 12/12 h light/dark cycle. Forty-two carotids-occluded rats were assigned to one of the three groups randomly (n = 14): (i) CCI control, (ii) SSTF-50: rats treated with 50 mg/kg SSTF, and (iii) SSTF-100: rats treated with 100 mg/kg SSTF. Sham-operated rats (n = 14) received the same surgical procedures without carotid artery ligation. All the rats were allowed to recover from surgery for 7 days. From Day 8 to Day 68 post surgery, the rats were given SSTF daily by intraperitoneal injection. The rats in the sham group and the CCI control group were given 0.9% normal saline only (10 ml/kg i.p., daily).
Assessment of learning and memory by Morris water maze
From Day 63 to Day 68 post surgery, spatial learning and memory abilities of rats were evaluated with Morris water maze (MWM) (training trials from Day 63 to Day 67 and probe trials on Day 68) as described previously [29,30]. Laboratory staff performing behavioral assessments had no knowledge of the group assignments at the time of test. The test includes two phases: the training trial and the probe trial. During a training trial phase, rats were allowed to swim from one quadrant and climb onto a hidden circular platform, where they remained for 10 s before being returned to a holding cage. The trials were always initiated from different positions in the tank. For each rat, the platform was located in the target quadrant constantly, but the point of immersion into the pool varied so that the rats were not able to predict the platform location. The rats were trained for 10 sessions in five consecutive days (two sessions each day). The latency (the time for rats to find the platform) was recorded in each trial and the mean latency for each training day was calculated. The probe trials were performed 1 day after the final training trail. For probe trail, the platform was removed, and the rats were placed in a new start position in the maze. The time for probe trials lasted 60 s. The time that the rats remained in the target quadrant and the movements of rats were recorded by a video camera connected to a computer running the program (HVS image; London, UK). The learning ability was evaluated in two aspects—searching time (in seconds) and path length.
Evaluation of ischemic infarct area
The rats (n = 5 from each group) were deeply anesthetized as described above and sacrificed by decapitation at 24 h after probe trail. The brains were quickly removed and cut into 1-mm thick coronal sections and stained with 2% 2,3,5-triphenyltetrazolium chloride solution at 37°C for 20 min in the dark and photographed. Infarct region was identified as the area of unstained tissue. The area of infarct and each hemisphere were measured by a morphological image analysis system. The relative infarct volume was calculated as a percentage of the total brain volume using the formula: . The average percentage of infarction volume was calculated for each group.
Western blot analysis
The rats (n = 9 from each group) were decapitated when the MWM recording was finished. Both the cortex and hippocampus were removed and stored at −80°C until use. The brain tissues were homogenized in lysis buffer (40 mM Tris–HCl, pH 7.6; 150 mM NaCl; 10 mM NaF; 1 mM Na3VO4; 5 mM ethylenediaminetetraacetic acid; 2 mM benzamidine; and 1 mM phenylmethylsulfonyl fluoride) using a Teflon glass homogenizer. The homogenate was centrifuged at 24,800 g for 30 min at 4°C and the protein concentration of the supernatant was estimated by BCA kit (Pierce Biotechnology, Rockford, USA). Equal amount of proteins (10 μg) were subject to 10% denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, the gels were electroblotted onto polyvinylidine difluoride membranes. Membranes were blocked with Tris-buffered saline containing 5% milk and 0.1% Tween 20 for 1 h and then incubated overnight with the primary antibody (Supplementary Table S1) at 4°C. Then the membranes were incubated with IRDye-conjugated anti-rabbit or anti-mouse immunoglobulin G secondary antibody (1:10,000) for 1 h at room temperature and visualized using the Odyssey Infrared Imaging System (Licor Biosciences, Lincoln, USA). The quantification of immunoblots was performed using the Quantity One program (Bio-Rad, Hercules, USA) and GAPDH was used as an internal loading control.
Statistical analysis
Data were expressed as the mean ± SEM and analyzed with SPSS. The escape latency and travel distance in the MWM were analyzed with two-way analysis of variance (ANOVA) and the Bonferroni post hoc test. Other data were analyzed with one-way ANOVA with multiple comparisons in Student–Newman––Keuls test. P < 0.05 was considered to be statistically significant.
Results
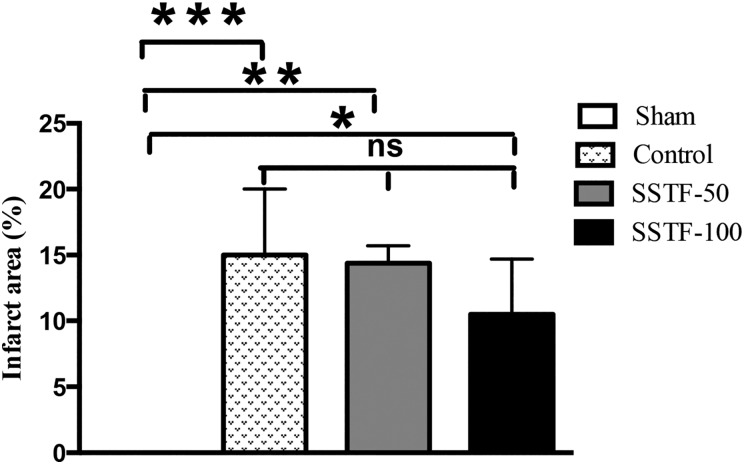
SSTF did not significantly affect infarct volume
BCCAo-induced neuronal damage in the cortex and hippocampus has been confirmed by magnetic resonance imaging studies [31]. In accordance with this result, extensive brain infarction was observed in the CCI rats after the occlusion (Fig. 1). The mean infarct volume in the CCI group reached 15% (15% ± 5%) after 60 days of normal saline administration. This was significantly different from the sham group, in which no obvious infarction was observed (P < 0.0001). The difference between the SSTF-50 group and the sham group was significant (P < 0.001), whereas no significant difference was observed between the SSTF-50 group and the CCI control group (14.4% ± 1.3% vs. 15% ± 5%; P > 0.05). Administration of 100 mg/kg SSTF slightly reduced infarct volume compared with the CCI controls (10.5% ± 4.2% vs. 15% ± 5%); however, it was not statistically different. The difference between the SSTF-100 group and the sham group was still significant (P < 0.05).
Figure 1.
SSTF did not decrease infarct area in the CCI rats Graph shows the quantification of infarct volume after 60 days of SSTF treatment. Data are shown as the mean ± SEM (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001, and ns: not significant. Sham, the sham group; Control, the CCI control group; SSTF-50, the group treated with 50 mg/kg of SSTF; and SSTF-100, the group treated with 100 mg/kg of SSTF.
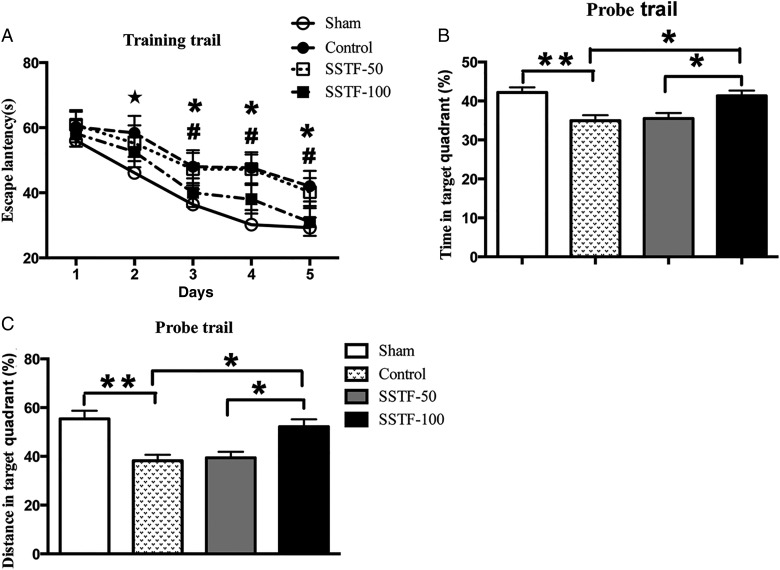
SSTF improved spatial learning and memory
MWM test showed that rats from four groups behaved efficiently at locating the platform on successive training trials. The escape latency shortened progressively in each group in a day-dependent manner (Fig. 2A). Tests on Day 1 showed no significant difference in the escape latency among the four groups (sham: 56.1 ± 5.0 s; control: 60.2 ± 4.7 s; SSTF-50: 60.9 ± 4.5 s; SSTF-100: 58.2 ± 4.1 s; P > 0.05). On Day 2, the rats in the CCI control group and the SSTF-treated group did not show significant difference in finding the platform compared with that on Day 1. While the rats in the sham group displayed shorter latency when compared with rats in other groups and the previous day (sham: 46.1 ± 4.9 s, P < 0.05; control: 58.4 ± 5.2 s; SSTF-50: 55.2 ± 5.5 s; SSTF-100: 52.6 ± 4.7 s). On Day 3, all groups showed significantly shortened latency to reach the platform when compared with Day 1 and Day 2 (sham: 36.4 ± 4.7 s; control: 48 ± 5.1 s; SSTF-50: 47.3 ± 5 s; SSTF-100: 40 ± 4.4 s; P < 0.05). On Day 4, group comparisons revealed that rats in the sham group as well as the SSTF-100 group displayed shorter escape latency when compared with rats in the CCI control group and the SSTF-50 group (sham: 30.2 ± 4.5 s; control: 47.7 ± 4.8 s; SSTF-50: 47.3 ± 4.5 s; SSTF-100: 38 ± 4.4 s; P < 0.01). Similar to Day 4, the group differences in latency on Day 5 were significant (sham: 29.6 ± 3.1 s; control: 42 ± 4.7 s; SSTF-50: 40.2 ± 4.2 s; SSTF-100: 31 ± 4.3 s; P < 0.01), whereas the difference between Day 4 and Day 5 in the same group was not significant. In probe trail, the platform was removed and the searching behavior for the platform was assessed (Fig. 2B,C). Results showed that CCI rats spent less time in the target quadrant (Fig. 2B), indicated by a lower percentage of searching time in target quadrant. The percentage of searching time in the sham group was notably higher when compared with that in the CCI controls (42.2% ± 1.3% vs. 34.9% ± 1.4%; P < 0.01), and the searching time of rats in the SSTF-100 group was also significantly increased compared with that in the CCI controls (41.3% ± 1.3%; P < 0.01). Group comparison revealed no significant difference between the CCI control group and the SSTF-50 group. Similar results were obtained in the traveled distance in probe trail among the groups (Fig. 2C). The percentage of traveled distance in the target quadrant in the CCI control group was significantly lower than that in the sham group (38.2% ± 2.4% vs. 55.4% ± 3.3%; P < 0.01). Significant difference was also observed between the CCI control group and the SSTF-100 group (P < 0.01), whereas no significant difference was observed between the CCI control group and the SSTF-50 group (38.2% ± 2.4% vs. 39.5% ± 2.4%; P > 0.05).
Figure 2.
SSTF treatment improved spatial learning and memory in VaD rats in MWM test Sixty days of SSTF (100 mg/kg) treatment reduced daily escape latency (A) in training trials from Day 3 (P < 0.05, performance of the sham group on Day 2 in comparison with that on Day 1; #P < 0.05, differences to the performance on Day 1 of the training trails within the same group; *P < 0.01, performance differences among the groups in each day). In probe trail, SSTF (100 mg/kg) treatment significantly increased the searching time (B) and travel distance (C) in the target quadrant. Data are shown as the mean ± SEM (n = 9). *P < 0.05 and **P < 0.01.
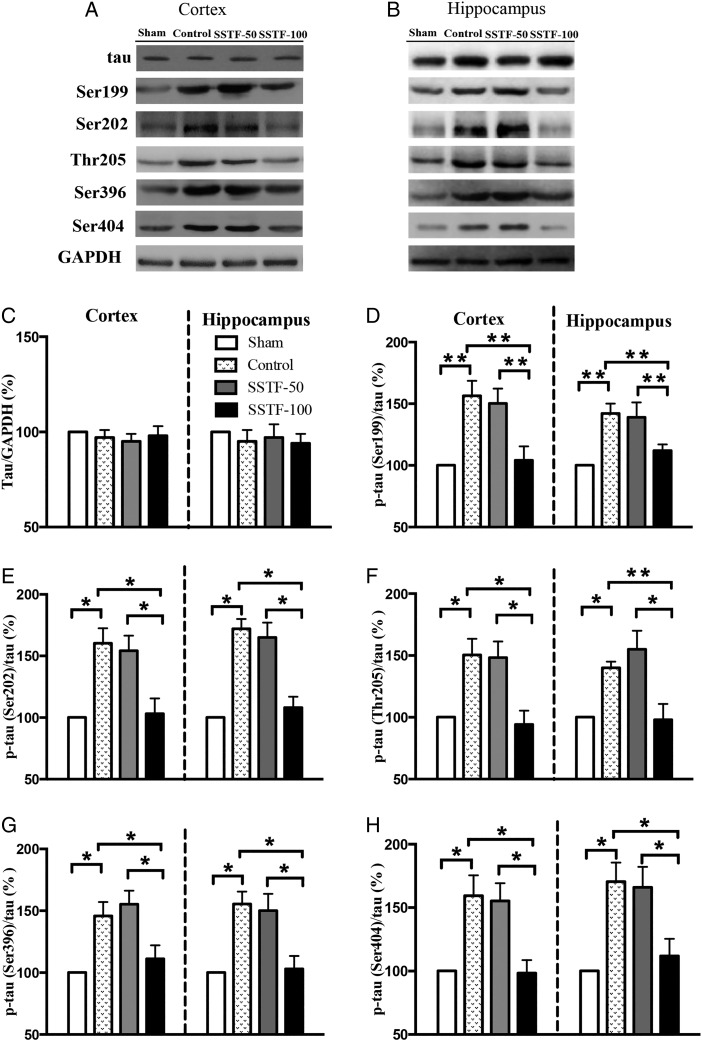
SSTF treatment reduced tau phosphorylation
Hyperphosphorylation of tau is a common feature of neurodegenerative tauopathies. However, the full extent of tau hyperhosphorylation in VaD patient is still uncertain. In this study, tau expression in the cortex and hippocampus was evaluated by western blot analysis on Day 68 post artery occlusion. Results showed that neither ischemia nor SSTF treatment altered the total level of tau in the cortex and hippocampus (Fig. 3A,B). Notably, the phosphorylated tau was increased after the ischemia induction, especially the tau phosphorylated at Ser202 (tauser202), Thr205 (tauthr205), Ser404 (tauser404), Ser199 (tauser199), and Ser396 (tauser396) was increased by 50%–60% in both the cortex and hippocampus (Fig. 3C–H; P < 0.05). Treatment with 50 mg/kg of SSTF did not reduce the level of the phosphorylated tau. In the CCI control group and SSTF-50 group, tauthr205 was increased by 50% and 48% in cortex (40% and 55% in hippocampus), τser404 was increased by 50% and 55% in cortex (70% and 65% in hippocampus), τser199 was increased by 59% and 56% in cortex (42% and 39% in hippocampus), and tauser396 was increased by 56% and 50% (55 and 50% in hippocampus), respectively. Treatment with 100 mg/kg of SSTF significantly inhibited the tau phosphorylation in comparison with the CCI group (P < 0.05), and the level of phosphorylated tau was restored to almost the same level of the sham group (tauser199 in cortex and hippocampus: 104% and 112%, tauser202: 103% and 108%, tauthr205: 94% and 98%, tauser396: 111% and 103%, tauser404: 98% and 112%, respectively; P > 0.05). The difference of phosphorylated tau in the cortex and hippocampus was not statistically significant (P > 0.05).
Figure 3.
SSTF treatment reduced tau phosphorylation in the VaD rat brain Representative immunoblots for the expression of tau and phosphorylated tau in the cortex (A) and hippocampus (B). Sixty days of SSTF (100 mg/kg) treatment did not alter the total tau (C), but significantly reduced levels of phosphorylated tau at sites Ser199 (D), Ser202 (E), Thr205 (F), Ser396 (G), and Ser404 (H) in the cortex and hippocampus. The total tau or phosphorylated tau was normalized to either GAPDH or total tau, respectively. The relative levels of total tau or phosphorylated tau of sham group were set at 100%. Data are shown as the mean ± SEM (n = 9). *P < 0.05 and **P < 0.01.
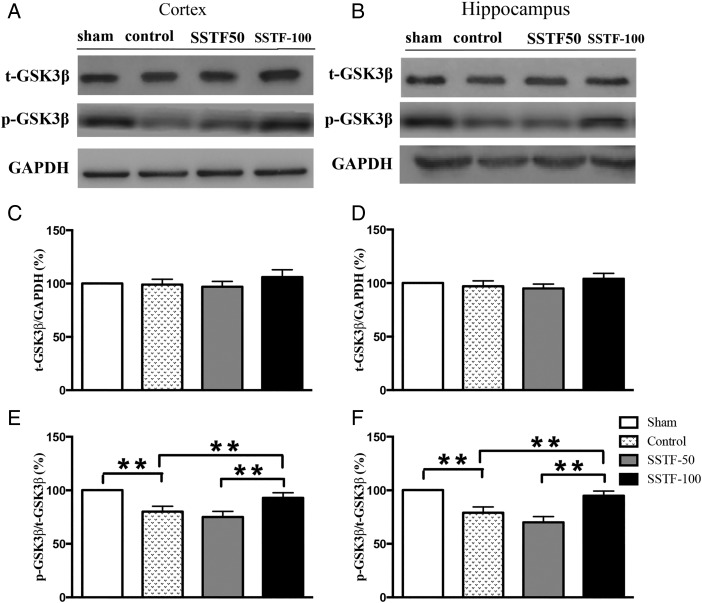
Glycogen synthase kinase 3β activity was reduced in SSTF-treated rats
Glycogen synthase kinase 3β (GSK3β) is the first identified kinase to phosphorylate tau. The kinase activity of GSK3β is regulated by Ser9 phosphorylation (GSK3βser9), which significantly decreases the active site availability [32]. To determine whether the increased tau phosphorylation in the CCI rats was related to the alteration of GSK3β activity, the expression levels of GSK3β and GSK3βser9 were examined. No significant difference of GSK3β expression was found among groups in both the cortex and hippocampus (Fig. 4A–D). The levels of GSK3βser9 in the cortex and hippocampus were quantified by normalizing to the expression of GSK3β. Statistical analyses revealed that the relative expression of GSK3βser9 among the groups was significantly different (Fig. 4E,F). GSK3βser9 in the CCI control group was decreased by 20% in both cortex (Fig. 4E; P < 0.01) and hippocampus (Fig. 4F; P < 0.01) compared with that in the sham group (the level of GSK3βser9 in the sham group was set as 100%). SSTF treatment did not affect the expression of GSK3β (Fig. 4A–D). However, the GSK3βser9 was significantly increased by SSTF-100 treatment. The relative levels of GSK3βser9 in the SSTF-100 group in both the cortex and hippocampus were similar to those in the sham group (92.9% and 94.8%; P > 0.05). SSTF treatment (50 mg/kg) did not significantly affect the GSK3βser9 level. There were no significant differences between the SSTF-50 and the CCI control group in both cortex and hippocampus (P > 0.05).
Figure 4.
SSTF increased GSK3β phosphorylation at Serine 9 Representative immunoblots of GSK3β and p-GSK3β in the rat cortex (A) and hippocampus (B). SSTF treatment (100 mg/kg) did not alter the GSK3β expression in the cortex (C) and the hippocampus (D), but significantly altered the GSK3β phosphorylation at Serine 9 in the cortex (E) and hippocampus (F). The expression of the total GSK3β or p-GSK3β was normalized to GAPDH or total GSK3β, respectively. The relative amount of GSK3β of the sham group was set as 100%. Data are shown as the mean ± SEM (n = 9). **P < 0.01.
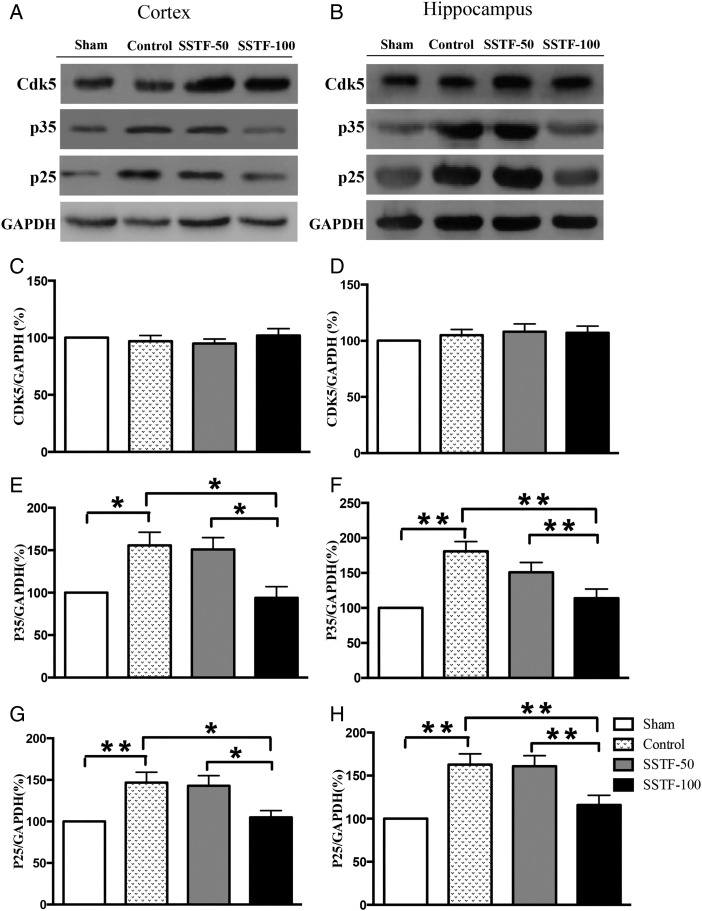
Cyclin-dependent kinase 5 activity was reduced in SSTF-treated rats
Cyclin-dependent kinase 5 (Cdk5) acts as a modulator of tau hyperphosphorylation via the inhibitory regulation of GSK3β in young mice [33]. However, Cdk5 was also identified as a major kinase mediating tau phosphorylation at sites characteristic for neurodegenerative tauopathies [34–37]. To determine the role of Cdk5 in tau hyperphosphorylation in VaD rats, the expression of Cdk5 and its regulators p35 and p25 in the cortex and hippocampus was examined. Our results showed that Cdk5 expression in the CCI control group was similar to that in the sham group on Day 68 post artery occlusion (Fig. 5A–D). This is the same in both the cortex and hippocampus, where SSTF treatment did not significantly affect the expression of Cdk5. However, the levels of p35 were significantly up-regulated after the occlusion in both the cortex and hippocampus. Compared with the level of p35 in the sham group, the expression of p35 was up-regulated by 55% in the cortex and 80% in the hippocampus in the CCI group (Fig 5E,F; P < 0.05). Treatment with 50 mg/kg SSTF slightly reduced p35 expression in the cortex and hippocampus. However, the differences were still statistically significant compared with sham group. Strikingly, the expression of p35 in the cortex and hippocampus was significantly down-regulated by the treatment of 100 mg/kg SSTF (Fig. 5E,F; P < 0.01) in comparison with CCI controls. In the same way, the expression of p25, a truncated form of p35, was increased by 40% in the cortex and 60% in the hippocampus of the CCI rats compared with that in the sham rats on Day 68 post operation (Fig. 5G,H; P < 0.01). Likewise, 50 mg/kg of SSTF did not significantly alter the expression levels of p25 in both the cortex and hippocampus, whereas 100 mg/kg of SSTF significantly down-regulated p25 expression in both the cortex and hippocampus (Fig. 5G,H; P < 0.01).
Figure 5.
SSTF reduced the expression levels of p35 and p25 Representative immunoblots showing the expression of Cdk5, p35 (Cdk5 activator), and p25 (p35 cleavage product) in the cortex (A) and the hippocampus (B). SSTF treatment (100 mg/kg) did not alter the expression of Cdk5 (C and D), but significantly reduced p35 and p25 in the VaD rat cortex (E and G) and hippocampus (F and H). The expression levels of Cdk5, p35, and p25 were normalized to GAPDH. The relative value of sham group was set as 100%. Data are shown as the mean ± SEM (n = 9). *P < 0.05 and **P < 0.01.
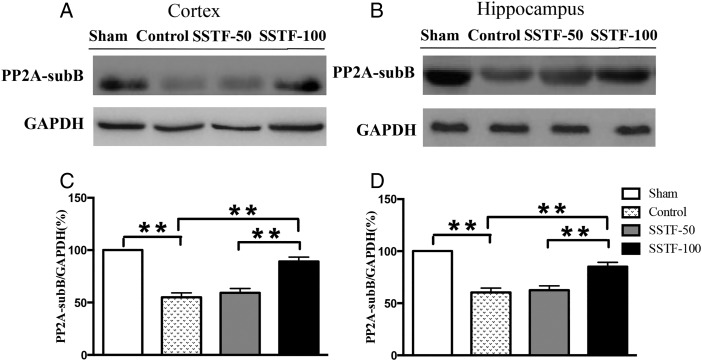
Protein phosphatase 2A was up-regulated in SSTF-treated rats
Protein phosphatase (PP) is a family of most important enzyme regulators of tau phosphorylation in the brain [38]. It is known that increased kinase activity promotes tau hyperphosphorylation. Comparably, dysregulated phosphatase activity also leads to tau pathology. Of all the phosphatases, PP2A was significantly reduced in AD patients, and pharmacological induction of PP2A by eicosanoyl-5-hydroxytryptamide resulted in dephosphorylation of α-synuclein at serine 129 and amelioration of behavioral deficits in an α-synuclein transgenic mouse model [39]. Similar results were observed in our VaD rats. Sixty-eight days after the occlusion, the expression of PP2A subunit B (PP2A-sub B) in the CCI control rats was significantly reduced in both the cortex and hippocampus (Fig. 6). In the cortex, the expression of PP2A-sub B was reduced to 55% of the sham level, which was 60% of the sham level in the hippocampus. Statistical analyses revealed significant difference between the CCI control and the sham group (P < 0.01). Rats treated with 100 mg/kg SSTF showed significant increase of PP2A-sub B in comparison with the CCI controls (P < 0.05). Treatment with 50 mg/kg SSTF did not show any noticeable effect on the expression of PP2A-sub B in both the cortex and hippocampus (Table 1).
Figure 6.
SSTF increased PP2A expression Representative immunoblots for the expression of PP2A subunit B (sub B) in the rat cortex (A) and hippocampus (B). Treatment with 100 mg/kg SSTF significantly increased PP2A-sub B expression in the VaD rat cortex (C) and hippocampus (D). The expression of PP2A-sub B was normalized to GAPDH. The relative levels of sham group were set as 100%. Data are shown as the mean ± SEM (n = 9). **P < 0.01.
Table 1.
Primary antibodies used in this study
| Antibody | Type | Dilution | Specificity | Phosphorylation sites | Company |
|---|---|---|---|---|---|
| pS199 | Ploy- | 1 : 1000 for WB | P-tau | Ser199 | Biosource |
| pS202 | Ploy- | 1 : 1000 for WB | P-tau | Ser202 | Biosource |
| pT205 | Ploy- | 1 : 1000 for WB | P-tau | Thr205 | Biosource |
| pS396 | Ploy- | 1 : 1000 for WB | P-tau | Ser396 | Biosource |
| pS404 | Ploy- | 1 : 1000 for WB | P-tau | Ser404 | Biosource |
| GSK3β | Ploy- | 1 : 1000 for WB | Total GSK-3β | Biovision | |
| p-GSK3β | Ploy- | 1 : 1000 for WB | p-GSK3β | Ser9 | Biovision |
| PP2A-sub B | Ploy- | 1 : 1000 for WB | TotalPP2A subunit B | Santa Cruz | |
| CDK5 | 1 : 1000 for WB | Rabbit anti-CDK5 | Cell Signaling Technology | ||
| p35/25 | 1 : 1000 for WB | Rabbit anti p35/25 | Cell Signaling Technology | ||
| Tau-5 | Mono- | 1 : 500 for WB | Total-tau | Abcam |
Discussion
BCCAo rats are the most commonly used animals for the modeling of chronic cerebral hypoperfusion. The pathophysiological changes after BCCAo include white matter damage, neuronal damage in the cortex and hippocampus, blood–brain barrier disruption, amyloidogenesis, and hyperphosphorylated tau aggregation [40,41]. Consistent with these descriptions, we observed extensive brain infarction in these rats after the occlusion (Fig. 1). Meanwhile, we observed severe impairment of spatial learning and memory in the CCI rats (Fig. 2). During the training trail in the MWM assessment, the latency in the disease controls extended significantly after ischemia when compared with the sham-operated rats (Fig. 2A). The searching time that is taken as an indicator of impaired spatial memory was significantly reduced in the CCI rats (Fig. 2B). The searching distance in target quadrant that is a parameter closely related to spatial learning was significantly decreased in the CCI rats, suggesting impaired ability of spatial learning in these rats (Fig. 2C). However, these changes were significantly attenuated in the rats treated with 100 mg/kg of SSTF. SSTF treatment ameliorated learning memory impairment as indicated by the curtailment of latency time during the training trial as well as the extension of searching time and searching distance in the target quadrant in the probe trial (Fig. 2B,C). SSTF (50 mg/kg) administration did not significantly affect the behavior of rats in probe trail, suggesting that 100 mg/kg might be the minimum dose required. Initially, we thought that SSTF might ameliorate the deficits of spatial learning and memory through reducing the brain infarction, because pretreatment with SSTF prevents cerebral ischemia-reperfusion injury [5,42]. However, in this study, the SSTF treatment did not significantly decrease the infarct volume in the brain (Fig. 1), suggesting that SSTF might exert beneficial effects on the animals via different pathways.
Tau hyperphosphorylation is one of the major pathologies of dementia contributing to the formation of Aβ and neurofibrillary tangles [35,43], which may exacerbate the ischemic neuronal damage and apoptosis after stroke. Abnormally high levels of intracellular tau are frequently observed in AD patients and may be directly implicated in neuron loss [43]. High tau inclusions were also detected in other neurodegenerative disorders such as multiple system atrophy, familial and sporadic Parkinson's disease and Down syndrome, indicating the crucial roles of tau phosphorylation in neurodegenerative diseases. However, a consensus has not been reached on the role of tau phosphorylation in patients with VaD. Our results showed that tau phosphorylation was significantly increased in the cortex and hippocampus of VaD rats (Fig. 3), suggesting that tau hyperphosphorylation may play a role in the impairment of spatial learning and memory in VaD rats. During memory formation, information on objects and events that we experience are processed in the cerebral cortex. Then, the information flows converge in hippocampus and are encoded by neural ensembles (place cells and timing cells) for spatial and temporal organization, which can be retrieved through prefrontal cortex–hippocampus interactions [44]. Clinically, patients with acute injures in cortex or hippocampus show different symptoms. However, we observed significant increase of phosphorylated tau in both the cortex and hippocampus after a global ischemia in VaD rats, suggesting that chronic responses to hypoperfusion in both the cortex and hippocampus may equivalently contribute to the impairment of spatial learning and memory in rats. Consistent with this, SSTF treatment significantly decreased the phosphorylation of tau similarly in both the cortex and hippocampus. Given that tau hyperphosphorylation is associated with disease progression, SSTF may enhance spatial learning and memory through reducing tau phosphorylation in VaD rats although the underlying mechanisms need to be further investigated.
The well-known disease-related epitopes of tau phosphorylation include AT8 (S199/S202/T205), 12E8 (S262/S356), AT180 (T231/S235), PHF-1 (S396/S404), AT100 (T22/S214/T217), S262, and S422 [45]. It is known that a few enzyme families, especially kinases, play pivotal roles in tau hyperphosphorylation. Kinases phosphorylate proteins and have been demonstrated to have different substrate preferences. For example, GSK3β, a major tau kinase in normal brains, phosphorylates (Ser/Thr)-(X3)-(pSer/pThr) with priming phosphorylation at +4 site in addition to (Ser/Thr)-Pro sequences, whereas another major tau kinase Cdk5 prefers the (Ser/Thr)-pro motif with a basic amino acid at the second C-terminal site. Recently, a phosphorylation map of tau in AD brain has been identified, including all major Cdk5 phosphorylation sites and major GSK3β phosphorylation sites. Of all the sites phosphorylated by multiple kinases, S202, T205, S235, and S404 are catalyzed by Cdk5, whereas S199, T231, S396, and S413 are catalyzed by GSK3β [37]. Our results showed no alteration of GSK3β protein in either the SSTF-treated rats or untreated rats, suggesting that the injury or treatment with SSTF does not alter the synthesis of GSK3β in the brain. However, phosphorylated GSK3β (p-GSK3β), the inactive form of GSK3β, was significantly down-regulated in VaD rats (Fig. 4), indicating an alteration of dynamical GSK3β. Decreased p-GSK3β in the CCI rats indicated enhanced transfer of a phosphate moiety to the substrate molecule tau, which was demonstrated by increased phosphorylation of tau at S199 and S396 (Fig. 3C,F). Strikingly, the decrease of p-GSK3β after CCI was reversed by the treatment with 100 mg/kg of SSTF, which in turn reduced tau phosphorylation. Similarly, we did not observe any difference in Cdk5 levels in either the cortex or hippocampus among all groups (Fig. 5). However, the kinase activity of Cdk5 is mainly determined by the available protein amounts of p35 in neurons. In the CCI control group, a significant increase of p35 was observed (1.6 folds; Fig. 5E), suggesting an increase in Cdk5 activity. p35 protein has a short half-life of only 30 min and is proteolytically cleaved by calpain to generate a truncated but active form, p25. p25 deregulates Cdk5 activity by prolonging its activation and changing its cellular location [35,46]. p25 accumulates in the brain of patients with AD, leading to aberrantly phosphorylated forms of tau [35,46]. In agreement with this, we observed increased p25 in VaD rats (Fig. 5A,B,G,H), suggesting an increased activity of Cdk5 after brain injury. It should be noted that SSTF administration decreased the levels of p35 and p25, which may ameliorate the activity of Cdk5 and contribute to the reduction of the tau phosphorylation at Ser202 and Ser 404 (Fig. 3D,G).
In the brain, kinases-triggered tau phosphorylation was counterbalanced by PP2A-catalyzed dephosphorylation [35,47]. A significant decrease in total PP2A activity in AD cortical and hippocampal homogenates has been reported [35,47,48], demonstrating that altered expression of PP2A is associated with AD pathology [47]. In this study, PP2A-sub B expression in the cortex and hippocampus was significantly down-regulated in VaD rats (Fig. 6), suggesting a deregulation of PP2A induced by ischemia. Notably, SSTF reversed the reduction of PP2A-sub B, suggesting that SSTF may reduce tau phosphorylation through increasing the PP2A regulation.
SSTF is a mixture of multiple compounds. Each component shows specific activity [1–3,49]. Therefore, the results obtained in this study reflected the combined activities of different components or an effect contributed by all components of SSTF acting synergistically/antagonistically. Further investigation needs to be done to clarify this issue. Recently, several small molecules and chemical compounds have been found to regulate the activities of GSK3β, Cdk5, or PP2A, suggesting that they may potentially be used in the treatment of AD [35,50,51]. These active compounds were shown to reduce oxidative stress, counteract neuronal death, and improve learning and memory. However, the efficacy varied when a single target was manipulated in each study. In contrast, our results showed that SSTF exerts strong inhibitory effects on tau phosphorylation in ischemia-induced VaD, most probably through regulating kinases-triggered phosphorylation and PP2A-catalyzed dephosphorylation. SSTF may be used as a promising candidate of drug discovery, which will help to develop novel therapeutic strategies for dementia.
Supplementary Data
Funding
This work was supported by the grants from Zhejiang Provincial Administration of Traditional Chinese Medicine (No. 2013ZB106) and Zhejiang Provincial Department of Health Project (No. 2014KYB198).
References
- 1.Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol 2015, 272: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pluta R, Jolkkonen J, Cuzzocrea S, Pedata F, Cechetto D, Popa-Wagner A. Cognitive impairment with vascular impairment and degeneration. Curr Neurovasc Res 2011, 8: 342–350. [DOI] [PubMed] [Google Scholar]
- 3.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 2007, 54: 162–180. [DOI] [PubMed] [Google Scholar]
- 4.Zhang N, Xing M, Wang Y, Liang H, Yang Z, Shi F, Cheng Y. Hydroxysafflor yellow A improves learning and memory in a rat model of vascular dementia by increasing VEGF and NR1 in the hippocampus. Neurosci Bull 2014, 30: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF, Du GH. Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett 2013, 546: 57–62. [DOI] [PubMed] [Google Scholar]
- 6.Miao G, Zhao H, Guo K, Cheng J, Zhang S, Zhang X, Cai Z et al. . Mechanisms underlying attenuation of apoptosis of cortical neurons in the hypoxic brain by flavonoids from the stems and leaves of Scutellaria baicalensis Georgi. Neural Regenerat Res 2014, 9: 1592–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai J, Chen L, Qiu YM, Li SQ, Xiong WH, Yin YH, Jia F et al. . Activations of GABAergic signaling, HSP70 and MAPK cascades are involved in baicalin's neuroprotection against gerbil global ischemia/reperfusion injury. Brain Res Bull 2013, 90: 1–9. [DOI] [PubMed] [Google Scholar]
- 8.Zhang SF, Dong YC, Zhang XF, Wu XG, Cheng JJ, Guan LH, Shang YZ. Flavonoids from Scutellaria attenuate okadaic acid-induced neuronal damage in rats. Brain Injury 2015, 29: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 9.Kim KC, Lee IK, Kang KA, Kim HS, Kang SS, Hyun JW. Baicalein (5,6,7-trihydroxyflavone) reduces oxidative stress-induced DNA damage by upregulating the DNA repair system. Cell Biol Toxicol 2012, 28: 421–433. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Lee SR. The effect of Baicalein on hippocampal neuronal damage and metalloproteinase activity following transient global cerebral ischaemia. Phytother Res 2012, 26: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, Cho GJ et al. . Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J 2003, 17: 1943–1944. [DOI] [PubMed] [Google Scholar]
- 12.Vepsalainen S, Koivisto H, Pekkarinen E, Makinen P, Dobson G, McDougall GJ, Stewart D et al. . Anthocyanin-enriched bilberry and blackcurrant extracts modulate amyloid precursor protein processing and alleviate behavioral abnormalities in the APP/PS1 mouse model of Alzheimer's disease. J Nutr Biochem 2013, 24: 360–370. [DOI] [PubMed] [Google Scholar]
- 13.Bastianetto S, Yao ZX, Papadopoulos V, Quirion R. Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid-induced toxicity. Eur J Neurosci 2006, 23: 55–64. [DOI] [PubMed] [Google Scholar]
- 14.Lu JH, Ardah MT, Durairajan SS, Liu LF, Xie LX, Fong WF, Hasan MY et al. . Baicalein inhibits formation of alpha-synuclein oligomers within living cells and prevents Abeta peptide fibrillation and oligomerisation. Chembiochem 2011, 12: 615–624. [DOI] [PubMed] [Google Scholar]
- 15.Onozuka H, Nakajima A, Matsuzaki K, Shin RW, Ogino K, Saigusa D, Tetsu N et al. . Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther 2008, 326: 739–744. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh D, Scheepens A. Vascular action of polyphenols. Mol Nutr Food Res 2009, 53: 322–331. [DOI] [PubMed] [Google Scholar]
- 17.Milner E, Zhou ML, Johnson AW, Vellimana AK, Greenberg JK, Holtzman DM, Han BH et al. . Cerebral amyloid angiopathy increases susceptibility to infarction after focal cerebral ischemia in Tg2576 mice. Stroke 2014, 45: 3064–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer JA. The Nun Study: risk factors for pathology and clinical-pathologic correlations. Curr Alzheimer Res 2012, 9: 621–627. [DOI] [PubMed] [Google Scholar]
- 19.Honjo K, Black SE, Verhoeff NP. Alzheimer's disease, cerebrovascular disease, and the beta-amyloid cascade. Can J Neurol Sci 2012, 39: 712–728. [DOI] [PubMed] [Google Scholar]
- 20.Murch SJ, Rupasinghe HP, Goodenowe D, Saxena PK. A metabolomic analysis of medicinal diversity in Huang-qin (Scutellaria baicalensis Georgi) genotypes: discovery of novel compounds. Plant Cell Rep 2004, 23: 419–425. [DOI] [PubMed] [Google Scholar]
- 21.He XL, Wang YH, Gao M, Li XX, Zhang TT, Du GH. Baicalein protects rat brain mitochondria against chronic cerebral hypoperfusion-induced oxidative damage. Brain Res 2009, 1249: 212–221. [DOI] [PubMed] [Google Scholar]
- 22.Song HR, Cheng JJ, Miao H, Shang YZ. Scutellaria flavonoid supplementation reverses ageing-related cognitive impairment and neuronal changes in aged rats. Brain Injury 2009, 23: 146–153. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SQ, Obregon D, Ehrhart J, Deng J, Tian J, Hou H, Giunta B et al. . Baicalein reduces beta-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer's disease transgenic mouse model. J Neurosci Res 2013, 91: 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shang Y, Cheng J, Qi J, Miao H. Scutellaria flavonoid reduced memory dysfunction and neuronal injury caused by permanent global ischemia in rats. Pharmacol Biochem Behav 2005, 82: 67–73. [DOI] [PubMed] [Google Scholar]
- 25.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE et al. . Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009, 68: 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You CL, Su PQ, Zhou XX. Study on effect and mechanism of Scutellaria baicalensis stem-leaf total flavonoid in regulating lipid metabolism. Zhongguo Zhong Yao Za Zhi 2008, 33: 1064–1066. [PubMed] [Google Scholar]
- 27.Chen SP, Zhao GQ, OPan HF, Han SY. Isolation of flavonoid compounds from stems and leaves of Scutellaria baicalensis Georgi by high-speed countercurrent chromatography. J Chengde Med Coll 2002, 19: 968–970. [Google Scholar]
- 28.Cechetti F, Worm PV, Pereira LO, Siqueira IR, Netto C. The modified 2VO ischemia protocol causes cognitive impairment similar to that induced by the standard method, but with a better survival rate. Braz J Med Biol Res 2010, 43: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 29.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 1984, 11: 47–60. [DOI] [PubMed] [Google Scholar]
- 30.Bromley-Brits K, Deng Y, Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp 2011. doi:10.3791/2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soria G, Tudela R, Marquez-Martin A, Camon L, Batalle D, Munoz-Moreno E, Eixarch E et al. . The ins and outs of the BCCAo model for chronic hypoperfusion: a multimodal and longitudinal MRI approach. PLoS One 2013, 8: e74631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res 2007, 32: 577–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem 2006, 281: 25457–25465. [DOI] [PubMed] [Google Scholar]
- 34.Sadleir KR, Vassar R. Cdk5 protein inhibition and Abeta42 increase BACE1 protein level in primary neurons by a post-transcriptional mechanism: implications of CDK5 as a therapeutic target for Alzheimer disease. J Biol Chem 2012, 287: 7224–7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shelton SB, Johnson GV. Cyclin-dependent kinase-5 in neurodegeneration. J Neurochem 2004, 88: 1313–1326. [DOI] [PubMed] [Google Scholar]
- 36.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL III. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol 2004, 63: 287–301. [DOI] [PubMed] [Google Scholar]
- 37.Kimura T, Ishiguro K, Hisanaga S. Physiological and pathological phosphorylation of tau by Cdk5. Front Mol Neurosci 2014, 7: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landrieu I, Smet-Nocca C, Amniai L, Louis JV, Wieruszeski JM, Goris J, Janssens V et al. . Molecular implication of PP2A and Pin1 in the Alzheimer's disease specific hyperphosphorylation of Tau. PLoS One 2011, 6: e21521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KW, Chen W, Junn E, Im JY, Grosso H, Sonsalla PK, Feng X et al. . Enhanced phosphatase activity attenuates alpha-synucleinopathy in a mouse model. J Neurosci 2011, 31: 6963–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun LH, Ban T, Liu CD, Chen QX, Wang X, Yan ML, Hu XL et al. . Activation of Cdk5/p25 and tau phosphorylation following chronic brain hypoperfusion in rats involves microRNA-195 down-regulation. J Neurochem 2015, 134: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 41.Villamil-Ortiz JG, Cardona-Gomez GP. Comparative analysis of autophagy and tauopathy related markers in cerebral ischemia and Alzheimer's disease animal models. Front Aging Neurosci 2015, 7: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao S, Kong W, Zhang S, Chen M, Zheng X, Kong X. Pretreatment with Scutellaria baicalensis stem-leaf total flavonoid prevents cerebral ischemia-reperfusion injury. Neural Regen Res 2013, 8: 3183–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serrano-Pozo A, Qian J, Monsell SE, Blacker D, Gomez-Isla T, Betensky RA, Growdon JH et al. . Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann Neurol 2014, 75: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 2013, 23: R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tenreiro S, Eckermann K, Outeiro TF. Protein phosphorylation in neurodegeneration: friend or foe? Front Mol Neurosci 2014, 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 1999, 402: 615–622. [DOI] [PubMed] [Google Scholar]
- 47.Sontag JM, Sontag E. Protein phosphatase 2A dysfunction in Alzheimer's disease. Front Mol Neurosci 2014, 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem 1995, 65: 732–738. [DOI] [PubMed] [Google Scholar]
- 49.Walle T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin Cancer Biol 2007, 17: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye Q, Shen Y, Zhou Y, Lv D, Gao J, Li J, Hu Y. Design, synthesis and evaluation of 7-azaindazolyl-indolyl-maleimides as glycogen synthase kinase-3beta (GSK-3beta) inhibitors. Eur J Med Chem 2013, 68: 361–371. [DOI] [PubMed] [Google Scholar]
- 51.Voronkov M, Braithwaite SP, Stock JB. Phosphoprotein phosphatase 2A: a novel druggable target for Alzheimer's disease. Future Med Chem 2011, 3: 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]