Abstract
Dysregulated release of neutrophil reactive oxygen species and proteolytic enzymes contributes to both acute and chronic inflammatory diseases. Therefore, molecular regulators of these processes are potential targets for new anti-inflammatory therapies. We have shown previously that MARCKS (Myristoylated Alanine Rich C-Kinase Substrate), a well-known PKC substrate protein, is a key regulator of neutrophil functions. In the current study we investigate the role of PKC-mediated MARCKS phosphorylation in neutrophil migration and adhesion in vitro. We report that treatment of human neutrophils with the δ-PKC inhibitor rottlerin significantly attenuates fMLF induced MARCKS phosphorylation (IC50 = 5.709 μM), adhesion (IC50 = 8.4 uM) and migration (IC50 = 6.7 uM); while α-, β- and ζ-PKC inhibitors had no significant effect. We conclude that δ-PKC mediated MARCKS phosphorylation is essential for human neutrophil migration and adhesion in vitro. These results implicate δ-PKC mediated MARCKS phosphorylation as a key step in the inflammatory response of neutrophils.
Keywords: neutrophil, chemotaxis, adhesion, migration, innate immunity, pkc signaling
Background
As essential components of the innate immune system, neutrophils provide a first line of defense against bacterial invasion through rapid accumulation in tissue, release of proteolytic enzymes, production of reactive oxygen species, and phagocytosis of microbial pathogens. The toxic substances released by neutrophils invariably result in some degree of host tissue injury and inflammation; but this so called “collateral damage” is normally limited by elimination of inciting pathogens and subsequent return to homeostasis. However, neutrophil responses that are misdirected or dysregulated can result in acute and chronic forms of inflammation that are debilitating and even life-threatening. This type of neutrophil mediated host injury occurs in diseases such as rheumatoid arthritis, ulcerative colitis, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [1]. Additional medications are desperately needed to treat these acute and chronic inflammatory conditions, and neutrophils, as the cause of damaging inflammation, are a viable target for therapeutic intervention [2]. For this reason, significant research efforts have focused on discerning essential cell signaling components involved in various neutrophil functions [3].
Protein Kinase C (PKC) is one such molecular regulator known to play a significant role in neutrophil inflammatory signaling [4]. Human neutrophils express several PKCs including conventional isoforms α- and β, novel isoform δ and atypical isoform ζ. These PKC isoforms are implicated in various neutrophil functions including adhesion, migration, oxidative burst, degranulation and apoptosis [5, 6]. Recently, δ-PKC has emerged as a key regulator of inflammation and promising target for anti-inflammatory therapy [7]. δ-PKC is activated by numerous inflammatory signals including host derived cytokines (i.e. TNFα and IL-1) and bacterial components such as LPS [6, 8, 9]. Studies targeting δ-PKC by use of a dominant negative peptide showed this PKC isoform to be integrally involved in adherence and migration of human neutrophils in vitro [10]. Intra-tracheal administration of this same inhibitor peptide significantly reduced inflammatory cell infiltration and pulmonary edema following cecal ligation and puncture in rats (an animal model of ARDS) [11]. However, the molecular basis for the beneficial effects seen with δ-PKC inhibition remains uncertain.
Myristoylated Alanine Rich C-Kinase Substrate (MARCKS) protein is another key regulator of neutrophil functions including migration, adhesion, degranulation and respiratory burst [12–14]. As a prominent protein kinase C substrate and known actin-binding protein, MARCKS has a demonstrated role in the regulation of cellular events requiring dynamic actin reorganization [15, 16]. In most resting cells, non-phosphorylated MARCKS is localized to the inner leaflet of the plasma membrane by hydrophobic insertion of the N-terminal myristoyl-moiety and electrostatic interactions of the basic, serine-rich effector domain (ED) [17]. Membrane bound MARCKS is believed to stabilize the cytoskeleton through actin cross-linking. Upon PKC phosphorylation of the ED, MARCKS is displaced to the cytosol and actin cross-linking is diminished, effectively relaxing the cellular cytoskeleton [18, 19]. Within the cytosol MARCKS is dephosphorylated by specific phosphatases, re-establishing MARCKS ability to both cross-link actin and return to the plasma membrane [20–22].
To better understand the role of MARCKS phosphorylation in neutrophil functions, we sought to determine which PKC isoforms regulate MARCKS in human neutrophils, and whether or not those same isoforms were required for neutrophil migration and adhesion. To that end, we investigated PKC isotypes α, β, δ and ζ using specific PKC isotype inhibitors Gö6976, CG53353, rottlerin and ζ pseudosubstrate, respectively. Using subcellular fractionation, we demonstrate that of the four isotypes examined, only δ-PKC translocates from cytosol to membrane in neutrophils in response to stimulation (fMLF and PMA). Because MARCKS phosphorylation is known to occur at the plasma membrane, this pattern of δ-PKC translocation is consistent with MARCKS regulation. We further report that the δ-PKC inhibitor rottlerin inhibits MARCKS phosphorylation in human neutrophils following fMLF stimulation in a concentration - dependent manner (IC50 = 5.709 μM). Interestingly, rottlerin pre-treatment also inhibits fMLF mediated human neutrophil migration and adhesion in a concentration - dependent manner (IC50 of 8.385 uM and 7.624 uM, respectively). We conclude that δ-PKC is the primary isotype regulating MARCKS phosphorylation in human neutrophils, and that δ-PKC mediated MARCKS phosphorylation is essential for neutrophil migration and adhesion. Previous research from our lab and others suggests that MARCKS and δ-PKC are both potential targets for future anti-inflammatory therapies. The results presented here shed further light on the integral relationship between these two important signaling molecules in the neutrophil’s response to inflammation.
Materials and Methods
Human Subjects
The human neutrophils utilized for this study were isolated from the peripheral blood of healthy, adult volunteers using the protocol approved by the Institutional Research Ethics Committee of North Carolina State University (IRB approval #616). Prior to donation, all participants provided written informed consent using the approved consent form.
Neutrophil isolation
Human neutrophils were isolated from whole blood using Ficoll gradient centrifugation of dextran-sedimented leukocyte rich plasma, as previously described. Briefly, approximately 6 ml of plasma was layered on 5 ml of sterile, endotoxin-free Ficoll-Paque solution and spun at 600 g for 20 minutes. Red blood cells were removed by hypotonic lysis and remaining neutrophils were washed once with HBSS. Cells were re-suspended in HBSS with 20 mM HEPES, 8.9 mM sodium bicarbonate, 1mM Ca 2+ and 1mM Mg 2+ prior to biochemical assays (HBSS++) and in HBSS with 1mM Ca 2+, 1mM Mg 2+ and 2% FCS (chemotaxis buffer) prior to adhesion and migration assays. With this isolation method neutrophils routinely demonstrated greater than 98% viability as determined by trypan blue exclusion.
Fluorescence labeling of neutrophils
For migration and adhesion experiments, isolated neutrophils (1 × 107/ml in HBSS) were incubated with the fluorescent dye calcein am (Anaspec, Fremont, CA) at 2 ug/ml for 30 minutes at room temperature. Cells were then centrifuged at 1000 rpm for 8 min and resuspended in chemotaxis buffer to the appropriate final experimental concentration.
Antibodies and reagents
Ficoll-Paque Plus and Dextran T500 were from GE Healthcare (Sweden). Dimethyl sulfoxide (Me2SO), f-Met-Leu-Phe (fMLF), phorbol 12-myristate 13-acetate (PMA), Triton-X 100, pepstatin, HEPES, staurosporine, Protein kinase C-ζ pseudosubstrate and poly-l-lysine were from Sigma Chemical Co. (St. Louis, MO). Rottlerin, Gö6976 and CG53353 were from Calbiochem (Billerica, MA). Powdered phosphate-buffered saline (PBS) and Hank’s balanced salt solution (HBSS) were from Life Technologies (Grand Island, NY). Ethylenediamine tetraacetate dihydrate (EDTA) was from Fisher Scientific (Atlanta, GA). Leukotriene B4 (LTB4) was from Cayman Chemical (Ann Arbor, MI). Interleukin 8 (IL-8) was from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). Fetal calf serum (FCS) was obtained from Hyclone (Logan, UT). MARCKS, Phospho-MARCKS, PKC α, PKC δ, PKC ζ primary antibodies and anti-rabbit HRP-conjugated secondary antibody were obtained from Cell Signaling (Beverly, MA). PKC β was from Invitrogen (Frederic, MD). Diisopropylfluorophosphate (DFP) was from BD Biosciences (San Diego, CA).
Lysate preparation and western blot assay
Purified human neutrophils (2.5 × 107 cells/ml) were suspended in HBSS++ and incubated with or without PKC isoform inhibitors for 15 to 30 min with varying concentrations in microcentrifuge tubes and incubated at 37°C. Cells were then stimulated with 100 nM fMLF, 50 ng/mL PMA or appropriate vehicle control (VC) for the indicated time periods during incubation at 37°C. Incubated cells were immediately transferred onto ice and lysed with ice cold RIPA buffer [1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 5 mM sodium pyrophosphate and 50 mM sodium fluoride] containing protease inhibitors [1mM phenylmethanesulphonylfluoride (PMSF), 100μg/mL pepstatin, 1mM iodoacetamide, and 10 μg/mL aprotinin/leupeptin] for 30 minutes on ice. After lysis, cell solutions were spun at 13,400 g for 5 minutes. Supernatants were collected and protein concentrations were measured using BCA Protein Assay Reagent (Pierce, Rockford, IL). Cell lysate was mixed with 5X sample buffer [25% glycerol, 2% SDS, 60mM Tris-HCL (pH 6.8), 5% β-mercaptoethanol, 0.1% bromophenol blue in diH2O] and boiled for 5 minutes. Equal amount of protein was analyzed in 10% SDS-PAGE. Resolved samples were transferred to Immobilon-P PVDF membrane (Millipore, Billerica, MA) and blocked for 1 hour with 5% non-fat dry milk in tris buffered saline with Tween-20 (TBS/T; 136 μM NaCl, 20 μM Tris-base (pH: 7.6) and 0.1% Tween-20 v/v) prior to overnight incubation with the 1:1000 dilution of phospho MARCKS or MARCKS primary antibody in 5% BSA in TBS/T at 4°C. Membranes were washed in TBS/T and incubated with 1:4000 dilution of anti-rabbit horseradish peroxidase (HRP) secondary antibody (Cell Signaling Technology, Danvers, MA) in 5% non-fat dry milk in TBS/T for 1 hour with gentle agitation. Membranes were washed at room temperature in TBS/T three times for 5 mins and developed using enhanced chemiluminescence and radiograph film. Films were scanned and the density of the bands was measured with densitometric software (Scanalytics, Fairfax, VA).
Subcellular fractionation
Subcellular fractionation assay was performed as previously described [12]. Briefly, stimulated cells were lysed by sonication in lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM DTT). The lysate was centrifuged at 800g for 10 min to remove cell debris and nuclei. Supernatant was then centrifuged at 20,000g for 1 hour. The supernatant was collected as the cytosolic fraction and the remaining pellet was regarded as the membrane fraction and resuspended in lysis buffer containing 1% Triton X-10. Protein concentration was determined by Protein 660 (Pierce, Rockford, IL) and samples were diluted in 5x sample buffer and boiled for 5 minutes. Equal protein concentrations were loaded onto a 10% SDS-PAGE and western blots were performed, as described above.
Neutrophil chemotaxis assay
Neutrophil chemotaxis assays were performed using the 8 micron pore size Neuro Probe ChemoTx® system as previously described [12]. Briefly, each upper well was loaded with 5 × 104 calcein-labeled neutrophils in 25 ul of chemotaxis buffer. Cells were allowed to migrate for 1 hour at 37° C toward lower wells containing chemoattractants (100 nM fMLF, 100 nM LTB4 or 100 ng/mL IL-8) in PBS or vehicle controls (DMSO and EtOH). Following the hour migration, non-migrated cells were removed from the top of the membrane with a cell scraper. Following membrane removal, fluorescence of the bottom wells was measured (485 nm excitation, 530 nm emission) using an fMax fluorescence plate reader (Molecular Devices). Percent migration was calculated by dividing the florescence of the experimental bottom wells by the fluorescence of bottom wells containing a known number of cells (5 × 104 cells). Treatment groups were tested in triplicate.
Neutrophil adhesion assay
To assess fMLF mediated adhesion, 1.2 × 105 calcein-labeled neutrophils (60 ul) from the various treatment groups were aliquoted into individual wells of Immulon2HB flat bottom 96-well plates (Thermo Fischer Scientific) previously coated with 5% FCS in sterile PBS, as previously described [12]. The plates were then placed in a 37°C incubator while the cells settled for 10 minutes. Following the addition of fMLF (100 nM final concentration) or vehicle control (DMSO) the plate was floated in the 37°C water bath for 3 minutes. Following incubation, neutrophil adhesion was assessed using an fMax fluorescence plate reader (Molecular Devices). After an initial fluorescence reading (485 nm excitation, 530 nm emission) the plates were gently dumped in a single inverted motion; the wells were filled with 150 ul sterile PBS and another fluorescence reading was obtained. This procedure was repeated for a total of three washes to remove non-adhered cells. Fluorescence after each washing was divided by the initial fluorescence to calculate percent adhesion. The first wash that demonstrated 10% adhesion of non-stimulated cells or less (second wash on average) was considered the final result. Treatment groups were tested in triplicate.
Statistical analysis
Films were scanned and the density of the bands was measured with densitometric software (SigmaScan 5). Results from 3 identical biochemical studies were analyzed by a student t-test with a p < 0.05 considered statistically significant. Migration and adhesion data are reported as mean ± SEM. Results were analyzed by one way repeated measures ANOVA (Holm-Sidak multiple comparison testing) assuming equal variance, with p < 0.05 considered statistically significant.
Results
δ-PKC translocates from cytosol to plasma membrane upon neutrophil stimulation
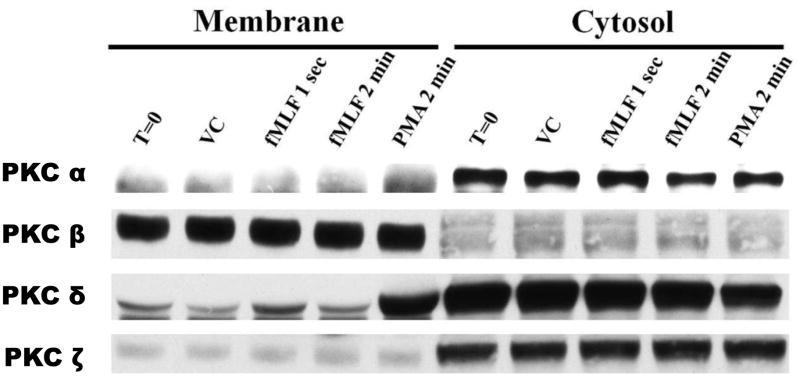
Circulating neutrophils, initially distant from the primary site of pathogen invasion, are recruited to areas of inflammation and/or infection by a gradient of intermediate- (i.e. IL-8 and LTB4) and end-stage chemoattractants (i.e. fMLF) [23]. This signaling, which occurs through transmembrane G-protein coupled chemoattractant receptors (GPCR), activates various isoforms of protein kinase C [5]. Translocation of PKC from the cytosol to the membrane compartments upon cell stimulation is indicative of intracellular PKC activation [24, 25]. To determine PKC isoform activation in human neutrophils, we performed sub-cellular fractionation of cell lysates following stimulation with either 100 nM fMLF or 50 ng/mL PMA. In unstimulated human neutrophils, α-, δ- and ζ-PKC isoforms were primarily located within the cytosolic fraction, while β-PKC was partitioned to the membrane (Figure 1). Following stimulation, δ-PKC was the only isoform that translocated from the cysotol to the membrane. Additionally, translocation of δ-PKC was extremely rapid in response to the physiologically relevant chemoattractant fMLF (Figure 1). Of the four PKC isoforms investigated, δ-PKC was the only isoform that appeared activated upon stimulation of human neutrophils with either 100 nM fMLF or 50 ng/ml PMA.
Figure 1. δ-PKC translocates from cytosol to membrane upon neutrophil stimulation.
Isolated human neutrophils were stimulated with fMLF (100 nM), PMA (50 ng/mL) or VC (DMSO) at 37°C for the indicated times. Prepared lysates were subsequently divided into membrane and cytosolic fractions using ultracentrifugation. Western blot analysis was performed to detect α-, β-, δ- and ζ-PKC in the two fractions. Data are representative of three separate experiments using different neutrophil donors.
MARCKS is transiently phosphorylated upon fMLF stimulation of primary human neutrophils
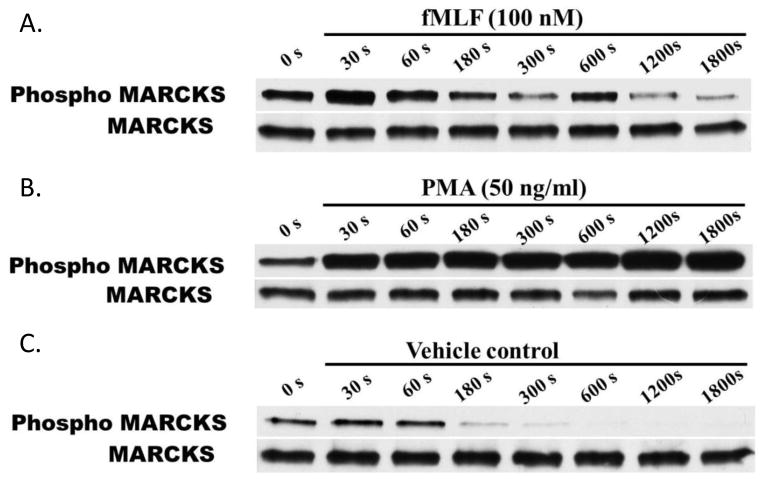
MARCKS plays a key role in human neutrophil migration [12] and its bi-directional movement between the plasma membrane and the cytosol is a well described aspect of its function [26]. Early studies by Thelen et al. show that the translocation of MARCKS between the plasma membrane and the cytosol in human neutrophils is dependent upon reversible PKC-phosphorylation of MARCKS ED [16]. Consistent with previous reports, we observed rapid phosphorylation of MARCKS following non-specific PKC activation with PMA (Figure 2A). However, with this receptor-independent form of activation, there was no evidence of subsequent MARCKS de-phosphorylation. This finding is consistent with the non-physiologic PKC activation seen with PMA, which is a synthetic analog of the second messenger diacylglycerol (DAG). With physiologically relevant activation using fMLF (100 nM) rapid MARCKS phosphorylation (30 seconds) was followed by return to baseline phosphorylation levels by 3 minutes (Figure 2B). A second phosphorylation peak was observed at 10 minutes. Thus, stimulation of human neutrophils with 100 nM fMLF caused a physiologically relevant pattern of MARCKS phosphorylation, which was both rapid and transient. Based on these results, we chose 30 seconds fMLF stimulation as the time point for maximal MARCKS phosphorylation for the remaining biochemical studies.
Figure 2. MARCKS is rapidly phosphorylated upon stimulation of human neutrophils.
Isolated human neutrophils were stimulated with (A) 100 nM fMLF, (B) 50 ng/mL PMA or (C) VC (DMSO) at 37°C for times ranging from 30 – 1800 seconds. Western blot analysis of lysates was performed to detect phosphorylated MARCKS (Phospho MARCKS) and total MARCKS protein (MARCKS). Data are representative of three separate experiments using different neutrophil donors.
PKC-mediated MARCKS phosphorylation is required for human neutrophil chemotaxis
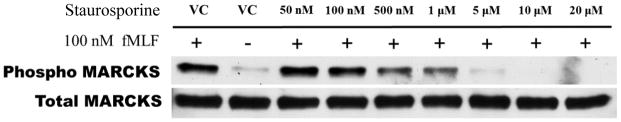
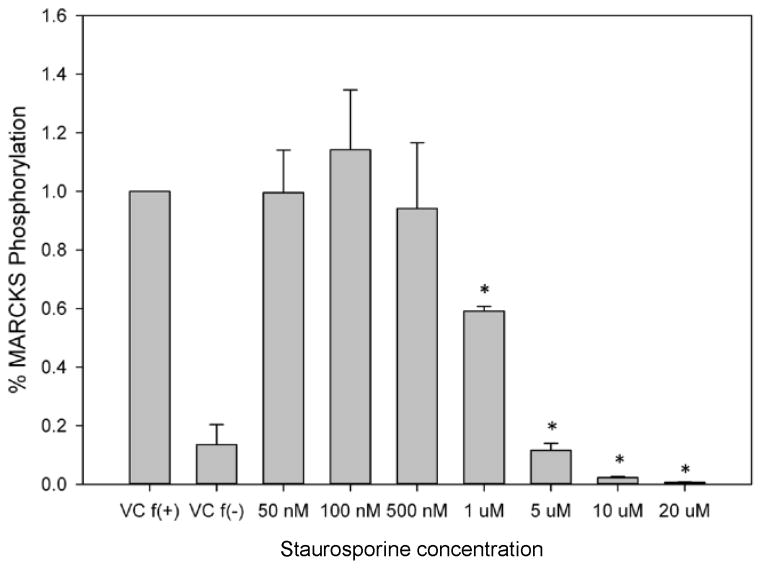
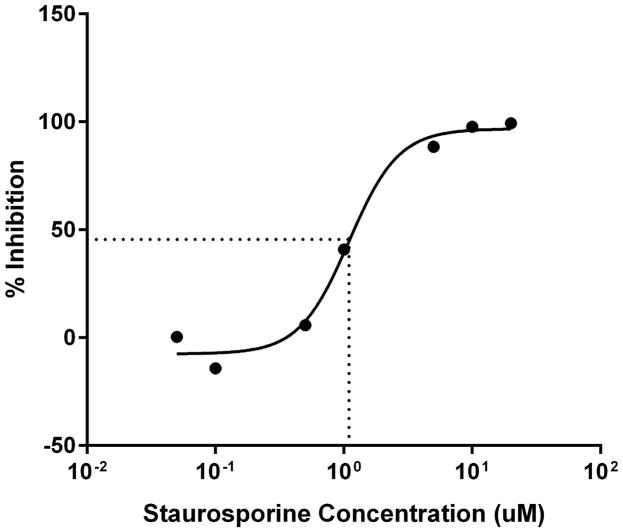
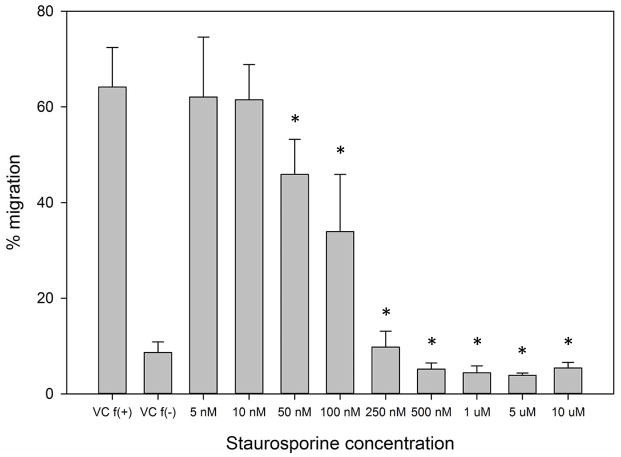
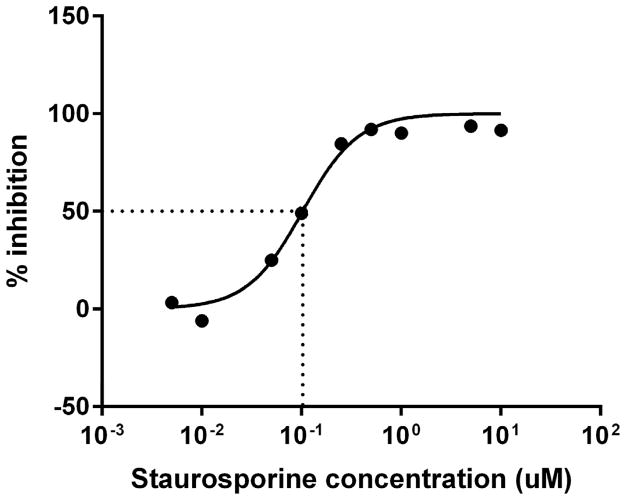
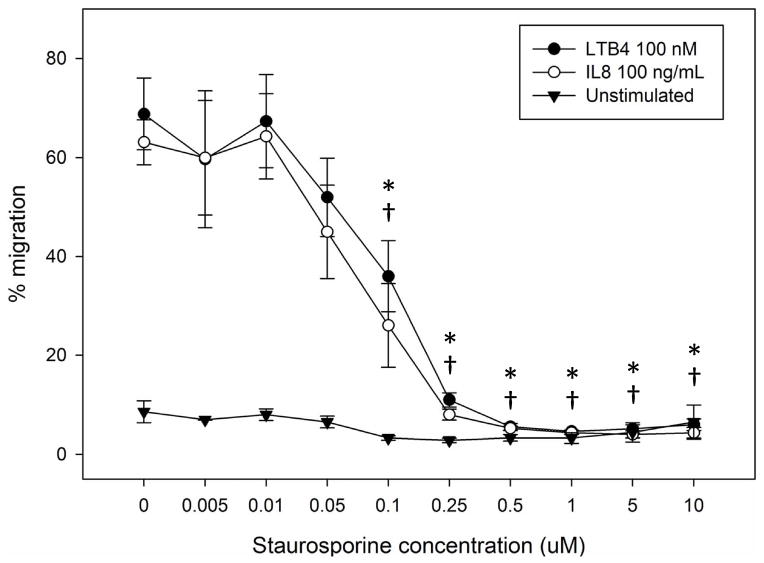
Next, we sought to determine the requirement for MARCKS phosphorylation in migrating human neutrophils. As shown in Figure 3A, pretreatment of human neutrophils with the pan-PKC inhibitor staurosporine attenuated fMLF mediated MARCKS phosphorylation in a concentration - dependent manner, with significant inhibition seen at concentrations of 1.0 uM and greater (Figure 3B). From this result, we calculated that the staurosporine IC50 for fMLF-induced MARCKS phosphorylation is 1.094 μM (Figure 3C). Staurosporine treatment also significantly attenuated fMLF, LTB4 and IL-8 induced migration of human neutrophils in a concentration - dependent manner (Figures 3D and 3F). Staurosporine inhibition of neutrophil migration for both intermediate (LTB4 and IL-8) and end-stage (fMLF) chemoattractants was significant at concentrations of 100 nM and greater. The staurosporine IC50 for fMLF-induced migration is 0.1027 uM (Figure 3E). Our findings indicate that the staurosporine IC50 for inhibition of migration is 10-fold lower than the IC50 calculated for the inhibition of MARCKS phosphorylation in human neutrophils. The lower inhibitory concentration for migration compared to MARCKS phosphorylation may potentially be explained by non-specific effects of pan-PKC inhibition on other signaling pathways and regulatory molecules in addition to MARCKS. It is interesting to note that in previous reports staurosporine failed to inhibit fMLF and IL-8 induced human neutrophil adhesion and migration in vitro [27], but these findings were reported as “data not shown”. Without knowing the concentrations of staurosporine examined, it is difficult to speculate on the reasons for the difference between this previous report and our findings.
Figure 3. PKC-mediated MARCKS phosphorylation is required for human neutrophil chemotaxis.
(A–C) Isolated human neutrophils were pretreated at 37°C for 30 minutes with indicated concentrations of the pan-PKC inhibitor staurosporine or VC (Me2SO) and then stimulated with 100 nM fMLF. (A) Representative dose response experiment (30 second fMLF stimulation). Western blot analysis of equal protein amounts from prepared lysates was performed to detect phosphorylated MARCKS (Phospho MARCKS) and total MARCKS protein (MARCKS). (B) Densitometry of phospho MARCKS and total MARCKS bands was determined and “% MARCKS phosphorylation” was calculated by dividing the phospho MARCKS band by the total MARCKS band. Data are presented as the mean ± SE. (*) indicates significant (p<0.05) difference in % MARCKS phosphorylation compared to VC f(+). (C) IC50 value (1.094 μM) was calculated by plotting percent inhibition of MARCKS phosphorylation against log10 staurosporine concentration (uM). (D–F) Chemotaxis of calcein-labeled human neutrophils pretreated with indicated concentrations of staurosporine was measured using an 8 micron ChemoTx plate (Neuro Probe Inc.) (D) % neutrophil migration toward 100 nM fMLF or VC (Me2SO) was calculated by dividing fluorescence of migrated cells by fluorescence of total cells. Data are presented as the mean ± SE. (*) indicates significant (p<0.05) difference in % migration compared to VC f(+). (E) IC50 value (0.1027 μM) was calculated by plotting percent inhibition of neutrophil migration against log10 staurosporine concentration (uM). (F) % neutrophil migration toward 100 nM LTB4, 100 ng/mL IL-8 or VC (EtOH)) was calculated by dividing fluorescence of migrated cells by fluorescence of total cells. Data are presented as the mean ± SE. * indicates significant (p<0.05) difference in % migration of LTB4 vs. VC (“0 uM” staurosporine), † indicates significant (p<0.05) difference in % migration of IL-8 vs. VC (“0 uM” staurosporine). (A–F) All data are representative of at least three separate experiments using different neutrophil donors.
δ-PKC regulates MARCKS phosphorylation, migration and adhesion in human neutrophils
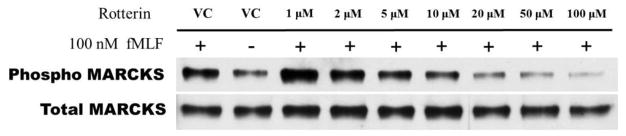
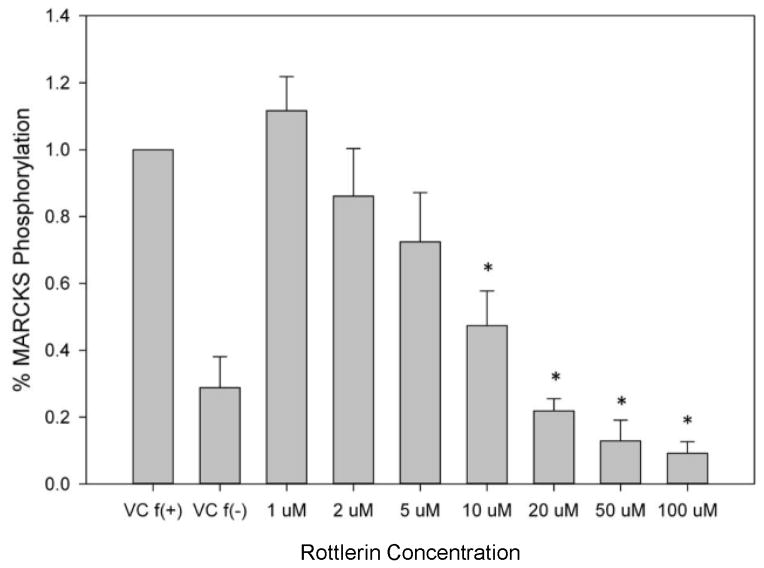
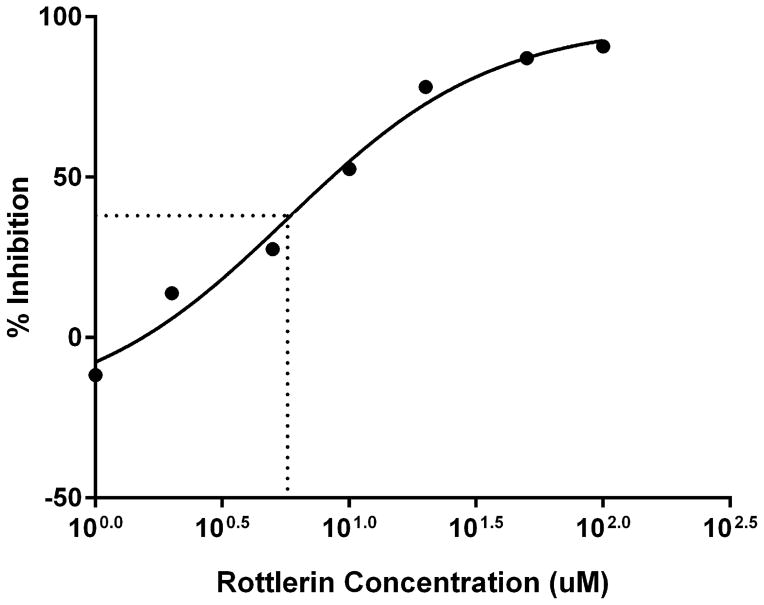
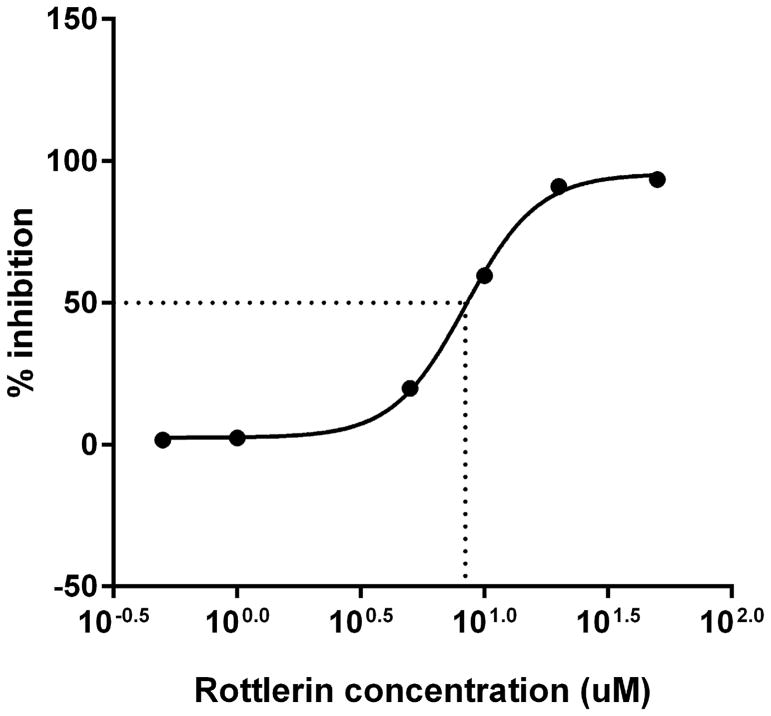
Treatment of human neutrophils with the pan-PKC inhibitor staurosporine inhibited both MARCKS phosphorylation and human neutrophil migration (Figure 3), verifying PKC-mediated MARCKS phosphorylation is an essential event for neutrophil chemotaxis. Neutrophils express several different PKC isotypes including the conventional α- and β-PKC, the novel δ-PKC, and the atypical ζ-PKC [24]. In the next series of experiments we utilized specific PKC isoform inhibitors to identify the PKC isoform(s) responsible for phosphorylating MARCKS in human neutrophils. Using Western blot analysis, we observed that treatment of human neutrophils with the δ-PKC inhibitor rottlerin significantly attenuated fMLF induced MARCKS phosphorylation at concentrations of 10 uM and greater (Figures 4a and 4B). Based on these results, we determined the rottlerin IC50 for fMLF-induced MARCKS phosphorylation of human neutrophils is 5.709 μM (Figure 4C). Thus, δ-PKC appears to be required for fMLF-induced MARCKS phosphorylation in human neutrophils.
Figure 4. δ-PKC regulates MARCKS phosphorylation, migration and adhesion in human neutrophils.
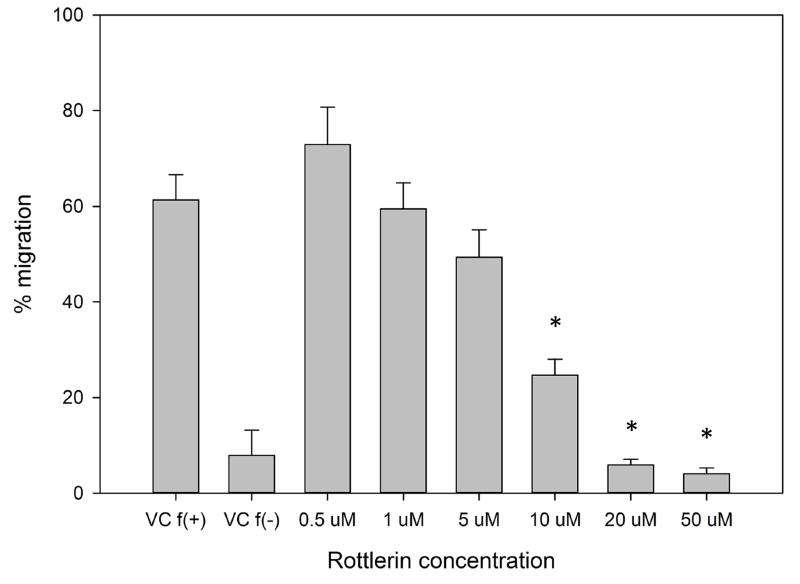
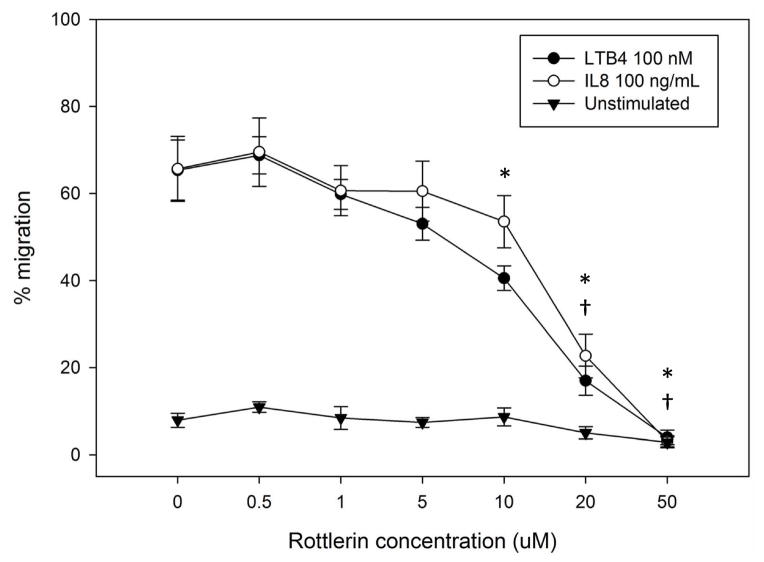
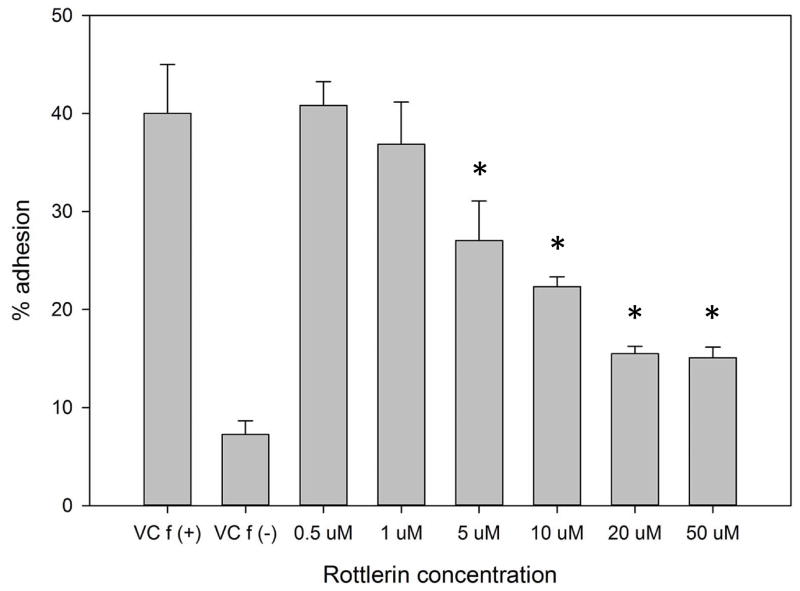
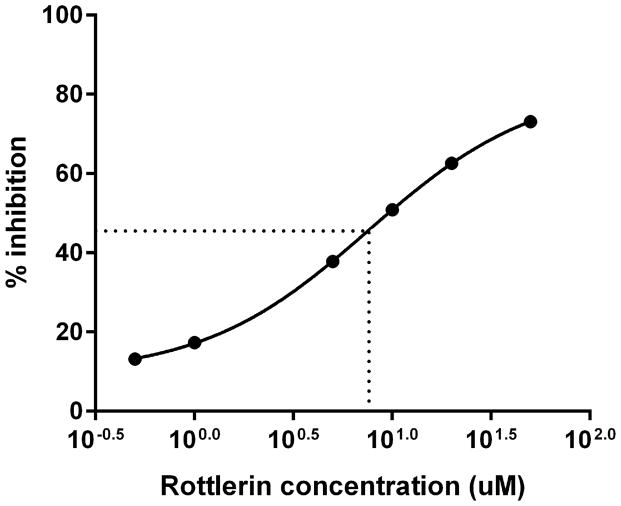
(A–C) Isolated human neutrophils were pretreated at 37°C for 30 minutes with indicated concentrations of the δ-PKC inhibitor rottlerin or VC (Me2SO) and then stimulated with 100 nM fMLF. (A) Representative dose response experiment (30 second fMLF stimulation). Western blot analysis of equal protein amounts from prepared lysates was performed to detect phosphorylated MARCKS (Phospho MARCKS) and total MARCKS protein (MARCKS). (B) Densitometry of phospho MARCKS and total MARCKS bands was determined and “% MARCKS phosphorylation” was calculated by dividing the phospho MARCKS band by the total MARCKS band. Data are presented as the mean ± SE. (*) indicates significant (p<0.05) difference in % MARCKS phosphorylation compared to VC f(+). (C) IC50 value (5.709 μM) was calculated by plotting percent inhibition of MARCKS phosphorylation against log10 rottlerin concentration (uM). (D–F) Chemotaxis of calcein-labeled human neutrophils pretreated with indicated concentrations of rottlerin was measured using an 8 micron ChemoTx plate (Neuro Probe Inc.) (D) % neutrophil migration toward 100 nM fMLF or VC (Me2SO) was calculated by dividing fluorescence of migrated cells by fluorescence of total cells. Data are presented as the mean ± SE. (*) indicates significant (p<0.05) difference in % migration compared to VC f(+). (E) IC50 value (8.385 μM) was calculated by plotting percent inhibition of neutrophil migration against log10 rottlerin concentration (uM). (F) % neutrophil migration toward 100 nM LTB4, 100 ng/mL IL-8 or VC (EtOH)) was calculated by dividing fluorescence of migrated cells by fluorescence of total cells. Data are presented as the mean ± SE. (*) indicates significant (p<0.05) difference in % migration compared to VC (“0 uM” rottlerin). (G–H) Adhesion of calcein-labeled human neutrophils pretreated with indicated concentrations of rottlerin using an Immulon 2HB plate coated with 5% FCS. (G) Neutrophil adhesion was stimulated with 100 nM fMLF for 3 minutes at 37°C. Fluorescence of wells after 2 washes was divided by the initial fluorescence to calculate % adhesion. Data are presented as the mean ± SE. (*) indicates significant (p<0.05) difference in % adhesion compared to VC f(+). (H) IC50 value (7.624 μM) was calculated by plotting percent inhibition of neutrophil adhesion against log10 rottlerin concentration (uM). (A–H) All data are representative of at least three separate experiments using different neutrophil donors.
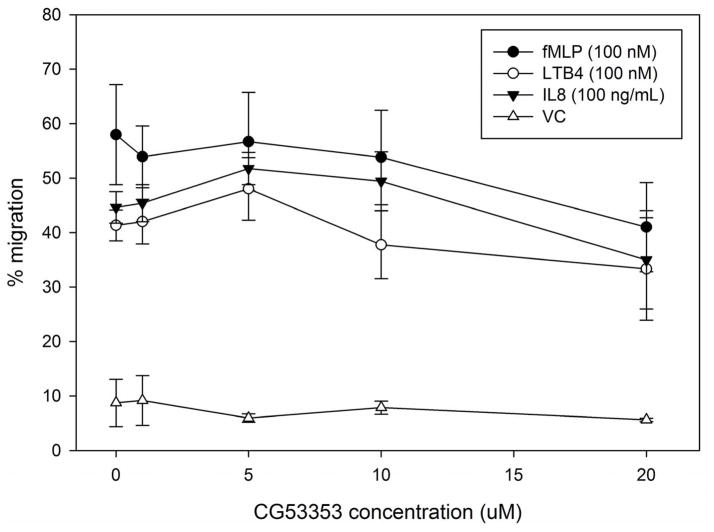
Next, we investigated the effect of δ-PKC inhibition on neutrophil migration. As with fMLF-induced MARCKS phosphorylation, rottlerin concentrations of 10 uM and greater significantly inhibited both fMLF- and LTB4-induced neutrophil chemotaxis (Figure 4D and 4F). Interestingly, significant inhibition of IL-8 mediated neutrophil chemotaxis was only achieved with rottlerin concentrations of 20 uM and greater, suggesting IL-8 mediated chemotaxis may be less dependent on δ-PKC and/or MARCKS (Figure 4F). The rottlerin IC50 for fMLF-induced migration was found to be 8.385 uM (Figure 4E).
In order to emigrate from the vasculature into tissues, neutrophils must adhere to the endothelium [28]. We next sought to determine whether δ-PKC mediated MARCKS phosphorylation was required for neutrophil adhesion. Consistent with our findings on MARCKS phosphorylation and migration, rottlerin concentrations of 10 uM and greater significantly inhibited fMLF-induced adhesion of human neutrophils (Figure 4G) with an IC50 of 7.624 uM (Figure 4H). We conclude from this series of experiments that δ-PKC mediated MARCKS phosphorylation is required for human neutrophil migration and adhesion.
PKC isoforms alpha, beta, and zeta do not regulate MARCKS phosphorylation in human neutrophils
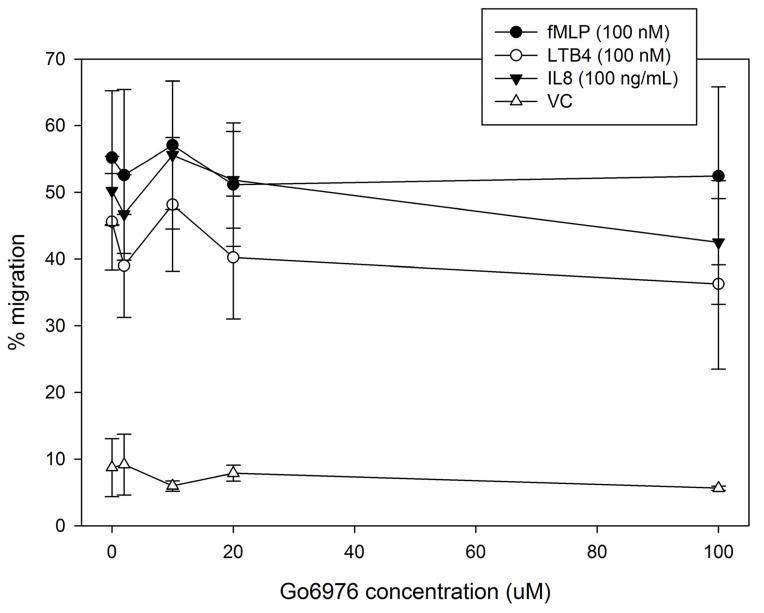
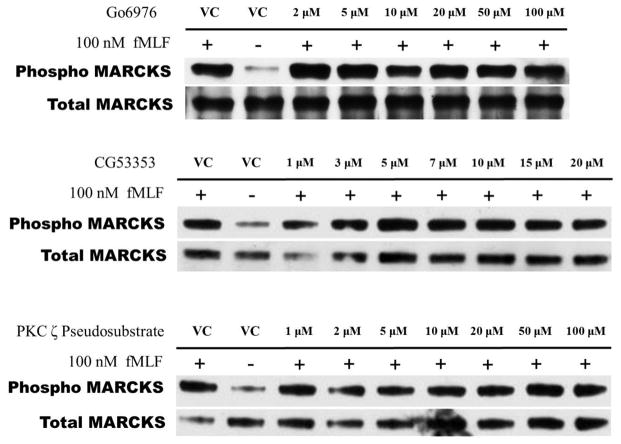
As neutrophils express several isotypes of PKC, we next evaluated whether inhibition of α-, β- or ζ-PKC would affect fMLF-induced MARCKS phosphorylation in human neutrophils. Based on Western blot analysis of lysates from fMLF-stimulated human neutrophils, the specific PKC isotype inhibitors Gö6976, CG53353 and PKC ζ pseudosubstrate had no effect on MARCKS phosphorylation. Indicating that δ-PKC is the only PKC-isotype responsible for fMLF-induced MARCKS phosphorylation in human neutrophils.
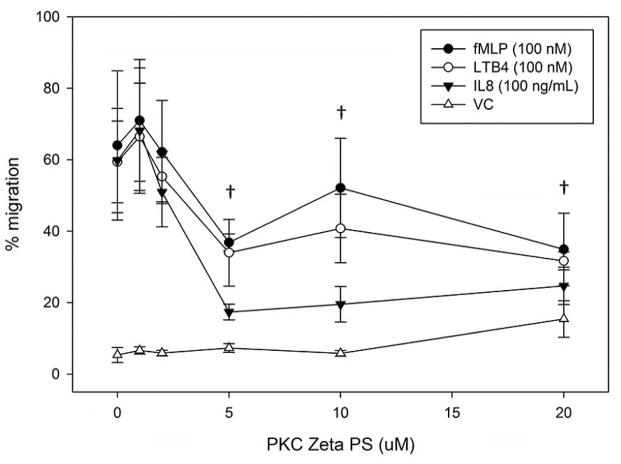
ζ-PKC plays a role in human neutrophil migration that is downstream or independent of MARCKS phosphorylation
Utilizing the same inhibitors as our phosphorylation study, we next investigated the requirement for α-, β- and ζ-PKC isotypes in LTB4, IL-8 and fMLF mediated human neutrophil migration. Inhibitors of α- and β-PKC (Gö6976 and CG53353, respectively) had no effect on LTB4, IL-8 or fMLF mediated neutrophil chemotaxis (Figure 6A and 6B); but the ζ-PKC pseudosubstrate inhibitor did significantly inhibit IL-8 induced neutrophil migration at concentrations of 5 uM and greater (Figure 6C). These findings are consistent with previous reports implicating the atypical ζ-PKC, and not classical PKC isoforms, in the regulation of neutrophil migration and adhesion [27, 29, 30]. Based on our findings, it appears that ζ-PKC plays a role in IL-8 mediated neutrophil migration that is either independent or downstream of δ-PKC mediated MARCKS phosphorylation.
Figure 6. ζ-PKC plays a role in IL-8 mediated chemotaxis of human neutrophils.
(A–C) Chemotaxis of calcein-labeled human neutrophils pretreated with indicated concentrations of (A) α-, (B) β- and (C) ζ-PKC inhibitors or vehicle control (Me2SO) was measured using an 8 micron ChemoTx plate (Neuro Probe Inc.) % neutrophil migration toward 100 nM fMLF, 100 nM LTB4, 100 ng/mL IL-8 or VC was calculated by dividing fluorescence of migrated cells by fluorescence of total cells and is presented as the mean ± SE. † indicates significant (p<0.05) difference in % migration of IL-8 vs. VC (“0 uM” staurosporine). Data are representative of at least three separate experiments using different neutrophil donors.
Discussion
The goal of the current study was to determine the PKC isoform(s) responsible for MARCKS phosphorylation in human neutrophils, and to evaluate the requirement for MARCKS phosphorylation in neutrophil chemotaxis in vitro
Using a combination of biochemical analysis and in vitro migration and adhesion assays, we investigated four PKC isotypes known to be expressed in human neutrophils: alpha (α), beta (β), delta (δ) and zeta (ζ). We conclude that δ-PKC is the isotype responsible for MARCKS phosphorylation in fMLF-stimulated human neutrophils and that MARCKS phosphorylation is essential for in vitro neutrophil chemotaxis. These conclusions are supported by the following experimental results: 1. δ-PKC, but not other PKC isotypes, redistributed from the cytosol to the membrane following fMLF stimulation of isolated human neutrophils (Figure 1). Translocation is consistent with PKC activation. 2. Pretreatment of human neutrophils with the δ-PKC inhibitor rottlerin, but not other PKC isotype inhibitors (Figure 5), prevented fMLF-induced MARCKS phosphorylation in a concentration - dependent manner (Figure 4A). 3. The range of rottlerin concentrations that blocked MARCKS phosphorylation in fMLF stimulated neutrophils also significantly attenuated fMLF-induced migration (Figure 4D) and adhesion (Figure 4G). Taken together, these findings provide strong evidence that δ-PKC is essential for neutrophil migration and adhesion. Importantly, this work also sheds new light on how δ-PKC might be regulating these important neutrophil functions – through the phosphorylation of a key substrate, MARCKS. Additional studies are needed to determine whether δ-PKC mediated MARCKS phosphorylation is relevant to neutrophil functions in vivo.
Figure 5. PKC isoforms alpha, beta, and zeta do not regulate MARCKS phosphorylation in human neutrophils.
(A–C) Isolated human neutrophils were pretreated at 37°C with indicated concentrations of (A) α-, (B) β- and (C) ζ-PKC inhibitors or vehicle control (Me2SO) and then stimulated with 100 nM fMLF. Western blot analysis of equal protein amounts from prepared lysates was performed to detect phosphorylated MARCKS (Phospho MARCKS) and total MARCKS protein (MARCKS). Data are representative of at least three separate experiments using different neutrophil donors.
The protein kinase C (PKC) family encompasses 10–15 isoforms (depending on species) that are activated by phospholipid second-messengers downstream from a variety of surface receptor interactions. PKC activation is associated with translocation of the enzyme from the cytosol to various membrane compartments [25], depending on cell type. Following activation, PKCs phosphorylate serine and threonine residues on a large number of proteins [31]. The large family of PKC enzymes is divided into three subfamilies: conventional PKCs (cPKC), novel PKCs (nPKC) and atypical PKCs (aPKC). The first two subfamilies, cPKC and nPKC, have high sequence homology and are similarly activated by phorbol esters, as well as the lipid-derived second messenger diacylglycerol (DAG). In addition to DAG, cPKCs also require calcium for their activation [5]. Atypical PKC isoforms share less sequence homology and do not respond to DAG, calcium or phorbol esters; but are instead activated by other membrane lipid components such as phosphatidic acid (PA) or phosphatidylinositol trisphosphates (PIP3) [32, 33].
PKC isoforms expressed in human neutrophils include two conventional isoforms (α- and β-PKC), one novel isoform (δ-PKC) and one atypical isoform (ζ-PKC) [5, 34, 35]. Several different studies have linked one or more of these PKC isoforms to a wide array of cellular processes including migration, apoptosis, adhesion and respiratory burst [9, 36–41]. To date, the most convincing evidence supports a role for novel (δ-PKC) and atypical (ζ-PKC) PKC isoforms in neutrophil functions including migration, adhesion and respiratory burst. In 1996, Kent et al. demonstrated that δ-PKC is activated in fMLF stimulated human neutrophils [24]. This is in agreement with our results, which showed δ-PKC translocation from the cytosol to the membrane following stimulation of human neutrophils with 100 nM fMLF (Figure 1). The study by Kent et al. also showed translocation of β-PKC from cytosolic to membrane fractions following fMLF neutrophil stimulation [24]. This finding is in contrast to the current study results (Figure 1). However, it is possible that this discrepancy could be explained by differences in buffer conditions. The HBSS++ used to suspend cells in this study contains 1 mM calcium. Added calcium in the extracellular environment can lead to increased intracellular calcium [42], and as a member of the conventional PKC subfamily, β-PKC is sensitive to calcium activation [43]. We therefore suggest that added calcium in the cell suspension media may have resulted in early β-PKC activation and subsequent accumulation within the membrane fraction, even prior to fMLF stimulation. We also saw differences in our ζ-PKC translocation results compared to a previous report by Laudanna et al. [27]. Based on our subcellular fractionation and Western blot analysis of isolated human neutrophils, ζ-PKC was primarily located in the cytosol and showed no evidence of subcellular redistribution with either fMLF or PMA stimulation. In contrast, Laudanna et al. report that ζ-PKC did translocate from the particulate fraction in resting neutrophils to the light membrane fraction in fMLF or PMA stimulated neutrophils [27]. Because we used a different method of subcellular fractionation, it is possible that there was ζ-PKC translocation that we did not detect.
Further evidence for the involvement of δ-PKC in neutrophil inflammatory functions comes from studies that utilize δ-PKC gene deficient mice. Neutrophils from these mice show markedly reduced adhesion in vitro following fMLF or IL-8 stimulation, compared to wild type neutrophils [44]. In addition, neutrophil adhesion, migration, superoxide generation and degranulation are reduced in δ-PKC null mice in vivo [44]. Additional in vivo studies have utilized a δ-PKC specific inhibitor peptide (TAT-PKCδ) to examine the role of this PKC isotype in neutrophil mediated lung injury. Intra-tracheal instillation of this dominant negative peptide significantly attenuates neutrophil infiltration, chemokine expression and pulmonary edema following cecal-ligation and puncture induced sepsis in rats [11]. From these studies it is clear that δ-PKC is activated during sepsis-induced pulmonary inflammation [11], and that δ-PKC plays an important role in neutrophil recruitment to the inflamed lung [10], but the molecular mechanisms of this regulation remain uncertain.
Like δ-PKC, previous study results have also implicated MARCKS as an important regulator of various neutrophil functions. In 2006, Takashi et al. demonstrated that inhibition of MARCKS, using a MARCKS specific inhibitor peptide known as MANS, caused a decrease in PMA-mediated neutrophil degranulation in vitro [13]. MANS mediated MARCKS inhibition also abrogated ozone-[45] and elastase [46] induced airway neutrophilia in mice in vivo. Subsequent studies by our laboratory utilized the MANS peptide to show that MARCKS function is essential for in vitro neutrophil functions including migration, adhesion and respiratory burst [12]. Most recently, work by Nordone et al. [47] showed that MANS treatment of LPS-stimulated canine neutrophils in vitro significantly attenuates production of inflammatory cytokines including IL-8 and TNFα. Interestingly, the MANS peptide, which is thought to act through inhibition of the MARCKS amino-terminus, has no known effect on MARCKS phosphorylation. But based on studies in several different cell types, MARCKS phosphorylation is a key step in regulating a variety of cellular events including spreading [26, 48, 49], adhesion [50, 51], motility [52–54], neurosecretion [55], membrane trafficking [41, 56–58] and tumorigenesis [59, 60]. Also, the PKC isotype responsible for MARCKS phosphorylation varies depending on cell type [41, 61–66]. In this study we hypothesized that MARCKS phosphorylation in human neutrophils would be regulated by a specific PKC isotype. We further hypothesized that MARCKS phosphorylation would be required for neutrophil functions (i.e. migration and adhesion) in vitro. To our knowledge, this is the first study to concurrently investigate PKC activation and MARCKS phosphorylation in human neutrophil functions in vitro.
Using rottlerin to inhibit δ-PKC activation, we observed concentration-dependent inhibition of MARCKS phosphorylation (Figure 4A), migration (Figure 4D) and adhesion (Figure 4G) in fMLF stimulated human neutrophils with markedly similar IC50 concentrations of 5.074 uM (Figure 4C), 8.385 uM (Figure 4E) and 7.624 uM (Figure 4H), respectively. Inhibitors of α-, β- and ζ-PKC had no effect on MARCKS phosphorylation (Figure 5A–C). Furthermore, we demonstrate that LTB4 and IL-8 induced migration are also dependent on δ-PKC mediated MARCKS phosphorylation (Figure 4F). It is worth noting, however, that significant inhibition of IL-8 induced migration requires a higher rottlerin concentration (i.e. 20 uM) than inhibition of migration induced by fMLF or LTB4 (i.e. 10 uM). This difference in inhibitory effect may reflect variability in the importance of certain PKC isotypes depending on the signal of activation. One perceived drawback of inhibitor studies, like the one reported here, is the potential for “off-target” effects of inhibitors. In the current report we utilized rottlerin as an inhibitor of δ-PKC. Previous reports identify rottlerin as a specific δ-PKC inhibitor with an IC50 of 3.0 to 6.0 uM. Rottlerin also reportedly inhibits conventional (IC50 for α- and β-PKC = 30–42 μM), as well as atypical (IC50 for ζ-PKC = 80–100 μM) PKC isoforms [67, 68]. Based on these reports, rottlerin mediated δ-PKC inhibition is 5 to 10 times more potent than conventional PKC inhibition and almost 30 times more potent than atypical PKC inhibition. But critics of rottlerin claim that this inhibitor causes non-specific δ-PKC inhibition due to mitochondrial uncoupling and reduced levels of cellular ATP [69]. However, a recent study using dermal fibroblasts shows that rottlerin-induced up-regulation of mitochondrial uncoupling agents is limited. Moreover, this study showed agreement in specificity among three methods of δ-PKC inhibition (i.e.δ-PKC siRNA knock-down, δ-PKC inhibitory peptide and rottlerin treatment) based on RNA and microarray analysis [70]. While we feel there is ample evidence to support the use of rottlerin as a specific δ-PKC inhibitor [71–73], we plan to further investigate MARCKS phosphorylation in neutrophils with more therapeutically relevant methods of δ-PKC inhibition in vivo.
Another key finding of the current study was the observation that ζ-PKC inhibition had no effect on MARCKS phosphorylation in human neutrophils (Figure 5C), but did significantly inhibit IL-8 mediated chemotaxis (Figure 6C). We also observed a downward trend of fMLF and LTB4 induced migration with ζ-PKC inhibition, but this was not significant. Our findings are in agreement with previous reports that ζ-PKC is a key regulator of motility in hematopoietic cells [29]. Several studies have shown that treatment of isolated human neutrophils with ζ-PKC pseudosubstrate inhibitor peptide significantly attenuates IL-8 induced adhesion [27]. Specifically, results from Chakrabarti et al. point to a roll for ζ-PKC in the regulation of the β2-integrin CD11b (also known as MAC-1) affinity and avidity [74]. We take great interest in the finding that ζ-PKC attenuates IL-8 mediated neutrophil chemotaxis in vitro with no apparent effect on MARCKS phosphorylation and plan to perform follow up investigations.
As presented here, our study results show that δ-PKC activation is essential for MARCKS phosphorylation in human neutrophils, and that inhibition of MARCKS phosphorylation correlates with a decrease in neutrophil migration and adhesion. Our results also show that ζ-PKC, which had no observed effect on MARCKS phosphorylation, plays a key role in neutrophil migration, at least toward the intermediate chemoattractant IL-8. Based on the known physiology of the signaling components involved, we propose the following narrative to explain our observations. In neutrophils, δ-PKC (a novel PKC isotype) is activated downstream of GPCR signaling by the secondary messenger DAG [75]. Activation of δ-PKC involves translocation to the plasma membrane [24], where the substrate MARCKS is localized in resting cells [16]. δ-PKC phosphorylation of MARCKS ED disrupts the electrostatic interaction with the plasma membrane and displaces MARCKS to the cytosol [26, 76, 77]. This PKC-mediated regulation of MARCKS has at least two significant effects on the cell. 1. MARCKS phosphorylation decreases actin cross-linking and allows for dynamic actin reorganization [17, 18, 22, 26, 49, 51, 58, 78–80]. 2. MARCKS phosphorylation displaces it from the membrane and effectively liberates pools of phosphoinositides (i.e. PIP2 and PIP3) to be involved in additional cell signaling [51, 52, 66, 79, 81–83]. We propose that an increase in unsequestered PIP3, as occurs with MARCKS phosphorylation, may facilitate the activation of ζ-PKC [84], potentially placing the role of this atypical PKC isotype downstream of δ-PKC mediated MARCKS phosphorylation.
Conclusions
We conclude that novel isotype δ-PKC is responsible for MARCKS phosphorylation in human neutrophils following fMLF stimulation and that MARCKS phosphorylation is essential for neutrophil migration and adhesion. Additionally, we find that ζ-PKC, which is an essential signaling component in IL-8 induced neutrophil migration, may be activated downstream of δ-PKC mediated MARCKS phosphorylation. Ultimately, our goal is to better understand the complex molecular interactions regulating neutrophil migration and adhesion in an effort to identify potential targets for new anti-inflammatory therapies. We submit that the current report contributes valuable information regarding two key components of neutrophil inflammatory functions, δ-PKC and MARCKS. To our knowledge, this study is the first report suggesting these two proteins may be integrally linked in the regulation of neutrophil migration. We plan to validate the importance of these in vitro findings with additional studies in vivo.
Acknowledgments
We would like to thank all of our volunteer blood donors for making this work possible.
Footnotes
Competing Interests
KBA holds 150,000 founders shares of a start-up biotech company, BioMarck, and serves as a scientific consultant and member of the scientific advisory board without monetary compensation. KBA receives over $100,000 yearly in research grants from National Institutes of Health (NIH) and U.S. Environmental Protection Agency. KBA is Editor-in-Chief of the American Journal of Respiratory Cell and Molecular Biology and receives a stipend of <$100,000 per year from the American Thoracic Society for this. The remaining authors have declared that no competing interests exist.
Authors’ contributions
MKS carried out the neutrophil chemotaxis and adhesion studies, performed statistical analysis of these studies and calculated relevant IC50 values. EJS carries out the biochemical studies, performed statistical analysis of these studies, and calculated relevant IC50 values. Together, MKS and EJS drafted the manuscript. KBA participated in the design and helped to draft the manuscript. SLJ conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Mary K. Sheats, Email: mkpeed@ncsu.edu.
Eui Jae Sung, Email: euijae.sung@nih.gov.
Kenneth B. Adler, Email: kbadler@ncsu.edu.
Samuel L. Jones, Email: sam_jones@ncsu.edu.
References
- 1.Malech HL, Gallin JI. Current concepts: Immunology. neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–94. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 2.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil’s role in tissue injury. J Leukoc Biol. 2011 Mar;89(3):359–72. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signaling in inflammation. Nat Rev Drug Discov. 2009 Jun;8(6):480–99. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009 Apr;21(2):317–24. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Bertram A, Ley K. Protein kinase C isoforms in neutrophil adhesion and activation. Arch Immunol Ther Exp (Warsz) 2011 Apr;59(2):79–87. doi: 10.1007/s00005-011-0112-7. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick LE, Lee JY, Haines KM, Campbell DE, Sullivan KE, Korchak HM. A role for PKC-delta and PI 3-kinase in TNF-alpha-mediated antiapoptotic signaling in the human neutrophil. Am J Physiol Cell Physiol. 2002 Jul;283(1):C48–57. doi: 10.1152/ajpcell.00385.2001. [DOI] [PubMed] [Google Scholar]
- 7.Ramnath RD, Sun J, Bhatia M. PKC delta mediates pro-inflammatory responses in a mouse model of caerulein-induced acute pancreatitis. J Mol Med (Berl) 2010 Oct;88(10):1055–63. doi: 10.1007/s00109-010-0647-9. [DOI] [PubMed] [Google Scholar]
- 8.Page K, Li J, Zhou L, Lasvovskaia S, Corbit KC, Soh JW, et al. Regulation of airway epithelial cell NF-kappa B-dependent gene expression by protein kinase C delta. J Immunol. 2003 Jun 1;170(11):5681–9. doi: 10.4049/jimmunol.170.11.5681. [DOI] [PubMed] [Google Scholar]
- 9.Kilpatrick LE, Sun S, Li H, Vary TC, Korchak HM. Regulation of TNF-induced oxygen radical production in human neutrophils: role of delta-PKC. J Leukoc Biol. 2010 Jan;87(1):153–64. doi: 10.1189/jlb.0408230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondrinos MJ, Zhang T, Sun S, Kennedy PA, King DJ, Wolfson MR, et al. Pulmonary endothelial protein kinase C-delta (PKC delta) regulates neutrophil migration in acute lung inflammation. Am J Pathol. 2014 Jan;184(1):200–13. doi: 10.1016/j.ajpath.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick LE, Standage SW, Li H, Raj NR, Korchak HM, Wolfson MR, et al. Protection against sepsis-induced lung injury by selective inhibition of protein kinase C-delta (delta-PKC) J Leukoc Biol. 2011 Jan;89(1):3–10. doi: 10.1189/jlb.0510281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert RE, Neuder LE, Park J, Adler KB, Jones SL. Myristoylated alanine-rich C-kinase substrate (MARCKS) protein regulation of human neutrophil migration. Am J Respir Cell Mol Biol. 2010 Jul;42(5):586–594. doi: 10.1165/rcmb.2008-0394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takashi S, Park J, Fang S, Koyama S, Parikh I, Adler KB. A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate inhibits degranulation of human leukocytes in vitro. Am J Respir Cell Mol Biol. 2006 Jun;34(6):647–52. doi: 10.1165/rcmb.2006-0030RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheats MK, Pescosolido KC, Hefner EM, Sung EJ, Adler KB, Jones SL. Myristoylated alanine rich C kinase substrate (MARCKS) is essential to beta2-integrin dependent responses of equine neutrophils. Vet Immunol Immunopathol. 2014 Aug 15;160(3–4):167–176. doi: 10.1016/j.vetimm.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thelen M, Rosen A, Nairn AC, Aderem A. Tumor necrosis factor alpha modifies agonist- dependent responses in human neutrophils by inducing the synthesis and myristoylation of a specific protein kinase C substrate. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5603–7. doi: 10.1073/pnas.87.15.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–2. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- 17.Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002 Feb 15;362(Pt 1):1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nairn AC, Aderem A. Calmodulin and protein kinase C cross-talk: The MARCKS protein is an actin filament and plasma membrane cross-linking protein regulated by protein kinase C phosphorylation and by calmodulin. Ciba Found Symp. 1992;164:145,54. doi: 10.1002/9780470514207.ch10. discussion 154–61. [DOI] [PubMed] [Google Scholar]
- 19.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–22. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 20.Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993 Jan 25;268(3):1501–4. [PubMed] [Google Scholar]
- 21.Aderem A. The MARCKS brothers: A family of protein kinase C substrates. Cell. 1992 Nov 27;71(5):713–6. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- 22.Aderem A. Signal transduction and the actin cytoskeleton: The roles of MARCKS and profilin. Trends Biochem Sci. 1992 Oct;17(10):438–43. doi: 10.1016/0968-0004(92)90016-3. [DOI] [PubMed] [Google Scholar]
- 23.Futosi K, Fodor S, Mocsai A. Reprint of neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013 Dec;17(4):1185–97. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Kent JD, Sergeant S, Burns DJ, McPhail LC. Identification and regulation of protein kinase C-delta in human neutrophils. J Immunol. 1996 Nov 15;157(10):4641–7. [PubMed] [Google Scholar]
- 25.Mochly-Rosen D, Henrich CJ, Cheever L, Khaner H, Simpson PC. A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regul. 1990 Aug;1(9):693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Disatnik MH, Boutet SC, Pacio W, Chan AY, Ross LB, Lee CH, et al. The bi-directional translocation of MARCKS between membrane and cytosol regulates integrin-mediated muscle cell spreading. J Cell Sci. 2004 Sep 1;117(Pt 19):4469–79. doi: 10.1242/jcs.01309. [DOI] [PubMed] [Google Scholar]
- 27.Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998 Nov 13;273(46):30306–15. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2013 Aug;55(1):49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Xiao H, Liu M. Atypical protein kinase C in cell motility. Cell Mol Life Sci. 2013 Sep;70(17):3057–66. doi: 10.1007/s00018-012-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirai T, Chida K. Protein kinase c zeta (PKC zeta): Activation mechanisms and cellular functions. J Biochem. 2003 Jan;133(1):1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008 Oct;88(4):1341–78. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–32. [PubMed] [Google Scholar]
- 33.Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, et al. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998 Sep 24;8(19):1069–77. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 34.Balasubramanian N, Advani SH, Zingde SM. Protein kinase C isoforms in normal and chronic myeloid leukemic neutrophils. distinct signal for PKC alpha by immunodetection on PVDF membrane, decreased expression of PKC alpha and increased expression of PKC delta in leukemic neutrophils. Leuk Res. 1998 Jul;22(7):597–604. doi: 10.1016/s0145-2126(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 35.Smallwood JI, Malawista SE. Protein kinase C isoforms in human neutrophil cytoplasts. J Leukoc Biol. 1992 Jan;51(1):84–92. doi: 10.1002/jlb.51.1.84. [DOI] [PubMed] [Google Scholar]
- 36.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010 Apr 30;106(8):1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpenter AC, Alexander JS. Endothelial PKC delta activation attenuates neutrophil transendothelial migration. Inflamm Res. 2008 May;57(5):216–29. doi: 10.1007/s00011-007-7031-4. [DOI] [PubMed] [Google Scholar]
- 38.Fogh BS, Multhaupt HA, Couchman JR. Protein kinase C, focal adhesions and the regulation of cell migration. J Histochem Cytochem. 2014 Mar;62(3):172–84. doi: 10.1369/0022155413517701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray RD, Lucas CD, Mackellar A, Li F, Hiersemenzel K, Haslett C, et al. Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J Inflamm (Lond) 2013 Mar 21;10(1):12,9255-10-12. doi: 10.1186/1476-9255-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006 Mar;18(3):276–84. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Park JA, Crews AL, Lampe WR, Fang S, Park J, Adler KB. Protein kinase C delta regulates airway mucin secretion via phosphorylation of MARCKS protein. Am J Pathol. 2007 Dec;171(6):1822–30. doi: 10.2353/ajpath.2007.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohkura K, Hori H, Terada H. Effect of extracellular calcium on the intracellular calcium level of newborn rat skin basal cells. Biol Pharm Bull. 1998 Jan;21(1):1–4. doi: 10.1248/bpb.21.1. [DOI] [PubMed] [Google Scholar]
- 43.Majumdar S, Rossi MW, Fujiki T, Phillips WA, Disa S, Queen CF, et al. Protein kinase C isotypes and signaling in neutrophils. differential substrate specificities of a translocatable calcium- and phospholipid-dependent beta-protein kinase C and a phospholipid-dependent protein kinase which is inhibited by long chain fatty acyl coenzyme A. J Biol Chem. 1991 May 15;266(14):9285–94. [PubMed] [Google Scholar]
- 44.Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, et al. Neutrophil protein kinase cdelta as a mediator of stroke-reperfusion injury. J Clin Invest. 2004 Jul;114(1):49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damera G, Jester WF, Jiang M, Zhao H, Fogle HW, Mittelman M, et al. Inhibition of myristoylated alanine-rich C kinase substrate (MARCKS) protein inhibits ozone-induced airway neutrophilia and inflammation. Exp Lung Res. 2010 Mar;36(2):75–84. doi: 10.3109/01902140903131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster WM, Adler KB, Crews AL, Potts EN, Fischer BM, Voynow JA. MARCKS-related peptide modulates in vivo the secretion of airway Muc5ac. Am J Physiol Lung Cell Mol Physiol. 2010 Sep;299(3):L345–52. doi: 10.1152/ajplung.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Li J, D’Annibale-Tolhurst MA, Adler KB, Fang S, Yin Q, Birkenheuer AJ, et al. A myristoylated alanine-rich C kinase substrate-related peptide suppresses cytokine mRNA and protein expression in LPS-activated canine neutrophils. Am J Respir Cell Mol Biol. 2013 Mar;48(3):314–21. doi: 10.1165/rcmb.2012-0278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue L, Lu S, Garces J, Jin T, Li J. Protein kinase C-regulated dynamitin-macrophage-enriched myristoylated alanine-rice C kinase substrate interaction is involved in macrophage cell spreading. J Biol Chem. 2000 Aug 4;275(31):23948–56. doi: 10.1074/jbc.M001845200. [DOI] [PubMed] [Google Scholar]
- 49.Myat MM, Anderson S, Allen LA, Aderem A. MARCKS regulates membrane ruffling and cell spreading. Curr Biol. 1997 Aug 1;7(8):611–4. doi: 10.1016/s0960-9822(06)00262-4. [DOI] [PubMed] [Google Scholar]
- 50.Estrada-Bernal A, Gatlin JC, Sunpaweravong S, Pfenninger KH. Dynamic adhesions and MARCKS in melanoma cells. J Cell Sci. 2009 Jul 1;122(Pt 13):2300–10. doi: 10.1242/jcs.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi H, Shiraishi M, Fukami K, Tanabe A, Ikeda-Matsuo Y, Naito Y, et al. MARCKS regulates lamellipodia formation induced by IGF-I via association with PIP2 and beta-actin at membrane microdomains. J Cell Physiol. 2009 Sep;220(3):748–55. doi: 10.1002/jcp.21822. [DOI] [PubMed] [Google Scholar]
- 52.Kalwa H, Michel T. The MARCKS protein plays a critical role in phosphatidylinositol 4,5- bisphosphate metabolism and directed cell movement in vascular endothelial cells. J Biol Chem. 2011 Jan 21;286(3):2320–30. doi: 10.1074/jbc.M110.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosen A, Keenan KF, Thelen M, Nairn AC, Aderem A. Activation of protein kinase C results in the displacement of its myristoylated, alanine-rich substrate from punctate structures in macrophage filopodia. J Exp Med. 1990 Oct 1;172(4):1211–5. doi: 10.1084/jem.172.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S, Jang E, Kim JH, Kim JH, Lee WH, Suk K. Lipocalin-type prostaglandin D2 synthase protein regulates glial cell migration and morphology through myristoylated alanine-rich C-kinase substrate: Prostaglandin D2-independent effects. J Biol Chem. 2012 Mar 16;287(12):9414–28. doi: 10.1074/jbc.M111.330662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanimukai S, Hasegawa H, Nakai M, Yagi K, Hirai M, Saito N, et al. Nanomolar amyloid beta protein activates a specific PKC isoform mediating phosphorylation of MARCKS in Neuro2A cells. Neuroreport. 2002 Mar 25;13(4):549–53. doi: 10.1097/00001756-200203250-00037. [DOI] [PubMed] [Google Scholar]
- 56.Chappell DS, Patel NA, Jiang K, Li P, Watson JE, Byers DM, et al. Functional involvement of protein kinase C-betaII and its substrate, myristoylated alanine-rich C-kinase substrate (MARCKS), in insulin-stimulated glucose transport in L6 rat skeletal muscle cells. Diabetologia. 2009 May;52(5):901–11. doi: 10.1007/s00125-009-1298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siskova Z, Baron W, de Vries H, Hoekstra D. Fibronectin impedes “myelin” sheet-directed flow in oligodendrocytes: A role for a beta 1 integrin-mediated PKC signaling pathway in vesicular trafficking. Mol Cell Neurosci. 2006 Oct;33(2):150–9. doi: 10.1016/j.mcn.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Salli U, Saito N, Stormshak F. Spatiotemporal interactions of myristoylated alanine-rich C kinase substrate (MARCKS) protein with the actin cytoskeleton and exocytosis of oxytocin upon prostaglandin F2alpha stimulation of bovine luteal cells. Biol Reprod. 2003 Dec;69(6):2053–8. doi: 10.1095/biolreprod.103.017640. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Rotenberg SA. PhosphoMARCKS drives motility of mouse melanoma cells. Cell Signal. 2010 Jul;22(7):1097–103. doi: 10.1016/j.cellsig.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finlayson AE, Freeman KW. A cell motility screen reveals role for MARCKS-related protein in adherens junction formation and tumorigenesis. PLoS One. 2009 Nov 18;4(11):e7833. doi: 10.1371/journal.pone.0007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uberall F, Giselbrecht S, Hellbert K, Fresser F, Bauer B, Gschwendt M, et al. Conventional PKC-alpha, novel PKC-epsilon and PKC-theta, but not atypical PKC-lambda are MARCKS kinases in intact NIH 3T3 fibroblasts. J Biol Chem. 1997 Feb 14;272(7):4072–8. doi: 10.1074/jbc.272.7.4072. [DOI] [PubMed] [Google Scholar]
- 62.Song JC, Hrnjez BJ, Farokhzad OC, Matthews JB. PKC-epsilon regulates basolateral endocytosis in human T84 intestinal epithelia: Role of F-actin and MARCKS. Am J Physiol. 1999 Dec;277(6 Pt 1):C1239–49. doi: 10.1152/ajpcell.1999.277.6.C1239. [DOI] [PubMed] [Google Scholar]
- 63.Kim SS, Kim JH, Kim HS, Park DE, Chung CH. Involvement of the theta-type protein kinase C in translocation of myristoylated alanine-rich C kinase substrate (MARCKS) during myogenesis of chick embryonic myoblasts. Biochem J. 2000 Apr 1;347(Pt 1):139–46. [PMC free article] [PubMed] [Google Scholar]
- 64.Dulong S, Goudenege S, Vuillier-Devillers K, Manenti S, Poussard S, Cottin P. Myristoylated alanine-rich C kinase substrate (MARCKS) is involved in myoblast fusion through its regulation by protein kinase calpha and calpain proteolytic cleavage. Biochem J. 2004 Sep 15;382(Pt 3):1015–23. doi: 10.1042/BJ20040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugiya H, Satoh K, Matsuki-Fukushima M, Qi B, Guo MY, Fujita-Yoshigaki J. Role of protein kinase C-delta in isoproterenol-induced amylase release in rat parotid acinar cells. J Med Invest. 2009;56( Suppl):368–70. doi: 10.2152/jmi.56.368. [DOI] [PubMed] [Google Scholar]
- 66.Gadi D, Wagenknecht-Wiesner A, Holowka D, Baird B. Sequestration of phosphoinositides by mutated MARCKS effector domain inhibits stimulated ca (2+) mobilization and degranulation in mast cells. Mol Biol Cell. 2011 Dec;22(24):4908–17. doi: 10.1091/mbc.E11-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, et al. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994 Feb 28;199(1):93–8. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 68.Villalba M, Kasibhatla S, Genestier L, Mahboubi A, Green DR, Altman A. Protein kinase c theta cooperates with calcineurin to induce fas ligand expression during activation-induced T cell death. J Immunol. 1999 Dec 1;163(11):5813–9. [PubMed] [Google Scholar]
- 69.Soltoff SP. Rottlerin: An inappropriate and ineffective inhibitor of PKC delta. Trends Pharmacol Sci. 2007 Sep;28(9):453–8. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Wermuth PJ, Addya S, Jimenez SA. Effect of protein kinase C delta (PKC-delta) inhibition on the transcriptome of normal and systemic sclerosis human dermal fibroblasts in vitro. PLoS One. 2011;6(11):e27110. doi: 10.1371/journal.pone.0027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hendey B, Zhu CL, Greenstein S. Fas activation opposes PMA-stimulated changes in the localization of PKC delta: A mechanism for reducing neutrophil adhesion to endothelial cells. J Leukoc Biol. 2002 May;71(5):863–70. [PubMed] [Google Scholar]
- 72.Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, et al. Inhibition of PKC delta reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest. 2011 Jul;121(7):2709–22. doi: 10.1172/JCI45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yanase N, Hayashida M, Kanetaka-Naka Y, Hoshika A, Mizuguchi J. PKC-delta mediates interferon-alpha-induced apoptosis through c-jun NH(2)-terminal kinase activation. BMC Cell Biol. 2012 Mar 21;13(7):2121–137. doi: 10.1186/1471-2121-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakrabarti S, Patel KD. The atypical zeta (zeta) isoform of protein kinase C regulates CD11b/CD18 activation in human neutrophils. Microcirculation. 2008 Aug;15(6):555–67. doi: 10.1080/10739680801949732. [DOI] [PubMed] [Google Scholar]
- 75.Dewald B, Thelen M, Baggiolini M. Two transduction sequences are necessary for neutrophil activation by receptor agonists. J Biol Chem. 1988 Nov 5;263(31):16179–84. [PubMed] [Google Scholar]
- 76.Ohmori S, Sakai N, Shirai Y, Yamamoto H, Miyamoto E, Shimizu N, et al. Importance of protein kinase C targeting for the phosphorylation of its substrate, myristoylated alanine-rich C-kinase substrate. J Biol Chem. 2000 Aug 25;275(34):26449–57. doi: 10.1074/jbc.M003588200. [DOI] [PubMed] [Google Scholar]
- 77.Yang L, Glaser M. Formation of membrane domains during the activation of protein kinase C. Biochemistry. 1996 Nov 5;35(44):13966–74. doi: 10.1021/bi9610008. [DOI] [PubMed] [Google Scholar]
- 78.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006 Mar;18(3):276–84. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 79.Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000 Jun 26;149(7):1455–72. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–22. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 81.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: Interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–75. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 82.Wang J, Arbuzova A, Hangyas-Mihalyne G, McLaughlin S. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2001 Feb 16;276(7):5012–9. doi: 10.1074/jbc.M008355200. [DOI] [PubMed] [Google Scholar]
- 83.Glaser M, Wanaski S, Buser CA, Boguslavsky V, Rashidzada W, Morris A, et al. Myristoylated alanine-rich C kinase substrate (MARCKS) produces reversible inhibition of phospholipase C by sequestering phosphatidylinositol 4,5-bisphosphate in lateral domains. J Biol Chem. 1996 Oct 18;271(42):26187–93. doi: 10.1074/jbc.271.42.26187. [DOI] [PubMed] [Google Scholar]
- 84.Nakanishi H, Brewer KA, Exton JH. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993 Jan 5;268(1):13–6. [PubMed] [Google Scholar]