Abstract
Background.
Our objective was to characterize the relationship of low and high hemoglobin concentrations and normocytic, microcytic, and macrocytic anemia with cross-sectional cognition and change in cognition over a median of 6 years.
Methods.
Cross-sectional and prospective analyses of 13,133 participants (mean age 57 years, 56% women, 24% black, 10% anemia) in the Atherosclerosis Risk in Communities (ARIC) study (baseline 1990–1992) were carried out. Anemia was defined as hemoglobin concentrations lower than 13g/dl for men and lower than 12g/dl for women and was subclassified as normocytic (mean corpuscular volume [MCV] 80–100 fL), microcytic (MCV < 80 fL), and macrocytic (MCV > 100 fL). Cognition was assessed by delayed word recall test (DWRT), digit symbol substitution test (DSST), word fluency test (WFT), and global Z-score at Visit 2 (1990–1992) and Visit 4 (1996–1998). Adjusted linear regression models and splines were used.
Results.
Cross-sectionally, anemia overall was associated with lower cognitive test scores on DSST and global Z-score among men and women (all p < .05), but not on DWRT or WFT. Anemia subtypes were associated similarly with cognition, with strongest associations for the DSST and global Z-score. Additionally, elevated hemoglobin level was associated with nonsignificantly worse cognition in cross-sectional analyses, suggesting a nonlinear association of hemoglobin with cognition. In contrast, anemia overall and anemia subtypes were not associated with cognition in prospective analyses (all p > .05).
Conclusions.
There was a cross-sectional, but not prospective, nonlinear association between hemoglobin concentrations and cognitive function, although only associations of low levels of hemoglobin (anemia) were statistically significant. Subtypes of anemia had similar magnitudes of associations with cognition.
Keywords: Cognition, Anemia, Epidemiology, Cognitive aging
The prevalence of anemia, defined as hemoglobin concentrations lower than 13g/dl for men and lower than 12g/dl for women (1), increases with age, with approximately 6% prevalence among those aged 50–64 years and 11% prevalence among those aged ≥65 years (2). Among older individuals (age ≥ 65 years), anemia has been shown to be associated with cognitive impairment and with increased risk for incident dementia in some studies (3–6), but not in others (7). It has also been suggested that elevated hemoglobin concentrations (defined as hemoglobin concentrations >17.5g/dl for men and >15.5g/dl for women) may also be associated with impaired cognition and risk of incident dementia risk among older individuals, possibly due to hyperviscosity (5,6). Little is known, however, about the association of the entire spectrum of the hemoglobin distribution with cognition and change in cognitive function over time among both white and black individuals with mean age less than 65 years. Further, most published studies on the association of hemoglobin with cognition and dementia have been smaller (≤1,435 participants) (4,8,9) and cross-sectional in design (4). Additionally, little is known about the associations of different subtypes of anemia (normocytic, microcytic, and macrocytic) with cognitive function, all of which may be similarly associated with cognition due to chronic inadequate cerebral oxygenation.
Our objective was to characterize the relationship of the spectrum of hemoglobin concentrations with cognition at baseline and with change in cognitive function over a median of 6 years. We additionally sought to investigate relationships of anemia subtypes (normocytic, microcytic, and macrocytic) with cognitive function. We hypothesized that both low hemoglobin levels (ie, those consistent with a diagnosis of anemia) and elevated hemoglobin levels would be associated with lower cross-sectional cognitive function and with a greater decline in cognitive function over the 6-year follow-up period. Further, we hypothesized that the anemia subtypes of normocytic, microcytic, and macrocytic anemia would have similar associations with cognitive function and dementia risk.
Methods
Study Design
The Atherosclerosis Risk in Communities (ARIC) study is an ongoing, community-based prospective cohort study of 15,792 individuals that was designed to investigate the etiology and natural history of atherosclerosis, the etiology of clinical atherosclerotic diseases, and variation in cardiovascular risk factors, medical care, and disease by race, gender, location, and date (10). All individuals aged 45–64 years living in the four study communities (suburbs of Minneapolis, MN; Washington County, MD; Forsyth County, NC; and Jackson, MS) were eligible for enrollment in the study at Visit 1 (1987–1989), regardless of medical comorbidities, and study participants were selected via probability sampling (10). Four subsequent in-person visits have taken place in 1990–1992 (Visit 2), 1993–1995 (Visit 3), 1996–1998 (Visit 4), and 2011–2013 (Visit 5). The ARIC study has been approved by the Institutional Review Boards of all participating institutions. All participants gave written informed consent at each study visit.
Cognitive testing was first performed at ARIC Visit 2 (1990–1992). Therefore, ARIC Visit 2 serves as the baseline for the present study. Of the 14,348 participants who attended Visit 2, we excluded 91 who self-identified as other than white or black race or self-identified as black at the Minnesota or Maryland study centers, 59 who experienced a stroke prior to baseline, 134 missing hemoglobin data, 189 missing cognitive test data, and 742 missing data on covariates included in statistical models, leaving a total of 13,133 participants included in the present study.
Hemoglobin Measurement and Hemoglobin Category Definitions
Hemoglobin was measured from blood samples obtained at ARIC Visit 2 (1990–1992) using automated hematology analyzers: Coulter S+IV (calibration S-Cal, Beckman Coulter, Fullerton, CA) in Maryland and Mississippi, Coulter S+III and Coulter S+IV (calibration S-Cal, Beckman Coulter) in Minnesota, and Technicon H-6000 (calibration Fisher, Technicon Corporation, Tarrytown, NY) in North Carolina. Anemia was defined according to World Health Organization criteria: hemoglobin concentrations lower than 13g/dl for men and lower than 12g/dl for women (1). Anemia was subclassified as normocytic (mean corpuscular volume [MCV] 80–100 fL), microcytic (MCV < 80 fL), and macrocytic (MCV > 100 fL) (1). Normal hemoglobin was defined as hemoglobin concentrations of 13–17.5g/dl for men and of 12–15.5g/dl for women (1). Elevated hemoglobin was defined as hemoglobin concentrations higher than 17.5g/dl for men and higher than 15.5g/dl for women (1).
Measures of Cognitive Function
Cognition was assessed at ARIC Visit 2 (1990–1992) and Visit 4 (1996–1998) using three standard tests: the delayed word recall test (DWRT) (11), the digit symbol substitution test (DSST) from the Wechsler Adult Intelligence Scale-Revised (WAIS-R) (12), and the word fluency test (WFT) (13,14). Identical protocols were used at Visit 2 and Visit 4 and at all study centers. Trained examiners administered the cognitive tests in a fixed order during one session in a quiet room. Examiner performance was recorded and reviewed routinely to ensure consistency. Normative data derived from a healthy subsample of ARIC study participants have been previously published (15).
The DWRT (11) is a test of verbal learning and recent memory. Participants were given 10 common nouns that they were asked to learn by using each word in a sentence. Two exposures to the words were given. After a 5-minute delay, participants were given 1 minute to recall the words. The score for the DWRT is the number of words correctly recalled (range 0–10 words), and the mean ARIC normative scores ranged from 5.9 to 7.0 words, depending on race and education level (15).
The DSST (12) is a test of executive function, processing speed, and new learning. Participants were given 90 seconds to translate numbers to symbols using a key. The score for the DSST is the total of numbers correctly translated to symbols (range 0–93 points), with mean ARIC normative scores ranging from 26.3 to 50.8, depending on race and education level (15).
The WFT (13,14) is a test of executive function and language. Participants were given 1 minute per letter to generate as many words as possible beginning with the letters F, A, and S, avoiding proper nouns. The score for the WFT is the total number of acceptable words generated for the three letters. The mean ARIC normative scores range from 22.3 to 40.6 words, depending on race and education level (15).
We generated Z-scores for each cognitive test at Visit 2 and Visit 4. All Z-scores were standardized using the Visit 2 mean and standard deviation. We averaged individual test Z-scores from Visit 2 and Visit 4 to create a global cognition Z-score for each visit, which were standardized using the Visit 2 global Z mean and standard deviation.
Covariates
All covariates used in the regression models were assessed at Visit 2 (1990–1992), unless otherwise stated. All continuous variables were centered at median values. Covariates included age (years; continuous), sex (male; female), race/center (Minnesota whites; Maryland whites; North Carolina whites; North Carolina blacks; Mississippi blacks), education (<high school; high school or equivalent; >high school; assessed at Visit 1), income (<$35,000/year; ≥$35,000/year; not reported; assessed at Visit 1), cigarette smoking (current; former; never), alcohol consumption (current; former; never), body mass index (kg/m2; continuous), total cholesterol (mg/dl; continuous), high density lipoprotein cholesterol (mg/dl; continuous), diabetes (defined as self-reported physician diagnosis, medication use, fasting glucose ≥ 126mg/dl, or nonfasting glucose ≥ 200mg/dl), hypertension (defined as medication use, systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg), estimated glomerular function rate (calculated using the Chronic Kidney Disease Epidemiology Collaboration [CKD-Epi] formula (16); mL/min per 1.73 m2; continuous), and APOE ε4 genotype (0, 1, 2 ε4 alleles).
Statistical Analysis
Baseline characteristics of the study population are presented by hemoglobin category (anemia; normal hemoglobin; elevated hemoglobin) and by sex and were compared using Student’s t-tests, chi square tests, Wilcoxon rank sums tests, and Fisher exact tests. A priori, we decided to present all results stratified by sex due to the different definitions of anemia and elevated hemoglobin by sex. Linear regression models were used to assess the cross-sectional associations of hemoglobin category and anemia subtypes with cognitive test performance at Visit 2 (1990–1992). Plots of residuals versus predicted values were used to assess linearity. We also modeled the associations of continuous hemoglobin with cross-sectional cognitive test performance using a restricted cubic spline model. Linear regression models were also used to estimate associations with change in cognitive test scores over a median of 6 years among the n = 10,283 participants with cognitive test at both Visit 2 (1990–1992) and Visit 4 (1996–1998). To assess for possible survival bias in these analyses, we compared baseline characteristics of participants included and excluded from our cognitive function change analyses using Student’s t-tests, chi square tests, Wilcoxon rank sums tests, and Fisher exact tests. We additionally repeated our cross-sectional analyses restricted to the 10,283 participants with cognitive test data at both Visit 2 and Visit 4.
Our statistical models were adjusted for demographic (age, gender, race/field center), behavioral/socioeconomic (education, income, cigarette smoking status, alcohol consumption, body mass index), and cardiovascular and genetic risk factors (total cholesterol, high density lipoprotein cholesterol, diabetes, hypertension, estimated glomerular function rate, and APOE ε4 genotype). We formally tested for interaction by age, sex, and race.
All reported p values are two sided, and p values less than .05 were considered statistically significant. All analyses were performed using Stata Version 13 (StataCorp, College Station, TX).
Results
Baseline characteristics (ARIC Visit 2, 1990–1992) of the 13,133 participants by hemoglobin category and by sex are shown in Table 1. Overall, the mean age of participants was 57 years, 56% of participants were women, and 24% were black. A total of 1,343 participants (10%) were classified as having anemia (6% of men and 14% of women). Compared with those with normal hemoglobin, those with anemia were more likely to be black (47% vs 17%, p < .001 for men and 51% vs 24%, p < .001 for women), have less than high school education (31% vs 20%, p < .001 for men and 27% vs 21%, p < .001 for women), and have low estimated glomerular function rate (<60mL/min per 1.73 m2; 9% vs 2%, p < .001 for men and 5% vs 2%, p < .001 for women). Compared with those with normal hemoglobin, those with elevated hemoglobin were more likely to be current smokers (71% vs 24%, p < .001 for men and 64% vs 22%, p < .001 for women). Mean scores for the DWRT, the DSST, and the WFT fell into the mean ARIC-derived normative range for all hemoglobin and sex categories, suggesting that on average, participants had normal cognitive functioning at baseline (15).
Table 1.
Baseline Characteristics by Sex and by Hemoglobin Category*, ARIC Visit 2 (1990–1992), N = 13,133
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal Hemoglobin(n = 5,467) | Anemia(n = 350) | p ValueNormal Versus Anemia | Elevated Hemoglobin(n = 21) | p ValueElevated Versus Anemia | Normal Hemoglobin(n = 6,246) | Anemia(n = 993) | p ValueNormal Versus Anemia | Elevated Hemoglobin(n = 56) | p ValueElevated Versus Anemia | |
| Age (years), mean (SD) | 57.3 (5.7) | 58.3 (6.0) | .002 | 58.3 (5.4) | .426 | 56.8 (5.6) | 56.2 (5.9) | .003 | 59.6 (5.4) | <.001 |
| Race/center, % | <.001 | .470 | <.001 | .008 | ||||||
| Minneapolis, Minnesota whites | 30.1 | 13.7 | 28.6 | 26.7 | 14.6 | 30.4 | ||||
| Washington County, Maryland whites | 28.1 | 16.9 | 19.1 | 27.3 | 13.1 | 39.3 | ||||
| Forsyth County, North Carolina whites | 24.5 | 22.3 | 23.8 | 22.4 | 21.1 | 26.8 | ||||
| Forsyth County, North Carolina blacks | 2.0 | 6.0 | 4.8 | 2.5 | 6.0 | 1.8 | ||||
| Jackson, Mississippi blacks | 15.3 | 41.1 | 23.8 | 21.1 | 45.2 | 1.8 | ||||
| Education, % | <.001 | .066 | <.001 | .002 | ||||||
| <High school | 20.1 | 30.6 | 28.6 | 20.7 | 26.6 | 33.9 | ||||
| High School, GED, or vocational school | 27.1 | 35.1 | 52.4 | 46.7 | 37.8 | 53.6 | ||||
| College, graduate, or professional school | 42.8 | 34.3 | 19.1 | 32.6 | 35.7 | 12.5 | ||||
| Income, % | <.001 | .154 | <.001 | .065 | ||||||
| <$35,000/year | 42.3 | 58.0 | 61.9 | 55.5 | 61.2 | 64.3 | ||||
| ≥$35,000/year | 53.1 | 35.1 | 33.3 | 38.5 | 30.1 | 25.0 | ||||
| Not reported | 4.6 | 6.9 | 4.8 | 6.0 | 8.7 | 10.7 | ||||
| Body mass index (kg/m2), mean (SD) | 27.8 (4.2) | 26.9 (4.6) | <.001 | 28.6 (4.1) | .344 | 28.2 (6.1) | 28.0 (6.2) | .414 | 29.0 (6.6) | .291 |
| Cigarette smoking status, % | .530 | <.001 | <.001 | <.001 | ||||||
| Current smoker | 23.9 | 21.4 | 71.4 | 22.0 | 12.0 | 64.3 | ||||
| Former smoker | 47.8 | 50.6 | 19.1 | 28.4 | 29.7 | 7.1 | ||||
| Never smoker | 26.3 | 28.0 | 9.5 | 49.7 | 58.3 | 28.6 | ||||
| Alcohol use status, % | .035 | .229 | <.001 | .075 | ||||||
| Current alcohol use | 65.0 | 58.3 | 81.0 | 51.7 | 40.0 | 50.0 | ||||
| Former alcohol use | 23.9 | 29.1 | 19.0 | 17.7 | 20.9 | 17.9 | ||||
| Never alcohol use | 11.0 | 12.6 | 0.0 | 30.6 | 39.1 | 32.1 | ||||
| Total cholesterol (mg/dl), mean (SD) | 204.7 (37.1) | 198.3 (40.9) | .002 | 203.1 (35.9) | .847 | 215.2 (39.7) | 210.3 (44.6) | <.001 | 215.8 (40.8) | .900 |
| HDL cholesterol (mg/dl), mean (SD) | 42.0 (13.1) | 45.4 (16.2) | <.001 | 38.4 (11.2) | .209 | 54.9 (16.5) | 58.2 (18.4) | <.001 | 47.4 (14.0) | <.001 |
| Hypertension, % | 34.7 | 40.0 | .045 | 66.7 | .004 | 35.1 | 37.9 | .092 | 41.1 | .352 |
| Diabetes, % | 11.9 | 13.7 | .319 | 14.3 | .732 | 11.9 | 13.4 | .189 | 19.6 | .077 |
| eGFR <60mL/min per 1.73 m2, % | 1.6 | 9.4 | <.001 | 0.0 | .556 | 1.5 | 4.6 | <.001 | 1.8 | .855 |
| APOE ε4 genotype, % | .194 | .785 | .657 | .296 | ||||||
| 0 alleles | 69.3 | 66.0 | 66.7 | 69.4 | 68.2 | 76.8 | ||||
| 1 allele | 28.2 | 32.3 | 33.3 | 27.7 | 29.1 | 23.2 | ||||
| 2 alleles | 2.5 | 1.7 | 0.0 | 2.9 | 2.7 | 0.0 | ||||
| Cognitive test score, mean (SD) | ||||||||||
| Delayed word recall test (words) | 6.35 (1.49) | 5.93 (1.66) | <.001 | 6.10 (1.41) | .427 | 6.88 (1.46) | 6.70 (1.60) | <.001 | 6.64 (1.21) | .221 |
| Digit symbol substitution test (points) | 43.1 (13.1) | 34.6 (14.2) | <.001 | 39.4 (12.6) | .197 | 47.2 (14.1) | 41.7 (15.9) | <.001 | 45.3 (14.5) | .298 |
| Word fluency test (words) | 33.0 (12.6) | 29.1 (13.3) | <.001 | 30.6 (13.4) | .394 | 34.0 (12.2) | 31.6 (12.2) | <.001 | 31.7 (10.9) | .150 |
| Global Z-score (SDs) | −0.13 (0.96) | −0.64 (1.07) | <.001 | −0.39 (0.90) | .207 | 0.18 (0.97) | −0.12 (1.09) | <.001 | −0.02 (0.78) | .108 |
Notes: *Anemia defined as hemoglobin concentrations <13g/dl for men and <12g/dl for women. Normal hemoglobin defined as hemoglobin concentrations of 13–17.5g/dl for men and of 12–15.5g/dl for women. Elevated hemoglobin defined as hemoglobin concentrations >17.5g/dl for men and >15.5g/dl for women.
eGFR = estimated glomerular function rate; HDL = high density lipoprotein
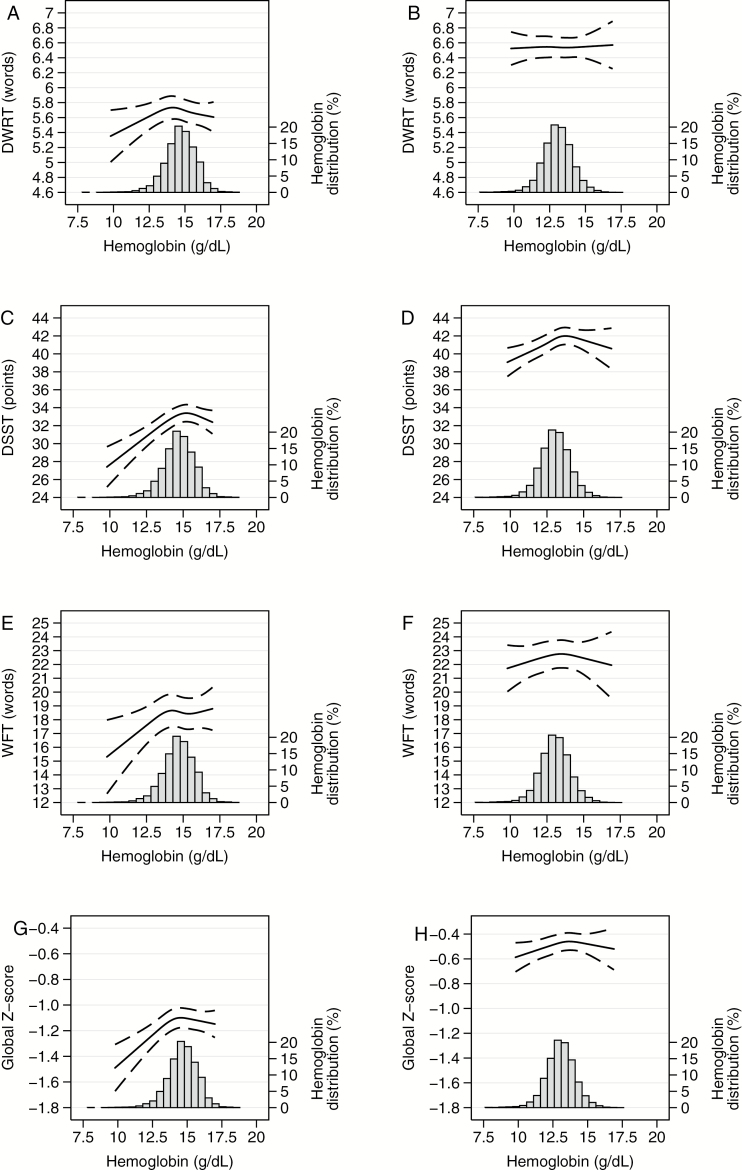
The cross-sectional associations of anemia and anemia subtypes with cognition are shown in Table 2. Anemia overall was cross-sectionally associated with lower cognitive test scores on the DSST (−1.97 points [95% confidence interval: −2.96, −0.97] for men and −1.00 points [95% confidence interval: −1.67, −0.32] for women) and global Z-score (−0.14 SDs [95% confidence interval: −0.22, −0.06] for men and −0.06 SDs [−0.11, −0.01] for women), but not on the DWRT or WFT. Normocytic anemia was associated with significantly worse performance on the DSST and global Z-score among men and women (all p < .05). Microcytic anemia was associated with significantly worse performance on the DWRT and global Z-score among men (all p < .05) and on the DSST among women (p < .05). Associations of macrocytic anemia showed similar patterns, but were limited by low power (n = 20 with macrocytic anemia). There was no evidence for interactions by age, sex, or race (all p interaction > .50). Figure 1 shows the continuous cross-sectional associations of hemoglobin with cognitive test score on the DWRT, the DSST, the WFT, and the global Z-score by sex. There was suggestion of a nonlinear association of hemoglobin with performance on the DWRT, the DSST, the WFT, and the global Z-score in men and on the DSST, the WFT, and the global Z-score in women. Consistent with the hemoglobin category analyses, lower hemoglobin concentrations were associated with significantly lower scores on the DSST and the global Z-score, compared with the median hemoglobin concentration, in both men and women. However, assessment of higher hemoglobin concentrations is limited by small numbers of participants with elevated hemoglobin (n = 56 women with hemoglobin level >15.5g/dl and n = 21 men with hemoglobin level >17.5g/dl) and was not statistically significant for any cognitive test (all p > .05) (Table 2).
Table 2.
Sex-stratified Adjusted* β-Coefficients (95% confidence intervals) for Cross-sectional Cognitive Test Scores (ARIC Visit 2, 1990–1992) by Hemoglobin Category and Anemia subtype†, N = 13,133
| Delayed Word Recall Test Z-Score | Digit Symbol Substitution Test Z-Score | Word Fluency Test Z-Score | Global Z-Score | |
|---|---|---|---|---|
| (words, per SD) | (points, per SD) | (words, per SD) | (per SD) | |
| Men | ||||
| All anemia (n = 350) | −0.15 (−0.31, 0.00) | −1.97 (−2.96, 0.97) | −1.05 (−2.26, 0.16) | −0.14 (−0.22, −0.06) |
| Microcytic anemia (n = 60) | −0.40 (−0.77, −0.04) | −2.18 (−4.49, 0.13) | −1.04 (−3.85, 1.77) | −0.22 (−0.41, −0.03) |
| Normocytic anemia (n = 279) | −0.07 (−0.25, 0.10) | −1.97 (−3.07, −0.87) | −1.11 (−2.44, 0.23) | −0.12 (−0.21, −0.03) |
| Macrocytic anemia (n = 11) | −0.84 (−1.68, −0.01) | −0.77 (−6.11, 4.70) | 0.35 (−6.14, 6.84) | −0.25 (−0.69, 0.19) |
| Normal hemoglobin (n = 5,467) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Elevated hemoglobin (n = 21) | 0.01 (−0.60, 0.62) | 1.89 (−1.98, 5.76) | 0.94 (−3.76, 5.64) | 0.09 (−0.22, 0.41) |
| Women | ||||
| All anemia (n = 993) | −0.02 (−0.12, 0.08) | −1.00 (−1.67, −0.32) | −0.72 (−1.45, 0.01) | −0.06 (−0.11, −0.01) |
| Microcytic anemia (n = 176) | −0.14 (−0.35, 0.07) | −1.48 (−2.97, −0.01) | −0.04 (−1.65, 1.57) | −0.09 (−0.20, 0.02) |
| Normocytic anemia (n = 808) | 0.00 (−0.10, 0.10) | −0.90 (−1.62, −0.17) | −0.89 (−1.68, −0.10) | −0.06 (−0.11, −0.01) |
| Macrocytic anemia (n = 9) | 0.28 (−0.62, 1.19) | −0.91 (−7.25, 5.43) | 1.68 (−5.20, 8.57) | 0.11 (−0.36, 0.58) |
| Normal hemoglobin (n = 6,246) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Elevated hemoglobin (n = 56) | −0.04 (−0.40, 0.33) | −0.17 (−2.73, 2.40) | −0.09 (−2.88, 2.70) | −0.02 (−0.21, 0.17) |
Notes: *Adjusted for age, gender, race/field center, education, income, cigarette smoking status, alcohol consumption, body mass index, total cholesterol, high density lipoprotein cholesterol, diabetes, hypertension, eGFR, and APOE ε4 genotype.
†Anemia defined as hemoglobin concentrations <13g/dl for men and <12g/dl for women. Normocytic anemia defined as anemia with MCV 80–100 fL. Microcytic anemia defined as anemia with MCV <80 fL. Macrocytic anemia defined as anemia with MCV >100 fL. Normal hemoglobin defined as hemoglobin concentrations of 13–17.5g/dl for men and of 12–15.5g/dl for women (reference). Elevated hemoglobin defined as hemoglobin concentrations >17.5g/dl for men and >15.5 for women.
Bolded data represent p < .05.
Figure 1.
Adjusted* restricted cubic spline model showing the continuous cross-sectional association of hemoglobin with cognitive test scores by gender, ARIC Visit 2, 1990–1992. (A) Men delayed word recall test (DWRT), (B) women DWRT, (C) men digit symbol substitution test (DSST), (D) women DSST, (E) men word fluency test (WFT), (F) women WFT, (G) men global Z-score, and (H) women global Z-score. The solid line represents the β-estimates and the dashed lines represent the 95% confidence intervals. Knots at 5th, 35th, 65th, and 95th percentiles. Restricted cubic spline centered at the 50th percentile and truncated at 0.5th and 99.5th percentile of hemoglobin. Histogram shows distribution of hemoglobin concentrations. *Adjusted for age, gender, race/field center, education, income, cigarette smoking status, alcohol consumption, body mass index, total cholesterol, high density lipoprotein cholesterol, diabetes, hypertension, eGFR, and APOE ε4 genotype. eGFR = estimated glomerular function rate.
In contrast to the cross-sectional results, anemia overall and elevated hemoglobin were not significantly associated with greater decline in performance on any of the cognitive tests over a median of 6 years in men and in women (all p > .05) (Table 3). Results were similarly not significantly associated with greater cognitive decline for anemia subtype analyses for both men and women (all p > .05) (Table 3). There were not any interactions by age, sex, or race (all p interaction > .50). However, as shown in Supplementary Table 1, compared with those who were included in the cognitive function change analyses (n = 10,283), the 2,850 participants excluded due to missing Visit 4 (1996–1998) cognitive test scores were older (58 years vs 57 years at Visit 2, p < .001), more likely to have less than high school education (33% vs 18%, p < .001), hypertension (46% vs 33%, p < .001), diabetes (20% vs 10%, p < .001), anemia (14% vs 9%, p < .001), and scored lower on all baseline cognitive tests (all p < .001). Cross-sectional analyses restricted to the 10,283 participants with cognitive test data at both Visit 2 and Visit 4 were slightly less precise due to smaller numbers, but not appreciably different from our main cross-sectional analyses (Supplementary Table 2).
Table 3.
Sex-stratified Adjusted* β-coefficients (95% confidence intervals) for Change in Cognitive Test Scores (ARIC Visit 2, 1990–1992 to ARIC Visit 4, 1996–1998) by Hemoglobin Category and Anemia subtype†, N = 10,283
| Delayed Word Recall Test Z-Score | Digit Symbol Substitution Test Z-Score | Word Fluency Test Z-Score | Global Z-Score | |
|---|---|---|---|---|
| (words, per SD) | (points, per SD) | (words, per SD) | (per SD) | |
| Men | ||||
| All anemia (n = 207) | −0.01 (−0.23, 0.20) | −0.05 (−0.95, 0.85) | 0.15 (−0.97, 1.28) | −0.01 (−0.09, 0.08) |
| Microcytic anemia (n = 43) | 0.08 (−0.39, 0.54) | −1.34 (−3.25, 0.58) | −0.63 (−3.06, 1.80) | −0.04 (−0.22, 0.13) |
| Normocytic anemia (n = 160) | −0.02 (−0.26, 0.22) | 0.27 (−0.74, 1.28) | 0.27 (−0.99, 1.53) | 0.01 (−0.09, 0.10) |
| Macrocytic anemia (n = 4) | −0.74 (−2.25, 0.77) | 0.83 (−5.38, 7.03) | 3.70 (−4.18, 11.59) | −0.06 (−0.62, 0.51) |
| Normal hemoglobin (n = 4,315) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Elevated hemoglobin (n = 12) | 1.02 (0.15, 1.90) | −1.07 (−4.66, 2.52) | 0.24 (−4.32, 4.80) | 0.26 (−0.07, 0.59) |
| Women | ||||
| All anemia (n = 726) | 0.00 (−0.12, 0.12) | 0.35 (−0.23, 0.93) | 0.08 (−0.55, 0.72) | 0.01 (−0.03, 0.06) |
| Microcytic anemia (n = 118) | 0.11 (−0.17, 0.40) | −0.26 (−1.59, 1.08) | 1.92 (0.46, 3.39) | 0.09 (−0.02, 0.20) |
| Normocytic anemia (n = 604) | −0.02 (−0.15, 0.12) | 0.47 (−1.16, 1.09) | −0.28 (−0.96, 0.40) | 0.00 (−0.05, 0.05) |
| Macrocytic anemia (n = 4) | −0.38 (−1.90, 1.14) | −0.86 (−7.92, 6.20) | 2.80 (−4.92, 10.5) | −0.04 (−0.61, 0.53) |
| Normal hemoglobin (n = 4,986) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Elevated hemoglobin (n = 37) | −0.04 (−0.54, 0.47) | −0.79 (−3.13, 1.55) | 0.21 (−2.35, 2.77) | −0.03 (−0.22, 0.16) |
*Adjusted for age, gender, race/field center, education, income, cigarette smoking status, alcohol consumption, body mass index, total cholesterol, high density lipoprotein cholesterol, diabetes, hypertension, eGFR, and APOE ε4 genotype.
†Anemia defined as hemoglobin concentrations <13g/dl for men and <12g/dl for women. Normocytic anemia defined as anemia with MCV 80–100 fL. Microcytic anemia defined as anemia with MCV <80 fL. Macrocytic anemia defined as anemia with MCV >100 fL. Normal hemoglobin defined as hemoglobin concentrations of 13–17.5g/dl for men and of 12–15.5g/dl for women (reference). Elevated hemoglobin defined as hemoglobin concentrations >17.5g/dl for men and >15.5g/dl for women.
Bolded data represent p < .05.
Discussion
In this community-based population of 13,133 white and black adults with mean baseline age of 57 years, low concentrations of hemoglobin (consistent with a diagnosis of anemia: hemoglobin concentrations <13g/dl for men and <12g/dl for women (1)) were associated with significantly lower cognitive test scores on the DSST and global Z-score at baseline. We also observed a nonsignificant association of higher concentrations of hemoglobin with lower cross-sectional cognitive test scores, suggesting a possible nonlinear association of hemoglobin with cognition. However, in contrast, we did not see significant results for 6-year change in cognition.
Our results are consistent with those from the Rush Memory and Aging Study (5,6), which reported nonlinear (U-shaped) associations of hemoglobin with cross-sectional cognitive function over a median of 3 years among individuals with mean age of 81 years at baseline. Further, similar to other cross-sectional studies (5,17–19), we found anemia to be associated with poorer global cognitive function, but this association may be specific to measures of processing speed and executive function (the DSST, in particular) and not to memory. This apparent selective involvement of domains typically involved in vascular cognitive impairment suggests that anemia may relate to cognitive function via vascular mechanisms rather than neurodegenerative mechanisms. Both low and high concentrations of hemoglobin may be associated with cognitive impairment by mechanisms resulting in inadequate cerebral oxygenation, which in turn may lead to impaired cerebral perfusion and cerebral function (ie, vascular mechanisms) (20). The response of the brain to anemia is vasodilation, with resultant increases in cerebral blood flow, in an attempt to compensate for the decrease in hemoglobin. Eventually, as hemoglobin concentrations fall, this compensation fails and cerebral blood flow becomes inadequate, sometimes leading to impaired cerebral function and ischemia (21). Both clinical (22,23) and subclinical (24) ischemia are known risk factors for cognitive decline and dementia. We saw similar associations of normocytic, microcytic, and macrocytic anemia with cognitive performance, which suggests a common mechanism. However, associations with microcytic anemia, likely representing iron deficiency anemia, may also be via oxidative stress in the brain, which has been associated with iron dysregulation (25). Elevated hemoglobin levels may represent hyperviscosity, hypovolemia, polycythemia vera, or pulmonary disease, all of which may lead to cerebral hypoxia and impaired cerebral function, which could then lead to impaired cognition (5,26). The incidence and prevalence of anemia and elevated hemoglobin both increase with age (27,28), suggesting that older individuals may be more at risk compared with younger individuals for cerebral hypoxia secondary to either low or high concentrations of hemoglobin. However, many types of anemia and elevated hemoglobin are treatable if identified (eg, iron deficiency anemia, vitamin B12 deficiency anemia, polycythemia vera) (27,28), but it remains unknown whether treatment of anemia and elevated hemoglobin has any impact on cognition and development of dementia.
In contrast to other prospective studies assessing change in cognitive function (6,29), we did not find evidence of an association of anemia with cognitive decline. However, this may be due to the relatively younger age of our participants (mean age 57 years), limited follow-up for cognitive decline (6 years), and likely selection biases whereby those participants who did not attend the follow-up visit were more likely to be older, were less educated, and have multiple vascular risk factors, which would put them at increased risk for cognitive decline. Alternatively, although we adjusted for likely confounders, the fact that we did not find associations with cognitive change may be due to unmeasured confounders in the observed cross-sectional relationships (that would not be present in a change analysis) (30), suggesting that anemia may not be directly associated with cognitive decline. This explanation would be consistent with prior research that has shown that education is not significantly associated with cognitive decline, although it is associated with cross-sectional cognitive function (31).
Certain limitations should be considered in the interpretation of our study. We had limited power to evaluate associations of elevated hemoglobin with cognitive function as only 56 women had hemoglobin concentrations higher than 15.5g/dl and 21 men had hemoglobin concentrations higher than 17.5g/dl in our study population. Therefore, these analyses on the association of elevated hemoglobin with cognition should be interpreted as exploratory and hypothesis generating. We were also unable to assess the etiology of anemia, but we were able to investigate potential differential associations of normocytic, microcytic, and macrocytic anemia (defined by MCV) with cognitive function and cognitive change, although our power was limited in assessing associations with macrocytic anemia (n = 20 participants).
Our study also has a number of strengths, including a large sample size of more than 13,000 white and black participants (mean age <65 years at baseline) with a median of 6 years of follow-up for cognitive test performance. Our study also had comprehensive measurement of confounders and data on three cognitive tests measured at two different time points, which allowed us to explore the association of hemoglobin with different cognitive domains.
In conclusion, in this community-based population, there was suggestion of a nonlinear association between hemoglobin concentrations and cross-sectional cognitive function, although only associations of low levels of hemoglobin (anemia) were statistically significant. Normocytic, microcytic, and macrocytic subtypes of anemia had similar associations with cross-sectional cognitive test scores, but we did not see any prospective associations of hemoglobin with cognitive function. Future large studies with long follow-up time are needed to further describe associations of both low and high hemoglobin concentrations with cognition and to further describe the associations of different anemia subtypes with cognition, in order to further understand mechanisms underlying these associations.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN26 8201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN26820110 0011C, and HHSN268201100012C). A.L.C.S. was supported by the NIH/NHLBI training grant T32HL007024.
Disclosures
All authors report no disclosures.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization; 2011. [Google Scholar]
- 2. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi:10.1182/blood-2004-05-1812 [DOI] [PubMed] [Google Scholar]
- 3. Andro M, Le Squere P, Estivin S, Gentric A. Anaemia and cognitive performances in the elderly: a systematic review. Eur J Neurol. 2013;20:1234–1240. doi:10.1111/ene.12175 [DOI] [PubMed] [Google Scholar]
- 4. Peters R, Burch L, Warner J, Beckett N, Poulter R, Bulpitt C. Haemoglobin, anaemia, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:18. doi:10.1186/1471-2318-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah RC, Wilson RS, Tang Y, Dong X, Murray A, Bennett DA. Relation of hemoglobin to level of cognitive function in older persons. Neuroepidemiology. 2009;32:40–46. doi:10.1159/000170905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah RC, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Hemoglobin level in older persons and incident Alzheimer disease: prospective cohort analysis. Neurology. 2011;77:219–226. doi:10.1212/WNL.0b013e318225aaa9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurella Tamura M, Wadley VG, et al. Hemoglobin concentration and cognitive impairment in the renal REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Gerontol A Biol Sci Med Sci. 2010;65:1380–1386. doi:10.1093/gerona/glq126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atti AR, Palmer K, Volpato S, Zuliani G, Winblad B, Fratiglioni L. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiol Aging. 2006;27:278–284. doi:10.1016/j.neurobiolaging.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 9. Beard CM, Kokmen E, O'Brien PC, Anía BJ, Melton LJ., 3rd Risk of Alzheimer’s disease among elderly patients with anemia: population-based investigations in Olmsted County, Minnesota. Ann Epidemiol. 1997;7:219–224. doi:10.1016/S1047-2797(97)00015-X [DOI] [PubMed] [Google Scholar]
- 10. ARIC Study Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11. Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–145. [DOI] [PubMed] [Google Scholar]
- 12. Wechsler D. Wechsler Adult Intelligence Scale-Revised (WAIS-R) Manual. New York, NY: Psychological Corp; 1987. [Google Scholar]
- 13. Spreen O, Benton A. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA) Manual of Instructions. Melbourne, Australia: University of Victoria; 1969. [Google Scholar]
- 14. Benton AL, Hamsher K. Multilingual Aphasia Examination. 2nd ed Iowa City, IA: AJA Associates; 1989. [Google Scholar]
- 15. Schneider AL, Sharrett AR, Gottesman RF, et al. Normative data for 8 neuropsychological tests in older blacks and whites from the atherosclerosis risk in communities (ARIC) study. Alzheimer Dis Assoc Disord. 2015;29:32–44. doi:10.1097/WAD.0000000000000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi:10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaves PH, Carlson MC, Ferrucci L, Guralnik JM, Semba R, Fried LP. Association between mild anemia and executive function impairment in community-dwelling older women: The Women’s Health and Aging Study II. J Am Geriatr Soc. 2006;54:1429–1435. doi:10.1111/j.1532-5415.2006.00863.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lucca U, Tettamanti M, Mosconi P, et al. Association of mild anemia with cognitive, functional, mood and quality of life outcomes in the elderly: the “Health and Anemia” study. PLoS One. 2008;3:e1920. doi:10.1371/journal.pone.0001920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng TP, Feng L, Niti M, Yap KB. Albumin, haemoglobin, BMI and cognitive performance in older adults. Age Ageing. 2008;37:423–429. doi:10.1093/ageing/afn102 [DOI] [PubMed] [Google Scholar]
- 20. Gottesman RF, Sojkova J, Beason-Held LL, et al. Patterns of regional cerebral blood flow associated with low hemoglobin in the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2012;67:963–969. doi:10.1093/gerona/gls121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13:R89. doi:10.1186/cc7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ihle-Hansen H, Thommessen B, Wyller TB, et al. Incidence and subtypes of MCI and dementia 1 year after first-ever stroke in patients without pre-existing cognitive impairment. Dement Geriatr Cogn Disord. 2011;32:401–407. doi:10.1159/000335361 [DOI] [PubMed] [Google Scholar]
- 23. Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O'Brien RJ. Effect of infarcts on dementia in the Baltimore Longitudinal Study of Aging. Ann Neurol. 2008;64:168–176. doi:10.1002/ana.21413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi:10.1056/NEJMoa022066 [DOI] [PubMed] [Google Scholar]
- 25. Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi:10.1111/j.1474-9726.2007.00294.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gottesman RF, Bahrainwala Z, Wityk RJ, Hillis AE. Neglect is more common and severe at extreme hemoglobin levels in right hemispheric stroke. Stroke. 2010;41:1641–1645. doi:10.1161/STROKEAHA.110.585265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(suppl. 7A):3S–10S. doi:10.1016/j.amjmed.2003.12.009 [DOI] [PubMed] [Google Scholar]
- 28. Deadmond MA, Smith-Gagen JA. Changing incidence of myeloproliferative neoplasms: trends and subgroup risk profiles in the USA, 1973–2011. J Cancer Res Clin Oncol. 2015. doi:10.1007/s00432-015-1983-5 [DOI] [PubMed] [Google Scholar]
- 29. Deal JA, Carlson MC, Xue QL, Fried LP, Chaves PH. Anemia and 9-year domain-specific cognitive decline in community-dwelling older women: The Women's Health and Aging Study II. J Am Geriatr Soc. 2009;57:1604–1611. doi:10.1111/j.1532-5415.2009.02400.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. doi:10.1093/aje/kwi187 [DOI] [PubMed] [Google Scholar]
- 31. Schneider AL, Sharrett AR, Patel MD, et al. Education and cognitive change over 15 years: the atherosclerosis risk in communities study. J Am Geriatr Soc. 2012;60:1847–1853. doi:10.1111/j.1532-5415.2012.04164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.