Abstract
Background.
Disability is a crucial health problem in aging. Identifying a biological contributory factor would be useful. Serum insulin-like growth factor-1 (IGF-1) plays an important role in the endocrine system and is associated with frailty. However, there is no consensus about the relationship between IGF-1 and disability. This study aimed to examine whether IGF-1 related to incident disability among older adults.
Methods.
The study included 4,133 older adults (mean age, 71.8±5.4 years) who were participants in the “Obu Study of Health Promotion for the Elderly” cohort study. We collected information on demographic variables, measured gait speed, Mini Mental State Examination score, and serum IGF-1 at baseline. During follow-up, incident disability was monitored by Long-Term Care Insurance certification.
Results.
Disability was observed in 212 participants during a mean follow-up duration period of 29.2 months. A log rank test indicated that lower levels of serum IGF-1 were related to incident disability (p = .004). A Cox hazard regression showed a lower quartile in IGF-1 related to disability compared with the highest quartile (Q4), even when adjusting for covariates including gait speed and Mini Mental State Examination score (Q1: hazard ratio = 1.72, 95% confidence intervals: 1.06–2.81; Q2: hazard ratio = 1.64, 95% confidence intervals: 0.99–2.71; Q3: hazard ratio = 1.31, 95% confidence intervals: 0.76–2.25). In the analysis, stratified by sex, there was also significant relationship between IGF-1 and disability among women, but not men.
Conclusions.
Lower serum IGF-1 was independently related to disability among older adults.
Keywords: IGF-1, Disability, Long-term care, Frailty
Disability is the one of the crucial adverse health problems among older adults. With the rising number of older adults, the number of disabled older adults is also increasing, and this will impact on health care costs (1). The absolute number of older people is increasing globally. The highest proportion of older people has been observed in Japan; the number of adults aged 65 years and older has almost doubled in the past two decades, reaching 23% of the population in 2010. Forty percent of Japan’s population will be older than 65 years of age in 2050 (2). The development of a strategy to detect a higher risk of disability and provide adequate intervention for the purposes of preventing or delaying the onset of disability in older adults is required (3).
Identification of biological factors related to disability will contribute to a better understanding of the mechanism of advanced aging. Among such measurable biomarkers, insulin-like growth factor-1 (IGF-1) is an important mediator of growth hormones. It has protective effects on neurobiological processes involving potent neurotrophic and neuroprotective actions (4,5) and contributes to promotion of skeletal muscle (6,7). Decreased serum IGF-1 is associated with various adverse health outcomes such as frailty (8), sarcopenia (9), and cognitive impairment (10,11), although most of these findings are from cross-sectional studies. A deficiency in IGF-1 is thought to be represented by biological changes, particularly in the endocrine system, that are caused by cumulative molecular and cellular damage and leads to frailty in older adults (12,13). However, there is a lack of evidence for an association between IGF-1 and disability among older adults. A better understanding of the associations of IGF-1 and disability would aid our understanding of the mechanism for preventing or delaying age-related disability because levels of serum IGF-1 are related to cognitive function (10,11), muscle mass, and strength (14,15).
The aim of this study was to examine whether serum IGF-1 is related to incident disability. Most of the biomarkers studies have used a cross-sectional design rather than longitudinal, and models based on cross-sectional designs do not accurately reflect biology (10). Thus, we designed a longitudinal study and hypothesized that lower levels of serum IGF-1 are related to a higher risk of incident disability.
Methods
Participants
Participants eligible for this study were participants in the population-based cohort of the Obu Study of Health Promotion for the Elderly. Recruitment was conducted via a letter sent to 14,313 individuals; 5,104 of these individuals participated in the Obu Study of Health Promotion for the Elderly in 2011–2012. A detailed protocol has been reported elsewhere (16). In the current study, we included participants who were independent of basic activities of daily living at baseline. This was confirmed by interview or not certified by Long-Term Care Insurance (LTCI) at any level. Participants were excluded based on a history of cerebrovascular disease, Parkinson’s disease, dementia, cancer, or cerebral vascular disorder. Participants who had missing values for these outcomes were also excluded. Finally, 4,133 participants were judged to be eligible for the study and completed assessments, including blood tests, at baseline. The Ethics Committee of the National Center for Geriatrics and Gerontology approved this study. Participants provided informed consent in accordance with the ethical policy.
Assessment of Disability
During the follow-up period, we monitored certification of LTCI for all participants. LTCI assists those in need of long-term care “to maintain dignity and an independent daily life routine according to each person’s own level of abilities” (17). The LTCI certifies a person as “Support Level 1 or 2” if he or she needs support for daily activities or “Care Level 1, 2, 3, 4, or 5” if he or she needs continuous care (18). Beneficiaries of the LTCI can use multiple services for which they are eligible, according to their care plan up to the maximum amount. They can use more services than covered as long as they pay all the costs for the services beyond the maximum level. The process of certification in LTCI has been described elsewhere (18,19). In summary, a trained local government official visits the home to evaluate nursing care needs using a questionnaire on current physical and mental status (73 items) and use of medical procedures (12 items). The results are entered into a computer to calculate the applicant’s standardized scores for the seven dimensions of physical and mental status, estimate time of care, and assign a care-needs level based on the total estimated care minutes. The Nursing Care Needs Certification Board, consisting of physicians, nurses, and other experts in health and social services appointed by a mayor, determines whether the initial assessment is appropriate, considering the applicant’s primary care physician’s statement and notes written by the assessor during the home visit. The board then makes a final decision about certification in LTCI. In this study, the outcome of disability was defined as a new requirement of LTCI service certified as any level. In the event that the follow-up could not be performed to assess for incident disability, this was treated as censored data, that is, moving out of the other city and death. We monitored this information that was updated monthly. We defined the follow-up period from the time we conducted the examination at baseline to 2014 (mean follow-up duration period: 29.2 months).
IGF-1
A blood assay was conducted at baseline; a detailed protocol for this has been described elsewhere (11). IGF-1 was quantitatively determined using an IGF-1 immunoradiometric assay “Daiichi” (TFB, Tokyo, Japan). Measurements were performed in duplicate and averaged to give a value in nanogram per milliliter. The assay was performed by SRL (Tokyo, Japan).
Covariates
Participants were interviewed and their physical and cognitive function was assessed at baseline. Demographic data were collected for age, sex, body mass index (weight/height2), educational history, and medication use in a face-to-face interview. Information about lifestyle, including sleep duration, and smoking and alcohol drinking status were obtained. Physical exercise habit was also assessed using the question “Do you engage in moderate levels of physical exercise or sports aimed at health?” rated on a five-point scale ranging from “no,” “less than 1 time/week,” “2–4 times/week,” “5–6 times/week,” to “every day.” Depressive symptoms were evaluated using the 15-item Geriatric Depression Scale (20). Gait speed was measured as an indicator of motor function. Participants were asked to walk on a straight walkway of 6.6 m in length on a flat floor with their usual gait speed. Gait time was measured over a 2.4-m distance between marks at 2.1 and 4.5 m from the start of the walkway, and the mean gait speed (meter/second) was calculated. Mini Mental State Examination score provided a measure of cognitive function (21).
Statistical Analysis
To examine the association of IGF-1 with subject characteristics, gait speed, and cognitive function, the participants were divided into quartiles based on levels of IGF-1 (Q1–Q4). Comparisons among these groups were conducted using analysis of variance or the χ2 test. Incidence rate were calculated in each groups (Q1–Q4). To examine the association of the level of IGF-1 with disability, Kaplan–Meier survival risk assessments were used to plot cumulative survival function, and the results for each group were compared using log rank tests. The objective variable was incident disability, and the explanatory variable was the level of IGF-1. Cox proportional hazards regression models were used to determine the hazard ratio (HR) of incident disability associated with IGF-1 level. Model 1 was the crude model. Model 2 added the following covariates: age, sex, body mass index, educational history, number of chronic diseases (hypertension, hyperlipidemia, and diabetes), number of medications used, habit of drinking, smoking, physical exercise, and Geriatric Depression Scale. Model 3 added Mini Mental State Examination score and gait speed to the variables in Model 2. In addition, the calculation of incidence rate and the Cox proportional hazards regression analysis were stratified by sex owing to potential differences (22,23). Each model in Cox proportional hazards regression analysis calculated the HR and 95% confidence intervals (CI) referring to the highest level of IGF-1 (Q4). Statistical analyses were performed using SPSS ver. 20 (IBM Corp., Armonk, NY). Statistical significance was set at p < .05 in all analyses.
Results
Participants were classified into quartiles based on IGF-1 levels (Q1: ≤82ng/mL, n = 1,052; Q2: 83–100ng/mL, n = 1,044; Q3: 101–119ng/mL, n = 1,008; Q4: ≥120ng/mL, n = 1,029). A comparison of characteristics between these groups is summarized in Table 1. The level of serum IGF-1 was significantly associated with demographic variables including age, sex, body mass index, medications used, educational history, Geriatric Depression Scale, and habit of drinking and smoking (all p < .05), but not with sleep duration (p = .068). Mini Mental State Examination and gait speed were also significantly associated with serum IGF-1 (p < .001).
Table 1.
Characteristics of Participants in Quartiles Based on IGF-1 Levels
| Variables | All | Level of IGF-1 (ng/mL) | p Value | |||
|---|---|---|---|---|---|---|
| Q1, ≤82 | Q2, 83–100 | Q3, 101–119 | Q4, ≥120 | |||
| Age, y | 71.8±5.4 | 74.2±6.1 | 71.8±5.3 | 71.0±4.8 | 70.2±4.5 | <.001 |
| Sex, % (women) | 52 | 64 | 56 | 48 | 41 | <.001 |
| BMI, kg/m2 | 23.4±3.1 | 22.8±3.3 | 23.4±3.1 | 23.5±2.9 | 24.1±2.9 | <.001 |
| Chronic diseases,* number | 1.0±0.8 | 0.9±0.8 | 1.0±0.8 | 1.0±0.8 | 1.1±0.8 | <.001 |
| Medications used, number | 1.9±2.0 | 2.0±2.1 | 1.9±2.0 | 1.8±1.9 | 1.9±1.9 | .018 |
| Education, y | 11.4±2.5 | 10.8±2.5 | 11.3±2.5 | 11.5±2.4 | 11.8±2.6 | <.001 |
| Smoking, % (current) | 10 | 8 | 9 | 13 | 11 | <.001 |
| Drinking alcohol, % (current) | 46 | 38 | 45 | 50 | 52 | <.001 |
| Habit of physical exercise, % (no) | 63 | 67 | 63 | 60 | 61 | .029 |
| Sleep duration, h | 7.7±1.3 | 7.8±1.4 | 7.7±1.2 | 7.7±1.2 | 7.8±1.2 | .068 |
| GDS, score | 2.8±2.6 | 3.1±2.6 | 2.7±2.6 | 2.7±2.6 | 2.6±2.4 | <.001 |
| MMSE, score | 26.3±2.7 | 25.9±2.9 | 26.3±2.7 | 26.3±2.7 | 26.5±2.5 | <.001 |
| Gait speed | 1.3±0.2 | 1.2±0.2 | 1.3±0.2 | 1.3±0.2 | 1.3±0.2 | <.001 |
Notes: BMI = body mass index; GDS = Geriatric Depression Scale; IGF-1 = insulin-like growth factor-1; MMSE = Mini Mental State Examination. Data are shown as mean ± SD or %. Variables were compared among IGF-1 levels (Q1–Q4).
*Chronic diseases included hypertension, hyperlipidemia, and diabetes.
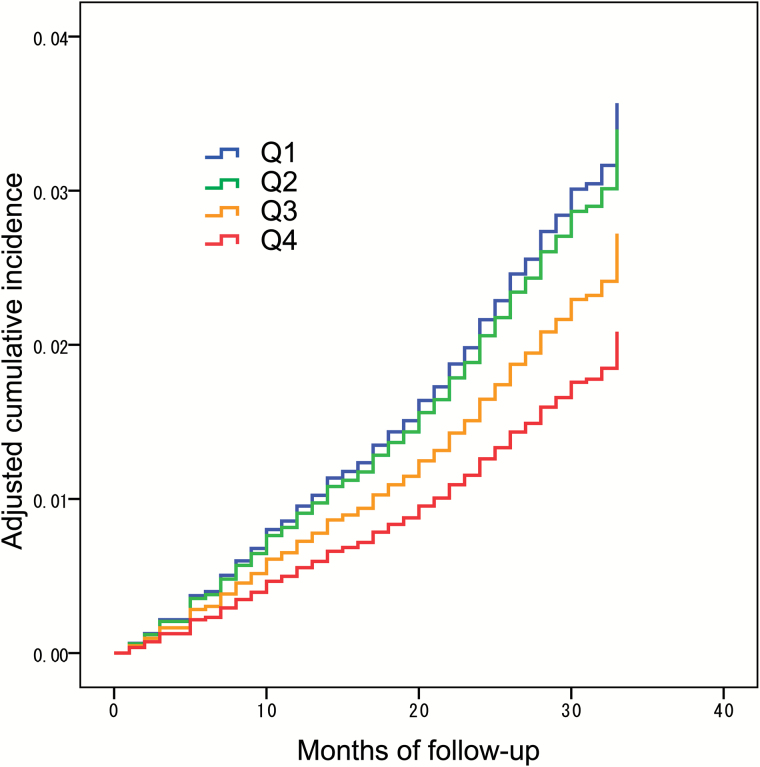
During the follow-up period, 212 participants were certified as having disability according to the LTCI (Q1: n = 99, 39.4 per 1,000 person-years; Q2: n = 55, 21.6 per 1,000 person-years; Q3: n = 36, 14.6 per 1,000 person-years; Q4: n = 22, 8.7 per 1,000 person-years), while participants with censoring was observed in each group (Q1: n = 24; Q2: n = 16; Q3: n = 18; Q4: n = 11). Mean follow-up duration in each group was as follows: (Q1: 28.7 months, Q2: 29.3 months, Q3: 29.4 months, Q4: 29.5 months). The results from a log rank test indicated that the level of serum IGF-1 related to disability (p = .004). Results of the Cox hazard regression analyses including all the participants are shown in Table 2. Model 1 (crude model) showed that the IGF-1 level in quartiles Q1 and Q2 was significantly related to incident disability relative to the Q4 quartile (Q1: HR = 4.40, 95% CI: 2.77–7.00; Q2: HR = 2.44, 95% CI: 1.49–4.01; Q3: HR = 1.59, 95% CI: 0.93–2.72). A multivariate model adjusting for demographic data (Model 2) also showed a relationship with disability compared with the Q4 quartile (Q1: HR = 1.91, 95% CI: 1.18–3.10; Q2: HR = 1.73, 95% CI: 1.05–2.86; Q3: HR = 1.42, 95% CI: 0.83–2.43). In addition, the final model, further adjusting for Mini Mental State Examination score and gait speed (Model 3), showed similar results as Model 2 (Q1: HR = 1.70, 95% CI: 1.06–2.81; Q2: HR = 1.64, 95% CI: 0.99–2.71; Q3: HR = 1.31, 95% CI: 0.76–2.25). The adjusted cumulative incidence (Model 3) in each group is shown in Figure 1.
Table 2.
Cox Proportional Hazards Regression Models Analyses of the Relationship Between Disability and IGF-1 Levels
| IGF-1 Level | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Q1 (lowest) | 4.40 (2.77–7.00) | <.001 | 1.91 (1.18–3.10) | .009 | 1.72 (1.06–2.81) | .028 |
| Q2 | 2.44 (1.49–4.01) | <.001 | 1.73 (1.05–2.86) | .032 | 1.64 (0.99–2.71) | .054 |
| Q3 | 1.59 (0.93–2.72) | .091 | 1.42 (0.83–2.43) | .200 | 1.31 (0.76–2.25) | .328 |
| Q4 (highest) | Reference | Reference | Reference | |||
Notes: BMI = body mass index; CI: confidence intervals; GDS = Geriatric Depression Scale; HR: hazard ratio; IGF-1 = insulin-like growth factor-1; MMSE = Mini Mental State Examination. Model 1: crude model; Model 2: adjusted for age, sex, BMI, number of chronic diseases (hypertension, hyperlipidemia, and diabetes), number of medications used, years of education, sleep duration, habit of alcohol drinking and smoking, habit of physical exercise, and GDS; Model 3: MMSE score and gait speed added to Model 2.
Figure 1.
Adjusted cumulative incident disability among groups (Q1–Q4). The cumulative incident was adjusted for age, sex, body mass index, educational history, number of chronic diseases, number of medications used, habit of drinking, smoking, physical exercise, Geriatric Depression Scale, Mini Mental State Examination, and gait speed in the Cox regression analysis (Model 3).
In the analysis stratified by sex, certified participants as having disability according to the LTCI were 75 in men (Q1: n = 26, 28.7 per 1,000 person-years; Q2: n = 20, 18.1 per 1,000 person-years; Q3: n = 18, 14.0 per 1,000 person-years; Q4: n = 11, 7.4 per 1,000 person-years) and 137 in women (Q1: n = 73, 45.4 per 1,000 person-years; Q2: n = 35, 24.2 per 1,000 person-years; Q3: n = 18, 15.3 per 1,000 person-years; Q4: n = 11, 10.7 per 1,000 person-years). The Cox hazard regression analyses were also conducted in each group stratified by sex (Table 3). Among women, the results were similar to results among the total samples showing that levels of IGF-1 were related with disability in all models. Among men, levels of IGF-1 were significantly associated with disability in the crude model (Model 1), but there were no significant associations in the adjusted models (Models 2 and 3).
Table 3.
Results of Cox Proportional Hazards Regression Models Analysis Among Men and Women Between Disability and IGF-1 Levels
| IGF-1 Level | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Q1 (lowest) | 3.93 (1.94–7.95) | <.001 | 4.08 (2.16–7.70) | <.001 | 1.39 (0.65–2.94) | .395 | 2.18 (1.14–4.19) | .019 | 1.29 (0.60–2.75) | .514 | 1.98 (1.03–3.82) | .041 |
| Q2 | 2.38 (1.13–5.01) | .022 | 2.24 (1.14–4.42) | .019 | 1.35 (0.63–2.89) | .435 | 1.94 (0.98–3.83) | .058 | 1.32 (0.61–2.83) | .477 | 1.82 (0.92–3.62) | .086 |
| Q3 | 1.91 (0.90–4.05) | .090 | 1.27 (0.59–2.73) | .544 | 1.46 (0.68–3.10) | .330 | 1.25 (0.58–2.71) | .566 | 1.47 (0.69–3.15) | .210 | 1.10 (0.51–2.39) | .809 |
| Q4 (highest) | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
Notes: BMI = body mass index; CI: confidence intervals; GDS = Geriatric Depression Scale; HR: hazard ratio; IGF-1 = insulin-like growth factor-1; MMSE = Mini Mental State Examination. Model 1: crude model; Model 2: adjusted for age, BMI, medications used, number of chronic diseases (hypertension, hyperlipidemia, and diabetes), years of education, sleep duration, habit of alcohol drinking and smoking, habit of physical exercise, and GDS; Model 3: MMSE score and gait speed added to Model 2.
Discussion
This prospective study revealed that serum IGF-1 levels are related to incident disability. Older adults with lower levels of IGF-1 had a significantly greater risk of disability. Although participants’ characteristics were different among groups divided according to serum IGF-1 levels, the relationship between serum IGF-1 levels and disability remained significant after adjusting for several covariates, including physical and cognitive function.
Our results suggest that IGF-1 is a marker of disability; this is in accordance with other limited evidence. Cappola and cowokers (23) showed that lower serum IGF-1 was related with a high risk for progressive disability in older women. Our results demonstrated sex differences; a significant association between IGF-1 and disability was observed among women even after adjusting for covariates, but this was not found in men. There are, however, discrepant results among studies. For example, a cross-sectional study revealed that IGF-1 among oldest old individuals (aged 80 years and older) was not associated with disability (24). The latter study further showed that functional impairments including physical and cognitive impairment were also not associated with IGF-1. However, these studies focused on a specific sample with different characteristics from the participants in our sample, such as sex and age. Our population study included a sufficient sample size, and the significant results remained after adjusting for covariates including demographic data, physical function, and cognitive function. Thus, our findings have expanded other results and provided further insight into understanding serum IGF-1 for age-related functional decline. In addition, the relationship between levels of IGF-1 and disability may differ by sex. Other studies have also reported sex differences in the role of IGF-1 for disability (22,23). Al-Delaimy and coworkers (22) suggested that sex differences in this regard might depend on the function of testosterone and/or estrogen. For example, testosterone administration elevated serum IGF-1 levels in men (25). However, there was no clear evidence for whether serum IGF-1 played a specific role among men or women. Further study would be required to clarify the sex differences for the effect of IGF-1 on disability.
Disability among older adults is thought to be one of the consequences of frailty (26–28). Decreased IGF-1 is considered to be caused by cumulative molecular and cellular damage and leads to frailty in older adults (12,13). Furthermore, lower serum IGF-1 is associated with and predictive of frailty among older adults (8,15,29,30). This can be explained by the effects of IGF-1 on age-related functional decline. Functional decline has been related to frailty and disability (12,31), but the cumulative evidence suggests that serum IGF-1 is associated with physical and cognitive function. Low IGF-1 levels have been associated with poor knee extensor muscle strength and slow walking gait speed (32). High IGF-1 levels have been associated with better physical function including gait speed among older adults with obesity, but not in non-obese individuals (33). On the contrary, lower levels of IGF-1 are predictive of cognitive decline (34,35), decreased IGF-1 levels over time are related to a decline in cognition (10), and lower levels of serum IGF-1 are a risk factor for Alzheimer’s disease and dementia (36,37). In addition, physical function has been shown to have an interrelationship with cognitive function (11,38). This relationship may partly depend on the role of IGF-1. Because blood-borne IGF-1 has major target organs such as muscle and the brain (39) and contributes to promotion of neuronal plasticity (4,5) and skeletal muscle (6,7), IGF-1 is thought to contribute to mediating the relationship between exercise and cognition (11,40). Maintaining IGF-1 levels may contribute to robust and healthy cognitive function among older adults. Thus, the association of IGF-1 with physical and cognitive function may contribute to incident disability.
Our study has several strengths; it had a sufficient sample size and used a prospective design. Other studies examining the relationship between IGF-1 and disability included smaller samples or used a cross-sectional design. Additionally, disability as a main outcome in this study was prospectively surveyed through data from a public insurance system. Most studies regarding disability have been performed using self-reported information or based on subjective judgment. Certification in LTCI was based on systematic assessment and required conference consensus for certification (18). Thus, the identification of disability in our study would be more objective and valid than that based on subjective judgment. On the contrary, our study had several limitations. First, our study did not include other biomarkers, for example, interleukin. There are other biomarkers that also have the potential to be associated with advanced aging. Also, IGFBP-3 is an important factor to consider because IGF-1 is regulated by the Insulin-like growth factor-binding protein-3 concentration (41). However, these measurements were not included in our study. Thus, further studies require a comparison between biomarkers. Additionally, other end point outcomes should be added in further studies. Although incident disability is an important adverse health outcome to explore, frailty or mortality should also be investigated.
Conclusion
Our population study showed that lower serum IGF-1 predicted incident disability among community-dwelling older adults. Further investigation is required to clarify the mechanism for decreased IGF-1 leading to disability.
Funding
This work was supported by the Health and Labor Sciences Research Grants (Comprehensive Research on Aging and Health); Grant-in-Aid for Challenging Exploratory Research (26560296); Grant-in-Aid for Scientific Research (B) (23300205); Grant-in-Aid for the Japan Society for the Promotion of Science Fellows (259435); and Research Funding for Longevity Sciences (22-16) from the National Center for Geriatrics and Gerontology.
Conflict of Interest
None.
Acknowledgments
We would like to thank the Obu city office for helping with participant recruitment.
References
- 1. Guralnik JM, Alecxih L, Branch LG, Wiener JM. Medical and long-term care costs when older persons become more dependent. Am J Public Health. 2002;92:1244–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamiya N, Noguchi H, Nishi A, et al. Population ageing and wellbeing: lessons from Japan’s long-term care insurance policy. Lancet. 2011;378:1183–1192. doi:10.1016/s0140-6736(11)61176-8 [DOI] [PubMed] [Google Scholar]
- 3. Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD; Interventions on Frailty Working Group. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–634. doi:10.1111/j.1532-5415.2004.52174.x [DOI] [PubMed] [Google Scholar]
- 4. Baker LD, Barsness SM, Borson S, et al. Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Arch Neurol. 2012;69:1420–1429. doi:10.1001/archneurol.2012.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. [DOI] [PubMed] [Google Scholar]
- 6. Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. doi:10.1146/annurev.ph.53.030191.001221 [DOI] [PubMed] [Google Scholar]
- 7. van Dam PS, Aleman A, de Vries WR, et al. Growth hormone, insulin-like growth factor I and cognitive function in adults. Growth Horm IGF Res. 2000;10(suppl B):S69–S73. [DOI] [PubMed] [Google Scholar]
- 8. Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women's Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64:243–248. doi:10.1093/gerona/gln026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volpato S, Bianchi L, Cherubini A, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci. 2014;69:438–446. doi:10.1093/gerona/glt149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanders JL, Ding V, Arnold AM, et al. Do changes in circulating biomarkers track with each other and with functional changes in older adults? J Gerontol A Biol Sci Med Sci. 2014;69:174–181. doi:10.1093/gerona/glt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doi T, Shimada H, Makizako H, et al. Association of insulin-like growth factor-1 with mild cognitive impairment and slow gait speed. Neurobiol Aging. 2015;36:942–947. doi:10.1016/j.neurobiolaging.2014.10.035 [DOI] [PubMed] [Google Scholar]
- 12. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi:10.1016/s0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. [DOI] [PubMed] [Google Scholar]
- 14. Léger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11:163–175B. doi:10.1089/rej.2007.0588 [DOI] [PubMed] [Google Scholar]
- 15. Mohamad MI, Khater MS. Evaluation of insulin like growth factor-1 (IGF-1) level and its impact on muscle and bone mineral density in frail elderly male. Arch Gerontol Geriatr. 2015;60:124–127. doi:10.1016/j.archger.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 16. Shimada H, Makizako H, Doi T, et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J Am Med Dir Assoc. 2013;14:518–524. doi:10.1016/j.jamda.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 17. Ministry of Justice. Long-Term Care Insurance Act (Kaigo hoken hō). 1997. [Google Scholar]
- 18. Tsutsui T, Muramatsu N. Japan’s universal long-term care system reform of 2005: containing costs and realizing a vision. J Am Geriatr Soc. 2007;55:1458–1463. doi:10.1111/j.1532-5415.2007.01281.x [DOI] [PubMed] [Google Scholar]
- 19. Tsutsui T, Muramatsu N. Care-needs certification in the long-term care insurance system of Japan. J Am Geriatr Soc. 2005;53:522–527. doi:10.1111/j.1532-5415.2005.53175.x [DOI] [PubMed] [Google Scholar]
- 20. Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 22. Al-Delaimy WK, von Muhlen D, Barrett-Connor E. Insulin like growth factor-1, insulin like growth factor binding protein-1, and cognitive function in older men and women. J Am Geriatr Soc. 2009;57:1441–1446. doi:10.1111/j.1532-5415.2009.02343.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–2025. doi:10.1210/jc.2002-021694 [DOI] [PubMed] [Google Scholar]
- 24. Adriaensen W, Matheï C, van Pottelbergh G, et al. Significance of serum immune markers in identification of global functional impairment in the oldest old: cross-sectional results from the BELFRAIL study. Age (Dordr). 2014;36:457–467. doi:10.1007/s11357-013-9558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi:10.1152/ajpendo.00362.2001 [DOI] [PubMed] [Google Scholar]
- 26. Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi:10.1001/archinternmed.2007.113 [DOI] [PubMed] [Google Scholar]
- 27. Ensrud KE, Ewing SK, Cawthon PM, et al. ; Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi:10.1111/j.1532-5415.2009.02137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc. 2014;62:721–726. doi:10.1111/jgs.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. [DOI] [PubMed] [Google Scholar]
- 30. Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf). 2005;63:403–411. doi:10.1111/j.1365-2265.2005.02355.x [DOI] [PubMed] [Google Scholar]
- 31. Kim J, Tanabe K, Yokoyama N, Zempo H, Kuno S. Objectively measured light-intensity lifestyle activity and sedentary time are independently associated with metabolic syndrome: a cross-sectional study of Japanese adults. Int J Behav Nutr Phys Act. 2013;10:30. doi:10.1186/1479-5868-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cappola AR, Bandeen-Roche K, Wand GS, Volpato S, Fried LP. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86:4139–4146. doi:10.1210/jcem.86.9.7868 [DOI] [PubMed] [Google Scholar]
- 33. Onder G, Liperoti R, Russo A, et al. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab. 2006;291:E829–E834. doi:10.1152/ajpendo.00138.2006 [DOI] [PubMed] [Google Scholar]
- 34. Kalmijn S, Janssen JA, Pols HA, Lamberts SW, Breteler MM. A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J Clin Endocrinol Metab. 2000;85:4551–4555. doi:10.1210/jcem.85.12.7033 [DOI] [PubMed] [Google Scholar]
- 35. Dik MG, Pluijm SM, Jonker C, Deeg DJ, Lomecky MZ, Lips P. Insulin-like growth factor I (IGF-I) and cognitive decline in older persons. Neurobiol Aging. 2003;24:573–581. [DOI] [PubMed] [Google Scholar]
- 36. Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T. Relationship between serum insulin-like growth factor-1 levels and Alzheimer’s disease and vascular dementia. J Am Geriatr Soc. 2005;53:1748–1753. doi:10.1111/j.1532-5415.2005.53524.x [DOI] [PubMed] [Google Scholar]
- 37. Westwood AJ, Beiser A, Decarli C, et al. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology. 2014;82:1613–1619. doi:10.1212/wnl.0000000000000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–851. doi:10.1016/j.arr.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 39. Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33:1690–1698. doi:10.1016/j.neurobiolaging.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 41. Froesch ER, Hussain MA, Schmid C, Zapf J. Insulin-like growth factor I: physiology, metabolic effects and clinical uses. Diabetes Metab Rev. 1996;12:195–215. doi:10.1002/(sici)1099-0895(199610)12:3<195::aid-dmr164>3.0.co;2-g [DOI] [PubMed] [Google Scholar]