Abstract
Study of the exacerbating effects of chemotherapeutics, such as doxorubicin, on the impairment of insulin metabolic signaling in aged skeletal muscle is very limited. Here, we tested the hypothesis that activation of sirtuin 1 deacetylase activity by resveratrol would prevent the disruption of insulin signaling and augmentation of catabolic markers induced by doxorubicin in aged skeletal muscle. Two- and 10-month-old senescence-accelerated mice (prone 8) were randomized to receive saline, doxorubicin, doxorubicin and resveratrol, or a combination of doxorubicin, resveratrol, and sirtinol or EX527. Doxorubicin reduced the sirtuin 1 activity without affecting the phosphorylation levels of IRS1Ser307, mTORSer2481, AktThr308/Ser473, membranous glucose transporter 4, protein abundance of PDK4, and enzymatic activity of pyruvate dehydrogenase in aged muscles. Intriguingly, resveratrol attenuated the doxorubicin-induced elevations of apoptotic and catabolic markers measured as Bax, caspase 3 activity, apoptotic DNA fragmentation, MuRF-1, ubiquitinated proteins, and proteasomal activity in aged muscles, whereas these beneficial effects were abolished on inhibition of sirtuin 1 by sirtinol or EX527. Markers of insulin signaling were not affected by doxorubicin or resveratrol in the senescent skeletal muscle. Nevertheless, the antiapoptotic and anticatabolic effects of resveratrol in aged skeletal muscle treated with doxorubicin were mediated in a sirtuin 1–dependent signaling manner.
Keywords: Apoptosis, SIRT1, Resveratrol, Doxorubicin
Decades after the discovery and application of doxorubicin in treating malignancies, it is still unknown whether doxorubicin would disrupt insulin signaling in the skeletal muscle. Previous studies have reported that doxorubicin increased the circulating levels of cholesterol and triglycerides (1). Thalidomide, an antiangiogenetic compound, was shown to accentuate insulin resistance in diabetic patients (2); however, whether this finding can be extrapolated to doxorubicin remains undetermined. There are also data suggesting that insulin receptor and insulin receptor substrate 1 (IRS1) were downregulated in doxorubicin-resistant breast cancer cells (3), but relevant data collected from skeletal muscle are still lacking. More importantly, factors including age and hyperglycemia were found to be the predictors of short-term survival in leukemia patients exposed to combined treatment with doxorubicin and other chemotherapeutics (4). Taken together, these findings bring forward the idea that manipulation of insulin signaling in the skeletal muscle of aged individuals undergoing doxorubicin chemotherapy might be clinically important.
Accumulating body of evidence raises the possibility that modulation of insulin and/or senescent signaling might alleviate multiorgan toxicity induced by doxorubicin. Metformin, a common antidiabetic prescription drug, was found to reduce the circulating level of malondialdehyde, a marker of lipid peroxidation, in adriamycin-treated mice (5). Overexpression of the thyroid hormone modulator D2 was reported to attenuate the reduction of cardiac function induced by doxorubicin, an effect observed in concomitant with increased phosphorylation of Akt at serine 473 and upregulation of glucose transporter 1 (GLUT1) (6). Furthermore, pharmacological activation of peroxisome proliferator–activated receptor δ, a transcription factor of various genes related to insulin sensitivity, reduced the senescence marker p16 and increased the total content of Bcl6 in cardiomyocytes challenged with doxorubicin (7). Considering that short-term physical exercise increased telomerase activity and alleviated doxorubicin-induced cardiomyopathy and apoptosis in mice (8), it is attempting to speculate that compounds that exhibit antihyperglycemic/prosurvival properties might ameliorate toxicity of doxorubicin in the aged skeletal muscle.
Recent studies suggest that resveratrol, which is a natural antioxidant present in grapes and red wine, improves insulin sensitivity. Resveratrol has been shown to reduce plasma insulin and fasting blood glucose, and attenuated islet apoptosis in mice fed with high-fat diet; in mice, these changes were paralleled by an increase in expression of sirtuin 1 (SIRT1), which is a longevity-related protein (9). Protein kinase B (Akt) is known to have dual roles in the regulation of insulin signaling and apoptotic cell death. Although the protein abundances of phospho-AktSer473 and proapoptotic Bim were reduced and elevated, respectively, in neuronal cultures in response to high-glucose exposure, these alterations were prevented by resveratrol treatment (10). It has also been reported that resveratrol elevated basal glucose uptake in cultured L6 myotubes in a dose-dependent manner (11) and upregulated SIRT1 and GLUT4 in the skeletal muscle of type 2 diabetic patients (12). Consistent data indicated that resveratrol increased membrane localization of GLUT4 in the soleus muscles of rats challenged with high-fat diet—an effect observed in concomitant with reduced fasting blood glucose (13). Nonetheless, it remains largely unknown whether doxorubicin would aggravate the disruption of insulin signaling in the aged skeletal muscle.
In the present study, we tested the hypothesis that doxorubicin would deteriorate insulin signaling in aged skeletal muscle and that the restoration of SIRT1 deacetylase activity by resveratrol would protect against the myotoxicity of doxorubicin in aged mice through concomitant modulation of insulin, apoptotic and catabolic signaling pathways.
Methods
Animals and Chemicals
Male senescence-accelerated mice (prone 8) obtained from the Chinese University of Hong Kong were used in this study. Animal husbandry was carried out according to our previous publication (14). All experimental procedures were approved by the Animal Subjects Ethics Subcommittee of the Hong Kong Polytechnic University. Resveratrol (R5010) and dimethyl sulphoxide (D2650) vehicle were purchased from Sigma Chemicals (St Louis, MO). To evaluate whether the modulating effects of resveratrol were mediated by SIRT1, this study involved the use of two specific SIRT1 inhibitors, namely sirtinol (205976, Santa Cruz Biotechnology, Santa Cruz, CA) and EX527 (E7034, Sigma).
Study Design
To study the effects of aging, 2-month old (young) and 10-month old (aged) senescence-accelerated mice (prone 8) were used (n = 5 per group). On Day 1, all mice were given a single intraperitoneal injection of 18mg/kg doxorubicin and vehicle (15) except the saline control (SC) group receiving the corresponding amount of saline. Resveratrol was then administered via the intraperitoneal injection route at 20mg/kg/day (16) for 3 consecutive days in the doxorubicin-treated mice. Dimethyl sulphoxide vehicle was given to the SC, and doxorubicin and vehicle groups accordingly. Animals assigned to combined treatment with sirtinol and EX527 received 2mg/kg/day sirtinol (17) and 5mg/kg/day EX527, respectively (based on our dose-optimization experiments). Both SIRT1 inhibitors were administered intraperitoneally immediately after the delivery of resveratrol. Twenty-four hours after the last injection of drug treatment, all animals were sacrificed by a lethal dose of ketamine (Alfasan, Woerden, The Netherlands). The gastrocnemius muscles were then rapidly excised and stored at −80°C until further analysis.
Immunoblotting
Cytoplasmic proteins were extracted from muscle homogenates according to the procedures previously reported by our laboratory (14). Membranous proteins were prepared by using a membrane protein extraction kit (K268-50, Biovision, Milpitas, CA). Equal amount of proteins (30 µg), as determined by Bradford assay, were subject to separation by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by transfer to polyvinylidene difluoride membranes (Immobilon P, Millipore, Billerica, MA) at 300 mA for 2 hours. The membranes were blocked in 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 for 1 hour at room temperature. After overnight incubation with the respective primary antibodies prepared in Tris-buffered saline with 0.1% Tween 20 containing 2% bovine serum albumin at 4°C, the membranes were washed and then incubated with appropriate secondary antibodies. Luminol reagent (NEL103001EA, Perkin Elmer, Waltham, MA) was applied and chemiluminescent signals were captured using a Kodak 4000R Pro camera. All data were normalized to the signal of β-tubulin.
This study involved the use of the following primary antibodies: anti-SIRT1 (15404, Santa Cruz Biotechnology), anti-GLUT4 (07-1404, Millipore), anti-Na+/K+ATPase (3010, Cell Signaling, Danvers, MA), anti-PDK4 (14495, Santa Cruz Biotechnology), anti-phospho-IRS1Ser307 (2381, Cell Signaling), anti-IRS1 (2382, Cell Signaling), anti-PDK1 (13037, Cell Signaling), anti-phospho-AktThr308 (4056, Cell Signaling), anti-Akt (9272, Cell Signaling), anti-phospho-mTORSer2481 (2974, Cell Signaling), anti-mTOR (2983, Cell Signaling), anti-phospho-AktSer473 (9271, Cell Signaling), anti-Bax (493, Santa Cruz Biotechnology), anti-MuRF-1 (32920, Santa Cruz Biotechnology), anti-ubiquitin (3936, Cell Signaling), and anti-β-tubulin (T0198, Sigma) and secondary antibodies: anti-rabbit IgG (7074, Cell Signaling), anti-mouse IgG (7076, Cell Signaling), and anti-goat IgG (2020, Santa Cruz Biotechnology).
SIRT1 Deacetylation Assay
Deacetylase activity of SIRT1 was measured by a fluorometric method in accordance with the instructions of the manufacturer (Cyclex, Nagoya, Japan). All data were normalized to the respective protein concentrations in the assay reactions.
Immunofluorescent Staining
Cryostat sections were prepared at a thickness of 10 µm. The slides were allowed to air-dry at room temperature for 1 hour followed by fixation with ice-cold acetone for 10 minutes at −20°C. After washing, the sections were blocked with 1% bovine serum albumin in 1× phosphate-buffered saline for 1 hour at room temperature and subject to overnight incubation with anti-GLUT4 antibody (07-1404, Millipore; 1:400) prepared in 1× phosphate-buffered saline containing 1% bovine serum albumin at 4°C. The samples were then washed and incubated with fluorescein-tagged secondary anti-rabbit antibody (FI-1000, Vector Laboratories, Burlingame, CA) for 1 hour at room temperature in a light-free environment. Cover slips were then sealed following the application of 4′,6-diamidino-2-phenylindole mounting medium (H1200, Vector Laboratories). All samples were kept in dark at 4°C until confocal microscopy using 20× objectives (Biological Research Microscope 80i, Nikon, Melville, NY).
Pyruvate Dehydrogenase Activity Assay
The activity of pyruvate dehydrogenase (PDH) was determined by a colorimetric method (K679-100, Biovision). All procedures were conducted with full adherence to the instructions of the manufacturer.
Caspase 3 Activity Assay
Protease activity of caspase 3 activity was assessed by a fluorometric approach involving the use of the caspase 3 substrate, DEVD-AFC (1007–200, Biovision) according to the recommendations of the manufacturer.
Cell Death ELISA Assay
Apoptotic DNA fragmentation was detected by a cell death ELISA assay kit (Roche Diagnostics, Indianapolis, IN). All procedures were adhered to the manufacturer’s instructions.
Proteasome Activity Assay
Proteasomal activity was measured based on the release of fluorescent AMC from AMC-tagged peptide substrate in the presence of proteolytic activity (K245-100, Biovision). All procedures were conducted as suggested by the manufacturer.
Statistical Analyses
Statistical analyses were conducted using SPSS 21.0 (IBM, Chicago, IL). A normality test was performed to examine data distributions. All data were expressed as means ± SEM. Comparisons were made using one-way analysis of variance followed by Tukey post hoc tests.
Statistical significance was considered at p < .05.
Results
Body Mass and Muscle Mass
Aged mice were generally heavier than young mice at baseline and posttreatment irrespective of the drug(s) administered (Table 1). The body mass was reduced by 8.8% and 5.3% in young and aged mice, respectively, in response to doxorubicin challenge, but these decreases were not reverted by resveratrol treatment (Table 1). Compared with young mice, the mass of medial gastrocnemius muscle in aged mice was 15.7% lower at baseline (Table 1). Doxorubicin decreased the muscle mass in young mice by 17.2% and in aged mice by 8.1% (Table 1). Resveratrol, but not in conjunction with sirtinol or EX527, mitigated the reduction of muscle mass induced by doxorubicin, although the myoprotection of resveratrol did not reach to statistical significance (young: p = .075; aged: p = .068) (Table 1). However, when the muscle mass was normalized to the respective body mass (ie, relative muscle mass), resveratrol significantly protected against the doxorubicin-induced muscle loss in both age groups in the absence of SIRT1 inhibition (Table 1).
Table 1.
Body Mass, Muscle Mass, and Relative Muscle Mass in Response to the Respective Treatment in Both Age Groups
| Young | Pretreatment | Posttreatment | Aged | Pretreatment | Posttreatment |
|---|---|---|---|---|---|
| Body mass (g) | |||||
| SC | 22.15±0.40 | 22.10±0.41 | SC | 25.83±0.39a | 25.83±0.40e |
| DV | 21.85±0.45 | 19.93±0.58b | DV | 26.00±0.35a | 24.63±0.31b,e |
| DR | 22.18±0.38 | 21.05±0.41 | DR | 26.23±0.48a | 24.85±0.40e |
| DRS | 21.43±0.41 | 20.03±0.60 | DRS | 25.95±0.57a | 24.58±0.73e |
| DRE | 21.65±0.35 | 19.80±0.60 | DRE | 26.58±0.36a | 25.18±0.50e |
| Wet weight of medial gastrocnemius muscle (g) | |||||
| SC | 0.051±0.0022 | SC | 0.043±0.0009a | ||
| DV | 0.042±0.0015b | DV | 0.040±0.0018b | ||
| DR | 0.048±0.0019(p = .075) | DR | 0.044±0.0009(p = .068) | ||
| DRS | 0.041±0.0009d | DRS | 0.039±0.0019d | ||
| DRE | 0.045±0.0026(p = .081) | DRE | 0.038±0.0009d | ||
| Muscle weight normalized to body weight (%) | |||||
| SC | 0.231±0.0067 | SC | 0.167±0.0041a | ||
| DV | 0.213±0.0119b | DV | 0.160±0.0062b,e | ||
| DR | 0.226±0.0099c | DR | 0.176±0.0060c,e | ||
| DRS | 0.204±0.0089d | DRS | 0.157±0.0091d,e | ||
| DRE | 0.218±0.0162d | DRE | 0.152±0.0057d,e | ||
Notes: DR = doxorubicin and resveratrol; DRE = DR with EX527; DRS = DR with sirtinol; DV = doxorubicin and vehicle; SC = saline control. Mice were randomly assigned to SC, DV, and DR with DRS or DRE groups.
a p < .05, aging effect.
b p < .05, doxorubicin effect.
c p < .05, resveratrol effect.
d p < .05, inhibitor effect.
e p < .05, aging effect within treatment group.
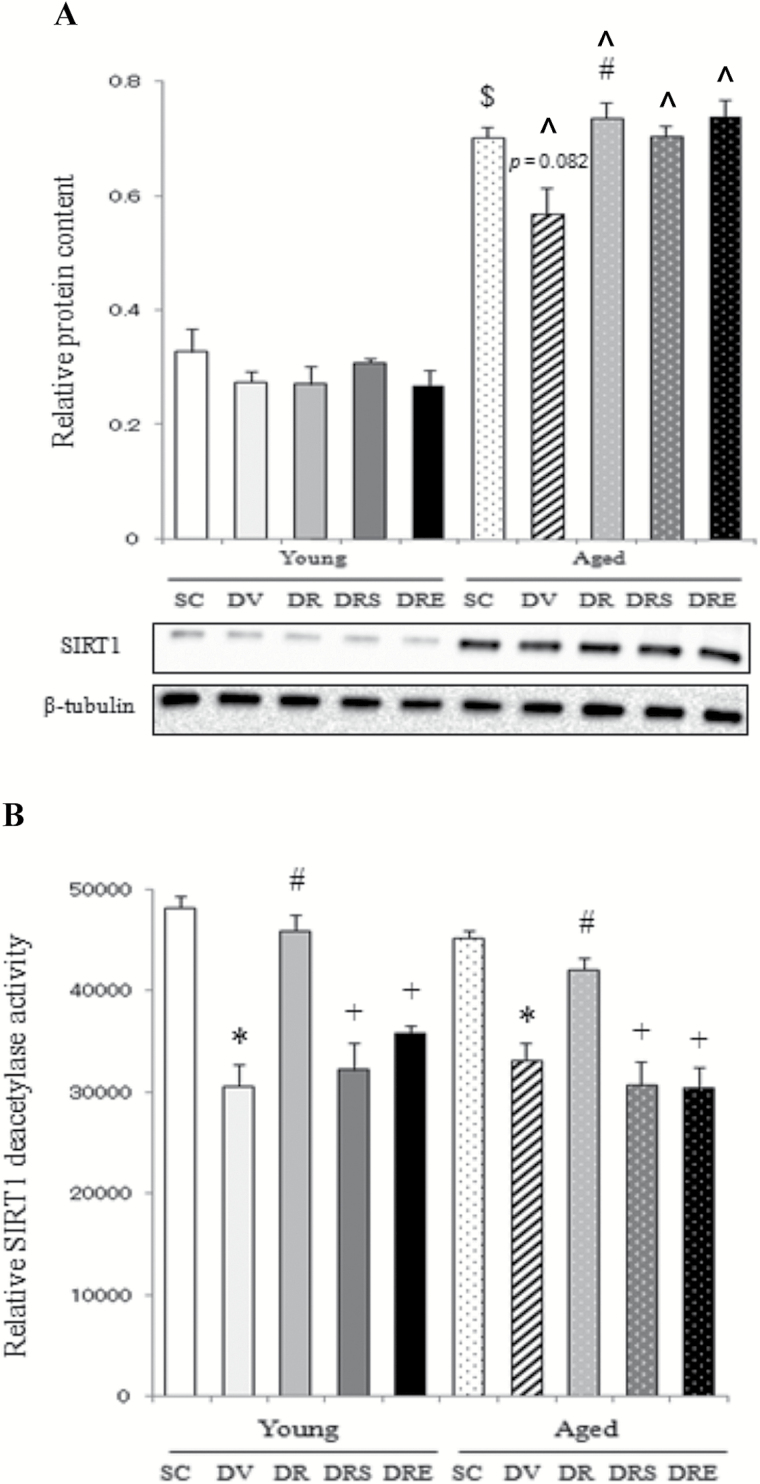
SIRT1 Expression and Activity
In young mice, the protein abundance of SIRT1 was not changed with any of the interventions (Figure 1A). The protein expression of SIRT1 was elevated significantly by 113% in the skeletal muscle of aged mice compared with young mice in the saline-treated control group (Figure 1A). Doxorubicin tended to downregulate SIRT1 by 19% (p = .082) in aged muscles, whereas this attenuation was blunted by resveratrol administration regardless of SIRT1 inhibition by sirtinol or EX527 (Figure 1A). Basal activity of SIRT1 in the aged skeletal muscle was not significantly different from that in the young counterparts (Figure 1B). The deacetylase activity of SIRT1 was reduced significantly in both young (36%) and aged muscles (29%) in response to doxorubicin challenge; these reductions were reversed by resveratrol, but not in combination with SIRT1 inhibitors sirtinol or EX527 (Figure 1B).
Figure 1.
Expression and activity of sirtuin 1 (SIRT1). Resveratrol increased the protein level of SIRT1 in doxorubicin-treated, aged skeletal muscle (A). Doxorubicin suppressed SIRT1 activity in the skeletal muscle of both age groups, but the activity of SIRT1 was restored in response to resveratrol treatment (B). $ p < .05, aging effect; *p < .05, doxorubicin effect; # p < .05, resveratrol effect; + p < .05, inhibitor effect; ^p < .05, aging effect within treatment group. Mice were randomly assigned to saline control (SC), doxorubicin and vehicle (DV), and doxorubicin and resveratrol (DR) with sirtinol (DRS) or EX527 (DRE) groups.
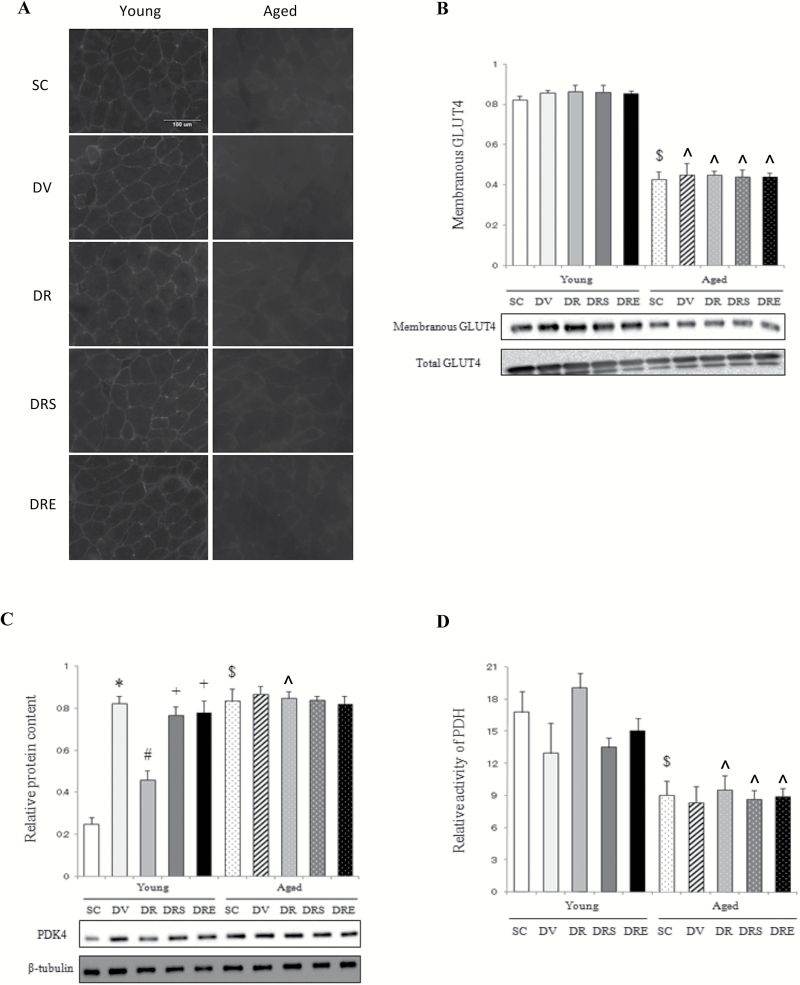
GLUT4 Localization and Glycolytic Activity of PDH
Immunofluorescence of membranous GLUT4 was reduced in aged muscles relative to young muscles in the SC group (Figure 2A). However, there were no intervention effects on membranous GLUT4 in the skeletal muscle of both age groups (Figure 2A). Our immunoblot analyses showed that the membranous content of GLUT4 was decreased significantly by 58% in aged muscles compared with young muscles of the SC group (Figure 2B). However, none of the drug treatments affected the membranous level of GLUT4 in both age groups (Figure 2B). The protein expression of PDK4, an inhibitor of the glycolytic enzyme PDH, was elevated remarkably by 238% in the skeletal muscle of aged mice (Figure 2C). Doxorubicin upregulated PDK4 remarkably by 231% in the skeletal muscle of young mice, but not of aged mice (Figure 2C). This doxorubicin-induced increase, however, was abrogated by combination treatment with resveratrol, but not in the presence of sirtinol or EX527 in young mice (Figure 2C). In the SC group, the enzymatic activity of PDH was 46% lower in aged mice compared with young mice (Figure 2D). However, the activity of PDH was not affected by any of the interventions in muscles of both age groups (Figure 2D).
Figure 2.
Markers of glucose usage. The membranous immunoreactivity of glucose transporter 4 (GLUT4) in aged muscles did not reduce further with doxorubicin treatment (A). Western blotting indicated that no intervention effects were observed on membranous GLUT4 in the skeletal muscle (B). Basal expression of PDK4 was elevated in aged muscles but remained unaffected in response to any of the drug treatments (C). The activity of pyruvate dehydrogenase (PDH) was decreased in aged mice (D). $ p < .05, aging effect; *p < .05, doxorubicin effect; # p < .05, resveratrol effect; + p < .05, inhibitor effect; ^p < 0.05, aging effect within treatment group. Mice were randomly assigned to saline control (SC), doxorubicin and vehicle (DV), and doxorubicin and resveratrol (DR) with sirtinol (DRS) or EX527 (DRE) groups.
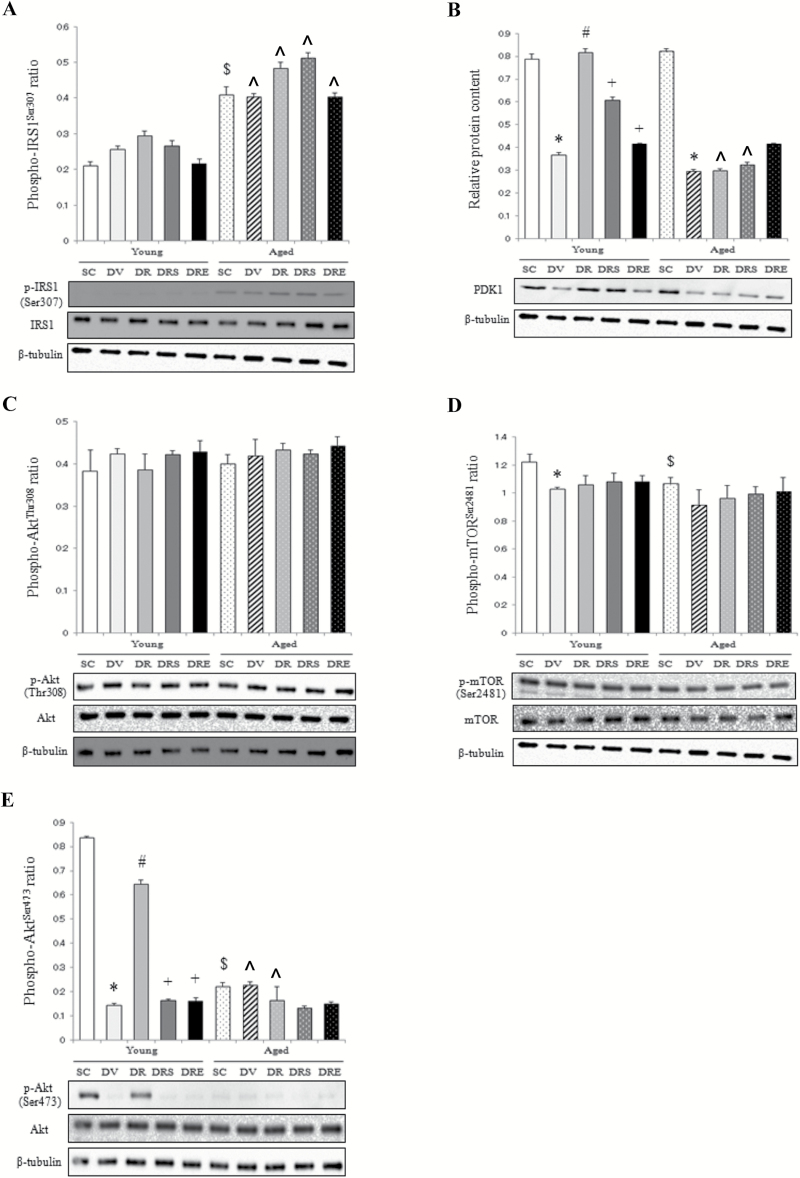
Insulin Signaling Markers
In the SC group, the phosphorylation status of IRS1 at serine 307 in aged muscles was 94% higher relative to young muscles (Figure 3A). The protein level of phospho-IRS1Ser307 in the aged muscle was not affected by any of the drug treatments (Figure 3A). In young mice, the protein content of PDK1 was reduced significantly by 53% in response to doxorubicin treatment, whereas this reduction was abolished by resveratrol, but not in combination with sirtinol or EX527 (Figure 3B). Doxorubicin also downregulated PDK1 by 64% in aged muscles, but this reduction was not affected by combined treatment with resveratrol (Figure 3B). However, the level of phosphorylated AktThr308 in both age groups did not change with any of the drugs studied (Figure 3C). The phosphorylation status of mTORSer2481 was reduced by 16% in doxorubicin-treated young muscles compared with the saline-treated counterparts, but this reduction was not affected by resveratrol administration (Figure 3D). The protein content of phospho-mTORSer2481 was 13% lower in aged muscles than in young muscles, but remained unchanged with any of the treatments (Figure 3D). In young muscles, doxorubicin reduced remarkably the content of phospho-AktSer473 by 83%, whereas this decrease was attenuated by resveratrol, but not in conjunction with the SIRT1 inhibitors (Figure 3E). The basal level of phospho-AktSer473 was 73% lower in aged mice compared with young mice (Figure 3E). No treatment effects were observed in the senescent muscles (Figure 3E).
Figure 3.
Markers of insulin signaling. The phosphorylation status of insulin receptor substrate 1 (IRS1) at Ser307 did not change with doxorubicin treatment (A). The protein level of PDK1 in aged muscles was reduced in response to doxorubicin administration (B). Doxorubicin did not have any effects on the protein abundances of p-AktThr308 (C), p-mTORSer2481 (D), and p-AktSer473 (E) in the aged skeletal muscle. $ p < .05, aging effect; *p < .05, doxorubicin effect; # p < .05, resveratrol effect; + p < .05, inhibitor effect; ^p < .05, aging effect within treatment group. Mice were randomly assigned to saline control (SC), doxorubicin and vehicle (DV), and doxorubicin and resveratrol (DR) with sirtinol (DRS) or EX527 (DRE) groups.
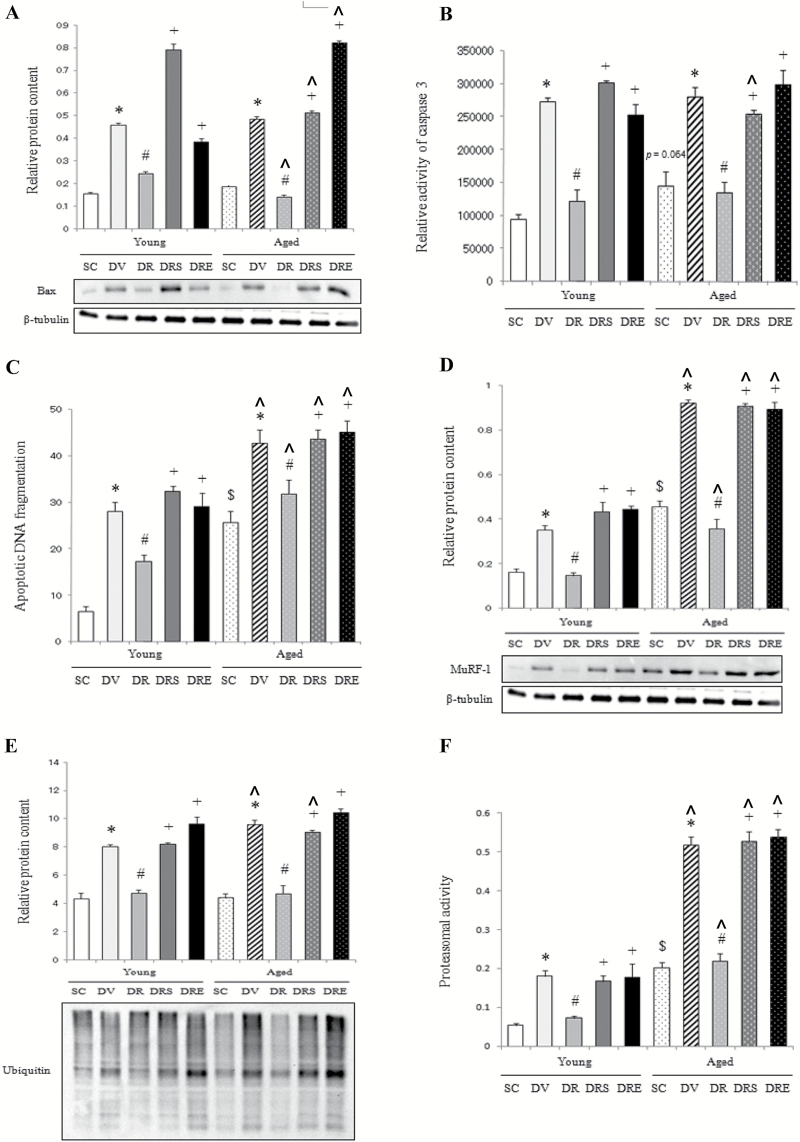
Apoptotic and Catabolic Markers
Basal protein expression level of Bax did not differ significantly between muscles of young and aged mice (Figure 4A). Doxorubicin elevated remarkably the protein abundance of Bax in the skeletal muscle of young and aged mice by 159% and 198%, respectively, whereas these doxorubicin-induced increases were prevented by resveratrol in the absence of SIRT1 inhibition (Figure 4A). Caspase 3 activity and apoptotic DNA fragmentation were 55% (p = 0.064) and 292% higher in aged mice compared with young mice (Figure 4B and C). Doxorubicin increased caspase 3 activity and apoptotic DNA fragmentation in both muscles of young (by 190% and 328%, respectively) and aged mice (by 93% and 68%, respectively), whereas these elevations were not seen in mice cotreated with resveratrol in the absence of SIRT1 inhibition (Figure 4B and C). Basal protein abundance of MuRF-1 in aged muscle was 180% higher compared with the young muscle (Figure 4D). The protein content of MuRF-1 was increased significantly by 116% and 102% in young and aged mice, respectively, in response to doxorubicin challenge, whereas these elevations were blunted by resveratrol, but not in combination with the SIRT1 inhibitors (Figure 4D). Protein ubiquitination was not significantly different between young and aged animals (Figure 4E). Doxorubicin elevated significantly the content of ubiquitinated proteins by 85% and 116% in young and aged muscles, respectively, whereas these increases were abolished by resveratrol only in the absence of the SIRT1 inhibitors (Figure 4E). The proteasomal activity was elevated remarkably by 279% in aged muscles relative to young muscles; this elevation was augmented further by 157% in response to doxorubicin treatment (Figure 4F). Resveratrol, but not in conjunction with any of the SIRT1 inhibitors, blunted the doxorubicin-induced increase in proteasomal activity in both age groups (Figure 4F).
Figure 4.
Apoptotic and catabolic signaling. Resveratrol prevented the doxorubicin-induced increases in Bax protein (A), caspase 3 activity (B) and apoptotic DNA fragmentation (C) in the aged muscle. The elevations of MuRF-1 (D), ubiquitinated proteins (E), and proteasomal activity (F) induced by doxorubicin treatment were abolished only by resveratrol, but not in the presence of sirtuin 1 inhibitors. $ p < .05, aging effect; *p < .05, doxorubicin effect; # p < .05, resveratrol effect; + p < .05, inhibitor effect; ^p < .05, aging effect within treatment group. Mice were randomly assigned to saline control (SC), doxorubicin and vehicle (DV), and doxorubicin and resveratrol (DR) with sirtinol (DRS) or EX527 (DRE) groups.
Discussion
Resveratrol Prevents Apoptotic Activation in Aged Muscles Challenged With Doxorubicin
Although intense efforts have been dedicated to the study of doxorubicin-induced cardiomyocyte apoptosis, relevant studies on doxorubicin toxicity in skeletal muscle are scarce. In agreement with a recent study showing that doxorubicin elevated the protein abundance of p53 and reduced cell viability in differentiated myotubes (18), our laboratory has further demonstrated that ghrelin, a stomach-derived peptide, antagonized the increases in Bax protein, TUNEL index, and apoptotic DNA fragmentation induced by doxorubicin in skeletal muscle (19). However, it remains largely unknown whether resveratrol would deactivate apoptotic signaling in the aged skeletal muscle challenged with doxorubicin. The present study provides the first evidence that resveratrol represses the doxorubicin-induced apoptosis measured as Bax, caspase 3 activity, and apoptotic DNA fragmentation in the aged skeletal muscle through the restoration of SIRT1 deacetylase activity. These data are in line with those reported in cardiomyocytes, in which the protein level of p53 was elevated with concomitant reduction in AMP-activated protein kinase activity (20) and SIRT1 transgenic mice manifested a reduction of cleavage of caspase 3 in response to doxorubicin stimulation (21). Furthermore, our data that pharmacological inhibition of SIRT1 by sirtinol or EX527 reversed the attenuation of apoptotic markers in muscles cotreated with doxorubicin and resveratrol support a previous study exhibiting that resveratrol enhanced SIRT1 activity and mitigated acetylation of p53 and Bax transactivation in doxorubicin-treated hearts (22). The findings of others and ours overall support the notion that activation of SIRT1 deacetylase activity may represent a promising therapeutic strategy to relieve doxorubicin-induced myotoxicity in aging individuals.
Resveratrol Blunted the Accentuation of Catabolic Pathway Induced by Doxorubicin
There are also data illustrating that increased catabolic signaling probably contributes to the toxicity of doxorubicin in skeletal muscle. It has been shown that the increase in mRNA level of MAFbx, an atrophy-related E3 ligase, was paralleled by reductions of protein contents of actin and myosin in C2C12 myotubes treated with doxorubicin (23). In contrast, these doxorubicin-induced alterations were abrogated by SS31, a cell-permeable peptide that reduces mitochondrial oxidants (23). These results agree with a recent report showing that doxorubicin increased carbonylation and degradation of cardiac myosin binding protein C in HL-1 cultures and spontaneously hypertensive rats (24). Previous research has also indicated that resveratrol inhibited total protein degradation in phorbol ester–treated myotubes (25), whereas the doxorubicin-induced elevation of cathepsin L, a lysosomal protease, was blunted in exercised-trained rats compared with sedentary controls (26). Here, we demonstrated for the first time that resveratrol suppressed the doxorubicin-induced elevations of MuRF-1, protein ubiquitination, and proteasomal activity in the aged skeletal muscle. These findings concur with the implications of anticatabolic interventions in myocardial toxicity of doxorubicin as suggested by the studies reporting that (1) doxorubicin upregulated MAFbx, MuRF-1, and ubiquitinated proteins in the heart (27) and (2) treatment with the proteasomal inhibitor MG132 ameliorated the reduction of cardiomyocyte surface area induced by doxorubicin (28). Our present findings suggest that further investigation of compounds that can modulate SIRT1/catabolic markers shall be granted for the alleviation of doxorubicin-induced toxicity in the skeletal muscle. This is thus of important clinical significance, provided that loss of muscle mass is evident in patients receiving doxorubicin therapy (29).
SIRT1 Activation by Resveratrol Does Not Affect Insulin Signaling in Aged Muscles
The therapeutic implications of resveratrol in experimental models of insulin resistance are well documented. This statement is well supported by the findings showing that resveratrol (6–11 weeks of intervention) elevated the protein abundances of GLUT4 and phospho-AktThr308 in the skeletal muscle of pigs fed with hypercholesterolemic diet (30) and upregulated GLUT4 and glycogen synthase in the skeletal muscle of db/db diabetic mice (31). Consistent findings indicated that piceatannol, a metabolite of resveratrol, attenuated the increase in blood glucose in db/db diabetic mice during glucose tolerance test (32). In contrast, another line of evidence suggested that resveratrol may confer divergent effects on markers of insulin signaling. Resveratrol suppressed the reduction of phospho-AktSer473 in insulin-resistant adipocytes, whereas in insulin-sensitive adipocytes, resveratrol attenuated the phosphorylation level of AktSer473 (33). It is also noteworthy that a 2-week resveratrol treatment restored the insulin-induced phosphorylation of AktSer473/Thr308 only in the liver, but not the skeletal muscle of animals fed with high-fat diet (33). Intriguingly, resveratrol abolished the elevation and reduction of phosphorylated AMPKThr172, which is an upstream activating signal of SIRT1, in the liver and skeletal muscle, respectively, induced by high-fat diet (33). Other findings exhibited that resveratrol supplementation lasting for 12 weeks did not affect insulin sensitivity and SIRT1 expression in the vastus lateralis muscle of non-obese women with normal glucose tolerance (34). These data raise the possibility that SIRT1 may not necessarily be a putative target mediating the hypoglycemic effects of resveratrol in the skeletal muscle. In this study, we demonstrated that resveratrol restored SIRT1 activity and attenuated apoptotic/catabolic markers, but had no effects on the Akt phosphorylation cascade and GLUT4 localization in doxorubicin-treated aged muscles. Our findings were supported by a recent study reporting that the phosphorylation levels of AktSer473/Thr308 were not enhanced by overexpression of SIRT1 in the skeletal muscle regardless of insulin stimulation (35). Corroborating data revealed that forced expression of SIRT1 mitigated ischemic reperfusion injury in the heart but failed to improve glucose uptake in the soleus muscle of mice challenged with high-fat diet (36). Consistently, 30-day intake of resveratrol has been demonstrated to increase total antioxidant status without affecting blood levels of glucose, insulin, and cholesterol in adult smokers (37). Taken together, it appears that acute resveratrol treatment may have more pronounced effects on stress responses rather than on insulin signaling cascade.
SIRT1 as a Target of Intervention Against Aging/Doxorubicin-Induced Muscle Atrophy
Activation of SIRT1 by resveratrol appears to be an attractive candidate to avert muscle loss associated with aging and repeated use of doxorubicin in cancer patients. This statement is supported by our data demonstrating that resveratrol alleviated the reduction of relative muscle mass, but not body mass induced by doxorubicin treatment in aged animals in the absence of SIRT1 inhibition. In fact, it has been shown that resveratrol hampered the hindlimb suspension–induced aggravation of muscle loss in senescent rats without affecting the body mass (38), thereby pointing out that skeletal muscle is an important site of action to mediate the anticatabolic effects of SIRT1 during muscle wasting. One should also be beware of the current observation that the SIRT1 inhibitors used in this study halted only the elevation of enzymatic activity, but not expression of SIRT1 in aged muscles cotreated with doxorubicin and resveratrol. Functional characterization of sirtinol and EX527 has unraveled that these compounds conferred significant inhibition of SIRT1 activity despite that the protein level of SIRT1 remained unchanged with escalating doses of these inhibitors (39), hence suggesting that targeting the enzymatic activity, but not the protein itself, might apparently be a more efficient strategy to modulate the downstream targets of SIRT1. Compelling evidence also showed that 2mg/kg sirtinol (ie, the dose adopted in this study) abrogated the curcumin-induced reduction of TUNEL-positive nuclei and elevation of SIRT1 protein in hearts exposed to ischemic injury (17). In the present study, the same dose of sirtinol was observed to restore the proapoptotic/procatabolic effects of doxorubicin, suggesting that 2mg/kg sirtinol was sufficient to abolish the myoprotective effects of SIRT1. Nevertheless, future research is needed to dissect the relative contribution of protein expression and deacetylase activity of SIRT1 in the orchestration of apoptotic and catabolic pathways in skeletal muscle through the use of suitable genetic approaches such as deacetylation-defective mutants.
Conclusion
The present study is the first attempt to unravel that the antiapoptotic/anticatabolic effects of resveratrol in the doxorubicin-challenged skeletal muscle of aged mice are SIRT1 dependent. Although it remains to be determined for the mechanisms underlying the observation that doxorubicin did not impair insulin signaling in the aged skeletal muscle, our data suggest that inhibition of apoptotic/catabolic pathways by resveratrol may represent an option of adjuvant therapy in doxorubicin-induced myotoxicity in aging individuals—effects that can be ascribed to the restoration of SIRT1 deacetylase activity.
Funding
This study was supported by The Hong Kong Polytechnic University (RPTL).
Conflict of Interest
The authors did not identify any conflicts of interest to declare.
Acknowledgments
The authors would like to acknowledge animal husbandry support received from the Centralized Animal Facilities of The Hong Kong Polytechnic University and the Laboratory Services Centre of The Chinese University of Hong Kong.
References
- 1. Venkatesan N, Venkatesan P, Karthikeyan J, Arumugam V. Protection by taurine against adriamycin-induced proteinuria and hyperlipidemia in rats. Proc Soc Exp Biol Med. 1997;215:158–164. [DOI] [PubMed] [Google Scholar]
- 2. Iqbal N, Zayed M, Boden G. Thalidomide impairs insulin action on glucose uptake and glycogen synthesis in patients with type 2 diabetes. Diabetes Care. 2000;23:1172–1176. [DOI] [PubMed] [Google Scholar]
- 3. Aljada A, Saleh AM, Al Suwaidan S. Modulation of insulin/IGFs pathways by sirtuin-7 inhibition in drug-induced chemoresistance. Diagn Pathol. 2014;9:94. doi:10.1186/1746-1596-9-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vu K, Busaidy N, Cabanillas ME, et al. A randomized controlled trial of an intensive insulin regimen in patients with hyperglycemic acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2012;12:355–362. doi:10.1016/j.clml.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aleisa AM, Al-Rejaie SS, Bakheet SA, et al. Effect of metformin on clastogenic and biochemical changes induced by adriamycin in Swiss albino mice. Mutat Res. 2007;634:93–100. [DOI] [PubMed] [Google Scholar]
- 6. Hong EG, Kim BW, Jung DY, et al. Cardiac expression of human type 2 iodothyronine deiodinase increases glucose metabolism and protects against doxorubicin-induced cardiac dysfunction in male mice. Endocrinology. 2013;154:3937–3946. doi:10.1210/en.2012-2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altieri P, Spallarossa P, Barisione C, et al. Inhibition of doxorubicin-induced senescence by PPARδ activation agonists in cardiac muscle cells: cooperation between PPARδ and Bcl6. PLoS One. 2012;7:e46126. doi:10.1371/journal.pone.0046126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Werner C, Hanhoun M, Widmann T, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52:470–482. [DOI] [PubMed] [Google Scholar]
- 9. Zhang J, Chen L, Zheng J, et al. The protective effect of resveratrol on islet insulin secretion and morphology in mice on a high-fat diet. Diabetes Res Clin Pract. 2012;97:474–482. [DOI] [PubMed] [Google Scholar]
- 10. Liu MH, Yuan C, He J, et al. Resveratrol protects PC12 cells from high glucose-induced neurotoxicity via PI3K/Akt/FoxO3a pathway. Cell Mol Neurobiol. 2015;35:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minakawa M, Kawano A, Miura Y, Yagasaki K. Hypoglycemic effect of resveratrol in type 2 diabetic model db/db mice and its actions in cultured L6 myotubes and RIN-5F pancreatic β-cells. J Clin Biochem Nutr. 2011;48:237–244. doi:10.3164/jcbn.10-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goh KP, Lee HY, Lau DP, Supaat W, Chan YH, Koh AF. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int J Sport Nutr Exerc Metab. 2014;24:2–13. [DOI] [PubMed] [Google Scholar]
- 13. Tan Z, Zhou LJ, Mu PW, et al. Caveolin-3 is involved in the protection of resveratrol against high-fat-diet-induced insulin resistance by promoting GLUT4 translocation to the plasma membrane in skeletal muscle of ovariectomized rats. J Nutr Biochem. 2012;23:1716–1724. [DOI] [PubMed] [Google Scholar]
- 14. Sin TK, Yu AP, Yung BY, et al. Modulating effect of SIRT1 activation induced by resveratrol on Foxo1-associated apoptotic signalling in senescent heart. J Physiol. 2014;592(Pt 12):2535–2548. doi:10.1113/jphysiol.2014.271387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu J, Zhang J, Xiang D, et al. Recombinant human interleukin-1 receptor antagonist protects mice against acute doxorubicin-induced cardiotoxicity. Eur J Pharmacol. 2010;643:247–253. [DOI] [PubMed] [Google Scholar]
- 16. Oktem G, Uysal A, Oral O, et al. Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp Toxicol Pathol. 2012;64:471–479. [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Duan W, Lin Y, et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013;65:667–679. [DOI] [PubMed] [Google Scholar]
- 18. Fortini P, Ferretti C, Pascucci B, et al. DNA damage response by single-strand breaks in terminally differentiated muscle cells and the control of muscle integrity. Cell Death Differ. 2012;19:1741–1749. doi:10.1038/cdd.2012.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu AP, Pei XM, Sin TK, et al. Acylated and unacylated ghrelin inhibit doxorubicin-induced apoptosis in skeletal muscle. Acta Physiol (Oxf). 2014;211:201–213. [DOI] [PubMed] [Google Scholar]
- 20. Wang S, Song P, Zou MH. Inhibition of AMP-activated protein kinase α (AMPKα) by doxorubicin accentuates genotoxic stress and cell death in mouse embryonic fibroblasts and cardiomyocytes: role of p53 and SIRT1. J Biol Chem. 2012;287:8001–8012. doi:10.1074/jbc.M111.315812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng W, Lu YB, Liang ST, et al. SIRT1 mediates the protective function of Nkx2.5 during stress in cardiomyocytes. Basic Res Cardiol. 2013;108:364. [DOI] [PubMed] [Google Scholar]
- 22. Zhang C, Feng Y, Qu S, et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res. 2011;90:538–545. [DOI] [PubMed] [Google Scholar]
- 23. Gilliam LA, Moylan JS, Patterson EW, et al. Doxorubicin acts via mitochondrial ROS to stimulate catabolism in C2C12 myotubes. Am J Physiol Cell Physiol. 2012;302:C195–C202. doi:10.1152/ajpcell.00217.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aryal B, Jeong J, Rao VA. Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity. Proc Natl Acad Sci USA. 2014;111:2011–2016. doi:10.1073/pnas.1321783111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wyke SM, Tisdale MJ. Induction of protein degradation in skeletal muscle by a phorbol ester involves upregulation of the ubiquitin-proteasome proteolytic pathway. Life Sci. 2006;78:2898–2910. [DOI] [PubMed] [Google Scholar]
- 26. Smuder AJ, Kavazis AN, Min K, Powers SK. Exercise protects against doxorubicin-induced markers of autophagy signaling in skeletal muscle. J Appl Physiol (1985). 2011;111:1190–1198. [DOI] [PubMed] [Google Scholar]
- 27. Sishi BJ, Loos B, van Rooyen J, Engelbrecht AM. Doxorubicin induces protein ubiquitination and inhibits proteasome activity during cardiotoxicity. Toxicology. 2013;309:23–29. [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto Y, Hoshino Y, Ito T, et al. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res. 2008;79:89–96. [DOI] [PubMed] [Google Scholar]
- 29. Tozer RG, Tai P, Falconer W, et al. Cysteine-rich protein reverses weight loss in lung cancer patients receiving chemotherapy or radiotherapy. Antioxid Redox Signal. 2008;10:395–402. [DOI] [PubMed] [Google Scholar]
- 30. Burgess TA, Robich MP, Chu LM, Bianchi C, Sellke FW. Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch Surg. 2011;146:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Do GM, Jung UJ, Park HJ, et al. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol Nutr Food Res. 2012;56:1282–1291. [DOI] [PubMed] [Google Scholar]
- 32. Minakawa M, Miura Y, Yagasaki K. Piceatannol, a resveratrol derivative, promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and suppresses blood glucose levels in type 2 diabetic model db/db mice. Biochem Biophys Res Commun. 2012;422:469–475. [DOI] [PubMed] [Google Scholar]
- 33. Kang W, Hong HJ, Guan J, et al. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012;61:424–433. [DOI] [PubMed] [Google Scholar]
- 34. Yoshino J, Conte C, Fontana L, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi:10.1016/j.cmet.2012.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White AT, McCurdy CE, Philp A, Hamilton DL, Johnson CD, Schenk S. Skeletal muscle-specific overexpression of SIRT1 does not enhance whole-body energy expenditure or insulin sensitivity in young mice. Diabetologia. 2013;56:1629–1637. doi:10.1007/s00125-013-2912-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White AT, Philp A, Fridolfsson HN, et al. High-fat diet-induced impairment of skeletal muscle insulin sensitivity is not prevented by SIRT1 overexpression. Am J Physiol Endocrinol Metab. 2014;307:E764–E772. doi:10.1152/ajpendo.00001.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bo S, Ciccone G, Castiglione A, et al. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20:1323–1331. [DOI] [PubMed] [Google Scholar]
- 38. Jackson JR, Ryan MJ, Hao Y, Alway SE. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1572–R1581. doi:10.1152/ajpregu.00489.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peck B, Chen CY, Ho KK, et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9:844–855. [DOI] [PubMed] [Google Scholar]