Abstract
Background:
Albuminuria is associated with cognitive impairment in people with type 2 diabetes mellitus (T2DM). The brain volume correlates of albuminuria in people with T2DM have not been well investigated.
Methods:
We examined 502 individuals with T2DM (9–12 years duration; mean age ~62 years) who had a brain MRI at baseline and at 40 months. Baseline MRI findings were examined by the presence or absence of albuminuria (≥30mg/g creatinine). Changes in MRI findings were examined by whether albuminuria was persistent, intermittent, or absent during follow-up.
Results:
At baseline, participants with albuminuria (28.7% of the cohort) had more abnormal white matter volume (AWMV) than participants without albuminuria on unadjusted analysis. This difference was attenuated with adjustment for systolic blood pressure, which was higher in participants with albuminuria than in those without albuminuria. During ~3.5 years of follow-up, participants with persistent albuminuria (15.8%) had a greater increase in new AWMV than participants without albuminuria (59.8%) or those with intermittent albuminuria on unadjusted analysis. This difference was attenuated with adjustment for age and systolic blood pressure. There were no significant differences in gray matter volume and total brain volume between participants with or without albuminuria at baseline or during follow-up. There was no significant effect modification of these findings by estimated glomerular filtration rate (eGFR) at baseline or change in eGFR during follow-up.
Conclusions:
In this diabetic cohort, baseline albuminuria and persistent albuminuria were not independently associated with any significant differences in brain volume measurements compared with participants without albuminuria.
Keywords: Albuminuria, Brain MRI, White matter volume, Gray matter volume
Older adults with type 2 diabetes mellitus (T2DM) are at increased risk of cognitive impairment as compared with similarly aged nondiabetic adults (1). Cerebral small vessel disease is believed to be a common cause of such impairment (2).
Albuminuria—a renal microvascular disorder—is associated with and predicts cognitive impairment in people with or without diabetes, suggesting that it may be a marker of cerebral microvascular disease (3–5). This hypothesis has biological plausibility because the renal and cerebral microcirculations share common factors. Both are characterized by low resistance (6). Loss of auto-regulation due to endothelial dysfunction exposes both vascular beds to high pressure, the exudation of protein into surrounding tissue, and the release of inflammation proteins (7,8). Pathologically, both the renal microvasculature in people with albuminuria and the cerebral microvasculature in people with cognitive impairment are characterized by tortuosity and thickened basement membranes (9).
In the ACCORD-MIND study, participants with persistent albuminuria and type 2 diabetes (T2DM) developed declines in information processing speed and auditory-verbal learning (Digit Symbol Substitution Test, Rey Auditory Verbal Learning Test, respectively) over ~3.5 years of follow-up compared with participants without albuminuria (10). Importantly, this finding was in participants who scored relatively high on the Mini-Mental State Examination, who had near-normal mean estimated glomerular filtration rate (eGFR ~90mL/min/1.73 m2), and was independent of baseline eGFR and annual rate of eGFR decline. Albuminuria was present in ~42% of the cohort at baseline, consistent with estimates from prior U.S. survey studies of diabetic adults of similar age (~62 years) (11).
MRI-derived brain structural correlates of impaired executive function in people with T2DM and albuminuria have been examined cross-sectionally, but few studies have examined this association prospectively. In the present study, we examine the associations between albuminuria at baseline and during follow-up with total brain volume (TBV), gray matter volume (GMV), and abnormal white matter disease volume (AWMV) on brain MRI in a subcohort of ACCORD-MIND participants. Because albuminuria can be associated with decline in eGFR, we further categorize our findings by the presence or absence of decreased eGFR. We hypothesize that albuminuria is independently associated with increased AWMV, a marker of cerebral small vessel disease (12), and with greater TBV and GMV atrophy.
Methods
The ACCORD trial was a randomized, multicenter, double 2×2 factorial design trial of 10,251 participants with T2DM who were at high risk for cardiovascular disease (CVD) events because of existing CVD or additional cardiovascular risk factors (13). The trial tested the effect on CVD outcomes of standard versus intensive therapeutic strategies aimed to reach physiologic goals for glycemic control, and within that trial, for blood pressure and LDL cholesterol. Renal exclusion criteria were (i) a dipstick result on a spot urine test >2+; (ii) a protein/creatinine ratio on a spot urine >700mg/dl; (iii) >1g protein/creatinine in a 24 hour urine; and (iv) a serum creatinine level >1.5mg/dl within 2 months of study participation.
ACCORD-MIND was an ancillary ACCORD study (14). It tested the hypothesis that persons randomized to an intensive glycemic therapeutic strategy targeting HbA1c <6% would have better cognitive function and a larger brain volume at 40 months follow-up than persons randomized to a standard strategy targeting HbA1c 7.0%–7.9%. The study found no differences in cognitive function between the two treatment approaches, but TBV was greater in those randomized to intensive glycemic therapy at 40 months follow-up (15).
The National Institute on Aging, in collaboration with the National Heart Lung and Blood Institute, sponsored the ACCORD-MIND trial. All participants signed informed consent. The ACCORD-MIND study adhered to the principles of the Declaration of Helsinki. The institutional review board or ethics committee at each participating center approved the ACCORD protocol. Separate informed consent was obtained for MIND.
MRI Subcohort
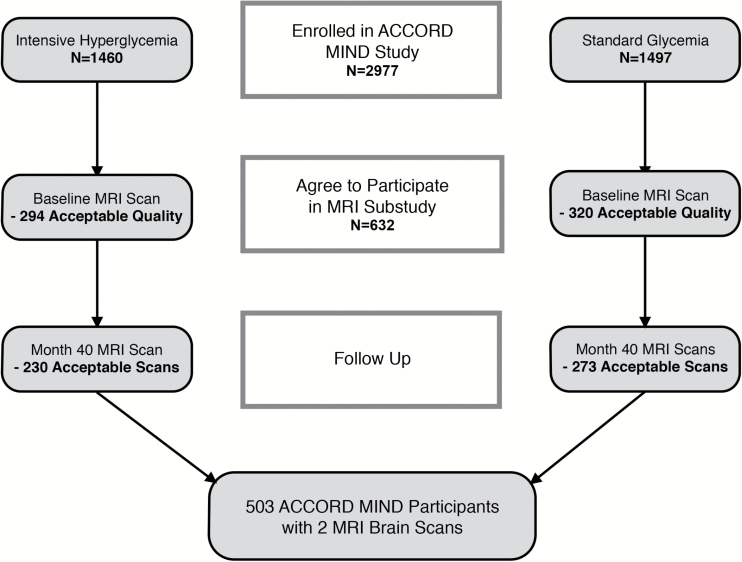
The MRI subcohort of ACCORD-MIND was recruited as previously described (14) (Figure 1). Scans were done twice: once at baseline, within 45 days after randomization; the second, at 40 months. There were 614 participants who had a baseline MRI scan; of these, 502 had a repeat MRI scan. For these analyses, we use only the 502 participants with two scans. Participants in the MRI subcohort had the same age, sex, ethnic distribution, alcohol consumption, systolic blood pressure (SBP), duration of known diabetes, cholesterol, and mean eGFR; had lower prevalence of CVD and albuminuria; and were less likely to be in the intensive glycemia and lipid arms of ACCORD than participants in the parent ACCORD-MIND cohort (Supplementary Table 1).
Figure 1.
Flow chart of the ACCORD-MIND MRI subcohort.
The standardized MRI scan protocol (14,15) and image analysis have been described (16,17). Monthly MRI quality control procedures followed the American College of Radiology’s MRI QC Program (http://www.acr.org/quality-safety/accreditation/mri). Performance of MRI scanners was stable across MRI sites throughout the duration of the study as reflected by the stability of intracranial volumes over time (baseline mean intracranial volume, 1,132.34cm3; follow-up mean intracranial volume, 1,132.32cm3; p = .47 by paired t test).
TBV, GMV, and AWMV for normal and abnormal tissue were estimated in cubic centimeters. T1-weighted volumetric MRI scans were first preprocessed according to a standardized protocol for alignment, removal of extra-cerebral tissue, and segmentation of brain parenchyma into gray matter, white matter, and cerebrospinal fluid. Following additional preprocessing steps, the lesion segmentation component of the algorithm was applied, based on local signal features (ie, T1, PD, T2, and FLAIR). White matter hyperintensities (also called, abnormal white matter volume [AWMV]) are most obvious on T2-weighted scans. They are often located next to the ventricles in the brain’s white matter and are believed to represent areas of small blood vessel disease.
Albuminuria, eGFR, and Annual eGFR Rate of Change
Urine albumin was measured at four time points: at baseline, at 24 and 48 months, and at the exit visit. The majority of participants had two or three postbaseline values (Supplementary Table 2). Albuminuria was defined as ≥30mg albumin/g creatinine. Owing to the small number of participants with macro-albuminuria (n = 22), albuminuria was not dichotomized into micro- and macro-albuminuria. Because albuminuria is often intermittent (18), participants were prospectively categorized by the presence or absence of albuminuria at baseline and on at least one follow-up urine test. Participants with albuminuria at baseline and on all the follow-up urine tests were considered to have persistent albuminuria. Those with no albuminuria at baseline or on any of the follow-up urine tests were considered to have no albuminuria. Participants with no albuminuria at baseline who developed albuminuria on at least one follow-up test were considered progressors. Those with albuminuria at baseline but none on follow-up were considered remitters.
Creatinine levels were measured a mean of 12 times during the study (Supplementary Table 2). eGFR was calculated using the CKD-Epi formula (19). eGFR levels at baseline were categorized into ≥ or <90mL/min/1.73 m2. This cutpoint was the mean eGFR value of participants without albuminuria at baseline. Annual change in eGFR is measured as the slope for the regression line of eGFR from baseline to Month 40 visit.
Statistical Methods
For the baseline analyses, we compare the mean and frequencies of MRI substudy participant characteristics and baseline brain volumes in those with and without albuminuria. Within the albuminuria groups, comparisons of brain volumes between those with eGFR ≥90 and <90mL/min/1.73 m2 were compared by t tests. For tables with all four albuminuria groups, three a priori pairwise comparisons with the no albuminuria group were performed adjusting for three multiple comparisons using the Bonferroni technique.
For the longitudinal analyses, changes in brain volumes from baseline to 40 months between the four albuminuria groups are presented. The least square means for changes in volumes is the same as the least square means for period effects in a repeated measures analysis of covariance model. Within the albuminuria groups, comparisons of brain volumes between those with eGFR ≥90 and <90mL/min/1.73 m2 were done. In Tables 3 and 5, an interaction term was added to the model between presence of albuminuria and eGFR ≥90 and <90mL/min/1.73 m2 and tested for significance.
Baseline analyses of brain MRI volumes are adjusted for age, sex, intracranial volume (to adjust for differences in head size), diabetes duration, prevalent CVD, SBP, and baseline eGFR. Analyses of MRI volume changes are additionally adjusted for incident CVD events, the treatment arms of the parent ACCORD study (intensive vs standard glycemia, lipid, and blood pressure control), for the annual rate of eGFR change, and for the interaction of baseline eGFR < or ≥ 90mL/min/1.73 m2.
Results
Baseline Characteristics of the MRI Cohort by the Presence or Absence of Albuminuria
Compared with participants without albuminuria, participants with albuminuria at baseline were older and were more likely to be men (Table 1). They had higher SBP, more history of hypertension, and a longer duration of T2DM. They did not differ in terms of smoking status, race distribution, alcohol intake, baseline cholesterol levels, mean eGFR, prevalent CVD, or assignments to the different arms of the ACCORD study. They also did not differ significantly with regard to subsequent annual rate of eGFR change.
Table 1.
Baseline Characteristics of ACCORD-MIND Participants Who Had a Baseline and 40 Months Brain MRI Categorized by the Presence or Absence of Albuminuria (≥30mg/g creatinine)
| Total Group | Albuminuria– | Albuminuria+ | p Value | |
|---|---|---|---|---|
| n = 502 | n = 358 (71.3%) | n = 144 (28.7%) | ||
| Age (y) | 62.2 (5.7) | 61.8 (5.5) | 63.2 (5.9) | .012 |
| Sex (% female) | 233 (46.4%) | 185 (51.7%) | 48 (33.3%) | .0002 |
| Smoking status | .52 | |||
| Never | 234 (46.7) | 168 (46.9%) | 67 (45.8%) | |
| Former | 203 (40.5) | 148 (41.3%) | 55 (27.1%) | |
| Current | 64 (12.8%) | 42 (11.7%) | 22 (15.4%) | |
| Race/ethnicity | .44 | |||
| African American | 87 (17.3) | 59 (16.5%) | 28 (19.4%) | |
| White | 340 (67.7%) | 244 (68.2%) | 96 (66.7%) | |
| Hispanic | 44 (8.8%) | 35 (9.8%) | 9 (6.2%) | |
| Other | 31 (6.2%) | 20 (5.6%) | 11 (7.6%) | |
| Alcoholic drinks per week | .42 | |||
| 0 | 368 (73.4%) | 266 (74.5%) | 102 (70.8%) | |
| 1–2 | 74 (14.8%) | 52 (14.6%) | 22 (15.3%) | |
| 3–7 | 39 (7.8%) | 28 (7.8%) | 11 (7.6%) | |
| 8 or more | 20 (4.0%) | 11 (3.1%) | 9 (6.2%) | |
| Systolic BP (mm Hg) | 134.8 (17.9) | 132.4 (17.6) | 140.8 (17.3) | <.0001 |
| History of HTN | 451 (89.8) | 315 (88.0) | 136 (94.4%) | .033 |
| Duration diabetes (y) | 10.0 (7.1) | 9.2 (6.9) | 11.9 (7.4) | .0002 |
| Cholesterol (mg/dl) | 181.9 (40.4) | 179.7 (39.7) | 187.3 (41.8) | .055 |
| LDL cholesterol (mg/dl) | 101.4 (32.4) | 100.2 (31.7) | 104.6 (34.0) | .17 |
| HDL cholesterol female (mg/dl) | 48.5 (12.3) | 48.3 (12.4) | 49.5 (12.2) | .55 |
| HDL cholesterol male (mg/dl) | 39.9 (10.2) | 39.7 (9.1) | 40.4 (12.1) | .57 |
| ACR (median, 25th, 75th IQ) | 12.1 (6.2–37.1) | 7.9 (5.1–13.) | 75.1 (45.5–201.9) | <.0001 |
| ACR >299 | 22 (4.4%) | 0 (0%) | 22 (15.2%) | <.0001 |
| eGFR (median IQ) mL/min/1.73 m2 | 88.5 (21.1) | 89.5 (20.5) | 85.9 (22.3) | .086 |
| History of CVD | 127 (25.3%) | 82 (22.9%) | 45 (31.2%) | .054 |
| Intensive lipid participant (%) | 101 (20.1%) | 77 (21.5%) | 24 (16.7) | .27 |
| Intensive BP participant (%) | 152 (30.3%) | 107 (29.9%) | 45 (31.2) | .83 |
| Intensive glucose participant (%) | 229 (45.6%) | 164 (45.8%) | 65 (45.1%) | .92 |
| ΔeGFR/y during trial, mean (SD) mL/min/1.73 m2/y | −3.8 (6.2) | −3.6 (6.2) | −4.1 (−5.1) | .41 |
Notes: ACR = albumin creatinine ratio; BP = blood pressure; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; HTN = hypertension.
Baseline MRI Findings
On unadjusted analysis, participants with albuminuria at baseline had significantly higher AWMV than participants without albuminuria (Table 2). There were no significant differences in TBV or GMV. With adjustment for SBP, the difference in AWMV between those with and without albuminuria was no longer significant. Adjustment for other covariates further attenuated this difference.
Table 2.
Baseline MRI Volumes Categorized by the Presence or Absence of Albuminuria
| No Albuminuria at Baseline | Albuminuria at Baseline | p Value | |
|---|---|---|---|
| n = 358 | n = 144 | ||
| Model I | |||
| Baseline AWMV | 1.80 (0.20) | 2.83 (0.32) | .008 |
| Baseline TBV | 921.1 (5.1) | 937.1 (8.0) | .09 |
| Baseline GMV | 464.2 (2.6) | 464.7 (4.0) | .93 |
| Model II | |||
| Baseline AWMV | 1.93 (0.22) | 2.87 (0.44) | .06 |
| Baseline TBV | 916.9 (5.5) | 936.2 (10.9) | .12 |
| Baseline GMV | 463.0 (2.8) | 461.6 (5.6) | .83 |
| Model III | |||
| Baseline AWMV | 1.96 (0.19) | 2.38 (0.31) | .27 |
| Baseline TBV | 925.8 (1.3) | 924.6 (2.1) | .63 |
| Baseline GMV | 465.6 (1.4) | 460.7 (2.3) | .08 |
| Model IV | 2.03 (0.19) | 2.23 (0.32 | .60 |
| Baseline AWMV | |||
| Baseline TBV | 925.8 (1.4) | 924.9 (2.2) | .73 |
| Baseline GMV | 465.4 (1.4) | 461.5 (2.3) | .17 |
Notes: AWMV = abnormal white matter volume; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; GMV = gray matter volume; SBP = systolic blood pressure; TBV = total brain volume.
Least square means (SE). Values are in cubic centimeters.
Model I: unadjusted.
Model II: adjusted for SBP.
Model III: adjusted for SBP, age, sex, and intracranial volume.
Model IV: adjusted for model III plus diabetes duration, prevalent CVD, and baseline eGFR.
When those with and without baseline albuminuria were categorized by eGFR ≥ or <90mL/min/1.73 m2 (Table 3), 60.8% of those with albuminuria had baseline eGFR of <90mL/min/1.73 m2. The mean eGFR of this group was 72.8mL/min/1.73 m2. Participants with albuminuria and eGFR <90mL/min/1.73 m2 had higher AWMV than participants with albuminuria and eGFR ≥90mL/min/1.73 m2 (3.40 [0.41] vs 1.97 [0.51] cc; p < .05) on unadjusted analysis. This finding was attenuated with adjustment for SBP. Among participants without albuminuria, adjustment for SBP led to significant differences between those with eGFR ≥ or <90mL/min/1.73 m2 for AWMV and TBV, but these differences were attenuated with further adjustments. There were no significant differences for GMV in either albuminuria groups when categorized by eGFR level. Interaction terms showed no significant differences in albuminuria groups when eGFR was ≥ or <90mL/min/1.73 m2.
Table 3.
Baseline MRI Volumes Categorized by Albuminuria Status and eGFR
| Variable | No ALB at Baseline | ALB at Baseline | |||
|---|---|---|---|---|---|
| eGFR ≥ 90 | eGFR < 90 | eGFR ≥ 90 | eGFR < 90 | Interaction‡ | |
| n = 174 | n = 180 | n = 56 | n = 87 | ||
| Model I | |||||
| Baseline total AWMV | 1.42 (0.29) | 2.13 (0.28) | 1.97 (0.51) | 3.40 (0.41)* | .51 |
| Baseline TBV | 931.4 (7.2) | 912.1 (7.1) | 945.6 (12.8) | 930.2 (10.2) | .84 |
| Baseline GMV | 468.2 (3.7) | 460.9 (3.6) | 469.7 (6.5) | 461.3 (5.2) | .91 |
| Model II | |||||
| Baseline total AWMV | 1.43 (0.29) | 2.31 (0.28)* | 1.82 (0.50) | 3.05 (0.41) | .65 |
| Baseline TBV | 931.4 (7.2) | 908.7 (7.2)* | 948.2 (12.8) | 934.4 (10.5) | .65 |
| Baseline GMV | 468.1 (3.7) | 469.7 (3.7) | 470.5 (6.5) | 462.5 (5.3) | .97 |
| Model III | |||||
| Baseline total AWMV | 1.71 (0.28) | 2.17 (0.28) | 2.04 (0.49) | 2.64 (0.41) | .85 |
| Baseline TBV | 923.6 (2.0) | 928.4 (2.0) | 922.5 (3.4)† | 925.9 (2.9) | .79 |
| Baseline GMV | 463.7 (2.0) | 468.6 (2.0) | 457.1 (3.6)† | 463/5 (3.0) | .93 |
| Model IV | |||||
| Baseline total AWMV | 1.78 (0.34) | 2.20 (0.32) | 2.03 (0.53) | 2.44 (0.45) | .99 |
| Baseline TBV | 924.4 (2.4) | 927.4 (2.3) | 923.7 (3.8) | 925.8 (3.2) | .86 |
| Baseline GMV | 463.5 (2.5) | 467.2 (2.4) | 458.7 (3.9) | 467.1 (3.9) | .74 |
| Duration of known DM (y) | 9.9 (0.5) | 8.7 (0.5) | 11.1 (0.9) | 12.3 (0.8)† | |
Notes: ALB = albuminuria; AWMV = abnormal white matter volume; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; GMV = gray matter volume; SBP = systolic blood pressure; TBV = total brain volume.
Least square means (SE). Values are in cubic centimeters. Several baseline eGFR measures were missing leading to slight differences in numbers from Table 2.
Model I: unadjusted.
Model II: adjusted for SBP.
Model III: adjusted for SBP, age, sex, and intracranial volume.
Model IV: adjusted for terms in Model III plus diabetes duration, prevalent CVD, and baseline eGFR.
*eGFR < 90 significantly (p < .05) different than the eGFR ≥ 90 group for the same level of ALB status.
†Any ALB different from No ALB (p < .05) at the same level of eGFR category.
‡Interaction between presence of ALB and GFR ≥ 90 or < 90.
Participants with albuminuria and eGFR <90mL/min/1.73 m2 had the longest duration of T2DM compared with other participants and significantly longer than participants without albuminuria and eGFR <90mL/min/1.73 m2.
Longitudinal Brain Volume Changes
In unadjusted models, participants with persistent albuminuria had the greatest increase of new AWMV of the four albuminuria groups (Table 4). The increase was significantly greater than the no albuminuria group. With adjustment for SBP, the increase in new AWMV in those with persistent albuminuria continued to be the highest of all the albuminuria groups and significantly higher than the no albuminuria group. Adjustment for other covariates abolished this significant difference. Decline in TBV and GMV occurred in all four albuminuria groups, but there were no significant differences across the groups.
Table 4.
Change in MRI Volumes From Baseline to 40 Months Categorized by Albuminuria Status
| No Albuminuria | Persistent Albuminuria | Remitters | Progressors | |
|---|---|---|---|---|
| n = 298 (59.5%) | n = 79 (15.8%) | n = 64 (12.8%) | n = 60 (12.0%) | |
| Model I | ||||
| AWMV change | 1.29 (0.16) | 2.54 (0.31)* | 0.80 (0.34) | 2.02 (0.35) |
| TBV change | −14.0 (1.0) | −17.7 (1.9) | −17.5 (2.1) | −16.0 (2.1) |
| GMV change | −19.7 (1.1) | −17.0 (2.2) | −19.6 (2.4) | −21.8 (2.5) |
| Model II | ||||
| AWMV change | 1.30 (0.16) | 2.44 (0.31)* | 0.79 (0.34) | 2.02 (0.35) |
| TBV change | −13.7 (0.9) | −18.0 (1.8) | −17.4 (2.0) | −16.0 (2.1) |
| GMV change | −19.5 (1.1) | −17.1 (2.2) | −19.6 (2.4) | −21.8 (2.5) |
| Model III | ||||
| AWMV change | 1.36 (0.16) | 2.18 (0.32) | 0.82 (0.34) | 2.01 (0.35) |
| TBV change | −14.0 (0.9) | −16.4 (1.8) | −17.7 (2.0) | −16.1 (2.0) |
| GMV change | −19.8 (1.1) | −16.0 (2.3) | −20.1 (2.5) | −21.5 (2.5) |
| Model IV | ||||
| AWMV change | 1.41 (0.16) | 2.10 (0.32) | 0.77 (0.34) | 1.92 (0.35) |
| TBV change | −14.3 (0.9) | −15.9 (1.8) | −17.4 (2.0) | −15.6 (2.0) |
| GMV change | −20.1 (1.1) | −15.3 (2.3) | −20.1 (2.5) | −21.2 (2.5) |
| Model V | ||||
| AWMV change | 1.42 (0.16) | 2.04 (0.34) | 0.77 (0.35) | 1.95 (0.36) |
| TBV change | −14.4 (0.9) | −15.1 (1.9) | −17.1 (2.0) | −14.5 (2.0) |
| GMV change | −20.1 (1.1) | −14.0 (2.4) | −20.0 (2.5) | −20.6 (2.6) |
Notes: AWMV = abnormal white matter volume; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; GMV = gray matter volume; SBP = systolic blood pressure; TBV = total brain volume.
Least square means (SE). Values are in cubic centimeters.
Model I: unadjusted.
Model II: adjusted for SBP.
Model III: adjusted for age, sex, SBP at baseline, treatment arms of ACCORD study (intensive vs standard glycemia, lipid, and blood pressure control), and intracranial volume.
Model IV: adjusted for terms in Model III plus diabetes duration, prevalent and incident CVD, baseline eGFR, and annual rate of eGFR change.
Model V: adjusted for terms in Model IV plus interaction term with baseline eGFR ≥ 90 vs eGFR < 90mL/min/1.73 m2.
*Significantly different from the No albuminuria group (p < .05).
When participants with no albuminuria and persistent albuminuria were categorized by baseline eGFR level ≥ or <90mL/min/1.73 m2 (Table 5), those with persistent albuminuria and eGFR <90mL/min/1.73 m2 had significantly more new AWMV than participants with persistent albuminuria and eGFR ≥90mL/min/1.73 m2 in unadjusted analyses. Participants with persistent albuminuria and eGFR <90mL/min/1.73 m2 also had more decline in TBV and GMV. However, interaction terms between albuminuria and eGFR ≥ or <90mL/min/1.73 m2 were not significant.
Table 5.
Change in MRI Volumes Categorized by Albuminuria Status and eGFR
| Variable | No ALB | Persistent ALB | Interaction‡ | ||
|---|---|---|---|---|---|
| eGFR ≥ 90 | eGFR < 90 | eGFR ≥ 90 | eGFR < 90 | ||
| n = 144 | n = 152 | n = 23 | n = 55 | ||
| Model I | |||||
| AWMV change | 1.25 (0.23) | 1.33 (0.22) | 1.70 (0.56) | 2.89 (0.37)* | .13 |
| TBV change | −12.8 (1.3) | −14.6 (1.3) | −10.8 (3.2) | −21.2 (2.1)* | .38 |
| GMV change | −18.1 (1.6) | −21.0 (1.6) | −7.2 (3.9)† | −21.3 (2.6)* | .12 |
| Model II | |||||
| AWMV change | 1.26 (0.23) | 1.36 (0.22) | 1.67 (0.55) | 2.78 (0.37) | .17 |
| TBV change | −12.7 (1.3) | −14.4 (1.3) | −11.1 (3.2) | −21.9 (2.2)* | .032 |
| GMV change | −18.0 (1.7) | −20.9 (1.6) | −7.4 (4.0)† | −21.8 (2.8)* | .032 |
| Model III | |||||
| AWMV change | 1.35 (0.23) | 1.34 (0.22) | 1.70 (0.56) | 2.59 (0.38)† | .25 |
| TBV change | −13.3 (1.3) | −14.7 (1.3) | −10.8 (3.2) | −19.3 (2.2) | .66 |
| GMV change | −18.3 (1.6) | −21.1 (1.6) | −7.1 (4.0)† | −20.5 (2.7) | .16 |
| Model IV | |||||
| AWMV change | 1.40 (0.27) | 1.37 (0.26) | 1.76 (0.57) | 2.48 (0.40)† | .28 |
| TBV change | −12.4 (1.5) | −16.1 (1.3) | −9.6 (3.3) | −19.7 (2.2)* | .74 |
| GMV change | −18.5 (1.7) | −21.3 (1.7) | −6.7 (4.1)† | −19.8 (2.8)* | .13 |
| ΔeGFR/y, mean (SD) mL/min/1.73 m2/y | −5.76 (0.8) | −3.12 (0.64)* | −5.05 (0.46) | −2.26 (0.46)* | |
Notes: ALB = albuminuria; AWMV = abnormal white matter volume; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; GMV = gray matter volume; SBP = systolic blood pressure; TBV = total brain volume.
Least square means (SE). Values are in cubic centimeters. Several baseline eGFR measures were missing leading to slight differences in numbers from Table 4.
Model I: unadjusted.
Model II: adjusted for SBP.
Model III: adjusted for age, sex, SBP, treatment arms of the study (intensive vs standard glycemia, lipid, and blood pressure control), and intracranial volume.
Model IV: adjusted for terms in Model III plus diabetes duration, prevalent and incident CVD, baseline eGFR, and change in eGFR/y.
*Mean for GFR categories different (p < .05) for the same level of ALB status.
†Mean for Persistent ALB different from No ALB (p < .05) for the same level of GFR category.
‡Interaction between presence of ALB and GFR ≥ 90 and < 90.
With adjustment for SBP, the statistically significant difference in the amount of new AWMV in those with persistent albuminuria and eGFR ≥ or <90mL/min/1.73 m2 was no longer significant. The differences in TBV and GMV, however, persisted and the interaction terms between albuminuria and eGFR status were significant. After further adjustments (including the annual change in eGFR during follow-up), there was still a persistent decline in TBV and GMV (~10 cc and 12 cc, respectively) among those with persistent albuminuria and eGFR <90mL/min/1.73 m2 as compared with those with eGFR >90mL/min/1.73 m2. However, the interaction term between albuminuria and eGFR status was not significant.
Discussion
In this study of middle-aged and older adults with T2DM, we report that albuminuria at baseline and its persistence during follow-up were not independently associated with increased AWMV when analyses were adjusted for SBP. Likewise, there were no significant differences in the decline in GMV or TBV whether albuminuria was present or not after the effects of elevated SBP and age were accounted for. These findings run contrary to our hypothesis that the association of albuminuria with early decline in executive function is related to brain volume changes. Rather it appears that the association of albuminuria with early declines in executive function is due to vascular risk factors shared by albuminuria and cognitive decline, that is, elevated SBP and advancing age (20,21).
Several cross-sectional studies have found positive associations between T2DM and the presence of AWMV (22–24), though not all studies agree (25,26). Two prospective studies have reported an increased rate of AWMV development in people with T2DM (23,27). With regard to albuminuria, several cross-sectional (3) and prospective studies (28,29) of people with T2DM have found that it increases AWMV, whereas other studies have not (26,30). Differences in cohort characteristics (eg, age, duration of T2DM, and racial mix) and factors that were adjusted for may explain some of these inconsistencies.
The role of albuminuria in TBV and GMV decline is also inconsistent in prior studies. In two cross-sectional studies (5,25), albuminuria was associated with lower GMV. In a cross-sectional study of diabetic African Americans, higher levels of albuminuria were also associated with lower GMV (24). On the other hand, a study of European Americans (78% with T2DM) (30) and from Holland (26) showed no difference in GMV in the presence of albuminuria. The latter results are similar to our own, in which 67% of the cohort is European American.
Two other of our findings should be noted. First, during follow-up, participants with persistent albuminuria had a smaller amount of GMV decline (~14 cc) compared with participants without albuminuria or intermittent albuminuria (~20 cc). When, however, the group with persistent albuminuria was categorized by the eGFR level at the time of baseline examination, a significant difference was found between the amount of GMV lost between those with intact eGFR versus those with decreased eGFR (−6.7 cc vs −19.8 cc; p < .05). This finding suggests that diminished eGFR enhanced the effect of albuminuria on GMV. Testing for interaction, however, was not statistically significant. Interaction testing (between albuminuria and intact versus diminished eGFR) was also not significant for AWMV increase or TBV decline. Other studies that have examined the interaction of albuminuria with reduced versus normal eGFR on brain volume measures have also reported nonsignificant interaction results (31–33).
A second finding was that the rate of TBV loss (~15 cc / 925 cc baseline volume over ~3.5 years = ~0.5% per year) and GMV loss (~20 cc / 463 cc baseline GMV volume per ~3.5 years = ~1.2%) in our cohort were consistent with brain volume loss associated with aging in healthy adults of 60 years of age (34). The losses were not excessive.
This study has several strengths. Our study included a prospective component that gauged change in brain volumes. We measured albuminuria over time; most studies rely on testing at one point in time only, not differentiating between persistent and intermittent albuminuria. Our cohort had a large number of participants relative to other brain MRI studies in T2DM. It consisted of people with normal or near-normal cognitive function, and a relatively narrow age range, thereby reducing variability in baseline factors and brain disease. Creatinine levels were measured centrally, reducing measurement variation due to use of multiple laboratories. Also our cohort consisted of people without significant renal disease (ie, eGFR > 60mL/min/1.73 m2). As such, our findings allowed us to reach conclusions regarding the effects of albuminuria not confounded by the effects of CKD. Last, we adjusted our analyses for eGFR levels at baseline and for eGFR changes over follow-up. Limitations should also be acknowledged. Our analyses were a posteriori and are subject to unidentified biases. There were few African Americans in our cohort who had albuminuria (n = 28), making it difficult to explore the effect of albuminuria on brain volumes in this group which may be more sensitive to the effects of albuminuria on the brain than European Americans (24,30). Last, our cohort was mostly restricted to individuals with normal or near-normal renal function and may not be applicable to individuals with advanced renal disease.
In conclusion, in this cohort of middle-aged and older individuals with T2DM, with declines in information processing speed and auditory-verbal learning, albuminuria was not associated with significant independent differences in brain volume measurements cross-sectionally or prospectively as compared with those without albuminuria. The decline in cognitive function in association with albuminuria appeared to be mediated by shared risk factors, such as elevated SBP and older age. Other factors—such as increased levels of inflammation factors and oxidative stress and metabolic disturbances that associate with albuminuria, brain dysfunction and diabetes (20,35), increased arterial stiffness (36), and the effects of obesity (37)—may also play a role.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
ACCORD-MIND was funded through an intra-agency agreement between NIA and NHLBI (AG-0002) and the NIA Intramural Research Program. ACCORD was funded by NHLBI (N01-HC-95178; N01-HC-95179; N01-HC-95180; N01-HC-95181; N01-HC-95182; N01-HC-95183; N01-HC-95184). The funding agencies had no role in the results reported here. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca, Bayer HealthCare, Closer Healthcare, GlaxoSmithKline, King Pharmaceuticals, Merck, Novartis, Novo Nordisk, Omron Healthcare, Sanofi-Aventis, and Schering-Plough.
Conflict of Interest
None of the authors of this paper has a conflict of interest (financial or intellectual) regarding the contents of this article.
Supplementary Material
Acknowledgments
J.I.B. conceived of the article, wrote the article, and helped with the analysis and interpretation of data. T.M.M. did the statistical analysis and contributed to the intellectual content of the article. A.M.M. contributed to data acquisition, the interpretation of data, the intellectual content of the article, and the design of the ACCORD-MIND study. R.N.B. contributed to the intellectual content of the article and the interpretation of MRI data. J.D.W. contributed to the intellectual content of the article and the design of ACCORD-MIND. A.S. contributed to the intellectual content of the article and the acquisition of data. L.J.L. contributed to the intellectual content of the article, the interpretation of data, writing of the article, and was the principle investigator of the ACCORD-MIND study. All the authors have read the final draft of the article and have given their approval to submit the article.
References
- 1. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. [DOI] [PubMed] [Google Scholar]
- 2. Jellinger KA. The pathology of “vascular dementia”: a critical update. J Alzheimers Dis. 2008;14:107–123. [DOI] [PubMed] [Google Scholar]
- 3. Barzilay JI, Fitzpatrick AL, Luchsinger J, et al. Albuminuria and dementia: a community study. Am J Kid Dis. 2008;52:216–226. doi:10.1053/j.ajkd.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Umemura T, Kawamura T, Umegaki H, et al. Association of chronic kidney disease and cerebral small vessel disease with cognitive impairment in elderly patients with type 2 diabetes. Dement Geriatr Cogn Dis Extra. 2013;3:212–222. doi:10.1159/000351424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knopman DS, Mosley TH, Jr, Bailey KR, Jack CR, Jr, Schwartz GL, Turner ST. Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci. 2008;271:53–60. doi:10.1016/j.jns.2008.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol (1985). 2008;105:1652–1660. doi:10.1152/japplphysiol.90549.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009;32:115–121. doi:10.1038/hr.2008.27 [DOI] [PubMed] [Google Scholar]
- 8. Knopman DS. Albuminuria and microvascular disease of the brain—a shared pathophysiology. Am J Epidemiol. 2010;171:287—289. doi:10.1093/aje/kwp429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. [DOI] [PubMed] [Google Scholar]
- 10. Barzilay JI, Lovato J, Murray AM, et al. Albuminuria and cognitive decline in people with diabetes and normal renal function. Clin J Am Soc Nephrol. 2013;8:1907–1914. doi:10.2215/CJN.11321112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. 2002;61:2165–2175. [DOI] [PubMed] [Google Scholar]
- 12. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi:10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- 13. ACCORD Study Group. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007; 99(suppl):21i–33i. [DOI] [PubMed] [Google Scholar]
- 14. Williamson JD, Miller ME, Bryan RN, et al. ; for the ACCORD Study Group. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol. 2007;99 (suppl):112i–122i. [DOI] [PubMed] [Google Scholar]
- 15. Launer LJ, Miller ME, Williamson JD, et al. ; ACCORD MIND investigators. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10:969–977. doi:10.1016/S1474-4422(11)70188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–837. [DOI] [PubMed] [Google Scholar]
- 17. Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–313. doi:10.1016/j.acra.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bach LA, Gilbert RE, Cooper ME, Tsalamandris C, Jerums G. Prediction of persistent microalbuminuria in patients with diabetes mellitus. J Diabetes Complications. 1993;7:67–72. [DOI] [PubMed] [Google Scholar]
- 19. Pugliese G, Solini A, Bonora E, et al. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) Study formula in subjects with type 2 diabetes. Atherosclerosis. 2011;218:194–199. doi:10.1016/j.atherosclerosis.2011.04.035 [DOI] [PubMed] [Google Scholar]
- 20. Barzilay JI, Peterson D, Cushman M, et al. The relationship of cardiovascular risk factors to microalbuminuria in older adults with or without diabetes mellitus or hypertension: the cardiovascular health study. Am J Kidney Dis. 2004;44:25–34. [DOI] [PubMed] [Google Scholar]
- 21. Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227. doi:10.1001/ jamaneurol.2014.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiner DE, Bartolomei K, Scott T, et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis. 2009;53:438–447. doi:10.1053/j.ajkd.2008.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Bresser J, Tiehuis AM, van den Berg E, et al. ; Utrecht Diabetic Encephalopathy Study Group. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care. 2010;33:1309–1314. doi:10.2337/dc09-1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sink KM, Divers J, Whitlow CT, et al. Cerebral structural changes in diabetic kidney disease: African America-Diabetes Heart Study MIND. Diabetes Care. 2015;38:206–212. doi:10.2337/dc14-1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta D, Pimentel DA, Núñez MZ, Abduljalil A, Novak V. Subclinical albuminuria is linked to gray matter atrophy in type 2 diabetes mellitus. Metabolism. 2014;63:1390–1397. doi:10.1016/j.metabol.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Bresser J, Reijmer YD, van den Berg E, et al. ; Utrecht Diabetic Encephalopathy Study Group. Microvascular determinants of cognitive decline and brain volume change in elderly patients with type 2 diabetes. Dement Geriatr Cogn Disord. 2010;30:381–386. doi:10.1159/000321354 [DOI] [PubMed] [Google Scholar]
- 27. van den Berg E, Reijmer YD, de Bresser J, Kessels RP, Kappelle LJ, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:58–65. doi:10.1007/s00125-009-1571-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anan F, Masaki T, Iwao T, et al. The role of microalbuminuria and insulin resistance as significant risk factors for white matter lesions in Japanese type 2 diabetic patients. Curr Med Res Opin. 2008;24:1561–1567. doi:10.1185/03007990802061818 [DOI] [PubMed] [Google Scholar]
- 29. Manschot SM, Biessels GJ, de Valk H, et al. ; Utrecht Diabetic Encephalopathy Study Group. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia 2007;50:2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murea M, Hsu FC, Cox AJ, et al. Structural and functional assessment of the brain in European Americans with mild-to-moderate kidney disease: Diabetes Heart Study-MIND. Nephrol Dial Transplant. 2015;30:1322–1329. doi:10.1093/ndt/gfv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol Dial Transplant. 2013;28:1810–1819. doi:10.1093/ndt/gfs470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sajjad I, Grodstein F, Kang JH, Curhan GC, Lin J. Kidney dysfunction and cognitive decline in women. Clin J Am Soc Nephrol. 2012;7:437–443. doi:10.2215/CJN.05330611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurella Tamura M, Muntner P, Wadley V, et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis. 2011;58:756–763. doi:10.1053/j.ajkd.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33:1987–2002. doi:10.1002/hbm.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Craft S, Foster TC, Landfield PW, Maier SF, Resnick SM, Yaffe K. Session III: Mechanisms of age-related cognitive change and targets for intervention: inflammatory, oxidative, and metabolic processes. J Gerontol A Biol Sci Med Sci. 2012;67:754–759. doi:10.1093/gerona/gls112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abbatecola AM, Barbieri M, Rizzo MR, et al. Arterial stiffness and cognition in elderly persons with impaired glucose tolerance and microalbuminuria. J Gerontol A Biol Sci Med Sci. 2008;63:991–996. [DOI] [PubMed] [Google Scholar]
- 37. Tucsek Z, Toth P, Tarantini S, et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi:10.1093/gerona/glu080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.