Abstract
Background:
This study examines the effects of mobility and cognition on mortality risk in women late in life.
Methods:
A prospective study was conducted among 1,495 women (mean age 87.6 years) participating in the Study of Osteoporotic Fractures Year 20 examination (2006–2008). Mobility (ascertained by Short Physical Performance Battery [SPPB]) was categorized as poor (SPPB 0–3, n = 312), intermediate (SPPB 4–9, n = 799), or good (SPPB 10–12, n = 384). Cognitive status (adjudicated based on neuropsychological tests) was classified as normal (n = 873), mild cognitive impairment (n = 354), or dementia (n = 268). Deaths (n = 749) were identified from Year 20 through July 31, 2014 (average follow-up 4.9 years).
Results:
There was not strong evidence of an interaction between mobility and cognition for prediction of mortality risk (p interaction term .16). Compared to women with good mobility, mortality risks were increased among women with intermediate mobility (hazard ratio [HR] 1.26, 95% confidence interval [CI] 1.02–1.57) and those with poor mobility (HR 1.64, 95% CI 1.24–2.16) after consideration of cognition and other mortality risk factors. Similarly, mortality risks were higher among women with mild cognitive impairment (HR 1.46, 95% CI 1.21–1.76) and those with dementia (HR 1.88, 95% CI 1.54–2.31) compared to women with normal cognition after consideration of mobility and other mortality risk factors.
Conclusions:
Among women late in life, 5-year mortality risk was substantially increased among women with deficits in mobility even after accounting for cognition and traditional prognostic indicators. Similarly, deficits in cognition were associated with increased 5-year mortality despite consideration of mobility and conventional risk factors.
Keywords: Physical function, Cognitive status, Death, Elderly women
Co-existing impairments in mobility and cognition are common late in life and accumulating evidence suggests a linkage between these two essential attributes of function (1). Several longitudinal studies have examined the dynamic relationship between age-related declines in mobility and cognition. Some have reported that reduced mobility is associated with the subsequent development of cognitive impairment (2–5) including dementia (6–8), whereas others have suggested that cognitive impairment predicts the development of mobility decline (9–11).
However, whether there is evidence of an interrelationship between mobility and cognition on risk of clinical outcomes in older adults including mortality is understudied. Several prospective studies in community-dwelling older adults have reported that mobility limitations are associated with an increased risk for mortality (12,13), but the impact of cognitive function on this association is unknown. Similarly, other studies have reported that mild cognitive impairment (MCI) (14–17) and dementia (17,18) are associated with increased risks of death, but the impact of mobility on these relationships is uncertain. Among a cohort of well-functioning younger older adults, one previous study (19) examined both measures of mobility and cognition and reported that slow gait speed and lower score on the Digit Symbol Substitution Test were independently associated with risk of mortality even after accounting for each other. To examine effects of mobility and cognition on risk of mortality in women late in life, we used a unique longitudinal data set comprised of 1,495 women (mean aged 87.6 years) participating in the Year 20 examination (2006–2008) of the Study of Osteoporotic Fractures with comprehensive measures of mobility and cognition who were followed prospectively for ascertainment of vital status.
Methods
Study Population
We studied participants enrolled in the Study of Osteoporotic Fractures, a multicenter prospective cohort study of community-dwelling women. From 1986 to 1988, a total of 9,704 women aged 65 years or older able to walk unassisted were recruited for participation in the initial visit from population-based listings in four areas (Baltimore, Maryland; Minneapolis, Minnesota; Portland, Oregon; and the Monongahela Valley, near Pittsburgh, Pennsylvania) of the United States (20). Black women were originally excluded because of their low incidence of hip fracture. Subsequently, at the Year 10 visit conducted between 1997 and 1998, 662 African American women were enrolled in the study, increasing total enrollment to 10,366 women. At each site, institutional review boards approved the study and written informed consent was obtained from all participants.
All active surviving women at three clinical centers (Minneapolis, Portland and Pittsburgh) were invited to participate in a Year 20 visit conducted between 2006 and 2008 (Supplementary Figure). A total of 2,368 women had at least minimal information collected at this visit and of these, 1,495 completed an examination performed in the clinic (n = 1016) or home (n = 479) that included a battery of lower extremity physical performance and neuropsychological tests.
Measures of Mobility
Mobility at the Year 20 examination was ascertained by administering the Short Physical Performance Battery (SPPB) (12,21), comprised of measures of standing balance, usual gait speed, and ability to rise from a chair five times without using the arms. Scores of 1–4 for each task were assigned based on quartiles of performance in the SPPB derivation cohort (12); participants were assigned a score of 0 for each task they were unable to complete. A summary score ranging from 0 to 12 was created for each participant by adding scores for each task and categorized as poor (score 0–3), intermediate (score 4–9), or good (score 10–12).
Assessment of Cognition
To assess cognition at the Year 20 examination, a battery of neuropsychological tests was administered including Trails B (22); the Modified Mini-Mental State Examination (3MS), a 100-point extended version of the MMSE (23); the California Verbal Learning Test (CVLT) Short Form (24); Digit Span (25); and category and verbal fluency tests (26).
Cognitive impairment at the Year 20 exam was determined in a two-step process (27). First, women were screened for impairment using the expanded neurological test battery. Women who screened negative were considered to have normal cognition. Women who screened positive had their clinical cognitive status adjudicated by a panel of experts. A diagnosis of dementia was made based on Diagnostic and Statistical Manual of Mental Disorders IV criteria (28). MCI was diagnosed using a modified Petersen Criteria (29,30). Participants were classified as having normal cognition, MCI, or dementia.
Mortality
Participants were followed from their Year 20 visit date until July 31, 2014 (mean [SD] follow-up 4.9 [2.0] years); deaths were identified by participant contacts every 6 months with confirmation by death certificates and using vital status information from the 2014 Medicare Master Beneficiary File.
Other Measurements
Each participant completed a questionnaire and was asked at the Year 20 visit about self-reported health, hospitalization in the past year, smoking status, whether she walked for exercise and ability to perform basic activities of daily living. Women were queried about a physician diagnosis of nine selected medical conditions including myocardial infarction, stroke, congestive heart failure, hip fracture, diabetes, arthritis, Parkinsonism, chronic obstructive pulmonary disease, and cancer excluding non-melanoma skin cancer. A comorbidity score for each participant was calculated as the sum of these comorbid conditions (range 0–9). Depressive symptoms were evaluated using the Geriatric Depression scale (31). Body mass index was calculated using measures of body weight and height. Participants were queried about race/ethnicity and education at the time of initial Study of Osteoporotic Fractures enrollment.
Statistical Analysis
Characteristics of the 1,495 participants at the Year 20 examination were compared across the three mobility phenotypes and across the three cognition phenotypes using chi-square tests (categorical variables) and analysis of variance (continuous variables).
Kaplan–Meier estimates of survival functions were used to depict survival experience by mobility phenotype stratified by cognition phenotype. The associations of combined mobility–cognition phenotypes (nine distinct combinations), mobility phenotypes, and cognition phenotypes with risk of mortality were analyzed using Cox proportional hazards models. We tested for evidence of an interaction between mobility and cognition for prediction of mortality to examine whether the associations of mobility phenotype with risk of death were modified by cognition phenotype (and vice versa). Analyses were then performed to determine the association of mobility phenotype with mortality without and with adjustment for cognition phenotype (and vice versa). Initial analyses were adjusted for age and site and then further adjusted for additional potentially relevant factors including race, education, health status, hospitalization in the prior year, smoking status, comorbidity score, whether the participant walked for exercise, activities of daily living impairment, depressive symptoms, and body mass index.
Results
Among the 1,495 women studied, mean age was 87.6 years at the Year 20 examination (Tables 1 and 2). A total of 173 women (11.6%) reported African American race and 401 (26.9%) had been hospitalized at least once in the past year. Mean (SD) SPPB score was 6.7 (3.5); mobility was classified as poor (SPPB score 0–3) in 312 women (20.9%), intermediate (SPPB score 4–9) in 799 women (53.4%) and good (SPPB score 10–12) in 384 women (25.7%). A total of 268 women (17.9%) were classified as having dementia, 354 women (23.7%) as having MCI, and 873 women (58.4%) as having normal cognition. The prevalence of dementia ranged from 6.5% among women with good mobility to 15.8% among women with intermediate mobility to 37.5% among women with poor mobility. Similarly, the prevalence of poor mobility ranged from 13.4% among women with normal cognition to 22.0% among women with MCI to 43.7% among women with dementia. However, substantial heterogeneity in combined mobility–cognition phenotypes was observed (Table 3). For example, among the 312 women with poor mobility, 62.5% had evidence of cognitive impairment (78 with MCI and 117 with dementia), but 37.5% had normal cognitive function. Among the 268 women with dementia, the vast majority (90.7%) had impaired mobility (126 with intermediate mobility and 117 with poor mobility), but 9.3% had good mobility.
Table 1.
Characteristics of 1495 Women at the SOF Year 20 Examination Overall and by Mobility Phenotype
| Characteristic | Overall | Good Mobility | Intermediate Mobility | Poor Mobility | |
|---|---|---|---|---|---|
| (n = 1,495) | (n = 384) | (n = 799) | (n = 312) | p-Value | |
| Age, years, mean (SD) | 87.6 (3.3) | 86.5 (3.1) | 87.7 (3.2) | 88.8 (3.6) | <.001 |
| African American, n (%) | 173 (11.6) | 55 (14.3) | 80 (10.0) | 38 (12.2) | .15 |
| Education, years, mean (SD) | 12.8 (2.6) | 13.0 (2.6) | 12.9 (2.6) | 12.3 (2.4) | <.001 |
| Health status, fair/poor/very poor, n (%) | 340 (22.8) | 35 (9.2) | 189 (23.7) | 116 (37.5) | <.001 |
| Hospitalization in year prior to Year 20 visit, n (%) | 401 (26.9) | 64 (16.7) | 223 (27.9) | 114 (36.9) | <.001 |
| Past smoker, n (%) | 473 (37.1) | 110 (28.7) | 268 (33.6) | 95 (30.7) | .63 |
| Current smoker, n (%) | 30 (2.0) | 10 (2.6) | 14 (1.8) | 6 (1.9) | |
| Comorbidity score (0–9), mean (SD) | 1.4 (1.2) | 0.9 (1.0) | 1.5 (1.2) | 1.9 (1.2) | <.001 |
| Walks for exercise, n (%) | 606 (41.6) | 210 (55.6) | 342 (43.9) | 54 (18.0) | <.001 |
| ≥1 ADL* difficulty, n (%) | 330 (22.4) | 15 (3.9) | 140 (17.8) | 175 (58.0) | <.001 |
| GDS score (0–15), mean (SD) | 2.5 (2.4) | 1.5 (1.7) | 2.6 (2.4) | 3.8 (2.7) | <.001 |
| BMI, kg/m2, mean (SD) | 26.5 (4.9) | 25.9 (4.2) | 26.5 (4.8) | 27.2 (5.8) | .003 |
| Mild cognitive impairment, n (%) | 354 (23.7) | 84 (21.9) | 192 (24.0) | 78 (25.0) | <.001 |
| Dementia, n (%) | 268 (17.9) | 25 (6.5) | 126 (15.8) | 117 (37.5) |
Notes: ADL = Activities of Daily Living; BMI, body mass index; GDS = Geriatric Depression scale; SOF = Study of Osteoporotic Fractures.
*ADLs assessed were bathing, dressing, and transferring.
Table 2.
Characteristics of 1,495 Women at the SOF Year 20 Examination Overall and by Cognition Phenotype
| Characteristic | Overall | Normal Cognition | Mild Cognitive Impairment | Dementia | p-Value |
|---|---|---|---|---|---|
| (n = 1,495) | (n = 873) | (n = 354) | (n = 268) | ||
| Age, years, mean (SD) | 87.6 (3.3) | 87.2 (3.1) | 87.8 (3.3) | 88.8 (3.8) | <.001 |
| African American, n (%) | 173 (11.6) | 86 (9.9) | 51 (14.4) | 36 (13.4) | .037 |
| Education, mean (SD) | 12.8 (2.6) | 13.1 (2.5) | 12.2 (2.6) | 12.5 (2.6) | <.001 |
| Health status, fair/poor/very poor, n (%) | 340 (22.8) | 185 (21.2) | 78 (22.2) | 77 (29.1) | .014 |
| Hospitalization in year prior to Year 20 visit, n (%) | 401 (26.9) | 224 (25.7) | 84 (23.9) | 93 (35.1) | .014 |
| Past smoker, n (%) | 473 (37.1) | 270 (30.9) | 116 (33.0) | 87 (32.8) | .42 |
| Current smoker, n (%) | 30 (2.0) | 15 (1.7) | 11 (3.1) | 4 (1.5) | |
| Comorbidity score (0–9), mean (SD) | 1.4 (1.2) | 1.4 (1.2) | 1.4 (1.2) | 1.5 (1.3) | .81 |
| Walks for exercise, n (%) | 606 (41.6) | 375 (43.9) | 149 (43.4) | 82 (31.7) | .002 |
| ≥1 ADL* difficulty, n (%) | 330 (22.4) | 140 (16.2) | 86 (24.7) | 104 (40.2) | <.001 |
| GDS score (0–15), mean (SD) | 2.5 (2.4) | 2.1 (2.1) | 3.0 (2.7) | 3.5 (2.7) | <.001 |
| BMI, kg/m2, mean (SD) | 26.5 (4.9) | 26.6 (4.8) | 26.3 (4.6) | 26.1 (5.3) | .23 |
| Intermediate mobility (SPPB 4–9), n (%) | 799 (53.4) | 481 (55.1) | 192 (54.2) | 126 (47.0) | <.001 |
| Poor mobility (SPPB 0–3), n (%) | 312 (20.9) | 117 (13.4) | 78 (22.0) | 117 (43.7) |
Notes: ADL = Activities of Daily Living; BMI = body mass index; GDS = Geriatric Depression scale; SOF, Study of Osteoporotic Fractures; SPPB, Short Physical Performance Battery.
*ADLs assessed were bathing, dressing, and transferring.
Table 3.
Associations of Combined Mobility–Cognition Phenotypes With Mortality Among 1,495 SOF Women
| Phenotype | N | Incidence Rate Per 100 Person-Years (95% CI)* | Relative Hazard (95% CI)† |
|---|---|---|---|
| Good mobility (SPPB 10–12), normal cognition | 275 | 6.3 (4.2–8.4) | 1.00 (referent) |
| Good mobility (SPPB 10–12), MCI | 84 | 9.2 (6.1–12.3) | 1.77 (1.20–2.61) |
| Good mobility (SPPB 10–12), dementia | 25 | 9.8 (2.9–16.6) | 2.04 (1.11–3.76) |
| Intermediate mobility (SPPB 4–9), normal cognition | 481 | 8.1 (7.0–9.2) | 1.59 (1.22–2.08) |
| Intermediate mobility (SPPB 4–9), MCI | 192 | 11.5 (9.3–13.8) | 2.41 (1.79–3.25) |
| Intermediate mobility (SPPB 4–9), dementia | 126 | 18.4 (12.9–24.0) | 3.24 (2.38–4.43) |
| Poor mobility (SPPB 0–3), normal cognition | 117 | 13.3 (10.1–16.6) | 2.84 (2.04–3.94) |
| Poor mobility (SPPB 0–3), MCI | 78 | 18.8 (13.2–24.3) | 3.63 (2.55–5.17) |
| Poor mobility (SPPB 0–3), dementia | 117 | 22.6 (17.1–28.1) | 4.53 (3.30–6.21) |
Notes: MCI = mild cognitive impairment; SOF = Study of Osteoporotic Fractures; SPPB = Short Physical Performance Battery.
*Adjusted for age.
†Adjusted for age and site.
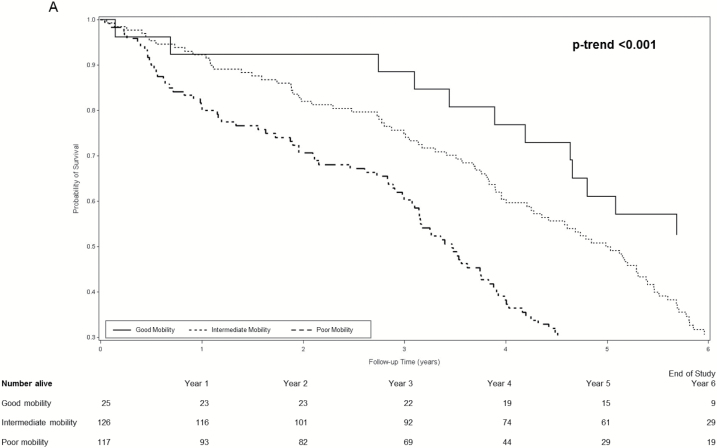
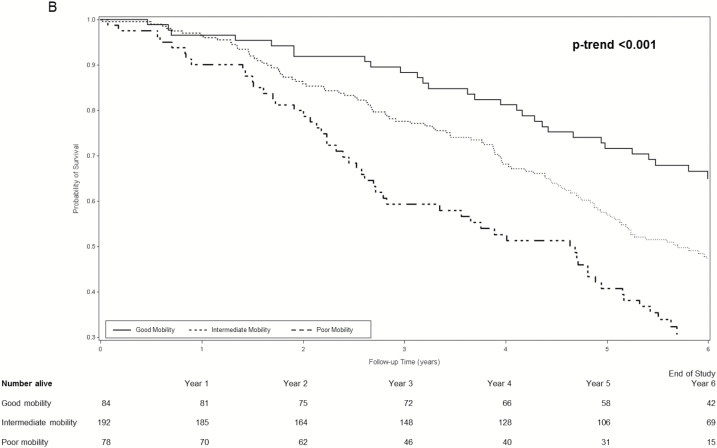
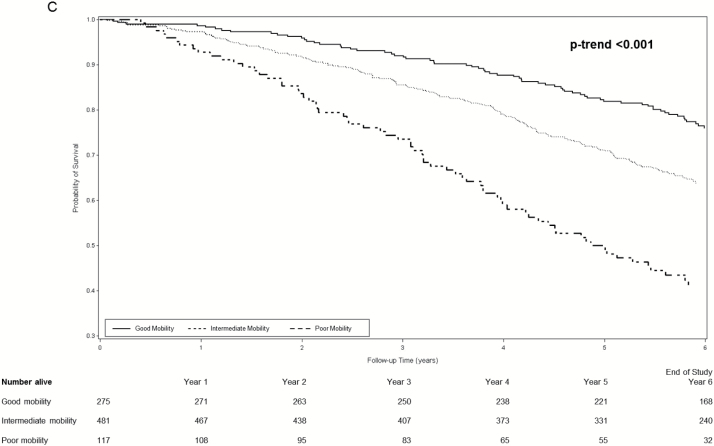
During an average follow-up of 4.9 years, 749 women (50.1%) died. Among women with dementia, survival curves for women with good, intermediate, and poor mobility separated early and the separation persisted throughout the subsequent 6 years (log rank test for trend <0.001 across mobility phenotypes) (Figure 1A). Separation of survival curves across mobility phenotypes was also observed among women with MCI (log rank test for trend <0.001 across mobility phenotypes) (Figure 1B) and among women with normal cognition (log rank test for trend <0.001 across mobility phenotypes) (Figure 1C).
Figure 1.
(A) Cumulative survival among women with dementia according to mobility phenotype. (B) Cumulative survival among women with mild cognitive impairment according to mobility phenotype. (C) Cumulative survival among women with normal cognition according mobility phenotype. *Probability of survival adjusted for age and site.
Age-adjusted incidence rates of mortality per 100 person-years increased in a graded manner across combined mobility–cognition phenotypes with a 3.6-fold higher rate among women with poor mobility/dementia (22.6, 95% confidence interval [CI] 17.1–28.1) compared with those with good mobility/normal cognition (6.3, 95% CI 4.2–8.4) (Table 3). Examination of mortality hazard ratios [HRs] according to combined phenotypes suggested the possible presence of an interaction between mobility and cognition for prediction of mortality (Table 3). For example, among women with poor mobility, MCI was associated with a 28% increase and dementia was associated with a 60% increase in the hazard of mortality (referent group normal cognition). However, among women with good mobility, the pattern appeared somewhat different as risk was elevated by 77% among women with MCI and doubled for those with dementia. However, due to the small number of women with good mobility/dementia (n = 25), the CI around the point estimate of risk in this group was wide. In addition, the interaction between mobility and cognition for the prediction of mortality did not reach the level of significance (p interaction term .16).
In a model adjusted for age and site, there was a graded association between poorer mobility and increased mortality that was slightly reduced after consideration of cognition HR intermediate vs. good mobility 1.54, 95% CI 1.25–1.88 and HR poor vs good mobility 2.39, 95% CI 1.89–3.03) (p-trend <.001) (Table 4). After additional adjustment for race, education, health status, hospitalization in the prior year, smoking status, comorbidity score, walking for exercise, activities of daily living impairment, depressive symptoms and body mass index, and after accounting for differences in cognition, the association of lower mobility with mortality was further attenuated but persisted (HR intermediate vs good mobility 1.26, 95% CI 1.02–1.57 and HR poor vs good mobility 1.64, 95% CI 1.24–2.16) (p-trend < .001).
Table 4.
Effect of Cognition Phenotype on Association of Mobility Phenotype With Mortality Among 1,495 SOF Women
| Relative Hazard (95% CI) | |||||
|---|---|---|---|---|---|
| Phenotype* | Mortality Rate Per 100 Person-Years (95% CI)† | Base Model‡ | Base Model‡ + Cognition | Multivariable Model§ | Multivariable Model§ + Cognition |
| Good mobility (SPPB 10–12) | 6.6 (5.3–7.8) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Intermediate mobility (SPPB 4–9) | 10.1 (9.1–11.0) | 1.62 (1.32–1.98) | 1.54 (1.25–1.88) | 1.34 (1.08–1.66) | 1.26 (1.02–1.57) |
| Poor mobility (SPPB 0–3) | 17.1 (14.7–19.5) | 2.86 (2.28–3.60) | 2.39 (1.89–3.03) | 1.84 (1.40–2.42) | 1.64 (1.24–2.16) |
Notes: SOF = Study of Osteoporotic Fractures; SPPB = Short Physical Performance Battery.
*Among the cohort, there were 384 women with good mobility, 799 women with intermediate mobility, and 312 women with poor mobility.
†Adjusted for age.
‡Adjusted for age and site.
§Adjusted for age, site, race, education, health status, hospitalization in the prior year, smoking status, comorbidity score, walking for exercise, ADL impairment, depressive symptoms, and body mass index.
Similarly, after consideration of multiple mortality risk factors and after accounting for differences in mobility, poorer cognition was associated in a graded manner with higher mortality (HR MCI vs normal cognition 1.46, 95% CI 1.21–1.76 and HR dementia vs normal cognition 1.88, 95% CI 1.54–2.31) (p-trend < .001) (Table 5).
Table 5.
Effect of Mobility Phenotype on Association of Cognition Phenotype With Mortality Among 1,495 SOF Women
| Relative Hazard (95% CI) | |||||
|---|---|---|---|---|---|
| Phenotype* | Mortality Rate Per 100 Person-Years (95% CI)† | Base Model‡ | Base Model‡ + Mobility | Multivariable Model§ | Multivariable Model§ + Mobility |
| Normal cognition | 7.9 (7.1–8.8) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| MCI | 12.1 (10.4–13.9) | 1.59 (1.34–1.90) | 1.51 (1.27–1.80) | 1.48 (1.23–1.79) | 1.46 (1.21–1.76) |
| Dementia | 18.2 (15.2–21.1) | 2.26 (1.89–2.71) | 1.90 (1.57–2.28) | 1.99 (1.63–2.44) | 1.88 (1.54–2.31) |
Notes: MCI = mild cognitive impairment; SOF = Study of Osteoporotic Fractures.
*Among the cohort, there were 873 women with normal cognition, 354 women with mild cognitive impairment, and 268 women with dementia.
†Adjusted for age.
‡Adjusted for age and site.
§Adjusted for adjusted for age, site, race, education, health status, hospitalization in the prior year, smoking status, comorbidity score, walking for exercise, ADL impairment, depressive symptoms, and body mass index.
Discussion
In this cohort of women in the 9th and 10th decades of life, the 5-year mortality risk was substantially increased among women with deficits in mobility even after accounting for cognition and traditional prognostic indicators of survival in late life. Similarly, both MCI and dementia were associated with increased 5-year mortality despite consideration of mobility and conventional mortality risk factors.
Several studies have reported associations between change in mobility performance and change in cognitive function in older adults (2–11), suggesting a linkage between these two processes with aging. In support of this hypothesis, we found in our cohort of women late in life that the prevalence of dementia substantially increased with worsening mobility and that the prevalence of poor mobility markedly increased with worsening cognition. At the same time, we also observed substantial heterogeneity in combined phenotypes of mobility–cognition suggesting a complex relationship between physical and cognitive functioning in aged populations. For example, nearly 4 of 10 women with poor mobility had normal cognition and nearly 1 of every 10 women with dementia had good mobility.
Mobility and cognition were each independent predictors of mortality in our study, even after accounting for each other and several mortality risk factors. Previous evidence suggests that mobility and cognition are important prognostic indicators of survival among older adults, though most prior studies have not evaluated the impact of the interrelationship between mobility and cognition on risk of mortality or adequately accounted for potential confounders. A pooled analysis of 34,485 community-dwelling older adults (13) indicated that 5-year and 10-year survival decreased across the full range of gait speed and observed that predicted survival based on age, sex, and gait speed was as accurate as that based on a more complex model, though this study did not consider the impact of cognition on the relationship. Similarly, SPPB score in cohort of older adults predicted 2-year risk of mortality independent of age and self-reported functional status (12). Prospective studies in community-dwelling aged populations have also reported an increased risk of mortality among those with MCI (14–17) and those with dementia (17,18) over short-term and long-term follow-up periods, but most of these studies have not considered the effect of accounting for mobility on the association. One prior study (19) of well-functioning younger older adults (mean age 70.4 years) found that slow gait and lower score on the Digit Symbol Substitution Test were each associated with 8-year risk of mortality, even after accounting for each other, prevalent cardiovascular disease, physical activity and grip strength. Our study expands on these earlier findings with its more comprehensive assessment of mobility and cognition in a population of women in late life unselected on the basis of function and its consideration of additional mortality risk factors.
Our results indicate that deficits in mobility and cognition in community-dwelling older women are strong predictors of life-expectancy with effects that are independent of more commonly assessed patient characteristics in the clinical practice setting including burden of serious medical conditions and history of recent hospitalization. Thus, both attributes of function may be critical to consider in medical decision making and in planning health care policies for the growing aged population. In addition, our results have implications for the design of future clinical trials of interventions including physical activity and pharmacologic treatments that should evaluate the effect of any intervention in slowing decline in both attributes of function and improving survival free of major disability. Such studies are clearly warranted given the growing societal burden of age-related deficits in mobility and cognition.
This study has a number of strengths including the well-characterized cohort of women late in life, comprehensive assessment of mobility, and cognition and consideration of key confounding and mediating factors. However, this study has several limitations. The cohort was comprised of women and the results may not be generalizable to men. However, women comprise more than two thirds of the growing population of adults aged 85 years and older in the United States (32). We did not find strong evidence for effect modification between mobility and cognition for mortality risk prediction, but our power to detect an interaction was limited. While our analyses accounted for several prognostic indicators of survival late in life, we acknowledge that residual confounding remains a possible explanation for our findings. We have included this limitation in the discussion section of the revised manuscript. Importantly, future analyses are needed to examine the effects of mobility and cognition on additional outcomes including risk of hospitalization and total health care costs.
In conclusion, both mobility and cognition are associated with mortality in women in their 9th and 10th decade of life even after accounting for each other and conventional predictive indicators. Both attributes of function should be considered when planning health care policies for the growing population of adults aged 85 years and older.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
Conflict of Interest
The authors report no declarations of interest.
Supplementary Material
References
- 1. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi:10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67:980–986. doi:10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi:10.1159/000111577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. doi:10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taniguchi Y, Yoshida H, Fujiwara Y, Motohashi Y, Shinkai S. A prospective study of gait performance and subsequent cognitive decline in a general population of older Japanese. J Gerontol A Biol Sci Med Sci. 2012;67:796–803. doi:10.1093/gerona/glr243 [DOI] [PubMed] [Google Scholar]
- 6. Abellan van Kan G, Rolland Y, Gillette-Guyonnet S, et al. Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J Gerontol A Biol Sci Med Sci. 2012;67:425–432. doi:10.1093/gerona/glr177 [DOI] [PubMed] [Google Scholar]
- 7. Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–1768. doi:10.1056/NEJMoa020441 [DOI] [PubMed] [Google Scholar]
- 8. Welmer AK, Rizzuto D, Qiu C, Caracciolo B, Laukka EJ. Walking speed, processing speed, and dementia: a population-based longitudinal study. J Gerontol A Biol Sci Med Sci. 2014;69:1503–1510. doi:10.1093/gerona/glu047 [DOI] [PubMed] [Google Scholar]
- 9. Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62(8):844–850. [DOI] [PubMed] [Google Scholar]
- 10. Soumaré A, Tavernier B, Alpérovitch A, Tzourio C, Elbaz A. A cross-sectional and longitudinal study of the relationship between walking speed and cognitive function in community-dwelling elderly people. J Gerontol A Biol Sci Med Sci. 2009;64:1058–1065. doi:10.1093/gerona/glp077 [DOI] [PubMed] [Google Scholar]
- 11. Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65(10):1093–1100. doi:10.1093/gerona/glq111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 13. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Bruijn RF, Akoudad S, Cremers LG, et al. Determinants, MRI correlates, and prognosis of mild cognitive impairment: the Rotterdam Study. J Alzheimers Dis. 2014;42(suppl 3):S239–S249. doi:10.3233/JAD-132558 [DOI] [PubMed] [Google Scholar]
- 15. Mossakowska M, Broczek K, Wieczorowska-Tobis K, et al. Cognitive performance and functional status are the major factors predicting survival of centenarians in Poland. J Gerontol A Biol Sci Med Sci. 2014;69:1269–1275. doi:10.1093/gerona/glu003 [DOI] [PubMed] [Google Scholar]
- 16. Sachs GA, Carter R, Holtz LR, et al. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Ann Intern Med. 2011;155:300–308. doi:10.7326/0003-4819-155-5-201109060-00007 [DOI] [PubMed] [Google Scholar]
- 17. Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2009;66:767–772. doi:10.1001/archneurol.2009.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Arch Neurol. 2005;62:779–784. doi:10.1001/archneur.62.5.779 [DOI] [PubMed] [Google Scholar]
- 19. Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625. doi:10.1111/j.1532-5415.2008.01856.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–668. [PubMed] [Google Scholar]
- 21. Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. [DOI] [PubMed] [Google Scholar]
- 22. Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Tucson, AZ: Neuropsychology Press; 1985. ISBN 9780934515023 [Google Scholar]
- 23. McDowell I, Kristjansson B, Hill GB, Hébert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50:377–383. [DOI] [PubMed] [Google Scholar]
- 24. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Second Edition (CVLT-II). San Antonio, TX: Psychological Corporation; 2000. ISBN 9780890420256 [Google Scholar]
- 25. Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: The Psychological Corporation; 1988. ISBN 9780154981004 [Google Scholar]
- 26. Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. New York, NY: Oxford University Press; 1991. ISBN 9780195054392 [Google Scholar]
- 27. Yaffe K, Middleton LE, Lui LY, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–636. doi:10.1001/archneurol.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.4th ed. Washington, DC: American Psychiatric Association; 2000. ISBN 9780890420256 [Google Scholar]
- 29. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 30. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 31. Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1/2):165–173. ISSN 0731-7115 [Google Scholar]
- 32. Werner CA. The older population 2010. https://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf . Issued November 1, 2011. Accessed September 29, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.