Abstract
Background
Several studies have been performed to investigate the relationship between psoriasis and epicardial fat tissue (EFT). However, the number of patients of every single study is relatively small.
Objectives
We carried out a meta-analysis to evaluate whether EFT is associated with psoriasis.
Methods
A search of PubMed, Ovid Embase, Ovid Medline, the Cochrane Library and Chinese BioMedical Literature Database (CBM) for controlled trials was done from inception to January 20th, 2016. Published trials that included a psoriasis group and a control group without psoriasis with data for at least epicardial fat tissue (EFT) were included. All statistical analyses were conducted using the Stata 12.0 (Stata Corporation, College Station, TX, USA).
Results
There were 5 trials involving 731 patients. Patients with psoriasis showed significantly higher EFT than control group (SMD: 0.86, 95 % CI: 0.27-1.46, P = 0.004).
Conclusions
Patients with psoriasis have higher EFT compared to control subjects without psoriasis.
Keywords: Epicardial fat tissue, Meta-analysis, Psoriasis
Background
Psoriasis is a chronic immune-mediated inflammatory disease which affects 2–4 % of the population worldwide [1]. It is characterized by sharply demarcated erythematous plaque lesions with silvery white scales. A number of literatures pointed that psoriasis is associated with several cardiometabolic co-morbidities and can increase the risk of cardiovascular disease (CVD) and cardiovascular mortality [2–6].
Epicardial fat tissue (EFT) is visceral fat around the heart and coronary vessels. Recent studies suggested that EFT play a significant role in the development of cardiovascular diseases through secreting several inflammatory adipocytokines (TNF-alpha, IL-6, adipocytokines, and leptin) in potential paracrine or endocrine mechanism [7, 8].
Psoriasis and EFT are both associated with CVD. Several studies have been performed to investigate the relationship between psoriasis and EFT [9–13]. But, the number of patients of every single study is relatively small. In this study, we play a meta-analysis to evaluate whether EFT is associated with psoriasis.
Materials and methods
The process of the meta-analysis was performed according to the Cochrane Collaboration recommendations [14]. The analytical results were reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [15].
Search strategy
A systematic search was made in the following databases: PubMed, Ovid Embase, Ovid Medline, the Cochrane Library and Chinese BioMedical Literature Database (CBM). The search used the terms “epicardial fat tissue”, “psoriasis [Mesh]” which were searched as text words and as exploded medical subject headings where possible. Free text words were searched combined with additional keywords: “epicardial adipose tissue”, “epicardial fat”, “epicardial adipose”. No language restrictions were applied. The search included literatures published until January 20th, 2016 with no lower date limit.
Data extraction and quality assessment
Data were abstracted and quality of studies was assessed independently by two reviewers (Xiaoxue Wang, Zexin Zhu). Disagreement on specific studies between the two reviewers was resolved by consensus. Data were extracted from each study including the sample size and mean difference ± SD in EFT, total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides in both psoriasis and non-psoriasis groups. Methodological study quality was assessed using the Strengthening of Reporting of Observational Studies in Epidemiology (STROBE) checklist of 22 items [16].
Including and excluding criteria
Studies investigating the association between EFT and psoriasis were considered for this meta-analysis. And the diagnosis of psoriasis was made according to clinical or pathological examination. The studies should include at least a psoriasis group and a control group. Abstracts, letters, and case reports and studies without sufficient formation on EFT were excluded. If the same samples were used in multiple publications, the most recent and/or the largest publication were included.
Statistical analyses
All statistical analyses were carried out using Stata 12.0 (Stata Corporation, College Station, TX, USA). Pooled standard mean difference (SMD) or weighted mean difference (WMD) with 95%CI were calculated using either the fixed-effects model or random-effects model. For each meta-analysis, the χ2 and I2 statistics were first calculated to assess the heterogeneity of the included studies. P < 0.1 and I2 > 50 % were considered significant. For P < 0.1 and I2 > 50 %, the random-effects model was utilized. Otherwise, data were evaluated using the fixed-effects model. The risk of publication bias in this study was evaluated by visual inspection of the symmetry of the funnel plot. The significance of the pooled SMD or WMD was rated by the Z-test. P < 0.05 was regarded as significant.
Results
Description of the studies
We have searched a total of 76 studies, and 5 studies left after excluded. The full-texts had been carefully evaluated. They were published from 2013 to 2015, all of them measured EFT in psoriasis and control group [9–13]. Totally 731 patients were enrolled in these studies. Among those, 349 patients with psoriasis compared with 382 subjects without psoriasis (Fig. 1).
Fig. 1.
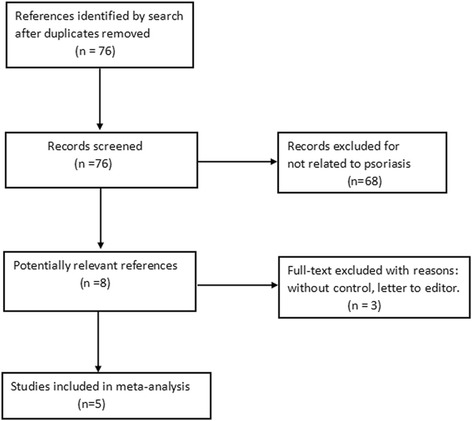
Flowchart showing selection of publications
The patients in each study are ranging from 63 to 302. EFT thickness was measured by echocardiography in 3 studies [9–11]. EFT was calculated as the area [12] or volume [13] by computed tomography (CT) in 2 studies. Disease severity of psoriasis was quantified using the Psoriasis Area and Severity Index (PASI) in all included studies. These characteristics and quality assessment assessed by the STROBE are listed in the Table 1.
Table 1.
The characteristics of studies included in the meta-analysis
| Study | Arms | Patients | Gender (M/F) | Age (year) | BMI (Kg/m2) | PASI score | Total cholesterol, mg/dl | Triglycerides, mg/dl | HDL, mg/dl | LDL, mg/dl | EFT | Measurement tool | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sen. et al. 2013 [9] | Psoriasis | 65 | 39/26 | 41.1 ± 3.3 | 25.9 ± 2.8 | 17.5 ± 5.7 | 190 ± 46 | 163 ± 78 | 33 ± 9 | 132 ± 33 | 7.3 ± 0.5 mm | Echocardiography | 20 |
| Non-psoriasis | 50 | 28/22 | 40.5 ± 3.8 | 25.5 ± 2.3 | 199 ± 37 | 172 ± 88 | 34 ± 7 | 121 ± 36 | 6.5 ± 0.5 mm | Echocardiography | |||
| Zehra. et al. 2014 [10] | Psoriasis | 31 | 14/17 | 42 ± 11.1 | 28.1 ± 5.6 | 6.1 ± 4 | 206.1 ± 34.8 | 165.4 ± 75.3 | 47.9 ± 8.0 | 125.1 ± 30.5 | 6.4 ± 2.6 mm | Echocardiography | 19 |
| Non-psoriasis | 32 | 13/19 | 41.1 ± 6.8 | 30.9 ± 9.9 | 198.6 ± 31.8 | 143.5 ± 60.6 | 43.3 ± 13.4 | 126.6 ± 28.0 | 5.1 ± 1.9 mm | Echocardiography | |||
| Ahmet. et al. 2014 [11] | Psoriasis | 115 | 62/53 | 33.6 ± 6.0 | 26.1 ± 3.1 | 3.8 ± 4.1 | 185.4 ± 37.4 | 125.3 ± 77.3 | 49.2 ± 12.9 | 129.4 ± 33.7 | 5.7 ± 1.2 mm | Echocardiography | 20 |
| Non-psoriasis | 60 | 28/32 | 32.5 ± 8.3 | 25.2 ± 3.2 | 179.1 ± 33.8 | 126.2 ± 67.0 | 48.4 ± 13.5 | 127.7 ± 26.1 | 4.1 ± 1.0 mm | Echocardiography | |||
| Balci .et al. 2014 [12] | Psoriasis | 38 | 26/12 | 42.2 ± 15.0 | 28.8 ± 3.9 | 9.8 ± 9.3 | 203.1 ± 33.7 | 150.2 ± 74.4 | 136.2 ± 27.7 | 37.6 ± 9.9 | 13.8 ± 8.4 cm2 | CT | 19 |
| Non-psoriasis | 38 | 26/12 | 39.8 ± 13.5 | 27.4 ± 4.2 | 185.2 ± 38.5 | 130.1 ± 62.6 | 124.4 ± 42.1 | 41.4 ± 8.9 | 9.7 ± 6.4 cm2 | CT | |||
| Torres. et al. 2015 [13] | Psoriasis | 100 | 64/36 | 47.4 ± 10.8 | 28.6 ± 4.99 | 12.8 ± 8.0 | 206.5 ± 39.9 | 126.7 ± 72.6 | 129.1 ± 38.2 | 51.5 ± 13.2 | 101.4 ± 55.52 ml | CT | 21 |
| Non-psoriasis | 202 | 130/72 | 54.4 ± 10.1 | 28.1 ± 4.17 | NA | NA | NA | NA | 92.2 ± 38.33 ml | CT |
Continuous variables were presented as mean ± standard deviation (SD), and categorical variables were presented as frequencies with percentages
BMI body mass index, PASI psoriasis area and severity index, LDL low- density lipoprotein, HDL high-density lipoprotein, CT computed tomography, NA not applicable
We compared the EFT, serum level of total cholesterol, LDL, HDL and triglycerides.
Epicardial fat tissue
All studies included reported EFT. Subgroup analysis was performed depending on different measurement of EFT (thickness, area and volume). Based on the great heterogeneity between trials (I2 = 92 %, P < 0.001), random effects meta-regression analysis was made. There was a significant difference in the SMD between psoriasis and non-psoriasis groups (0.86, 95 % CI: 0.27-1.46, P = 0.004) (Fig. 2).
Fig. 2.
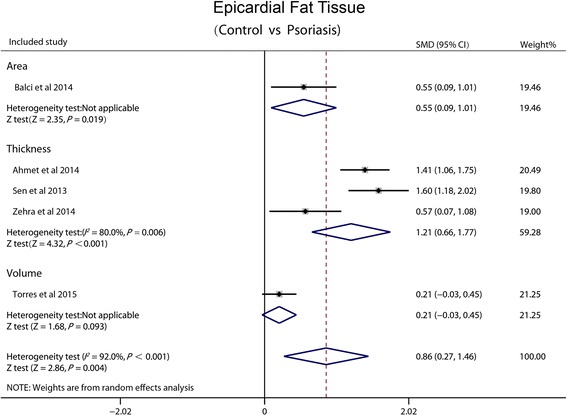
Forest plot showing standardized difference in means and 95 % CI of EFT between patients with psoriasis and control group
Total cholesterol
Four studies reported the total cholesterol in the serum. We made a fixed-effect pooled analysis based on the low heterogeneity among the 4 trials (I2 = 48.4 %, P = 0.121). There was no significant difference in the WMD between psoriasis and non-psoriasis groups (5.40 mg/dl, 95 % CI: −1.65-12.44, P = 0.18) (Fig. 3).
Fig. 3.
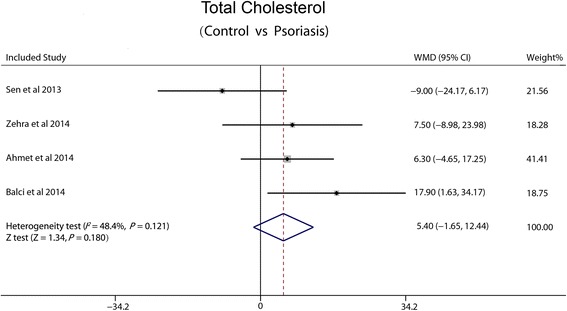
Forest plot showing difference in means and 95 % CI of total cholesterol between patients with psoriasis and control group
Low-density lipoprotein
Four studies included reported LDL. According to the low heterogeneity between trials (I2 = 0 %, P =0.421), fixed-effect pooled analysis was made. There was no significant difference in the WMD between psoriasis and non-psoriasis groups (4.69 mg/dl, 95 % CI: −1.39-10.77, P = 0.102) (Fig. 4).
Fig. 4.
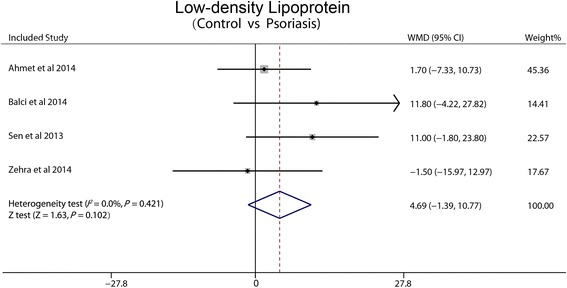
Forest plot showing difference in means and 95 % CI of LDL between patients with psoriasis and control group
High-density lipoprotein
Four studies reported HDL. Heterogeneity between trials (I2 = 57.6 %, P = 0.069) is high, so random effects meta-regression analysis was made. There was no significant difference in the WMD between psoriasis and non-psoriasis groups (−0.32 mg/dl, 95 % CI: −3.31-2.67, P = 0.833) (Fig. 5).
Fig. 5.
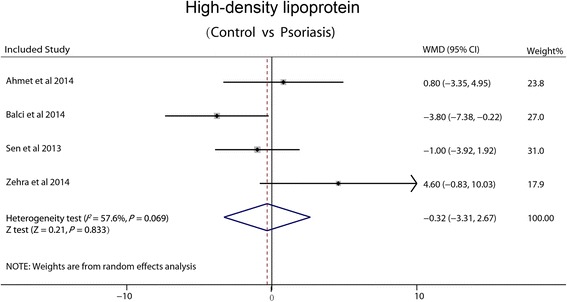
Forest plot showing difference in means and 95 % CI of HDL between patients with psoriasis and control group
Triglycerides
Four studies included reported triglycerides. Based on the low heterogeneity between trials (I2 = 0 %, P = 0.403), fixed-effect pooled analysis was made. There was no significant difference in the WMD between psoriasis and non-psoriasis groups (5.76 mg/dl, 95 % CI: −8.36-19.87, P = 0.42) (Fig. 6).
Fig. 6.
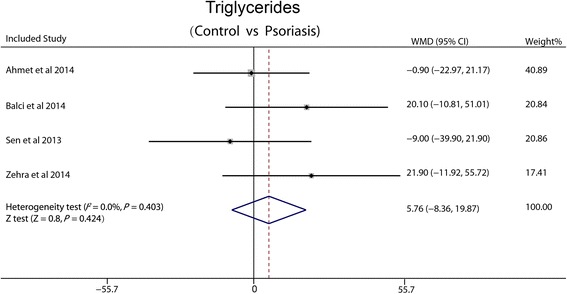
Forest plot showing difference in means and 95 % CI of triglycerides between patients with psoriasis and control group
Publication bias was not assessed because of limited studies.
Discussions
The results of this meta-analysis suggest that EFT increased significantly in psoriasis patients, compared with non-psoriasis patients. However, there was no significant difference in total cholesterol, LDL, HDL and triglycerides between the patients with and without psoriasis.
In recent years, many clinical studies found that psoriasis patients are under a high risk of CVD. Neimann et al. [17] conducted an epidemiology investigation of the relationship between psoriasis and coronary heart disease based on the General Practice Research Database (131,560 psoriasis patients and 465,252 non-psoriasis patients). This study showed that traditional risk factors for coronary heart disease such as diabetes, hypertension, hyperlipidemia, obesity and smoking are highly correlated with psoriasis. A prospective study showed that patients with psoriasis is under a higher relative risk (RR) of CVD compared with the general population [18]. Alexandroff et al. put forward that psoriasis is an independent risk factor for myocardial infarction (MI) [19]. CVD and psoriasis have similar histology and molecular immune pathological basis [20], based on Th1 cytokines (TNF alpha, IL - 2, IL - 10, IL - 12) [21].
Epicardial fat tissue (EFT) refers to the visceral fat deposition in heart, and it developed from brown adipose tissue in embryonic period. EFT has both protective effects and adverse effects [22]. Its physiological role has not been completely elucidated so far. Clinical research has proved that the EFT is associated with cardiovascular risk factors. Epicardial fat thickness in the metabolic syndrome population is significantly higher than that of control group [23, 24]. EFT is the source of cytokines which can induce mononuclear cells infiltration into the vascular intima. This process is an important step in the development of atherosclerosis. The expression of inflammatory gene in EFT is higher than that in the subcutaneous adipose tissue in cardiovascular disease patients. And EFT release more inflammatory mediators such as, interleukin, MCP - 1 and TNF alpha [25]. The thickness, area or volume of EFT can be measured and evaluated by two-dimensional echocardiography, CT or magnetic resonance imaging (MRI).
To our knowledge, there are few studies explored the relationship between EFT and psoriasis. However, the causes of increased EFT in psoriasis patients are still unknown. Chronic immune-mediated systemic inflammation may be liable for this phenomenon. This meta-analysis also showed that no significant difference was existed in the serum level of total cholesterol, LDL, HDL and triglycerides between psoriasis patients and control subjects. This demonstrates that increased EFT may be an independent risk factor for psoriasis.
There is no multi-center, large sample size study about the role of EFT in psoriasis published, and this meta-analysis is the first time to investigate the relationship between EFT and patients with psoriasis. This study may have several possible limitations. Firstly, three studies included [9, 10, 12] were small sample size. Secondly, all the studies were performed as the cross sectional design and the study groups were not under clinical follow-up. Thirdly, the gold standard in measuring EFT is MRI. Echocardiography is less accurate than CT or MRI, but echocardiography is a noninvasive, less costly and convenient method. Another possible limitation of this analysis is the great heterogeneity of EFT between trials. That may be caused by differences in measurement technology.
Conclusion
In conclusion, this meta-analysis has demonstrated that there is no significant difference in total cholesterol, LDL, HDL or triglycerides between psoriasis and control, but higher EFT in psoriasis group compared to control group. That may indicate that EFT is an independent risk factor of psoriasis. This might give an explanation for the relationship between psoriasis and elevated risk of CVD. Moreover, increased EFT can also provide a new clue for the future research of psoriasis.
Acknowledgements
We are grateful for the valuable suggestion from reviewers of this manuscript.
Authors’ contributions
WX carried out the EFT studies and drafted the manuscript. GZ helped perform the analysis with constructive discussions. ZZ performed the statistical analysis and helped write the manuscript. BY and YB participated in the data extraction. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–41. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 2.Husted JA, Thavaneswaran A, Chandran V, Eder L, Rosen CF, Cook RJ, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken) 2011;63(12):1729–35. doi: 10.1002/acr.20627. [DOI] [PubMed] [Google Scholar]
- 3.Friedewald VE, Cather JC, Gelfand JM, Gordon KB, Gibbons GH, Grundy SM, et al. AJC editor’s consensus: psoriasis and coronary artery disease. Am J Cardiol. 2008;102:1631–43. doi: 10.1016/j.amjcard.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–3. doi: 10.1001/archdermatol.2009.94. [DOI] [PubMed] [Google Scholar]
- 5.Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69(6):1014–24. doi: 10.1016/j.jaad.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Horreau C, Pouplard C, Brenaut E, Barnetche T, Misery L, Cribier, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):12–29. doi: 10.1111/jdv.12163. [DOI] [PubMed] [Google Scholar]
- 7.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2(10):536–43. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 9.Bulbul Sen B, Atci N, Rifaioglu EN, Ekiz O, Kartal I, Buyukkaya E, et al. Increased epicardial fat tissue is a marker of subclinical atherosclerosis in patients with psoriasis. Br J Dermatol. 2013;169(5):1081–6. doi: 10.1111/bjd.12569. [DOI] [PubMed] [Google Scholar]
- 10.Akyildiz ZI, Seremet S, Emren V, Ozcelik S, Gediz B, Tastan A, et al. Epicardial fat thickness is independently associated with psoriasis. Dermatology. 2014;228(1):55–9. doi: 10.1159/000354726. [DOI] [PubMed] [Google Scholar]
- 11.Bacaksız A, Tasal A, Sevgili E, Erdoğan E, Onsun N, Sönmez O, et al. Epicardial fat thickness in patients with psoriasis vulgaris. Turk Kardiyol Dern Ars. Turk Kardiyol Dern Ars. 2014;42(1):47–54. doi: 10.5543/tkda.2014.78949. [DOI] [PubMed] [Google Scholar]
- 12.Balci A, Celik M, Balci DD, Karazincir S, Yonden Z, Korkmaz I, et al. Patients with psoriasis have an increased amount of epicardial fat tissue. Clin Exp Dermatol. 2014;39(2):123–8. doi: 10.1111/ced.12216. [DOI] [PubMed] [Google Scholar]
- 13.Torres T, Bettencourt N, Mendonça D, Vasconcelos C, Gama V, Silva BM, et al. Epicardial adipose tissue and coronary artery calcification in psoriasis patients. J Eur Acad Dermatol Venereol. 2015;29(2):270–7. doi: 10.1111/jdv.12516. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 updated March 2011. Available at: http://www.handbook.cochrane.org. Accessed Dec 2015.
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2011;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 18.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 19.Alexandroff AB, Pauriah M, Camp RD, Lang CC, Struthers AD, Armstrong DJ. More thanskin deep: atherosclerosis as a systemic manifestation of psoriasis. Br J Dermatol. 2009;161(1):1–7. doi: 10.1111/j.1365-2133.2009.09281.x. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro J, Cohen AD, David M, Hodak E, Chodik G, Viner A, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case–control study. J Am Acad Dermatol. 2007;56(4):629–34. doi: 10.1016/j.jaad.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Gulliver W. Long-term prognosis in patients with psoriasis. Br J Dermatol. 2008;159(Suppl 2):2–9. doi: 10.1111/j.1365-2133.2008.08779.x. [DOI] [PubMed] [Google Scholar]
- 22.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40(7):442–5. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 23.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin-a key adipkine in the metabolic syndrome. Diabetes Obes Metab. 2006;8(3):264–80. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 24.Perseghin G, Lattuada G, De Cobelli F, Ntali G, Esposito A, Burska A, et al. Serum resitin and hepatic fat content in nondiabetic individuals. J Clin Endocrinol Metab. 2006;91(12):5122–5. doi: 10.1210/jc.2006-1368. [DOI] [PubMed] [Google Scholar]
- 25.Shimabukuro M. Cardiac adioposity and global cardiometabolic risk: new concept and clinical implication. Circ J. 2009;73(1):27–34. doi: 10.1253/circj.CJ-08-1012. [DOI] [PubMed] [Google Scholar]


