Abstract
Among the several mechanisms that contribute to MDR (multidrug resistance), the overexpression of drug-efflux pumps belonging to the ABC (ATP-binding cassette) superfamily is the most frequent cause of resistance to antifungal agents. The multidrug transporter proteins Cdr1p and Cdr2p of the ABCG subfamily are major players in the development of MDR in Candida albicans. Because several genes coding for ABC proteins exist in the genome of C. albicans, but only Cdr1p and Cdr2p have established roles in MDR, it is implicit that the other members of the ABC family also have alternative physiological roles. The present study focuses on an ABC transporter of C. albicans, Mlt1p, which is localized in the vacuolar membrane and specifically transports PC (phosphatidylcholine) into the vacuolar lumen. Transcriptional profiling of the mlt1∆/∆ mutant revealed a down-regulation of the genes involved in endocytosis, oxidoreductase activity, virulence and hyphal development. High-throughput MS-based lipidome analysis revealed that the Mlt1p levels affect lipid homoeostasis and thus lead to a plethora of physiological perturbations. These include a delay in endocytosis, inefficient sequestering of reactive oxygen species (ROS), defects in hyphal development and attenuated virulence. The present study is an emerging example where new and unconventional roles of an ABC transporter are being identified.
Keywords: ABC transporter, Candida albicans, MLT1, phosphotidylcholine, virulence
INTRODUCTION
Only a few Candida species exist in humans commensally, and they may become pathogenic when the microbiota is unbalanced, epithelial barriers are disrupted or the immune system is weakened. Various categories of drugs, such as azoles, polyenes, allylamines, echinocandins and pyrimidine analogues, are being used to combat Candida albicans infections. The prolonged use of antifungal agents increases the probability that Candida species develop tolerance not only to the drugs to which they are exposed, but also to several other drugs. This phenomenon of MDR (multidrug resistance) is supported by the different strategies adopted by Candida, which include target alteration and the overexpression of its gene products. Among the various mechanisms of MDR, enhanced drug extrusion by resistant Candida cells represents a prominent strategy. Rapid drug extrusion by resistant C. albicans cells is the result of overexpression of the drug-efflux pump-encoding genes CDR1 and CDR2, which belong to the ABC (ATP-binding cassette) and MDR1 gene families within the MFS (major facilitator superfamily) of transporters [1–4].
The C. albicans genome is composed of 26 genes that encode putative ABC superfamily proteins belonging to the ABCB, ABCC, ABCD, ABCF, ABCE and ABCG major subfamilies. Only two members of this superfamily, Cdr1p and Cdr2p, which belong to the ABCG subfamily, are involved in clinical MDR. However, the presence of large numbers of proteins in the ABC and MFS classes suggests that they may have distinct physiological roles. These transporter proteins, particularly those belonging to the ABC superfamily, perform diverse functions. The functional diversity of these proteins is reflected by additional roles in absorption, excretion, signal transduction and pathogenesis. For instance, ABC transporters such as ScPdr5 of Saccharomyces cerevisiae or Cdr1p and Cdr2p of C. albicans, are phospholipid translocators that maintain membrane asymmetry [5]. The S. cerevisiae transporter ScMdl1 is a peptide transporter, whereas ScSte6 exports a-factor pheromone [6,7]. The cryptococcal transporters CnItra1A and CnItra3C not only transport inositol but also affect its virulence [8]. The Cryptococcus neoformans ABC transporter gene CnAFR1 provides resistance to fluconazole and is involved in the delayed phagosomal maturation of phagosomes containing C. neoformans cells [9,10]. The GDP-mannose transporters CnGmt1 and CnGmt2 of C. neoformans are involved in capsule synthesis [11]. The loss of abcB from Aspergillus fumigatus invariably elicits increased azole susceptibility and decreased virulence [12]. In Dictyostelium discoideum, the ABC transporter AmtA acts as an ammonia transporter and regulates ammonia homoeostasis [13]. The ammonium transporter Ump2 of the plant pathogen Ustilago maydis also interacts with the signalling protein Rho1, which controls polarized growth [14]. The vacuole transporter CgCtr2 of the plant pathogen Colletotrichum gloeosporioides is involved in copper transport and affects its germination and pathogenicity [15].
The present study characterizes MLT1 of C. albicans belonging to the ABCC family. We demonstrate that, apart from PC (phosphatidylcholine) transport into the vacuolar lumen, the MLT1 levels affect endocytosis, sequestration of ROS (reactive oxygen species), hypha formation and virulence implying its unconventional roles.
MATERIALS AND METHODS
Materials
The growth media YEPD (yeast extract/peptone/dextrose), serum and LB broth (LB) were purchased from Himedia. YNB (yeast nitrogen base) medium was purchased from Difco. CuSO4, NiSO4, KCl, KNO3, FeCl3, CaCl2, MgCl2 and H2O2 were obtained from Qualigens. The drug MTX (methotrexate) and chemicals Ficoll-400, BSA, sodium azide, phosphocreatine, creatine kinase, quinacrine, DCFDA (2′,7′-dichlorofluorescein diacetate), sucrose, Tris buffer, DMSO and MES were purchased from Sigma. FM4-64 (N-(3-triethylammoniumpropyl)-4-{6-[4-(diethylamino)phenyl] hexatrienyl} pyridinium ibromide) and NBD-PC (1-myristoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine) were obtained from Life Technologies and Avanti Polar Lipids respectively. HRP (horseradish peroxidase)-conjugated anti-His monoclonal antibody was purchased from Santa Cruz Biotechnology. BCA protein estimation kit was obtained from G Biosciences. The oligonucleotides used in the present study, as listed in Table 1, were obtained from Sigma Genosys.
Table 1. List of primers used in the present study.
FP, forward primer; RP, reverse primer.
| Primer name | Primer sequence |
|---|---|
| YBT1 null FP | 5′-GCAGCTAATATAAACAAGTGATC-3′ |
| YBT1 null RP | 5′-CGACTGGAATATTGAAGTTAACGG-3′ |
| YBT1 del con FP | 5′-CTAAAGAAAAAGCCACTCAAG-3′ |
| YBT1 del con RP | 5′-CTTCGCGCCGTGCGGCCATC-3′ |
| MLT1-PacI FP | 5′-CGCGATTAATTAAATGAATGAACTGAATAGAGAACTTATC-3′ |
| MLT1-NotI RP | 5′-CGCGAGCGGCCGCAATCTATGTATCCACCTTCTTTGGC-3′ |
| MLT1-K710A FP | 5′-AAAGTTGGAAGTGGGGCATCTACTTTGATTAAG-3′ |
| MLT1-K710A RP | 5′-CTTAATCAAAGTAGATGCCCCACTTCCAACTTT-3′ |
| Hgt12 FP | 5′-GTTGTTATGGCTGTCTCCCAA-3′ |
| Hgt12 RP | 5′-CCACAAATAGCCCAACAAAGA-3′ |
| SIT1 FP | 5′-AATCCATTGGGGTTGAAAAAG-3′ |
| SIT1 RP | 5′-ACATCCGACAATCTGGCATAG-3′ |
| NAD4 FP | 5′-TGTATCTCCGCAGGTATAATGG-3′ |
| NAD4 RP | 5′-GCTAATAGCATTGAACCAGCAA-3′ |
| FGR46 FP | 5′-TGAACAAGGGCAGAAAGAAGA-3′ |
| FGR46 RP | 5′-GCAATCAGACGCAGTTGAAAT-3′ |
| AOX2 FP | 5′-CCTTCGTCATTTGCATTCATT-3′ |
| AOX2 RP | 5′-AATTTCCCAGGAACAGCAAGT-3′ |
| ROY1 FP | 5′-CAACTACTACCGTCGTTGGGA-3′ |
| ROY1 RP | 5′-ACTCGTCCAAATCTCCAAGGT-3′ |
| RTA4 FP | 5′-TTCTATAGCGGCTCAACGGTA-3′ |
| RTA4 RP | 5′-TGGTCGACCTCGTAAATCTTG-3′ |
| RTA2 FP | 5′-CAGTTTTGAAGCCAATGTGGT-3′ |
| RTA2 RP | 5′-TTATCGAACGTCGACCATAGG-3′ |
| CSH1 FP | 5′-GTCAAAGACGACGCAGAAGAC-3′ |
| CSH1 RP | 5′-TTTGCAACTCAACAAATTCCC-3′ |
| OYE32 FP | 5′-CCAATTGTCGATTACGCTCAT-3′ |
| OYE32 RP | 5′-TTCTAGCAGCAGCACCAAAAT-3′ |
| ACT1 FP | 5′-GGGTAGGGTGGGAAAACTTCA-3′ |
| ACT1 RP | 5′-TTGAAACCACTGCCGACAGA-3′ |
Growth media and strains
All of the yeast strains were grown and maintained in YEPD and YNB media according to the experiment requirements. Spot assays were performed in YEPD agar medium with or without indicated treatment. Glycerol stock of strains were made in 15% glycerol and maintained at −80°C and freshly revived in YEPD before use. Table 2 lists all of the strains used. All plasmids were maintained in the bacterial strain Escherichia coli DH5α as a host for the construction and propagation. E. coli cells were grown in LB medium containing 0.1 mg/ml ampicillin (Amresco).
Table 2. List of strains used in the present study.
| Strain | Genotype/description | Source/reference |
|---|---|---|
| WT(SC5314) | Wild-type strain | [59] |
| mlt1Δ/MLT1 (ST13-9) | ST13 derivative, Δmlt1-1::hisG/MLT1 | [43] |
| mlt1∆/∆ (ST13-63) | ST13-12 derivative, Δmlt1-1::hisG/Δmlt1-2::hisG | [43] |
| mlt1∆/∆::MLT1 (ST13-K2) | ST13-63 derivative, Δmlt1-1::hisG/MLT1-MPAR | [43] |
| C4GFP | CAI4 derivative, MLT1/MLT1::GFP-URA3 | [43] |
| AD-RP | AD1-8U− derivative (Mata,pdr1-3,ura3 his1, Δyor1::hisG, Δsnq2::hisG, Δpdr5::hisG, Δpdr10::hisG, Δpdr11::hisG, Δycf1::hisG, Δpdr3::hisG, Δpdr15::hisG, Δybt1:: kanMX) | The present study |
| AD-RP-Mlt1p-GFP | AD-RP cells harbouring MLT1 ORF fused with GFP integrated at the PDR5 locus | The present study |
| AD-RP-K710A Mlt1p-GFP | AD-RP-Mlt1p-GFP cells harbouring K710A mutation in MLT1 ORF and integrated at the PDR5 locus | The present study |
| AD-RP-Mlt1p-HIS | AD-RP cells harbouring MLT1 ORF fused with a His tag integrated at the PDR5 locus | The present study |
| AD-RP-K710A Mlt1p-HIS | AD-RP-Mlt1p-HIS cells harbouring K710A mutation in MLT1 ORF and integrated at the PDR5 locus | The present study |
MLT1 plasmid construction
The MLT1 gene was amplified using MLT1-PacI FP and MLT1-NotI RP primers (Table 1) from the genomic DNA of strain SC5314. The PCR product was inserted into PacI/NotI-digested pABC3GFP and pABC3His vectors [16]. Positive clones were confirmed by sequencing. Mutant variants of the WT (wild-type) MLT1 gene were made by site-directed mutagenesis using a QuikChange® Site-Directed Mutagenesis Kit from Agilent Technology following the manufacturer's instructions [2]. Mutations were introduced into plasmids pABC3-Mlt1-GFP and pABC3-Mlt1-His using the primers MLT1-K710A FP and MLT1-K710A RP (Table 1). The mutations were confirmed by sequencing. The mutated plasmids were maintained in E. coli DH5α cells.
Strain construction
AD-RP strain construction
A YBT1 gene deletion cassette along with the KanamaX selection marker gene was amplified from a YBT1-null strain using primers YBT1 null FP and YBT1 null RP (Table 1). To construct an AD-RP strain, S. cerevisiae AD1-8u− strain was transformed with the PCR product using the lithium acetate method, and transformants were selected on YEPD plates with 25 μg/ml geneticin (Amresco). Genomic DNA was isolated from the putative colonies, and gene deletion was confirmed by using YBT1-null confirmation primers (Table 1).
MLT1 overexpression strains
To construct strains overexpressing WT (pABC3-Mlt1-GFP and pABC3-Mlt1-His) and mutant variants (pABC3-Mlt1-K710A-GFP and pABC3-Mlt1-K710A-His), plasmids were digested with AscI. The resultant transformation cassettes were used to transform AD-RP cells using the lithium acetate method and selected for uracil prototrophy [16,17].
Bioinformatic analysis
The topology of Mlt1p was predicted using the online software TOPOCONS, and a cartoon was prepared [18,19]. Phylogenetic analysis was carried out using MEGA 6 software.
Spot dilution growth assays
The susceptibility of strains towards various drugs and chemicals was tested by serial dilution spot assays essentially as described previously [20]. In summary, the strains were grown overnight on YEPD agar plates, and cultures were diluted to a D600 of 0.1 in 0.9% saline. From this culture, further 5-fold serial dilutions were made, and 5 μl of cells from each dilution was spotted on to a YEPD agar plate with test chemicals for growth inhibition, and the plates were incubated at 30°C for 48 h. Each experiment was repeated two or three times. Representative images are shown.
NBD-PC-accumulation assay
The NBD-PC-transport study was carried out in early-exponential-phase cells, as described previously [21]. Early-exponential-phase cells in YNB medium were incubated with 10 μM NBD-PC for 30 min at 30°C with shaking (200 rev./min). Then, the cells were centrifuged at 2319.85 g and washed twice with YNB medium at room temperature. For the visualization of vacuoles, cells were resuspended in 2 ml of YEPD medium to which 20 μM FM4-64 was added and incubated for 1 h at 30°C with shaking. After incubation, the cells were washed twice with ice-cold YNB and sodium azide, and slides were prepared for fluorescence microscopy.
Mlt1p expression and ATPase activity assay
Purified vacuoles (100 μg) were separated by SDS/PAGE (8% gel) and transferred to membrane. The membrane was immunodetected with HRP-conjugated anti-His monoclonal antibody (Santa Cruz Biotechnology) as described previously [2]. After immunodetection, the membrane was stained with Ponceau S solution and used as a loading control.
For ATPase activity measurement, purified vacuoles (10 μg) were used in an enzymatic assay as described previously [22]. In summary, this assay couples ATP hydrolysis to the oxidation of NADH. The oxidized NADH can be measured as the loss of absorbance at 340 nm. The reaction mixture was incubated at 30°C for 1 h. The vacuole ATPase activity of the AD-RP strain was subtracted from both the WT and mutant version of Mlt1p-overexpressing strains to assess ATPase activity specific to Mlt1p.
In vitro NBD-PC-transport assay
Vacuoles were purified as described previously using a ficoll density gradient method from C. albicans WT (SC5314) and mlt1Δ/Δ mutant [23] and kept at 4°C. The NBD-PC-uptake assay was performed on freshly prepared vacuolar vesicles at 30°C, as described previously [21]. Uptake was studied in intact vacuoles, which were selected on the basis of limiting membrane staining with FM4-64.
Lipid analysis
Lipid extraction and analyses was performed using an ESI source on a triple quadrupole mass spectrometer (ESI–MS/MS) (API 4000, Applied Biosystems), using methods described previously [24]. All experiments were performed in triplicate and results are shown as means±S.E.M.
Transcriptome analysis
C. albicans strains were grown overnight in YEPD in biological duplicates. From primary cultures, cells at a D600 of 0.2 were inoculated into 15 ml of YEPD and allowed to grow for 6 h to reach exponential phase. The cells were pelleted down at 8228.48 g and washed with DEPC (diethyl pyrocarbonate)-treated water. RNA was isolated using an RNeasy Mini Kit (Qiagen) following the manufacturer's instructions. The concentration and purity of RNA samples were estimated using a nanodrop spectrophotometer and bioanalyser. Genotypic Technology performed microarray and data scanning. The threshold value was set to 2-fold to filter out only significantly affected genes. The transcription profile was analysed using the Go-Slim mapper and was annotated on the basis of biological processes [25].
Microarray accession number
The microarrays used in the present study along with complete transcriptome data can be accessed from the NCBI Gene Expression Omnibus database under accession number GSE70341.
FM4-64 endocytosis and live-cell imaging
C. albicans cells were stained with the lipophilic dye FM4-64. Cells were grown to exponential phase and incubated in 20 μM FM4-64 dye at static condition at room temperature. An aliquot of 50 μl was withdrawn at different time points to monitor the internalization of the stain.
To monitor time-lapse FM4-64 dye endocytosis in C. albicans, early-exponential-phase cells were mixed with 20 μM FM4-64 and fixed on microscopy slides with 2% agarose in YNB. The slide was kept inside a chamber that was maintained at 30°C for timelapse imaging under a Nikon Eclipse TiE microscope equipped with an Andor iXON3 EMCCD camera and controlled using Andor iQ2.7 software. Image acquisition was started 10 min after the addition of dye and was monitored for a total duration of 3 h with a frequency of one image capture every 3 min.
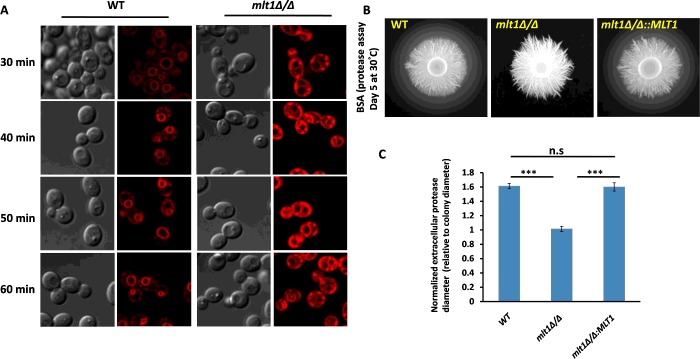
Analysis of secretory protease activity
Extracellular protease activity was assayed on YBD (yeast/BSA/dextrose) plates [26]. Overnight cultures were washed with PBS and set to a D600 of 1 for each strain. A total of 5 μl of culture was spotted on a YBD plate (BSA agar plate) and incubated at 30°C for 5 days. After 5 days, a plate image was captured by a Bio-Rad ChemiDoc™ XRS+ System, and the diameter of the halo zone surrounding the colony and colony diameter were measured using the quantification tool; halo diameters were normalized to the diameter of the fungal colony, where a value of 1 indicates no halo zone.
Measurement of ROS levels
The oxidant-sensitive probe DCFDA was used to measure endogenous ROS [27]. Cells were set to a D600 of 0.1 in YEPD medium and incubated for 4 h at 30°C with shaking at 200 rev./min. Then, a 4 mM final concentration of H2O2 was added, and the culture was grown for 2 h. The cells were then divided into two equal parts. One half of the cells was analysed with a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems) at 495 nm excitation and 529 nm emission (filter FL1). A total of 10000 events were considered. The remaining cells were used to prepare slides and were observed under confocal microscopy with a FITC filter.
Whole blood killing assay
C. albicans strains SC5314, mlt1Δ/Δ and mlt1Δ/Δ::MLT1 were grown overnight in YEPD at 30°C, re-inoculated into fresh YEPD and grown at 30°C to mid-exponential-phase. The cells were harvested in PBS and diluted to an appropriate concentration. Human whole blood was freshly drawn from healthy volunteers and anticoagulated with recombinant hirudin (Sarstedt), which does not influence complement activation. Immediately, yeast cells were added at a concentration of 106 cells/ml of whole blood and incubated at 37°C for 4 h. After incubation, samples were instantly diluted in ice-cold water and plated on YEPD agar.
Morphogenic studies
Hyphae were induced on solid and liquid media as described previously [28]. Briefly, overnight cultures of C. albicans strains were washed with PBS and set to a D600 of 1. A total of 10 μl of cells was spotted on solid medium (spider, 10% serum), and the plates were incubated at 37°C for 3 or 6 days. Images were taken under a Nikon SMZ 1500 microscope. The experiment was repeated at least three times. Representative images are shown. For a liquid hyphal assay, exponential-phase cells at a D600 of 1 were diluted to a D600 of 0.5 with different hypha-inducing media and incubated in a 12-well plate at 37°C. Images were captured at the indicated time points.
Mouse survival assay
For all mouse experiments, female BALB/c mice (6 weeks old; Charles River) were housed in ventilated cages with free access to food and water. Yeast strains were grown in individual tubes for 16 h under agitation at 30°C in YEPD medium. Each strain was subsequently diluted 100-fold in YEPD medium and grown overnight under agitation at 30°C. Overnight cultures were washed twice with PBS and resuspended in 5 ml of PBS. The concentration of each culture was measured by determination of the attenuance (D600), and each strain was diluted in PBS to the desired concentration.
For survival experiments with single strain infections, groups of seven to ten mice were used. The mice were injected through the lateral tail vein with 250 μl of a cell suspension containing 2×106 cells/ml. The weight and health of the animals were monitored daily. The post-infection day of natural death or killing of moribund animals was recorded for each mouse. Survival experiments were terminated at 15 days after infection.
Statistical analysis
Differences in secreted protease activity were compared by one-way ANOVA followed by Tukey's post-hoc test. P<0.001 is represented by ***. A two-tailed unpaired Student's t test was used for comparison of expression of Mlt1p between H2O2-treated and untreated cells, where P=0.007 is represented by ***. Statistically significant lipid changes were highlighted by the pattern recognition tools such as PCA (principal component analysis) using the software XLSTAT (Addinsoft). A statistical significance value of 0.05 was employed using Student's t test in lipid species changes.
Ethics statement
All survival assay animal experiments were performed at the University Hospital Center of Lausanne with approval through the Institutional Animal Use Committee, Affaires Vétérinaires du Canton de Vaud, Switzerland (authorization numbers 1734.2 and 1734.3), according to decree 18 of the federal law on animal protection. For all mice experiments, female BALB/c mice (6 weeks old) were housed in ventilated cages with free access to food and water.
Human peripheral blood was collected from healthy volunteers who gave written informed consent. This study was conducted according to the principles expressed in the Declaration of Helsinki. The blood donation protocol and use of blood for this study were approved by the institutional ethics committee of the University Hospital Jena (permission number 2207-01/08).
RESULTS
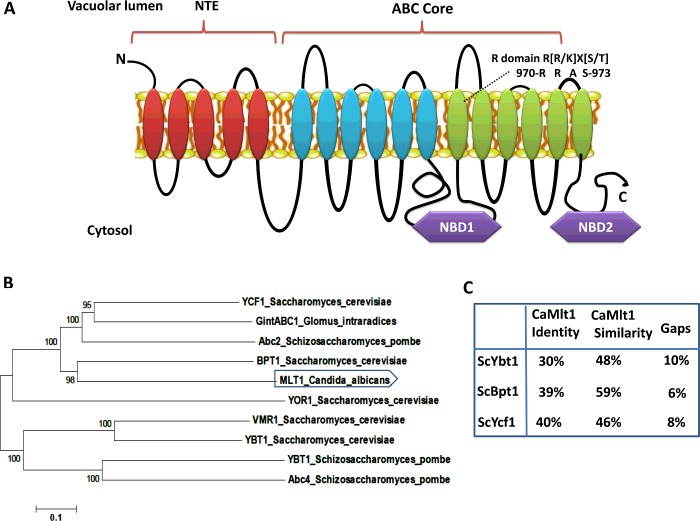
The topology prediction of Mlt1p suggests that its domain arrangement is similar to that of other MRPs (MDR proteins). It has a characteristic extra TMD (transmembrane domain) composed of five TMHs (transmembrane helices). Thus, similar to other proteins of the MRP subfamily, Mlt1p has a TMH5-(TMD-NBD)2 arrangement as depicted in Figure 1(A). Phylogenetic analysis of Mlt1p with functionally characterized fungal MRP (ABCC) subfamily members revealed its close resemblance to ScBpt1, a vacuolar transporter in S. cerevisiae (Figure 1B). Furthermore, BLASTp analysis with functionally characterized S. cerevisiae vacuolar transporters highlights that Mlt1p shows close sequence homology with S. cerevisiae ScBpt1p (39% identity, 59% similarity), ScYcf1p (40% identity, 46% similarity) and ScYbt1p (30% identity, 48% similarity) (Figure 1C).
Figure 1. Bioinformatic analysis of the Mlt1p transporter of C. albicans.
(A) Pictorial representation of the putative topology of Mlt1p as predicted by TOPCONS software showing typical domain arrangement of MRPs including a regulatory domain (R-domain) in TMH 12. NTE, N-terminal extension. (B) Phylogenetic analysis of C. albicans Mlt1p with yeast ABCC/MRP transporters of known functions. The analysis shows that the Mlt1p transporter is closest to the Bpt1 transporter of S. cerevisiae. The phylogenetic tree was generated using MEGA 6 software. Values at nodes represent bootstrap values signifying confidence levels. (C) BLASTp analysis of Mlt1p and S. cerevisiae vacuolar MRP transporters (ScYbt1, ScBpt1 and ScYcf1) of known functions.
Overexpression of Mlt1p in S. cerevisiae
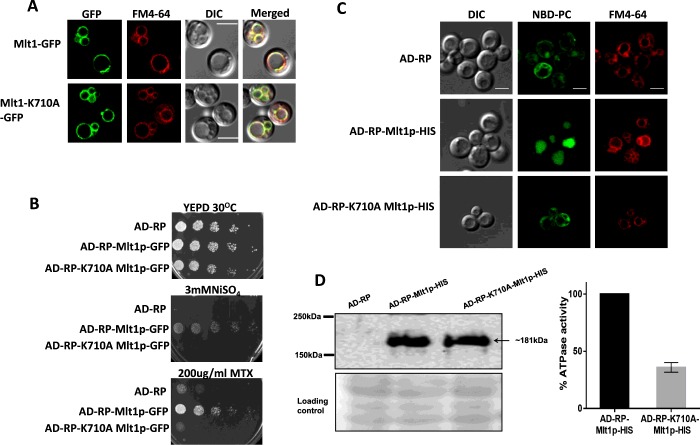
For functional characterization, we used the S. cerevisiae AD1-8u− strain as a heterologous expression system, which lacks seven major ABC transporters, including ScYor1p, ScSnq2p, ScPdr5p, ScPdr10p, ScPdr11p, ScYcf1p and ScPdr15p, thus resulting in hypersusceptibility to drugs [29]. This host expression system AD1-8u− was derivatized further by the deletion of ScYBT1 (an ion and drug transporter). The resulting strain was designated AD-RP. Because the well-characterized vacuolar transporters ScYcf1p and ScYbt1p are involved in drug transport, the deletion of YBT1 from the AD1-8u− background provided an overexpression system (AD-RP) with a cleaner background, ensuring minimum masking effects due to major ABC transporters of the host. Thus AD-RP, which is the derivative of AD1-8u−, not only lacks six PM (plasma membrane)-localized ABC transporters (ScSnq2p, ScPdr5p, ScPdr10p, ScPdr11p, ScYor1p and ScPdr15p), but also is also devoid of two major vacuolar transporters (ScYcf1p and ScYbt1). MLT1 was cloned into the vectors pABC3-GFP and pABC3-His and integrated into strain AD-RP to give strains AD-RP-Mlt1p-GFP and AD-RP-Mlt1p-HIS expressing Mlt1p from the PDR5 locus. This strain exhibits a pdr1-3 allele with a gain-of-function mutation in the transcription factor PDR1, resulting in constitutive high expression of Mlt1p [30]. The localization of overexpressed recombinant Mlt1p in AD-RP-Mlt1p-GFP was confirmed by confocal microscopy by employing the VM (vacuolar membrane)-specific fluorescent dye FM4-64 and was visualized using a TRITC filter (red fluorescence). The green fluorescence of GFP-tagged Mlt1p distinctly showed a rimmed appearance, specifically on the VM, which merged with the red fluorescence of FM4-64. The merged image clearly showed yellow fluorescence, which confirmed the localization of Mlt1p–GFP in the VM (Figure 2A, upper panel).
Figure 2. Expression, localization and characterization of WT Mlt1p and its mutant variant in a heterologous overexpressing strain.
(A) Fluorescence imaging by confocal microscope (right-hand panel) showing VM localization of Mlt1p–GFP and Mlt1p-K710A–GFP proteins with corresponding differential interference contrast (DIC) images, FM4-64 staining and merged images. Scale bar, 10 μm. (B) A comparison by spot dilution assays of susceptibilities of overexpressing strains AD-RP-Mlt1p-GFP, AD-RP-K710A Mlt1p-GFP with parental AD-RP strain. A 5-fold serial dilution of each strain was spotted on to NiSO4 and MTX at the indicated concentrations, in YEPD agar plates and grown for 48 h at 30°C. (C) NBD-PC accumulates in vacuolar lumen of WT Mlt1p-His-overexpressing strain (AD-RP-Mlt1p-HIS). Overexpression strains AD-RP-Mlt1p-HIS, AD-RP- K710A Mlt1p-HIS and parental strain AD-RP were grown to mid-exponential phase and incubated with NBD-PC. After incubation, cells were washed and stained with FM4-64. Samples were prepared for microscopy and photographed under a confocal microscope. Scale bar, 10 μm. (D) Immunoblot (left-hand panel) showing expression of Mlt1p-K710A–His and WT Mlt1p–His proteins. Vacuoles were isolated using the Ficoll gradient method and equal amounts of proteins (100 μg) were resolved by SDS/PAGE (8% gel) and then probed with anti-His antibody. After probing, membrane was stained with Ponceau S which is used as a loading control. ATPase activities (right-hand panel) in purified vacuolar vesicles from AD-RP-Mlt1p-HIS and AD-RP-Mlt1p-K710A-HIS strains were measured using an enzyme assay coupled to NADH oxidation at 340 nm. ATP hydrolysis is 60% reduced in Mlt1p-K710A-HIS mutants. Results are means±S.D. (n=3).
Mlt1p levels affect susceptibility to NiSO4 and MTX
We performed growth assays with the Mlt1p–GFP protein overexpression strain on solid agar medium containing different ions such as CuSO4, NiSO4, KCl, KNO3, FeCl3, CaCl2 and MgCl2. There was no growth difference between the Mlt1p–GFP protein-overexpressing strain (AD-RP-Mlt1p-GFP) and the parental strain (AD-RP) in the majority of the conditions tested (results not shown). However, the overexpression of Mlt1p–GFP resulted in resistance to NiSO4 compared with the parental AD-RP strain. Figure 2(B) depicts that the Mlt1p–GFP protein overexpressing strain AD-RP-Mlt1p-GFP could grow on up to 3 mM NiSO4 compared with the parental strain AD-RP, which showed no growth at this concentration. Notably, the growth of the strain overexpressing Mlt1p–GFP protein (AD-RP-Mlt1p-GFP) remained unaffected in the presence of various other compounds (results not shown). However, it did show increased resistance towards MTX compared with the parental AD-RP strain (Figure 2B).
Mlt1p transports NBD-PC into the vacuolar lumen
We examined whether Mlt1p of C. albicans could transport phospholipids into vacuoles. For this, we exploited fluorescent NBD-PC to monitor PC accumulation inside vacuoles. Because fluorescence overlaps between the NBD tag of PC and the GFP tag of Mlt1p, we performed the NBD-PC-accumulation assays by using the Mlt1p–His-tagged overexpression strain (AD-RP-Mlt1p-HIS) and comparing it with the parental AD-RP strain (Figure 2C). It was evident that the fluorescent NBD-PC specifically accumulated within the vacuolar lumen of AD-RP-Mlt1p-HIS cells, which was confirmed further by the vacuole-specific dye FM4-64, whereas the parental strain AD-RP showed no NBD-PC fluorescence in the vacuolar lumen (Figure 2C).
Deletion of MLT1 affects NiSO4 and MTX susceptibility
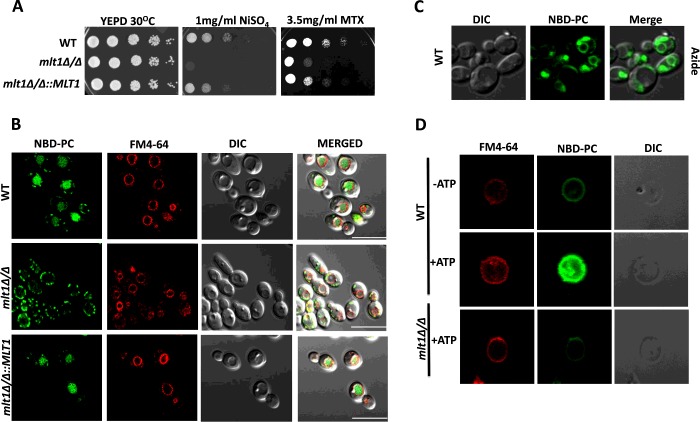
As mentioned above, the S. cerevisiae MLT1-overexpressing strain showed resistance to NiSO4 and MTX. We therefore addressed whether the deletion of MLT1 in C. albicans would show the reverse phenotype. Serial dilution assays of C. albicans WT mlt1∆/∆ and mlt1∆/∆::MLT1 isolates on NiSO4 and MTX revealed that MLT1 was contributing to NiSO4 and MTX resistance. The susceptibility towards both compounds in mlt1∆/∆ was partially reversed in revertant isolates mlt1∆/∆::MLT1 (Figure 3A).
Figure 3. Mlt1p levels are required to maintain growth in the presence of NiSO4, methotrexate and for accumulation of NBD-PC into the vacuolar lumen.
(A) Comparison of growth by spot dilution assays of C. albicans WT (SC5314), mlt1Δ/Δ and mlt1Δ/Δ::MLT1 cells. A 5-fold serial dilution of each strain was spotted on to NiSO4 and MTX at the indicated concentrations, in YEPD agar plates and grown for 48 h at 30°C. (B) Deletion of MLT1 results in the loss of accumulation of NBD-PC in the vacuolar lumen. C. albicans WT (SC5314), mlt1Δ/Δ and mlt1Δ/Δ::MLT1 cells were grown to mid-exponential phase and incubated with NBD-PC. After incubation, cells were washed and stained with FM4-64. Samples were prepared for microscopy and photographed under a confocal microscope. Scale bar, 10 μm. (C) NBD-PC accumulation into the vacuolar lumen of C. albicans is energy-dependent. Sodium azide treatment was given to mid-exponential-phase WT C. albicans (SC5314) cells before their incubation with NBD-PC. The cells were photographed under a confocal microscope. (D) NBD-PC accumulation into the vacuolar lumen is energy-dependent. Vacuoles were isolated from WT and mlt1Δ/Δ mutant C. albicans using a Ficoll gradient ultracentrifuge-based method. Equal amounts (25 μg) of purified vacuolar vesicles were then incubated at 30°C for 30 min in buffer containing 10 μM NBD-PC and 10 μM FM4-64. A transport assay was carried out under two conditions: one in the absence of energy source ATP and another in the presence of 5 mM ATP. After incubation, samples were washed with ice-cold Tris/sucrose buffer containing 3% fatty-acid-free BSA and observed under a confocal microscope. In the presence of ATP, the isolated vacuoles from WT C. albicans showed NBD-PC accumulation. There was no NBD-PC accumulation in the absence of ATP in WT cells and in vacuoles isolated from mlt1Δ/Δ mutant. DIC, differential interference contrast.
MLT1 from C. albicans is involved in the transport of NBD-PC
Examination of the NBD-PC-accumulation capacity of vacuoles in C. albicans strains revealed that WT C. albicans was able to accumulate NBD-PC into the vacuolar lumen (Figure 3B, top panel); however, the mlt1∆/∆ strain did not show any accumulation of NBD-PC, as was evident from the absence of NBD-PC fluorescence within the vacuolar lumen. Indeed, complementation of the null strain with a single allele of MLT1 could restore NBD-PC accumulation within the vacuolar lumen (Figure 3B).
NBD-PC transport by Mlt1p into vacuoles is energy-dependent
Mlt1p is an ABC transporter, and it is expected that it drives ATP hydrolysis. We checked the energy-dependence of NBD-PC accumulation using two independent approaches. In the first instance, we performed the NBD-PC-accumulation assay in WT C. albicans (SC5134) cells treated with sodium azide. As depicted in Figure 3(C), it is clear that, following sodium azide treatment, which inhibited ATP hydrolysis, the WT C. albicans cells lost their ability to accumulate NBD-PC into the vacuolar lumen. We also used the AD-RP-Mlt1p-HIS and AD-RP-Mlt1p-GFP strains and changed a well-conserved and critical lysine residue of the Walker A motif of NBD1 (G704KVGSGKS711) to alanine by site-directed mutagenesis. This conserved lysine residue has been reported to be important for ATP catalysis in many studies [21,31,32], and replacement of it with alanine (K710A) severely reduced ATPase activity (Figure 2D, right-hand panel) and abolished NBD-PC accumulation into vacuoles (Figure 2C). As expected, the mutant variant (K710A)-overexpressing strain also displayed enhanced susceptibility to NiSO4 and MTX (Figure 2B). Confocal images of a strain expressing AD-RP-K710A Mlt1p-GFP confirmed that the K710A mutation did not affect Mlt1p localization. This eliminated the possibility that the inability to accumulate NBD-PC and susceptibility to NiSO4 and MTX in the Mlt1p mutant variant could be due to mislocalization of the mutant variant protein (Figure 2A, lower panel). We performed Western blotting with vacuoles isolated from the AD-RP-Mlt1p-HIS and AD-RP-K710A Mlt1p-HIS strains to show that K710A Mlt1p–His was expressed and localized properly (Figure 2D, left-hand panel).
Mlt1p could transport NBD-PC into the vacuolar lumen in vitro
To strengthen the above results that Mlt1p transports NBD-PC and that this process is energy-dependent, we performed an in vitro NBD-PC-transport assay in a cell-free system using isolated vacuoles. For this, we grew C. albicans and the mlt1∆/∆ mutant to exponential phase in YEPD medium and isolated vacuoles by density gradient centrifugation [23]. FM4-64 staining was performed to assess the integrity of the vacuoles. It is evident from Figure 3(D) that the isolated vacuoles were intact and could accumulate NBD-PC in the presence of 5 mM ATP, whereas this was not the case with vacuoles isolated from the mlt1∆/∆ strain (Figure 3D, bottom panel).
Disruption of MLT1 leads to altered lipid homoeostasis
Lipid homoeostasis by ABC transporters is very important in different organisms. For example, a large number of human genetic disorders related to ABC transporters are due to defects in lipid transport [33]. In the yeast S. cerevisiae, MRP family transporters such as ScYbt1 and ScYor1 are involved in PC and PE (phosphatidylethanolamine) translocation respectively [21,29]. ScYbt1 is able to accumulate PC into the lumen of the vacuole and is involved in phospholipid metabolism in S. cerevisiae cells [21]. Mlt1p, which translocates PC into the vacuolar lumen, could also affect lipid homoeostasis. We explored this possibility by performing high-throughput MS-based lipidome analysis of WT and mlt1∆/∆ strains using ESI–MS/MS.
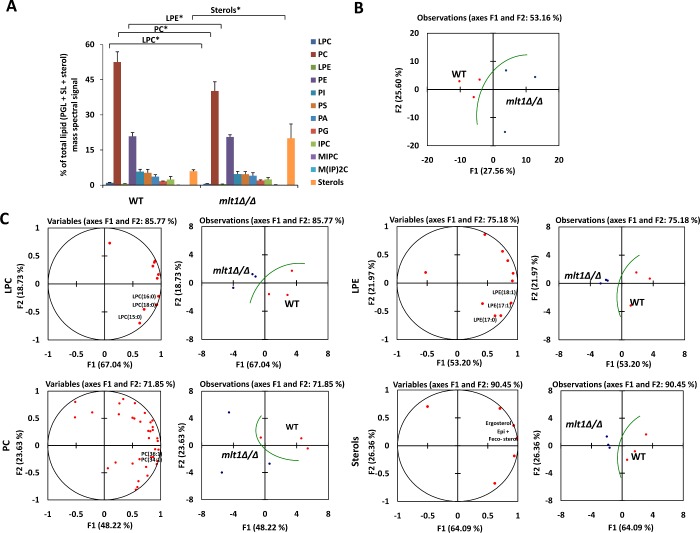
Our analysis showed that the lipid profile of the mlt1∆/∆ mutant was indeed different from that of WT (Figures 4A and 4B). The mlt1∆/∆ mutant showed depletion of PC, LPC (lysophosphatidylcholine) and LPE (lysophosphatidylethanolamine) content compared with WT (Figure 4A). These data suggest that deletion of MLT1 alters the overall phospholipid homoeostasis of the cell. A closer look at the lipid species in the mlt1∆/∆ mutant and WT using the PCA tool suggested significant modulation of molecular lipid species. The most notable differences were observed among mono-unsaturated (LPE18:1, LPE17:1, PC34:1 and PC36:1) and saturated (LPC16:0, LPC15:0, LPE17:0, LPC18:0) lipid species (Figure 4C).
Figure 4. Deletion of Mlt1p results in altered lipid homoeostasis.
(A) Lipid profile of mlt1∆/∆ mutant compared with WT. The values represent the percentage of total PGL (phosphoglycerides)+SL (sphingolipids)+sterol mass spectral signal. Results are means±S.E.M. (n=3). *P<0.05 (Student's t test). (B) PCA analysis of all lipid species analysed shows that the three replicates are grouped into WT (red) and mlt1∆/∆ mutant (blue); and that their lipid profiles are different. Factors F1 (x-axis) and F2 (y-axis) represent the two most variable principal components. (C) PCA analyses of LPC, LPE, PC and sterols. PCA loading plots (left) represent the contribution of individual species to the sample variability. PCA plots showing the grouping of replicates are on the right. LPC, lysophosphatidylcholine; PC, phosphatidylcholine; LPE, lysophosphatidylethanolamine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; PA, phosphatidic acid; PG, phosphatidylglycerol; IPC, inositol phosphorylceramide; MIPC, mannosylinositol phosphorylceramide; M(IP)2C, mannosyldi-inositol phosphorylceramide.
The mlt1∆/∆ strain transcriptome revealed differential expression of genes related to lipid homoeostasis, endocytosis, oxidative stress, hyphal development, biofilm formation and virulence
Because ABC transporters perform various physiological roles, we conducted a genome-wide transcriptome analysis of mlt1∆/∆ in comparison with the WT. The transcription profile was analysed using CGD Go-slim mapper and was annotated on the basis of biological processes (Supplementary Figures S1A and S1B). The comparative transcriptomic profile revealed that the mlt1∆/∆ strain showed up- and down-regulation (≥2-fold) of 33 and 63 genes respectively (Figure 5). Notably, the differentially regulated transcripts in the mlt1∆/∆ mutant included genes involved in endocytosis (ROY1 and APS2), oxidoreductase (OYE32, AOX2 and NAD4), lipid metabolism (RTA2), hyphal development (SSU1, RTA4, DAD48, HGT12 and FGR46), biofilm formation, pathogenesis and adherence (CSH1, TRY6, SIT1 and HWP1) as shown in Figure 5. The expression of some randomly selected genes was validated by semi-quantitative reverse transcription (RT)–PCR (Supplementary Figure S1C).
Figure 5. The comparative transcriptomic profile of mlt1Δ/Δ mutant.
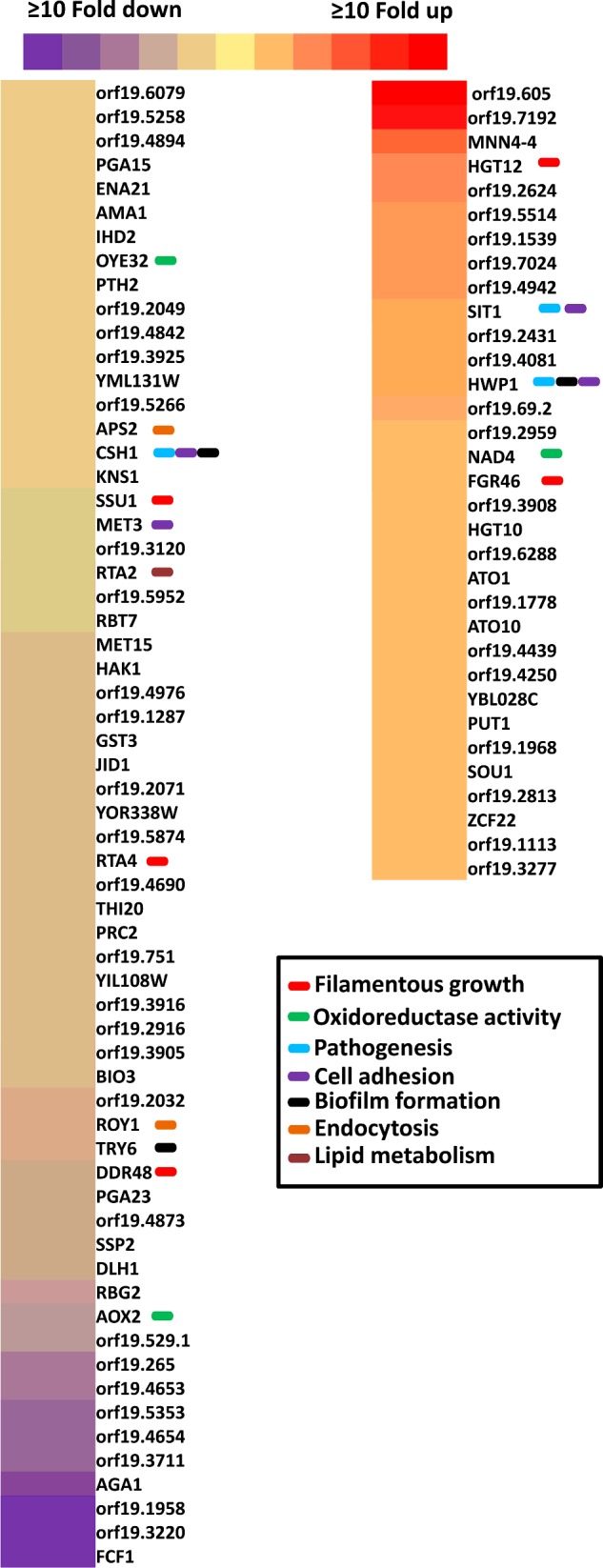
The genes with ≥2-fold change are shown. The intensity of the colour represents the extent of change in the expression values with red for up-regulation and violet for down-regulation as per the scale. The affected genes involved in endocytosis, oxidoreductase, lipid metabolism, hyphal development, biofilm formation, pathogenesis and adherence are labelled with a colour box. The different colour box represents different gene categories.
The mlt1∆/∆ mutant shows a delay in endocytosis
Transcriptome data analysis revealed that gene products involved in endocytosis were down-regulated in the mlt1∆/∆ mutant. We investigated endocytosis in the WT and the mlt1∆/∆ mutant using FM4-64, a lipophilic dye commonly used to visualize endocytosis by confocal microscopy [34]. The FM4-64 dye stained the VM distinctly in WT after 30 min of exposure (Figure 6A). However, in the mlt1∆/∆ mutant, even after 60 min, most of the dye remained restricted either to the PM or between the VM and PM. The delay in FM4-64 endocytosis was confirmed by timelapse microscopy of WT and mlt1∆/∆ mutant strains. WT cells began to show FM4-64 staining of the VM from 9 min onwards, which became distinctly visible within 30 min. In contrast with WT, the mlt1∆/∆ cells began to show FM4-64 staining of VM only after 90 min of incubation (Supplementary Figure S2).
Figure 6. mlt1Δ/Δ mutant shows a delay in endocytosis of the lipophilic dye FM4-64 and loss of secreted protease activity.
(A) Endocytosis process was studied using FM4-64 dye in WT and mlt1Δ/Δ mutant of C. albicans. Exponential-phase cells were incubated in 20 μM FM4-64 for 30, 40, 50 or 60 min under static conditions and monitored for internalization of the stain. In the case of WT, most of the dye was internalized by the endocytosis process and can be seen at the VM, whereas in the mlt1Δ/Δ mutant, the dye remained restricted either at the PM or between the VM and PM. (B) Overnight cultures were spotted on to BSA agar plates and grown at 30°C for 5 days. The zone of BSA degradation surrounding the fungal colony represents the secreted protease activity. Representative images are shown for WT, mlt1Δ/Δ and mlt1Δ/Δ::MLT1 strains. (C) The quantification of secreted protease activity was made by measuring the diameter of the degradation halo surrounding the fungal colony and was normalized to the diameter of the colony itself, thus a ratio of 1 indicates a lack of detectable halo. Results are means±S.E.M. Halo size is shown as the average for n=7 replicates. ***P<0.001 for WT compared with mlt1Δ/Δ and mlt1Δ/Δ compared with mlt1Δ/Δ::MLT1 strains calculated by one-way ANOVA followed by Tukey's post-hoc test.
The mlt1∆/∆ mutant displays reduced secretory protease activity
A previous study revealed that vacuolar proteins such as Vph1 could affect the release of secretory proteases in C. albicans [22]. We explored whether the Mlt1p vacuolar transporter levels could affect protease secretion. For this, an in vitro BSA plate assay for secretory protease activity was performed, wherein the amount of extracellular secreted enzyme activity was quantified by the size of haloes surrounding fungal colonies corresponding to a zone of BSA degradation [26]. As depicted in Figure 6(B), in comparison with WT, no halo of protein degradation appeared in the mlt1∆/∆ mutant, indicating a deficiency in secretory protease activity or secretion (Figure 6C). Notably, the defect in secretory protease activity/secretion was not associated with any change in vacuolar lumen pH as measured by quinacrine pH dye (results not shown).
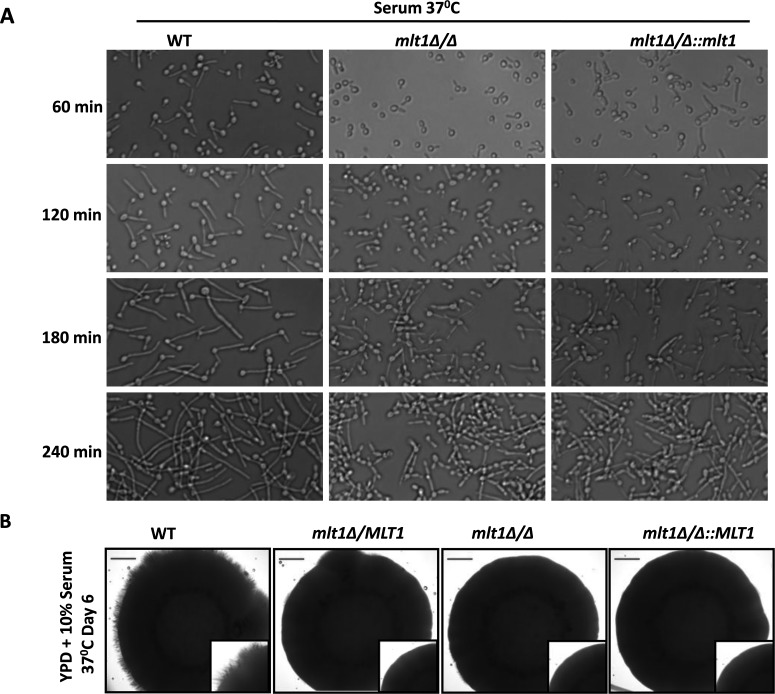
Deletion of MLT1 affects filamentous growth in C. albicans
Our microarray data showed a differential expression of hyphal development-related genes (SSU1, RTA4, DAD48, HGT12 and FGR46) and prompted us to evaluate the hyphal development ability of the mlt1∆/∆ mutant in various hypha-inducing conditions. In liquid hypha-inducing medium (serum, spider and RPMI 1640), we performed a time-course study of hypha formation. Up to 60 min, it was observed that only a few cells from the mlt1∆/∆ mutant showed hypha formation. Only at later time points (120, 180 and 240 min) could hyphae be observed, although their lengths were shorter than those of both WT and mlt1∆/∆::MLT1 cells. Thus deletion of MLT1 delayed hypha formation (Figure 7A, and Supplementary Figures S3B and S3C). Notably, in solid YEPD and 10% serum medium, MLT1 becomes haploinsufficient, as the single allele null strain mlt1Δ/MLT1 also showed absence of hypha formation (Figure 7B). In spider solid medium, the single allele mutant (mlt1Δ/MLT1) showed hyphae but of shorter length (Supplementary Figure S3A). However, in embedded agar conditions, deletion of MLT1 did not affect hypha formation (results not shown).
Figure 7. Deletion of MLT1 affects hyphal growth in C. albicans.
(A) C. albicans WT (SC5314), mlt1Δ/Δ and mlt1Δ/Δ::MLT1 were incubated in liquid YEPD medium containing 10% serum at 37°C and images were captured after 60, 120, 180 or 240 min of incubation. (B) MLT1 mutant becomes haploinsufficient on solid hypha-inducing medium. C. albicans WT (SC5314), MLT1/mlt1Δ, mlt1Δ/Δ and mlt1Δ/Δ::MLT1 strains were spotted on to YEPD containing 10% serum agar plates and incubated at 37°C for 6 days. Scale bar, 100 μm.
Yeast to hyphal switching can be considered an important virulence factor as mutants that are defective in filament formation show attenuated virulence [35]. The formation of hyphae and their active penetration are involved in host epithelial cell damage [36]. Because the mlt1Δ/Δ mutant was defective in hyphal formation, we compared the oral epithelial cell-damaging ability of WT, mlt1Δ/Δ and mlt1∆/∆::MLT1 cells. However, we did not observe any significant difference in epithelial cell damage between these different cell types (results not shown).
The transcriptome profile and the hyphal formation data indicated that the mlt1∆/∆ mutant could be defective in biofilm formation compared with WT. Different genes involved in biofilm formation, such as CSH1, TRY6 and HWP1, are differentially expressed in the mlt1Δ/Δ mutant compared with WT. However, a close comparison of the biofilm-formation capacity of WT, mlt1Δ/Δ and mlt1∆/∆::MLT1 strains in a rat catheter model did not show significant differences (results not shown). This may suggest that the observed down-regulation of gene expression in mlt1Δ/Δ could be a transient effect not directly associated with MLT1. Alternatively, since biofilm formation involves a complex regulatory network, the presence of overlapping genes could mask any detectable phenotype due to altered expression of few genes in mlt1Δ/Δ cells.
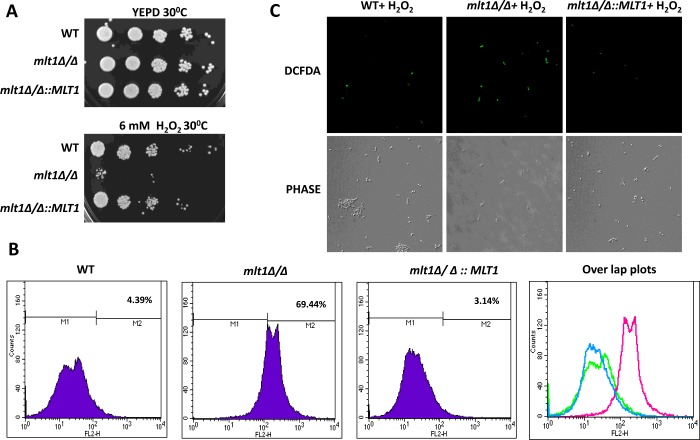
MLT1 deletion results in hypersusceptibility to oxidative stress and higher killing by human whole blood
We observed that some oxidoreductase activity-related genes (OYE32, AOX2 and NAD4) are differentially regulated in the mlt1∆/∆ mutant. We checked the survival of the mlt1∆/∆ mutant in the presence of 6 mM H2O2 by serial dilution assays. Figure 8(A) depicts that, in contrast with WT and mlt1∆/∆::MLT1, the mlt1∆/∆ strain was unable to grow at the H2O2 concentrations tested. This finding was well supported by ROS measurements where it was evident using DCFDA, an oxidant-sensitive probe, that the fluorescence of the dye was higher in mlt1∆/∆ cells than in WT and mlt1∆/∆::MLT1 cells (Figure 8B). The enhanced level of ROS in mlt1∆/∆ cells was also reflected in fluorescence images that were examined simultaneously in another batch of cells withdrawn from the same suspension. It was apparent that mlt1∆/∆ cells displayed enhanced fluorescence compared with WT and mlt1∆/∆::MLT1 cells (Figure 8C), thus suggesting that the MLT1-null strain was not able to sequester ROS efficiently.
Figure 8. MLT1 mutant is susceptible to oxidative stress by H2O2 and shows low ROS sequestration.
(A) Comparison of growth by spot dilution assays of C. albicans WT (SC5314), mlt1Δ/Δ and mlt1Δ/Δ::MLT1 cells. A 5-fold serial dilution of each strain was spotted on to 6 mM H2O2-containing YEPD agar and YEPD control and grown for 48 h at 30°C. (B) Measurement of ROS generation by FACS analysis using DCFDA in WT (SC5314), mlt1Δ/Δ and mlt1Δ/Δ::MLT1 cells after treatment with sub-lethal concentration 4 mM H2O2 for 2 h. n=10000 cells for each sample. (C) Confocal images of aliquots of samples used in FACS to measure ROS by DCFDA in WT (SC5314), mlt1Δ/Δ and mlt1Δ/Δ::MLT1.
Once C. albicans enters the host bloodstream, neutrophils and macrophages play an important role in killing the fungus. ROS generation is one of the key mechanisms in the killing of pathogens by neutrophils. Although the percentage of killing in whole human blood did not show a large difference, there was a noticeable trend of higher killing after 4 h in the case of the mlt1Δ/Δ mutant (91.1%) compared with WT (81.1%) and mlt1∆/∆::MLT1 (83.1%) strains (Figure 9A). This may also explain the lower ROS-sequestering efficiency of MLT1-null strains (Figures 8B and 8C). However, we did not observe any difference in the killing of WT and mlt1Δ/Δ mutant by human primary macrophages (results not shown).
Figure 9. mlt1Δ/Δ mutant shows attenuation in virulence.
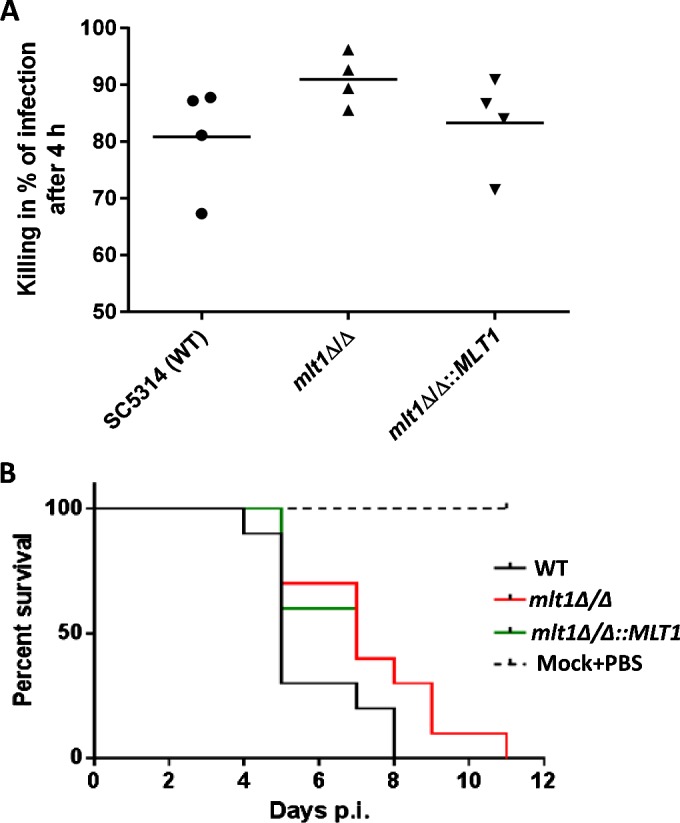
(A) Killing assay for C. albicans WT (SC5314), mlt1Δ/Δ and mlt1Δ/Δ::MLT1 cells in human whole blood after 4 h showing higher tendency of killing by the mlt1Δ/Δ mutant. (B) Survival curve of mice infected with mlt1Δ/Δ mutant shows attenuation in virulence as the mice infected with mutant strains survived up to 11 days post-infection compared with the WT C. albicans strains where all of the mice succumbed to infection within 8 days. Virulence was partially restored when a single copy of the MLT1 gene was added back to mutants. p.i., post-infection.
To determine the infection capacity of the mlt1∆/∆ mutant, a mouse survival assay was performed. Immunocompetent BALB/c mice were infected with WT (SC5314), mlt1Δ/Δ and mlt1∆/∆::MLT1 cells. A marked difference in the survival rate of mice infected with WT and mlt1Δ/Δ strains was observed. Some 70% of mice infected with WT C. albicans died on day 5, peaking to 100% death at day 8. In contrast with WT, among mice infected with the mlt1Δ/Δ mutant, only 30% death was recorded at day 5, reaching 100% only after day 11 (Figure 9B). Notably, mice infected with the revertant mlt1∆/∆::MLT1 showed survival closer to the mlt1Δ/Δ mutant. This could be because the presence of both of the MLT1 alleles is required for maintenance of the virulence trait as both alleles must be retained in order to maintain proper hypha formation (Figure 7).
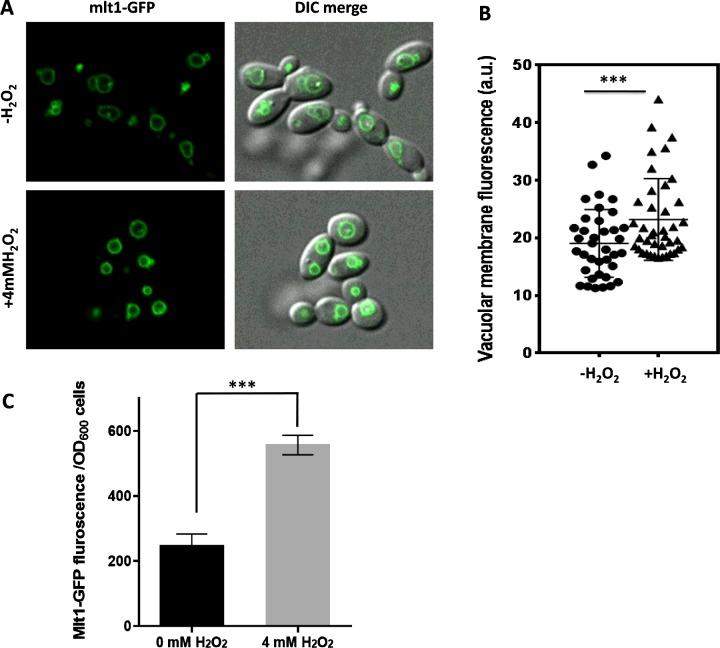
H2O2 exposure enhances the level of Mlt1p–GFP
Because Mlt1p helps in the survival of C. albicans under H2O2 stress, we explored whether oxidative stress could also influence the expression of Mlt1p. For this, the fluorescence of GFP in GFP-tagged Mlt1p, which was expressed from its chromosomal locus in C. albicans, was quantified by two methods: by measuring ROI (region of interest) intensity using confocal microscopy and by a quantitative estimation of fluorescence using a spectrofluorimeter. The ROI mean intensity of the VM was calculated for approximately 40 cells from different fields. Figures 10(A) and 10(B) depict a statistically significant (P=0.007) increase in ROI mean intensity in H2O2-treated cells. The Mlt1p–GFP fluorescence quantified using a spectrofluorimeter further supported the confocal microscopy data, revealing an increase of ∼2-fold in the fluorescence intensity following H2O2 treatment of C. albicans (Figure 10C).
Figure 10. H2O2 treatment induces the expression of Mlt1p–GFP.
(A) Exponential-phase cells of C. albicans expressing GFP-tagged Mlt1p from its chromosomal locus were grown further for 2 h with or without 4 mM H2O2. The cells were then washed with PBS and observed under a fluorescence microscope. Representative images are shown. (B) ROI intensity measured using NIS elements AR are plotted. Results are means±S.E.M. (n=37 for untreated and n=40 for treated samples). Two-tailed unpaired Student's t test was used. ***P=0.007. (C) Equal numbers of cells (D600 of 1) were set in PBS buffer in aliquots of samples treated for 2 h with or without 4 mM H2O2 and quantification of Mlt1p–GFP fluorescence was measured using a spectrofluorimeter. Results are means±S.D. ***P<0.0004 for mutant compared with WT strains as measured by unpaired Student's t test.
DISCUSSION
The members belonging to the MRP subfamily of ABC transporters from different organisms have different physiological roles such as transport of PC and PE lipids, ions, bile salt, glutathione (GS)-conjugated compounds, leukotriene C4, glycocholic acid, cyclic nucleotides and ade2 pigments; and in vacuole fusion and oxidative stress. [21,29,37–40]. Except for the PM-localized ScYor1, all of the remaining MRP members (ScYbt1, ScYcf1, ScBpt1, ScNft1 and ScVmr1) in S. cerevisiae are localized in the VM and transport different substrates, including ions, bile salt, GS-conjugated compounds and phosphoglycerides [21,41,42]. The commensal pathogenic C. albicans has four MRP family members, MLT1/orf19.5100, orf19.6478, orf19.6382, and orf19.1783, but none has been assigned any functional role. However, Mlt1p is one of the members of the MRP subfamily in C. albicans that is localized in the VM [43]. Our functional analysis in the present study revealed that contrary to an earlier report, which suggested the absence of a ScYBT1 homologue in C. albicans [44], MLT1 appears to be a functional homologue of this transporter. For instance, similarly to ScYBT1, MLT1 deletion not only results in complete loss of NBD-PC accumulation in the vacuolar lumen, but it also promotes enhanced susceptibility to NiSO4 and MTX.
We observed that MLT1 levels are critical for withstanding oxidative stress in C. albicans. Thus not only are mlt1Δ/Δ cells susceptible to H2O2 treatment, but also they have elevated levels of ROS, implying lower efficiency of the null strain in its sequestration. Of note, the ScYCF1 transporter is known to be involved in oxidative stress [39]; our data also indicate that MLT1 has such a role, a notion that is well reinforced by transcriptome data showing differential regulation of oxidative-stress-related genes (OYE32, AOX2 and NAD4) in mlt1Δ/Δ cells. This result would imply that MLT1 not only is able to functionally complement ScYBT1, but also can also partially perform the function of ScYCF1. Hence the vacuolar transporter MLT1 is capable of performing dual functions, which are otherwise performed by two independent proteins in S. cerevisiae. This situation can probably justify the existence of fewer members of the MRP subfamily in the C. albicans genome compared with that of S. cerevisiae. Interestingly, the inventory of ABC proteins in the C. albicans genome did not identify any homologue of ScYBT1; it did, however, indicate two putative ORFs, orf19.6478 and orf19.5100/MLT1, as ScYCF1 homologues. Although the former remains uncharacterized, we now report that part of the function of S. cerevisiae ScYCF1 is performed by MLT1. Together, the data from the present and previous studies rule out the existence of real homologues of ScYBT1 and ScYCF1 in C. albicans, which apparently is taken over by MLT1 in parts.
Further functional analysis revealed that MLT1 levels affect cellular endocytosis. For instance, as revealed by timelapse confocal microscopy, mlt1Δ/Δ cells displayed a significant delay in endocytosis (Figure 6A and Supplementary Figure S2). The involvement of an ABC transporter in endocytosis appears to be a novel function, but it is not surprising because, in different organisms, several members of the ABC family have diverse non-transport functions such as in metabolism, germination, pathogenicity, biofilm architecture, polarized growth, capsule synthesis and oxidative stress [8,11,12,15,39,45–48]. Notably, hyphal development in C. albicans is already known to be linked to endocytosis with proteins such as Vrp1, Vps1, Vph1, Pep12, Rvs161 and Rvs167 affecting endocytosis and hyphal development [22,49–53].
The behaviour of the mlt1Δ/Δ mutant with regard to hyphal development could also explain an earlier study wherein an Mlt1p–GFP fusion protein was used to study the effect of the Rab GTPase on Golgi-to-vacuole trafficking. It was reported that vps21Δ/Δ (vacuolar protein sorting Rab GTPase) cells of C. albicans showed defective hyphal formation on agar medium, and this phenotype was enhanced when another GTPase (YPT52) was also deleted in the vps21Δ/Δ background. However, the deletion of YPT52 alone in the WT did not affect hyphal formation [54]. Interestingly, in the double knockout (vps21Δ/Δ, ypt52Δ/Δ), a significant amount of Mlt1p was mislocalized. Considering our observations that deletion of even a single allele of MLT1 (mlt1Δ/MLT1) can lead to a defect in hyphal development, it is thus plausible that the potentiation of hyphal defects by YPT52 deletion in the vps21Δ/Δ null background could have been caused by Mlt1p mislocalization. Interestingly, the mlt1Δ/Δ mutant shows a delay in endocytosis and in parallel differential expression of hyphal development-related genes. Additionally, the mlt1Δ/Δ mutant showed attenuated virulence in an immunocompetent mouse model. Thus our results also support the well-established correlation between endocytosis, hyphal development and virulence.
Generally, phenotypes such as altered vesicle trafficking, oxidative stress and attenuated virulence are associated with defects in ergosterol biosynthesis in C. albicans [55–58]. Additionally, these phenotypes are also mimicked in vacuolar endocytosis mutants [22,49–51,53]. However, in the case of the mlt1∆/∆ mutant, its high ergosterol content suggests that these phenotypes might be independent of the ergosterol pathway (Supplementary Table S1). High ergosterol content could rather be a compensatory change. Interestingly, this consideration emphasizes further that the role of Mlt1p is more attributable to PC transport into the vacuolar lumen.
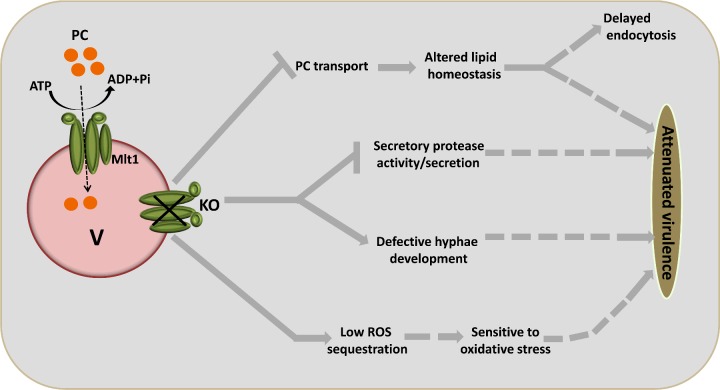
In conclusion, Mlt1p transports PC into the vacuolar lumen, and it affects lipid homoeostasis, thus leading to delayed endocytosis, defects in hyphae and biofilm, susceptibility to drugs, protease activity/secretion, survival of oxidative stress and killing by whole blood, together culminating in attenuated virulence (Figure 11). The present study not only supports the well-known role of ABC transporters in MDR, but also provides strong evidence in support of multiple functional roles of a member of the ABC transporter superfamily in C. albicans.
Figure 11. Proposed model for vacuolar Mlt1p transporter function and its roles in different phenotypes.
Mlt1p which is localized to the VM transports the PC analogue NBD-PC into the vacuolar lumen in an energy-dependent manner and maintains lipid homoeostasis. Thus MLT1 deletion may lead to defects in endocytosis and virulence. Deletion of MLT1 also negatively affects various virulence related traits such as hyphae formation, secretory protease activity/secretion and sensitivity to oxidative stress. Thus all of these phenotypes show their cumulative effect to attenuated virulence in the mlt1Δ/Δ mutant.
Acknowledgments
We acknowledge Dr Gerwald A. Kohler for providing Candida albicans Mlt1p mutant strains. We acknowledge Advance Instrumentation Research Facility (AIRF), Jawaharlal Nehru University, for providing instrumental support and Mr Ashok Kumar Sahu and Ms Tripti Panwar for confocal microscopy and live-cell imaging. We are thankful to Dr K. Natarajan for providing the S. cerevisiae YBT1-null strain. We also thank Mr Kaushal Kumar Mahto for helping with lipid preparation and Ms Meghna Gupta for helping with data representation. The help of Dr Sanjiveeni Dhamgaye, Dr Peer Abdul Haseeb Shah and Mr Atanu Banerjee for discussion during the work is highly appreciated.
Abbreviations
- ABC
ATP-binding cassette
- DCFDA
2′,7′-dichlorofluorescein diacetate
- GS
glutathione
- HRP
horseradish peroxidase
- LPC
lysophosphatidylcholine
- LPE
lysophosphatidylethanolamine
- MDR
multidrug resistance
- MFS
major facilitator superfamily
- MRP
MDR protein
- MTX
methotrexate
- NBD-PC
1-myristoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine
- PC
phosphatidylcholine
- PCA
principal component analysis
- PE
phosphatidylethanolamine
- PM
plasma membrane
- ROI
region of interest
- ROS
reactive oxygen species
- TMD
transmembrane domain
- TMH
transmembrane helix
- VM
vacuolar membrane
- WT
wild-type
- YBD
yeast/BSA/dextrose
- YEPD
yeast extract/peptone/dextrose
- YNB
yeast nitrogen base
AUTHOR CONTRIBUTION
Nitesh Kumar Khandelwal, Ashutosh Singh, Bernhard Hube, David Andes, Dominique Sanglard and Rajendra Prasad designed the experiments. Nitesh Kumar Khandelwal, Philipp Kaemmer, Toni Förster, Alix Coste and David Andes performed the experiments. Nitesh Kumar Khandelwal, Ashutosh Singh, Bernhard Hube, David Andes, Dominique Sanglard, Alok Kumar Mondal, Neeraj Chauhan, Rupinder Kaur, Christophe d'Enfert and Rajendra Prasad analysed the data with input from the other authors. Bernhard Hube, David Andes, Dominique Sanglard and Rajendra Prasad contributed reagents/materials. Nitesh Kumar Khandelwal, Ashutosh Singh, Bernhard Hube, Dominique Sanglard, Alok Kumar Mondal and Rajendra Prasad wrote the paper with input from the other authors. All authors read and approved the final paper.
FUNDING
The work has been supported, in part, by the Department of Biotechnology, Ministry of Science and Technology, Government of India [grant numbers BT/01/CEIB/10/III/02, BT/PR7392/MED/29/652/2012 and BT/PR14879/BRB10/885/2010 (to R.P.)]. N.K.K. acknowledges the University Grants Commission, India, for a senior research fellowship award. Financial Support from the Indo–Swiss Joint Research Programme [grant number 122 917 (to D.S. and R.P.)], for supporting N.K.K.’s visit to CHUV, Lausanne, is acknowledged.
References
- 1.White T.C., Holleman S., Dy F., Laurence F., Stevens D.A., Mirels L.F. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 2002;46:1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla S., Saini P., Jha S., Ambudkar V., Prasad R., Ambudkar S.V. Functional characterization of Candida albicans ABC transporter Cdr1p. Eukaryot. Cell. 2003;2:1361–1375. doi: 10.1128/EC.2.6.1361-1375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T.T., Znaidi S., Barker K.S., Xu L., Homayouni R., Saidane S., Morschhäuser J., Nantel A., Raymond M., Rogers P.D. Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot. Cell. 2007;6:2122–2138. doi: 10.1128/EC.00327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanglard D., Ischer F., Monod M., Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 5.Smriti, Krishnamurthy S., Dixit B.L., Gupta C.M., Milewski S., Prasad R. ABC transporters CdrLp, Cdr2p and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast. 2002;19:303–318. doi: 10.1002/yea.818. [DOI] [PubMed] [Google Scholar]
- 6.Young L., Leonhard K., Tatsuta T., Trowsdale J., Langer T. Role of the ABC transporter Mdl1 in peptide export from mitochondria. Science. 2001;291:2135–2138. doi: 10.1126/science.1056957. [DOI] [PubMed] [Google Scholar]
- 7.Ketchum C.J., Schmidt W.K., Rajendrakumar G.V., Michaelis S., Maloney P.C. The yeast a-factor transporter Ste6p, a member of the ABC superfamily, couples ATP hydrolysis to pheromone export. J. Biol. Chem. 2001;276:29007–29011. doi: 10.1074/jbc.M100810200. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Liu T.B., Delmas G., Park S., Perlin D., Xue C. Two major inositol transporters and their role in cryptococcal virulence. Eukaryot. Cell. 2011;10:618–628. doi: 10.1128/EC.00327-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanguinetti M., Posteraro B., La Sorda M., Torelli R., Fiori B., Santangelo R., Delogu G., Fadda G. Role of AFR1, an ABC transporter-encoding gene, in the in vivo response to fluconazole and virulence of Cryptococcus neoformans. Infect. Immun. 2006;74:1352–1359. doi: 10.1128/IAI.74.2.1352-1359.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orsi C.F., Colombari B., Ardizzoni A., Peppoloni S., Neglia R., Posteraro B., Morace G., Fadda G., Blasi E. The ABC transporter-encoding gene AFR1 affects the resistance of Cryptococcus neoformans to microglia-mediated antifungal activity by delaying phagosomal maturation. FEMS Yeast Res. 2009;9:301–310. doi: 10.1111/j.1567-1364.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- 11.Cottrell T.R., Griffith C.L., Liu H., Nenninger A.A., Doering T.L. The pathogenic fungus Cryptococcus neoformans expresses two functional GDP-mannose transporters with distinct expression patterns and roles in capsule synthesis. Eukaryot. Cell. 2007;6:776–785. doi: 10.1128/EC.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul S., Diekema D., Moye-Rowley W.S. Contributions of Aspergillus fumigatus ATP-binding cassette transporter proteins to drug resistance and virulence. Eukaryot. Cell. 2013;12:1619–1628. doi: 10.1128/EC.00171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshino R., Morio T., Yamada Y., Kuwayama H., Sameshima M., Tanaka Y., Sesaki H., Iijima M. Regulation of ammonia homeostasis by the ammonium transporter AmtA in Dictyostelium discoideum. Eukaryot. Cell. 2007;6:2419–2428. doi: 10.1128/EC.00204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul J.A., Barati M.T., Cooper M., Perlin M.H. Physical and genetic interaction between ammonium transporters and the signaling protein Rho1 in the plant pathogen Ustilago maydis. Eukaryot. Cell. 2014;13:1328–1336. doi: 10.1128/EC.00150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barhoom S., Kupiec M., Zhao X., Xu J.R., Sharon A. Functional characterization of CgCTR2, a putative vacuole copper transporter that is involved in germination and pathogenicity in Colletotrichum gloeosporioides. Eukaryot. Cell. 2008;7:1098–1108. doi: 10.1128/EC.00109-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamping E., Monk B.C., Niimi K., Holmes A.R., Tsao S., Tanabe K., Niimi M., Uehara Y., Cannon R.D. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot. Cell. 2007;6:1150–1165. doi: 10.1128/EC.00091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gietzt R.D., Schiestls R.H., Willems A.R., Woods R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 18.Bernsel A., Viklund H., Hennerdal A., Elofsson A. TOPCONS: consensus prediction of membrane protein topology. Nucleic Acids Res. 2009;37:465–468. doi: 10.1093/nar/gkp363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsirigos K.D., Peters C., Shu N., Kall L., Elofsson A. The TOPCONS web server for consensus prediction of membrane protein topology and signal peptides. Nucleic Acids Res. 2015;43:W401–W407. doi: 10.1093/nar/gkv485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay K., Kohli A., Prasad R. Drug susceptibilities of yeast cells are affected by membrane lipid composition. Antimicrob. Agents Chemother. 2002;46:3695–3705. doi: 10.1128/AAC.46.12.3695-3705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulshan K., Moye-Rowley W.S. Vacuolar import of phosphatidylcholine requires the ATP-binding cassette transporter ybt1. Traffic. 2011;12:1257–1268. doi: 10.1111/j.1600-0854.2011.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raines S.M., Rane H.S., Bernardo S.M., Binder J.L., Lee S.A., Parra K.J. Deletion of vacuolar proton-translocating ATPase Voa isoforms clarifies the role of vacuolar pH as a determinant of virulence-associated traits in Candida albicans. J. Biol. Chem. 2013;288:6190–6201. doi: 10.1074/jbc.M112.426197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bankaitis V.A., Johnson L.M., Emr S.D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc. Natl. Acad. Sci. U.S.A. 1986;83:9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahto K.K., Singh A., Khandelwal N.K., Bhardwaj N., Jha J., Prasad R. An assessment of growth media enrichment on lipid metabolome and the concurrent phenotypic properties of Candida albicans. PLoS One. 2014;9:e113664. doi: 10.1371/journal.pone.0113664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle E.I., Weng S., Gollub J., Jin H., Botstein D., Cherry J.M., Sherlock G. GO::TermFinder: open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics. 2004;20:3710–3715. doi: 10.1093/bioinformatics/bth456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross I.K., De Bernardis F., Emerson G.W., Cassone A., Sullivan P.A. The secreted aspartate proteinase of Candida albicans: physiology of secretion and virulence of aproteinase-deficient mutant. J. Gen. Microbiol. 1990;136:687–694. doi: 10.1099/00221287-136-4-687. [DOI] [PubMed] [Google Scholar]
- 27.Dhamgaye S., Devaux F., Vandeputte P., Khandelwal N.K., Sanglard D., Mukhopadhyay G., Prasad R. Molecular mechanisms of action of herbal antifungal alkaloid berberine, in Candida albicans. PLoS One. 2014;9:e104554. doi: 10.1371/journal.pone.0104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad T., Saini P., Gaur N.A., Ram A., Khan L.A., Haq Q.M.R., Vishwakarma R.A. Functional analysis of CaIPT1, a sphingolipid biosynthetic gene involved in multidrug resistance and morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 2005;49:3442–3452. doi: 10.1128/AAC.49.8.3442-3452.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Decottignies A., Grant A.M., Nichols J.W., De Wet H., McIntosh D.B., Goffeau A. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 1998;273:12612–12622. doi: 10.1074/jbc.273.20.12612. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K., Niimi M., Niimi K., Holmes A.R., Yates J.E., Decottignies A., Monk B.C., Goffeau A., Cannon R.D. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 2001;45:3366–3374. doi: 10.1128/AAC.45.12.3366-3374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frelet A., Klein M. Insight in eukaryotic ABC transporter function by mutation analysis. FEBS Lett. 2006;580:1064–1084. doi: 10.1016/j.febslet.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Ren X.-Q., Furukawa T., Haraguchi M., Sumizawa T., Aoki S., Kobayashi M., Akiyama S. Function of the ABC signature sequences in the human multidrug resistance protein 1. Mol. Pharmacol. (2004;65:1536–1542. doi: 10.1124/mol.65.6.1536. [DOI] [PubMed] [Google Scholar]
- 33.Paulusma C.C., Oude Elferink R.P.J. Diseases of intramembranous lipid transport. FEBS Lett. 2006;580:5500–5509. doi: 10.1016/j.febslet.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 34.Vida T.A., Emr S.D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo H.J., Köhler J.R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G.R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/S0092-8674(00)80358-X. [DOI] [PubMed] [Google Scholar]
- 36.Wächtler B., Wilson D., Haedicke K., Dalle F., Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One. 2011;6:e17046. doi: 10.1371/journal.pone.0017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein M., Mamnun Y.M., Eggmann T., Schüller C., Wolfger H., Martinoia E., Kuchler K. The ATP-binding cassette (ABC) transporter Bpt1p mediates vacuolar sequestration of glutathione conjugates in yeast. FEBS Lett. 2002;520:63–67. doi: 10.1016/S0014-5793(02)02767-9. [DOI] [PubMed] [Google Scholar]
- 38.Kruh G.D., Belinsky M.G. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 39.Lee M.E., Singh K., Snider J., Shenoy A., Paumi C.M., Stagljar I., Park H.O. The Rho1 GTPase acts together with a vacuolar glutathione S-conjugate transporter to protect yeast cells from oxidative stress. Genetics. 2011;188:859–870. doi: 10.1534/genetics.111.130724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasser T.L., Lawrence G., Karunakaran S., Brown C., Fratti R.A. The yeast ATP-binding cassette (ABC) transporter Ycf1p enhances the recruitment of the soluble SNARE Vam7p to vacuoles for efficient membrane fusion. J. Biol. Chem. 2013;288:18300–18310. doi: 10.1074/jbc.M112.441089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z.S., Szczypka M., Lu Y.P., Thiele D.J., Rea P.A. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J. Biol. Chem. 1996;271:6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- 42.Sharma K.G., Mason D.L., Liu G., Rea P.A., Bachhawat A.K., Michaelis S. Localization, regulation, and substrate transport properties of Bpt1p, a Saccharomyces cerevisiae MRP-type ABC transporter. Eukaryot. Cell. 2002;1:391–400. doi: 10.1128/EC.1.3.391-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theiss S., Kretschmar M., Nichterlein T., Hof H., Agabian N., Hacker J., Köhler G.A. Functional analysis of a vacuolar ABC transporter in wild-type Candida albicans reveals its involvement in virulence. Mol. Microbiol. 2002;43:571–584. doi: 10.1046/j.1365-2958.2002.02769.x. [DOI] [PubMed] [Google Scholar]
- 44.Kovalchuk A., Driessen A.J.M. Phylogenetic analysis of fungal ABC transporters. BMC Genomics. 2010;11:177. doi: 10.1186/1471-2164-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer F.L., Wilson D., Jacobsen I.D., Miramón P., Große K., Hube B. The novel Candida albicans transporter Dur31 is a multi-stage pathogenicity factor. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop A.C. Ph.D. Thesis. Pittsburgh, PA, U.S.A: Duquesne University; 2013. Transport and Metabolism of Glycerophosphodiesters by Candida albicans. [Google Scholar]
- 47.Shah A.H., Singh A., Dhamgaye S., Chauhan N., Vandeputte P., Suneetha K.J., Kaur R., Mukherjee P.K., Chandra J., Ghannoum M.A., et al. Novel role of a family of major facilitator transporters in biofilm development and virulence of Candida albicans. Biochem. J. 2014;460:223–235. doi: 10.1042/BJ20140010. [DOI] [PubMed] [Google Scholar]
- 48.Tran P.N., Brown S.H.J., Mitchell T.W., Matuschewski K., McMillan P.J., Kirk K., Dixon M.W.A., Maier A.G. A female gametocyte-specific ABC transporter plays a role in lipid metabolism in the malaria parasite. Nat. Commun. 2014;5:4773. doi: 10.1038/ncomms5773. [DOI] [PubMed] [Google Scholar]
- 49.Barelle C.J., Richard M.L., Gaillardin C., Gow N.A.R., Brown A.J.P. Candida albicans VAC8 is required for vacuolar inheritance and normal hyphal branching. Eukaryot. Cell. 2006;5:359–367. doi: 10.1128/EC.5.2.359-367.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornet M., Bidard F., Schwarz P., Costa D., Blanchin-roland S., Dromer F. Deletions of endocytic components VPS28 and VPS32 affect growth at alkaline pH and virulence through both RIM101 pathways in Candida albicans. Infect. Immun. 2005;73:7977–7987. doi: 10.1128/IAI.73.12.7977-7987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernardo S.M., Khalique Z., Kot J., Jones J.K., Lee S.A. Candida albicans VPS1 contributes to protease secretion, filamentation, and biofilm formation. Fungal Genet. Biol. 2008;45:861–877. doi: 10.1016/j.fgb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Douglas L.M., Martin S.W., Konopka J.B. BAR domain proteins Rvs161 and Rvs167 contribute to Candida albicans endocytosis, morphogenesis, and virulence. Infect. Immun. 2009;77:4150–4160. doi: 10.1128/IAI.00683-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palanisamy S.K.A., Ramirez M.A., Lorenz M., Lee S.A. Candida albicans PEP12 is required for biofilm integrity and in vivo virulence. Eukaryot. Cell. 2010;9:266–277. doi: 10.1128/EC.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnston D.A., Tapia A.L., Eberle K.E., Palmer G.E. Three prevacuolar compartment Rab GTPases impact Candida albicans hyphal growth. Eukaryot. Cell. 2013;12:1039–1050. doi: 10.1128/EC.00359-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukherjee P.K., Chandra J., Kuhn D.M., Ghannoum M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003;71:4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorpe G.W., Fong C.S., Alic N., Higgins V.J., Dawes I.W. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6564–6569. doi: 10.1073/pnas.0305888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y.Q., Gamarra S., Garcia-Effron G., Park S., Perlin D.S., Rao R. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog. 2010;6:e1000939. doi: 10.1371/journal.ppat.1000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Landolfo S., Zara G., Zara S., Budroni M., Ciani M., Mannazzu I. Oleic acid and ergosterol supplementation mitigates oxidative stress in wine strains of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2010;141:229–235. doi: 10.1016/j.ijfoodmicro.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 59.Mullaney E.J., Hamer J.E., Roberti K.A., Yelton M.M., Timberlake W.E. Primary structure of the trpC gene from Aspergillus nidulans. Mol. Gen. Genet. 1985;199:37–45. doi: 10.1007/BF00327506. [DOI] [PubMed] [Google Scholar]