Current knowledge about the glycosylation of matrix metalloproteinases (MMPs) and the inhibitors of metalloproteinases (TIMPs) is reviewed. Whereas structural and functional aspects of the glycobiology of many MMPs is unknown, research on MMP-9 and MMP-14 glycosylation reveals important functional implications, such as altered inhibitor binding and cellular localization. This, together with the fact that MMPs contain conserved and many potential attachment sites for N-linked and O-linked oligosaccharides, proves the need for further studies on MMP glycobiology.
Keywords: glycosylation, MMPs, TIMPs
Abstract
Matrix metalloproteases (MMPs) are crucial components of a complex and dynamic network of proteases. With a wide range of potential substrates, their production and activity are tightly controlled by a combination of signalling events, zymogen activation, post-translational modifications and extracellular inhibition. Slight imbalances may result in the initiation or progression of specific disease states, such as cancer and pathological inflammation. As glycosylation modifies the structures and functions of glycoproteins and many MMPs contain N- or O-linked oligosaccharides, we examine, compare and evaluate the evidence for whether glycosylation affects MMP catalytic activity and other functions. It is interesting that the catalytic sites of MMPs do not contain O-linked glycans, but instead possess a conserved N-linked glycosylation site. Both N- and O-linked oligosaccharides, attached to specific protein domains, endow these domains with novel functions such as the binding to lectins, cell-surface receptors and tissue inhibitors of metalloproteases (TIMPs). Validated glycobiological data on N- and O-linked oligosaccharides of gelatinase B/MMP-9 and on O-linked structures of membrane-type 1 MMP/MMP-14 indicate that in-depth research of other MMPs may yield important insights, e.g. about subcellular localizations and functions within macromolecular complexes.
INTRODUCTION
In recent years, research on matrix metalloproteases (MMPs) has grown exponentially due to increasing interest from biomedical scientists, in both academia and industry. The scientific excitement about MMPs originates from their involvement in several physiological and pathological processes. When first discovered, MMPs were defined as secreted proteases, able to degrade extracellular matrix (ECM) proteins (e.g. collagens and elastins) during ECM remodelling events [1,2]. This vision has now been broadened because MMPs also modulate intracellular, pericellular and extracellular signalling pathways and networks, by activating or inactivating other molecules such as chemokines, proteases, protease inhibitors, cell-surface receptors and intracellular proteins [3–5]. Small changes in the activity, specificity or levels of MMPs can result in the development of both localized and systemic pathological conditions such as cancer [6], inflammation [7,8], autoimmune diseases [9,10], vascular diseases [7] and neurological disorders [11–14]. Therefore, MMPs are tightly controlled enzymes and this control is hierarchically organized at various levels. At the cellular level, MMP expression is transcriptionally regulated by signalling pathways, triggered through a wide range of receptors, e.g. MMP production is mediated by mitogen-activated protein kinase (MAPK) pathways, which are triggered by mitogens, phorbol esters, cellular stress signals and inflammatory cytokines [15]. Whereas, originally, these mechanisms of action were proven by demonstration of specific transcription factors acting on genetic cis elements in the promoter regions of MMP genes, more recently, epigenetic control mechanisms have been added in the form of DNA methylation/demethylation and histone acetylation/deacetylation events. Furthermore, once MMP mRNAs have been synthesized, their levels are altered post-transcriptionally by many control RNA mechanisms, including miRNAs [1,16–22].
During and after mRNA translation, several modifications occur that alter specific parts of a protein/enzyme after synthesis, thereby inducing an extra level of structural and functional diversity. A great variety of co- and post-translational modifications exist, so that almost all amino acids can be altered by one of these processes. One such modification is glycosylation [23]. By a complex series of enzymatic steps, oligosaccharides can be attached to glycoproteins. Most often, glycans are formed by common N-glycosidic and O-glycosidic bonds. N-Glycans are attached to asparagine residues and the O-glycans can be linked to every amino acid with a hydroxy functional group (often serine or threonine). Whereas N-glycans are attached during translation [24], O-glycans are attached in the Golgi and trans-Golgi complexes after protein folding [25]. An important tuning function of N-linked sugars in the endoplasmic reticulum (ER) is protein folding [23,26]. Once folded, the glycoproteins move through the Golgi system, where a series of transferases sequentially attach and modify, at the luminal side, O-linked sugar structures on serines or threonines. Throughout the present review we follow the paradigm that oligosaccharides impose fine-tuning functions on glycoproteins, including MMPs [27–31].
Once synthesized, most MMPs exist as inactive pro-enzymes, which become active on proteolytic removal of the pro-peptide or modification of the chelating cysteine residue, located in the pro-domain [7]. Proteolytic removal of pro-peptides is done by many proteases, including plasmin, meprins, furins and even activated MMPs. Modification of the chelating cysteine residue includes processes such as S-nitrosylation and S-glutathiolation. Both modes of activation can happen intracellularly and extracellularly [4]. The core function of the cysteine residue in this activation process is known as the cysteine-switch mechanism [32]. Once in the activated state, MMP activity can be blocked by a range of inhibitors. Whereas in plasma the main inhibitor of MMP activity is α2-macroglobulin [33], in tissues four MMP inhibitors, named the tissue inhibitors of metalloproteases (TIMPs) [34], execute such a function.
Finally, MMPs that possess one or more free cysteine residues may form disulfide bonds in both a homotypical and a heterotypical way, e.g. MMP-9 forms a covalent complex with neutrophil gelatinase B-associated lipocalin [35,36]. Although the functional consequences of this complex formation are not yet clear, it proves that MMPs may form covalent complexes via disulfide bridges. This mechanism was also discovered for homotypical interactions in MMP-9 trimers. The covalent trimer of MMP-9 molecules results in a new structural entity that is differentially inhibited by TIMP-1, when compared with the monomer [37]. The relationship between structure and function is critical for biology. Therefore, in the present review, we evaluate the presence and effects of both O-glycans and N-glycans on the functions of MMPs.
MMP STRUCTURE
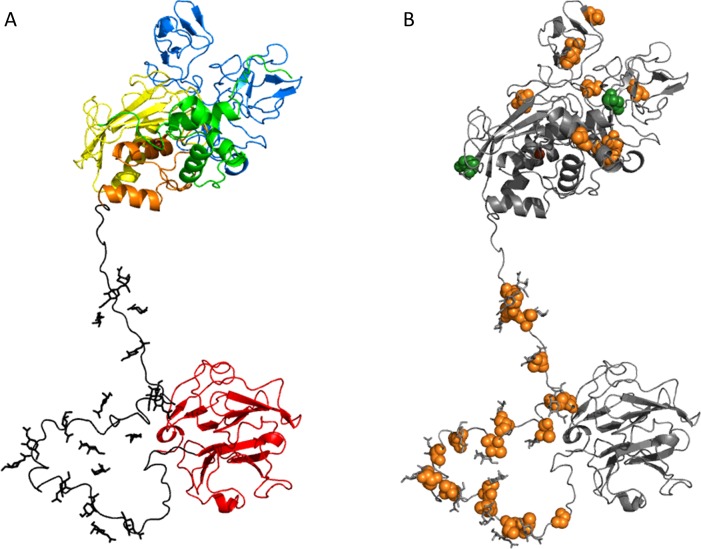
MMPs are multi-domain enzymes with designated functions for each domain (Figure 1A) [1]. The pro-peptide domain is shared by all MMPs and functions as a regulator of enzyme activity. This domain contains a signature amino acid sequence (PRCXXPD), in which the conserved cysteine residue can interact with the catalytic Zn2+ ion and thereby keep the enzymes inactive. On proteolytic removal of the pro-peptide or chemical modification of the conserved cysteine residue, MMPs become active, via the ‘cysteine switch’ [32]. The catalytic part is formed by the catalytic domain and the Zn2+-binding domain, and is highly conserved within the MMP family [38]. The fibronectin repeats are present only in gelatinases (MMP-2 and MMP-9) and they assist these enzymes in the catalysis of large substrates such as gelatins [39]. Membrane-type MMPs (MT-MMPs) are characterized by having a membrane anchor and some MMPs also have a cytoplasmic tail at the C-terminus. Several MMPs also have a linker domain that is situated between the Zn2+-binding domain and the haemopexin domain. In MMP-9 the linker domain is rich in the amino acids serine, threonine and proline, and was found to be highly O-glycosylated (Figure 1B). For this reason this domain was renamed the O-glycosylated domain [40]. Finally, the haemopexin domain is present in several MMPs and has a range of functions. Although this domain assists in binding to substrates, it also mediates binding to inhibitors and cell-surface receptors, and was found to induce autoactivation of the enzyme [41–43]. Consequently, soluble MMPs, such as MMP-9, may become attached to cell surfaces by protein–protein interactions, e.g. MMP-9 binds to α4β1 integrin [42], or by protein–sugar interactions, e.g. MMP-9 binds to cell-surface galectins (see below) [44].
Figure 1. Domains and glycosylation of pro-MMP-9.
(A) Multi-domain structure of pro-MMP-9 in a refined model of MMP-9 based on compositional and site-specific glycan analysis, sedimentation data and atomic force microscopy structures (PDB codes 1L6J and 1ITV). The MMP-9 domains are shown in different colours: pro-peptide (green), active site (yellow), three fibronectin repeats (blue), Zn2+-binding domain (orange), OG domain (black), haemopexin domain (red) and Zn2+ (brown). (B) Localization in the pro-MMP-9 model of the validated N-glycosylation positions Asn38 and Asn120 (dark green) and the possible O-glycosylated sites (orange) predicted by NetOGlyc 4.0: Ser26, Ser66, Ser250, Ser257, Thr258, Thr259, Ser273, Ser298, Thr316, Thr317, Thr455, Thr456, Thr457, Thr458, Thr462, Thr466, Thr470, Thr474, Ser478, Thr482, Thr486, Ser490, Thr494, Thr498, Ser502, Thr503, Thr505, Thr506 and Ser510.
GLYCOSYLATION
Glycosylation is one of the many post-transcriptional modifications that can be found in MMPs. The implications of glycosylation in this protease family are just starting to be understood. N-Glycosylation occurs when the consensus sequences Asn-Xaa-Ser or Asn-Xaa-Thr (Xaa is any amino acid except proline) are generated in the ER. A large enzyme complex [45] transfers en bloc and at the luminal side a dolichol pyrophosphate-linked branched oligosaccharide (GlcNAc2Man9Glc3) to the nascent protein [46]. The biosynthesis of this oligosaccharide and the topology of the enzymes involved are complex. For many of these enzymes, rare genetic defects have been discovered and classified as congenital diseases of glycosylation (CDGs). The fact that these CDGs are rare diseases points towards the importance of N-linked glycosylation in biology [47]. Furthermore, some organisms (e.g. most bacteria) live without these enzymes, resulting in a lack of N-linked glycosylation patterns. This finding places glycosylation as a next step in evolution. Indeed, by diversification of proteins into glycoproteins, a basis is formed for the generation of new molecules and eventually new species.
Glycans attached to proteins are often the first point of contact between molecules in cellular interactions, making these modifications essential for the correct physiological functioning of molecules. O-Glycosylation has several physiological functions [25], e.g. mucins are heavily O-glycosylated molecules which function as a protective layer in epithelia and control properties such as the interactions with the environment and the immune system (cell binding through lectins) [48]. Recognition of sugars by lectins is a common theme in immune functions, as observed for selectins and siglecs [49]. Alterations in O-glycosylation patterns have also been associated with diseases. As an example, the glycosylation pattern in cancer cells varies significantly from that of normal cells [50,51], and therefore aberrant O-glycosylation is considered to be a hallmark of cancer. Cancer-associated O-glycans are often truncated, highly sialylated and less sulfated, and contain N-acetylgalactosamine (GalNAc) and Galβ1–3GalNAc, also known as Tn and T antigens because they can trigger an immune response [25,48]. In addition, N-acetylgalactosaminyltransferase 14 (GALNT14), which catalyses the initial step of O-glycosylation, is heterogeneously expressed in, for example, breast cancers, and was associated with invasion and migration of breast cancer cells [52]. Moreover, a similar effect was witnessed for β1,3-N-acetylglucosaminyltransferase-8 (β3GnT8), which catalyses the formation of polylactosamine on β1–6-branched N-glycans in U251 glioma cells [53].
MMPs AND THEIR GLYCOSYLATIONS
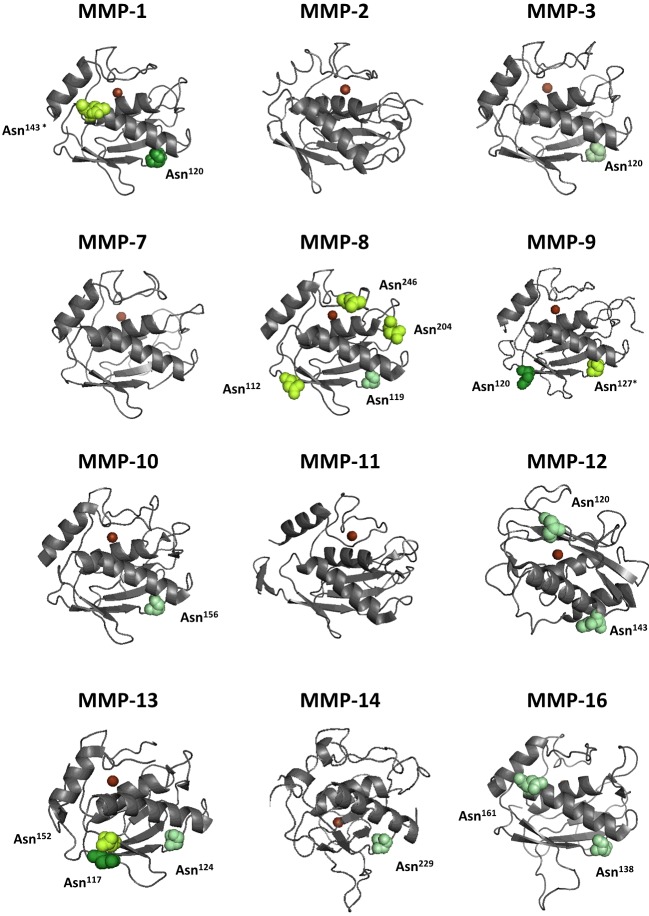
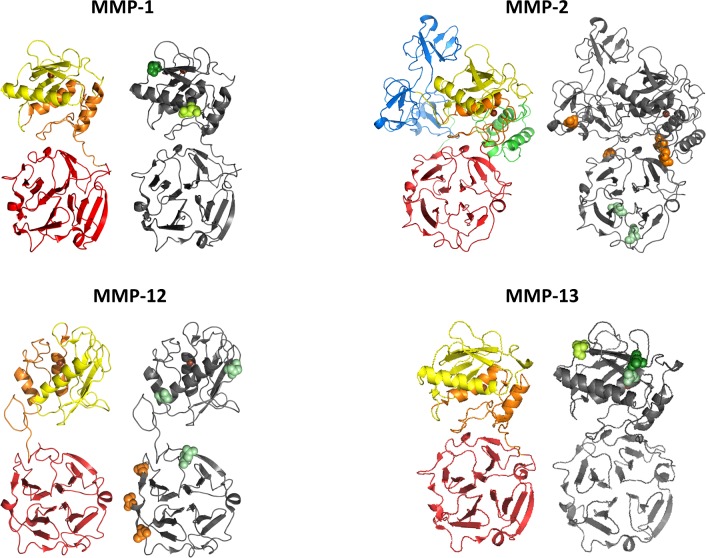
In this section we list separately what is known about the glycosylation of each MMP. For MMP-7, -8, -10, -11, -12, -15, -16, -24 and -25 no literature is available on glycosylation. We used the NetNGlyc and NetOGlyc tools [54] to estimate the glycosylation status of these MMPs. An alignment of all MMPs with their potential and proven glycosylation sites can be found in Supplementary Figure S1, which illustrates that all MMPs have potential glycosylation sites. Across the MMP family, conserved glycosylation sites appear mainly as N-glycosylations in the active site, a feature that becomes even more evident when comparing MMP active sites in 3D (Figure 2). In contrast, glycosylation might also introduce an extra level of interfamily diversification by conferring different functionalities to otherwise similar protein domains. This becomes evident when comparing glycosylation patterns of the haemopexin domains of, for example, the gelatinases MMP-2 and MMP-9 (Figure 1), and other MMPs such as MMP-1, MMP-12 and MMP-13, as exemplified in Figure 3.
Figure 2. Comparisons and conservation of glycosylation in the active sites of MMPs.
The active sites of MMPs consist of the catalytic domain and the Zn2+-binding domain, which are both devoid of O-linked oligosaccharides (see Table 1). Validated N-glycosylation (dark green), predicted by the NetNGlyc1.0 Server but not yet validated (lime green), and potential sequences not predicted by the program (pale green) are shown in the crystal structures of the catalytic domains of MMPs [PDB codes 966C (MMP-1), 1CK7 (MMP-2), 1SLN (MMP-3), 1MMR (MMP-7), 2OY4 (MMP-8), 1GKC (MMP-9), 1Q3A (MMP-10), 1HV5 (MMP-11), 4H49 (MMP-12), 23PJT (MMP-13), 1BUV (MMP-14) and 1RM8 (MMP-16)]. Zn2+ is indicated in brown. In human MMP-1, Asn143 and in human MMP-9, Asn127 are not occupied (asterisks).
Figure 3. Comparison of domain structures and glycosylation sites of MMPs.
Specific domains endow proteins with specific functions and there are variations in glycosylation sites and patterns within the MMP family. Consequently, glycosylation superimposes additional functionalities on MMPs. Domains, and validated and predicted glycosylation sites, are indicated in the crystal structures of MMP-1, pro-MMP-2, MMP-12 and MMP-13 (PDB codes 2CLT, 1CK7, 3BAO and 4FU4, respectively). The following domains are shown: pro-peptide (green), active site (yellow), three fibronectin repeats (blue), Zn2+-binding domain (orange), haemopexin domain (red) and Zn2+ (brown). Validated N-glycosylated sites are indicated in dark green, sites predicted by the NetNGlyc1.0 program are in lime green, and potential sequences not predicted by the program are in pale green. The O-glycosylated sites predicted by NetOGlyc 4.0 are indicated in orange.
MMP-1
MMP-1 is secreted by human skin fibroblasts and from, for example, HT-1080 fibrosarcoma cells in two forms: a less abundant N-glycosylated form (∼57 kDa) and a more abundant non-glycosylated form (∼52 kDa). Two potential N-glycosylation sites are found at Asn120 and Asn143; however, only glycosylation of Asn120 has been experimentally proven [55–57] (Figures 2 and 3). In fibroblasts, these N-glycans are mainly α2,3-sialylated complex-type diantennary glycans, whereas, in HT-1080 fibrosarcoma cells, the N-glycosylation pattern is more heterogeneous with diantennary glycans carrying Lewis X, LacdiNAc, sialylated LacdiNAc and GalNAcβ1,4(Fucα1,3)GlcNAc [57]. Many of these glycan structures contain motifs that are recognized by selectins and thus may have biological consequences, e.g. the α1,3-fucosylated LacdiNAc structure inhibits E-selectin-mediated cell adhesion [58]. Therefore, it is thought that glycosylated MMP-1 may bind to the surface of activated cells through a selectin/glycan interface and therefore contribute to tumour cell invasion and angiogenesis. By comparison of glycosylated and non-glycosylated MMP-1, it was shown that both enzyme forms have similar substrate specificity, specific activity and are equally well inhibited by TIMP-1 [57].
MMP-2
GALNT14 catalyses the initial step of the common form of O-glycosylation and is increased in breast cancers [59]. Increased GALNT14 expression results in up-regulation of MMP-2 in MCF-7 cells, and silencing of GALNT14 results in decreased expression of MMP-2 [52]. Although the mechanism behind this observation is unclear, if MMP-2 would be O-glycosylated, it may be better stabilized, secreted and protected against degradation, and thus increase to higher steady-state levels than aglycosyl MMP-2. Site-specific analysis is not available, but the pro-peptide of MMP-2 has two potential O-glycosylated sites, namely Ser32 and Thr96 (see Supplementary Figure S1). The catalytic domain has no predicted glycosylation sites and the haemopexin domain has two potential sites for N-glycosylation (Asn573 and Asn642). More potential O-glycosylation sites are located in the fibronectin domain (Thr262) and between the Zn2+-binding and haemopexin domains, in the so-called linker domain (Thr458 and Thr460). A structural model of pro-MMP-2, with indication of the potential glycosylation sites, is shown in Figure 3.
MMP-3
Similar to MMP-1, MMP-3/stromelysin-1 is also secreted as a glycosylated (60 kDa) and a non-glycosylated (57 kDa) form in human skin fibroblasts [60]. However, there are no reports on the exact site and structure of glycosylations of stromelysin-1. Computational analysis revealed several potential glycosylation sites. One potential N-glycosylation site is located in blade III of the haemopexin domain (Asn398). In addition, there are three possible O-glycosylated residues: Ser57 (in the pro-peptide), Ser269 and Thr277 (in the linker domain). Based on the consensus sequence for N-glycosylations, Asn120 is also a potential glycosylation site, but was not predicted by the computational analysis (Figure 2).
MMP-9
Of all MMPs, MMP-9 is most extensively glycosylated. An estimated 85% of the human neutrophil gelatinase B sugars are O-linked and attached to 14 potential O-glycosylation sites (Figure 1). These sites are all clustered in a single stretch of 50 amino acids (full-length MMP-9 is ∼700 amino acids) which contains a proline-rich section (P445RPEPEPRPP) followed by eight Pro-Thr and three Pro-Ser couples (14 potential O-glycosylation sites), each spaced by two residues, thereby being a highly probable attachment region for clustered O-linked glycans [1,25,29,40,61]. On the basis of the presence of O-linked glycans in this short stretch, we called this segment the O-glycosylated domain and suggested that the glycosylation would elongate this structure in the form of a corkscrew [1,40]. Subsequent analysis by small-angle X-ray spectroscopy provided further experimental evidence for this mucin-like structure [62], and this helped to generate a better model of the MMP-9 monomer [1]. Chemically, these O-glycans consist of a mixture of type 1 core disaccharide (Galβ1–3GalNAc), but foremost elongated type 2 core structures with Galβ1–4GlcNAc (N-acetyl-lactosamine) extensions, with or without sialic acid or fucose [63].
The pro-MMP-9 sequence also contains three potential N-glycosylation sites, one in the pro-peptide (Asn38-Leu-Thr) and two in the active site (Asn120-Ile-Thr and Asn127-Tyr-Ser). Experimentally it was proven that only Asn38 (in the pro-peptide) and Asn120 (in the active site) are glycosylated [40]. By performing HLPC-based experiments, Rudd et al. [29] showed that more than 95% of the N-linked glycans of natural neutrophil MMP-9 are partially sialylated core-fucosylated biantennary structures, with and without outer-arm fucose linked to N-acetylglucosamine (GlcNAc). Recombinant pro-MMP-9, derived from Sf9 insect cells, also contains Man3GlcNAc2 structures with core fucosylation [40]. These results are in line with the data obtained with natural human MMP-9 [30] and recombinant MMP-9 expressed in HeLa cells [64].
To study the function of the attached oligosaccharides, various tests were used. In vitro tests showed that the activation rate of pro-MMP-9 by MMP-2 and MMP-3 and the catalytic activity of MMP-9 towards gelatin were not changed after N-deglycosylation [30]. However, desialylation (with sialidase from Streptococcus sp.) alters the interaction of MMP-9 with TIMP-1, consequently lowering MMP-9's inhibition by TIMP-1. After desialylation, the net activity of MMP-9 is increased significantly in the presence of equimolar or excess amounts of TIMP-1 [30]. An N120S point mutant showed reduced secretion by retention in the ER, probably due to stronger binding of aglycosyl MMP-9 to ER-resident calreticulin. This points to a possible function of the oligosaccharide in secretion, although influence by modification of the amino acid cannot be excluded. In addition, the oligosaccharide at position 38 helps in proper protein folding and secretion. In the latter the function of the oligosaccharide can be mimicked by small, but not large, amino acids. Also in this case the secretion effect cannot be fully attributed to the sugar side chain [65]. More functions for the N-linked glycans should be explored, e.g. the influence on MMP-9 stability, resistance against proteolysis/degradation, and interactions with its substrates or ECM components. Also, the finding that the pro-peptide of MMP-9 contains one N-linked glycan opens the possibility that this sugar is involved in pro-MMP-9 activation and activity.
For O-linked glycans, several functions have been suggested: extending and increasing the rigidity of a polypeptide chain, recognition, modulation of the activity of signalling molecules and enzymes, increasing stability and protection against proteases [25]. None of these possible functions have been experimentally proven for MMP-9. Protection against proteolysis might be important for MMP-9 because it is released at inflammatory sites, where other proteases are likely to be abundant. MMP-9-deletion mutants, lacking the O-glycosylated domain, show similar activation by MMP-3. In addition, the catalytic activity towards several known substrates was not altered, on deletion of the O-glycosylated domain, but these mutants were unable to bind to Helix pomatia agglutinin, a lectin specific for serine- or threonine-linked GalNAc [40]. This suggests that lectin binding, as proposed for galectins, may contribute to anchoring MMP-9 to cell-surface-bound galectins or other supramolecular complexes. For an illustration of this, the reader is referred to a published model of MMP-9 and four molecules of galectin-3 [44]. At present, the unique O-glycosylated domain is also suggested to function as a protease-resistant spacer that extends the enzyme, separating the haemopexin from the other domains. Thereby, the abundant glycosylation of the O-glycosylated domain has considerable implications for the domain organization of MMP-9 [63]. The presence of this O-glycosylated domain results in completely different structures between gelatinase A/MMP-2 and gelatinase B/MMP-9 [61]. More specifically, the O-glycosylated domain co-determines the bioavailability of active MMP-9, together with the haemopexin domain, by correctly orienting the haemopexin domain for inhibition by TIMP-1 and internalization by low-density lipoprotein receptor-related protein (LRP)-1 and LRP-2/megalin [40]. In addition, the O-glycosylated domain lends the MMP-9 molecule a high degree of interdomain flexibility believed to be important in finding cleavage sites on long substrates [66].
MMP-9 glycosylation has been associated with several pathological conditions, e.g. by comparison of MMP-9 glycans from MCF-7 breast cancer cells, THP-1 myeloid leukaemia cells and natural neutrophils, Fry et al. [67] revealed cancer-associated glycoforms that exhibit decreased binding to galectin-3. It needs to be stressed here that the glycosylation patterns differed between normal and transformed myeloid cells (neutrophils compared with THP-1 cells) and between various types of cancers (leukaemic THP-1 compared with MCF-7 breast cancer cells). O-linked glycans of neutrophil MMP-9 are mainly galactosylated core 2 structures, 46% of which are ligands for galectin-3; 11% contained two to three N-acetyl-lactosamine repeating units which are high-affinity ligands for this lectin. Glycans provide MMP-9 with both high-affinity and high-avidity interactions with galectin-3. In contrast, the O-linked glycans released from MMP-9 expressed in MCF-7 and THP-1 cells are sialylated core 1 structures, of which only 10% are ligands for galectin-3 and contained only a single N-acetyl-lactosamine repeat. Consequently, these cancer-associated glycoforms bind galectin-3 with significantly reduced affinity and avidity. The fact that it has been suggested that galectin-3 tethers MMP-9 to the cell surface under normal conditions implies that, in the tumour environment, altered glycans could allow cells to detach from the ECM, providing evidence that MMP-9 contributes to cancer-associated processes of invasion and metastasis [67].
A feature of endometriosis is the existence of autoantibodies against endometrial and serum antigens. Certain carbohydrate moieties, including the disaccharide Thomsen–Friedenreich antigen (Galβ1–3GalNAc), a type-1-core O-linked oligosaccharide, are crucial for endometriotic sera to bind their antigens [68]. Multiple T antigens are present on MMP-9 and, although no data were shown, it was mentioned that natively glycosylated pro-MMP-9 multimers reacted to serum from patients with endometriosis [69]. Nevertheless, structural comparisons of the glycans present on monomeric and trimeric MMP-9, after recombinant expression in insect cells, showed that both forms contain the same type of glycosylations [37]. Additional studies will be needed to determine whether monomers and multimers always carry similar oligosaccharides or whether structural differences are imposed under specific conditions in particular cell types.
As conclusions for MMP-9 one can summarize that: (i) the presence of both N- and O-linked structures has been defined and validated; (ii) alterations of these structures lead to functional differences in the interactions with TIMP-1 and galectin-3; and (iii) this information can be placed in a biological context. In particular, the O-glycosylated domain of gelatinase B/MMP-9 yields a completely different structure from gelatinase A/MMP-2, and contributes to subcellular localizations, and as a binding partner in macromolecular complexes.
MMP-13
MMP-13 contains two asparagine residues predicted to be N-glycosylated by the NetNGlyc 1.0 program: Asn117 and Asn152. The presence of glycans was experimentally validated at Asn117 [70]. Two serines with potential O-glycosylation are Ser24 and Ser62 (see Supplementary Figure S1). No functions of MMP-13 glycosylation have been described. A structural model of MMP-13, with an indication of potential glycosylation sites, is shown in Figure 3.
MMP-14/MT1-MMP
Membrane-type 1 MMP (MT1-MMP), also referred to as MMP-14, has only two potential N-glycosylated sequences at Asn229 (catalytic domain) and Asn311 (linker domain). In addition, the proline-rich hinge region of MMP-14 contains six potential O-glycosylation sites: Thr291, Thr299, Thr300, Ser301, Ser304 and Thr313. By performing enzymatic deglycosylation experiments, site-directed mutagenesis and lectin precipitation assays, Wu et al. [71,72] presented experimental evidence that MMP-14 contains O-linked complex carbohydrates on Thr291, Thr299, Thr300 and/or Ser301. Ser304 is not glycosylated and the O-glycosylation pattern of MMP-14 is influenced by a dileucine motif (Leu571-Leu572) in the cytoplasmic tail of the protein [73]. In contrast to most other MMPs, MMP-14 is activated intracellularly in the trans-Golgi network, thereby being an important trigger of proteolytic cascades [74,75]. Zymogen activation and interstitial collagenase activity are not impaired in glycosylation-defective MMP-14. However, loss of O-glycosylation prevents proper interaction of MMP-14 with TIMP-2, the inability to recruit TIMP-2 to the cell surface and, consequently, defective formation of the MMP-14–TIMP-2–pro-MMP-2 trimeric activation complex (in COS-7 cells) [71]. As this complex mediates activation of pro-MMP-2 [76], aberrant glycosylation of MMP-14 has a direct effect on MMP-2 and consequently also on pericellular proteolysis [71]. In a second study, using a different cell system, it was suggested that this effect can also be due to increased autolysis of glycosylation-deficient MMP-14 [77]. Indeed, the MMP-14 hinge region, containing the O-linked glycans, and in particular the region around Gly284 and Gly285, is highly susceptible to proteolysis [78], e.g. at the cell surface, MMP-14 undergoes autocatalysis to form a membrane-associated 44-kDa form, lacking the catalytic domain [73].
Several authors postulated that O-glycosylation protects MMP-14 from autocatalysis by increasing its stability [77], although in other studies similar autocatalysis products in O-glycosylated and aglycosyl forms were seen [73]. It was suggested that this dissimilarity is due to a differential affinity of the detection antibody used (anti-hinge antibody) depending on the glycosylation status of MMP-14. However, a similar stabilizing effect of O-glycosylation was reported for MMP-9 [64]. MMP-14 is also found as an intermediate form, suggesting that it is an incomplete glycosylated pro-enzyme form, which is present mostly intracellularly. The intermediate is transported inefficiently to the plasma membrane, suggesting that N-glycosylation affects MMP-14 cell-surface presentation [77].
Alterations in glycosylation of MMP-14 were found between different cell types [77] and different cancer cell lines [71]. Also, in vivo, mouse prostate tumour-associated MMP-14, and MMP-14 from mouse prostate epithelial cells deficient in the phosphatase and tensin homologue deleted on chromosome 10 (PTEN), have a slightly higher molecular mass than normal mouse prostate MMP-14, due to differential O-glycosylation. This results in the accumulation of MMP-14 at the cell surface, because of either reduced autocatalytic processing or diminished internalization of the enzyme [79].
As a conclusion, also in the case of MMP-14, the presence of O-linked oligosaccharides has been validated and is important for interactions with TIMP-2. The latter is known as a critical event in cell-surface proteolysis of cancer cell invasion and metastasis.
MMP-17/MT4-MMP
MMP-17 is anchored in membranes by glycosylphosphatidylinositol and has two predicted N-glycosylation sites at Asn137 and Asn318, located in the catalytic domain and hinge region, respectively. Deglycosylation studies show that MMP-17 is modified by N-glycosylation. Treatment with tunicamycin (an inhibitor of N-linked glycosylation) causes a significant reduction in overall MMP-17 expression levels: the electrophoretic abundance of the 57-kDa form, and to a lesser extent the 72-kDa form, of MMP-17 is reduced, compared with the untreated control. Although 11 potential O-glycosylation sites are present, enzymatic treatment to modify O-glycans (O-glycanase and sialidase A) had no effect on MMP-17 functions, in line with the suggestion that, in this system, MMP-17 is not modified with O-linked glycans [80].
MMP-23/CA-MMP
MMP-23 or cysteine array MMP (CA-MMP) has four potential N-glycosylation sites (Asn93, Asn149, Asn233 and Asn317) and eight potential O-glycosylation sites (see Supplementary Figure S1). By performing experiments with tunicamycin, it was shown that N-glycosylation contributes 6–10 kDa to the molecular mass of cell-associated MMP-23/CA-MMP [81].
INDIRECT EFFECTS OF GLYCOSYLATION ON MMPs
Both molecules that function upstream (regulators) and those that function downstream (e.g. substrates) of MMPs are subject to glycosylation. Alterations in the glycosylation status of these molecules might have direct or indirect implications on MMP function. Important regulators of MMP function are the TIMPs, which are endogenous inhibitors of the proteolytic activity of MMPs. Inhibition is accomplished by the co-ordination of the catalytic Zn2+ ion of the MMP active site with the N-terminal cysteine residues of each TIMP [82,83]. Of the four human TIMPs, TIMP-1 and TIMP-3 contain N-linked glycans. TIMP-1 has highly heterogeneous fucosylated N-linked oligosaccharides attached to two asparagine residues in the N-terminal domain: Asn30 and Asn78 [84]. In colon cancer cells, aberrant glycosylation of TIMP-1 has a direct effect on MMP-2 and MMP-9 by interfering with their interaction [85]. Highly glycosylated TIMP-1 with extensive outer fucosylation is expressed by human embryonic kidney (HEK)-293 cells, resulting in a reduced binding and inhibitory activity to MMPs [86]. Similar effects were observed for TIMP-3 [87–89]. These data provide evidence that extensive glycosylation and outer-arm fucosylation can interfere with the inhibitory capacity of TIMPs.
Other glycosylated upstream regulators of MMPs include the ECM metalloprotease inducer (EMMPRIN)/CD147/basigin [90,91] and reversion-inducing cysteine-rich protein with Kazal motifs (RECK) [92]. Glycosylation of EMMPRIN is crucial for the formation of homophilic EMMPRIN interactions [93]. Native glycosylated CD147 exists exclusively as oligomers in solution and directly stimulates MMP production more efficiently than non-glycosylated prokaryotic CD147 [94]. In fibroblasts N-glycosylation of EMMPRIN is critical for the induction of MMP-2 [95]. The glycosylation status of EMMPRIN might also play a role in the regulation of MMPs in atherosclerotic lesions and plaque stabilization [96].
Molecules that act downstream of MMPs include cell-surface receptors such as β1 integrin and substrates of catalysis by MMPs. In this context, it was found that glycosylation of β1 integrin affects its association with MMP-14, and this most probably supports localization of proteases on tumour cells towards the invading front [97]. Some MMP substrates are also glycosylated, e.g. collagen II is extensively glycosylated [98,99], and these glycans might determine the cleavage sites by MMP-9 on collagen II [100]. Glycosylation of substrates might impair their cleavage by MMPs, e.g. glycosylated interferon (IFN)-β is more resistant to proteolysis by MMP-9 than recombinant IFN-β from bacteria [101]. In the retina, cyclic-nucleotide-gated (CNG) channels are essential for phototransduction in photoreceptors. On glycosylation, these CNG channels are resistant to modification by MMPs [102].
DISCUSSION
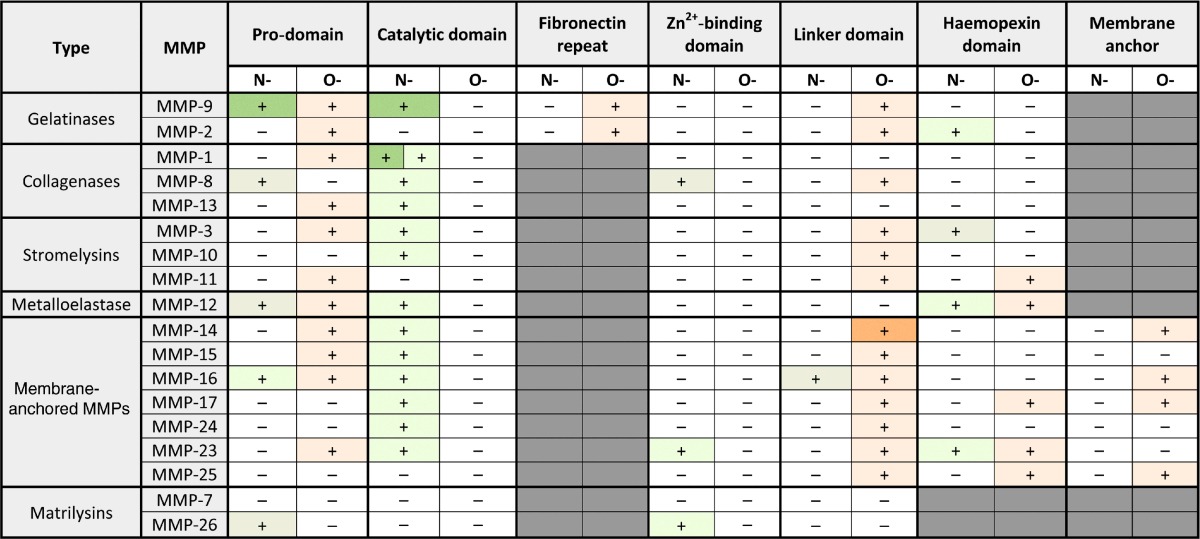
By analysing the N- and O-glycosylated sites predicted by the NetNglyc1.0 and NetOGlyc4.0 programs, and by comparing the available literature, a number of conclusions may be drawn about the glycobiology of MMPs. First, the linker domains have abundant potential O-glycosylation sites, whereas the catalytic domains (active site and Zn2+-binding domain) have only potential N-glycosylation sites. In addition, the membrane anchors and the cytoplasmic domains of the MT-MMPs have only potential O-glycosylated sites (Table 1). It is also clear from Table 1 that glycosylation results in an additional level of heterogeneity in an otherwise highly similar family of proteins. Figure 3, with indications for MMP-1, MMP-2, MMP-12 and MMP-13, clearly illustrates this: although the amino acid sequences of the members of the MMP family are highly conserved, the predicted glycosylation patterns are rather heterogeneous. However, two N-glycosylation sites appear to be conserved for most MMPs and these are situated in the active sites (see Figure 2 and Supplementary Figure S1). The conservation of these N-glycans strengthens the likelihood that the glycan has a potential biological function and stimulates further investigations on MMP glycosylation. A third paradigm resulting from the present review is about the role of MMP glycosylation in the interaction with TIMPs. Experiments with glycosylation-deficient production lines for MMPs and TIMPs, rather than with site-directed mutagenesis (which not only deletes the N-linked sugar but also alters the protein backbone), will yield the products for study of in-depth additional examples of MMP/TIMP glycobiology. As oligosaccharides protect glycoproteins against proteolysis and MMPs are proteases, substrate glycosylation needs to be considered in all biological systems. Finally, oligosaccharides provide recognition functions and this may radically alter the affinities of MMPs to substrates, receptors, inhibitors and macromolecular complexes in cellular systems.
Table 1. Overview of the glycosylation sites of MMPs per domain.
Green: N-glycosylation: validated (dark green), non-validated (light green). Orange: O-glycosylation: validated (dark orange) non-validated (light orange). Note that the active site, consisting of the catalytic domain and Zn2+-binding domain, is devoid of any predicted O-linked glycosylation.
Current information about structural and functional aspects of MMP glycosylation is fragmentary, skewed, and in need of more examples with biological and medical implications. Whereas other post-translational modifications, such as phosphorylation [103] and acetylation [104], even the attachment of a single GlcNAc as an O-linked monosaccharide [105] are well understood, our knowledge about oligosaccharide attachment to glycoproteins, and hence to MMPs and TIMPs, remains fragmentary. Reasons behind this lack of knowledge are the heterogeneity of attached glycan structures and the difficulty in analysing the structures and functions in-depth. To understand this, it is critical to understand the impact of glycosylation on protein structures. In insect cells, the MMP-9 active site is glycosylated at Asn120 with an N-linked glycan structure (Man3GlcNAc2) with core fucosylation (Figure 4). At the molecular level, the addition of this (rather small) glycan results in the addition of an extra width of approximately 25 Å (1 Å=0.1 nm) to an active site of an approximate width of 37 Å. Furthermore, the orientation of the glycan, relative to the active site, is flexible and allows a high degree of space filling. In structural terms, the N-linked oligosaccharide represents a space that is comparable to the volume of the active site.
Figure 4. Three-dimensional model of the catalytic domain of MMP-9 with attached N-linked glycosylation.
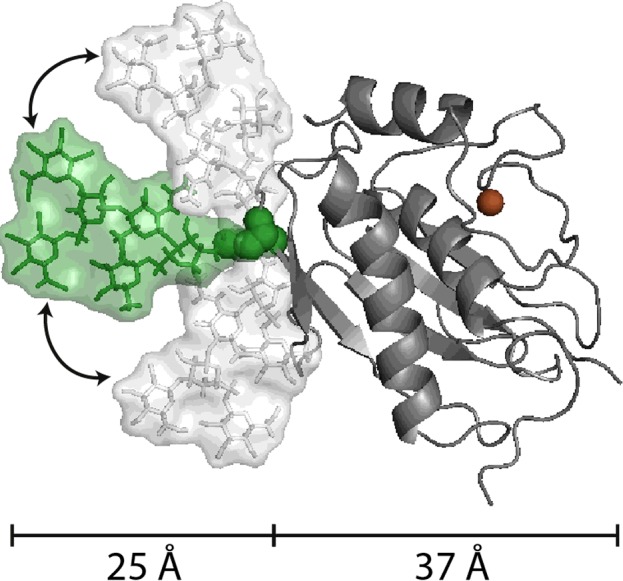
The N-glycosylation presented here is a small structure (Man3GlcNAc2) with a core fucosylation and is attached to Asn120 of MMP-9. This structure is typically produced by insect cells and is considerably smaller than the N-glycans produced by human cells. The two-headed arrows indicate that the relative position of the glycan towards the active site is rather flexible and, in addition, moves beyond the plane of the three conformers illustrated. The figure exemplifies well the relative volumes occupied by N-linked sugars (in green and white) attached to MMPs (catalytic site in grey, Zn2+ in brown).
The analytical aspect is being solved with high-throughput platforms [28,106] and the heterogeneity issue has been tackled with the definition of glycoforms and glycotypes [107]. Meanwhile, it has been well established that specific glycoforms of a molecule may be pathogenic [108] or disease-limiting [109]. Detailed insights into the structure and functions of oligosaccharides on glycoproteins have been well defined for clinically used glycoproteins, e.g. immunoglobulins, erythropoietin, tissue-type plasminogen activator and recombinant cytokines, but MMPs have not reached this status. Predictably, from the moment that a first recombinant MMP will be used in practical applications, this gap in our knowledge will be rapidly filled.
For nine MMPs the glycosylation status has not yet been studied (MMP-7, MMP-8, MMP-10, MMP-11, MMP-12, MMP-15, MMP-16, MMP-24 and MMP-25), and even for previously studied MMPs the information remains partial. Our knowledge on MMP glycobiology is also skewed towards that of MMP-9 and MMP-14, although these molecules represent prototypical MMPs studied in inflammation and cancer biology. The structures of N- and O-linked carbohydrates have been defined in natural [29,63] and recombinant [40] MMPs, and have been compared in various molecular forms of MMP-9, such as monomers and homotrimers [37]. In addition, of all possible functions of N- and O-linked oligosaccharides attached to glycoproteins [23,25], several have already been probed for MMP-9 and MMP-14, but not for other MMPs. The possible functions defined in glycobiology research include: folding and 3D structure [26], conferring resistance against proteolytic attack [101,110], interference with binding to molecular partners (negatively by limiting protein–protein interactions; positively by enhancing lectin binding) [111], alterations of dynamic stability, and specific activities of enzymes [110] and signalling molecules [112], and evolutionary diversification. MMP-9 and MMP-14 possess both catalytic and non-catalytic signalling activities [113]. Therefore, a future challenge is to decipher whether and how the sugars of MMPs contribute to these activities and whether specific glycoforms dominate specific functions, as is the case for immunoglobulins.
A third reason for the remaining gaps in our knowledge about MMP glycobiology is the fact that academic research is driven by economy. In the biomedical field, research of biomarkers for diagnostic use and of disease targets for drug development for common diseases is most critical. Many MMPs are associated with common diseases, such as cancer and inflammation, and thus fulfil market criteria. For glycobiology, however, a commonly misused way to get around the heterogeneity and complexity problem has been the argument that CDGs are rare diseases. Recent technological advancements prove that this reasoning is shortsighted, in fact wrong, and that glycan profiling as one of the next great challenges is becoming a reality [114–116]. Reciprocally, it has been shown that commonly used medications alter glycosylation [117]. Such examples provide the necessary basis to enhance activities, in both academia and the private sector, towards better glycobiology research. Finally, once the beneficial effects of specific MMPs [118–120] and TIMPs become better established, interest will grow, and up-to-date platforms will be used to define the structural details and functional consequences of MMP and TIMP oligosaccharides.
Acknowledgments
G. Opdenakker dedicates this work to Professor Raymond A. Dwek on the occasion of his 75th birthday.
Abbreviations
- CA-MMP
cysteine array MMP
- CDG
congenital disease of glycosylation
- CNG
cyclic-nucleotide-gated
- ECM
extracellular matrix
- EMMPRIN
ECM metalloprotease inducer
- ER
endoplasmic reticulum
- GalNAc
N-acetylgalactosamine
- GALNT14
N-acetylgalactosaminyltransferase 14
- GlcNAc
N-acetylglucosamine
- IFN
interferon
- LRP
low-density lipoprotein receptor-related protein
- MMP
matrix metalloprotease
- MT-MMP
membrane-type MMP
- TIMP
tissue inhibitor of metalloproteases
References
- 1.Vandooren J., Van den Steen P.E., Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit. Rev. Biochem. Mol. Biol. 2013;48:222–272. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 2.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler G.S., Overall C.M. Updated biological roles for matrix metalloproteinases and new ‘intracellular’ substrates revealed by degradomics. Biochemistry. 2009;48:10830–10845. doi: 10.1021/bi901656f. [DOI] [PubMed] [Google Scholar]
- 4.Cauwe B., Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2010;45:351–423. doi: 10.3109/10409238.2010.501783. [DOI] [PubMed] [Google Scholar]
- 5.Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J., Van den Steen P.E., Sang Q.X., Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 8.Vandenbroucke R.E., Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014;13:904–927. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- 9.Opdenakker G., Van Damme J. Cytokine-regulated proteases in autoimmune diseases. Immunol. Today. 1994;15:103–107. doi: 10.1016/0167-5699(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 10.Milner J.M., Cawston T.E. Matrix metalloproteinase knockout studies and the potential use of matrix metalloproteinase inhibitors in the rheumatic diseases. Curr. Drug Targets Inflamm. Allergy. 2005;4:363–375. doi: 10.2174/1568010054022141. [DOI] [PubMed] [Google Scholar]
- 11.Huntley G.W. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandooren J., Van Damme J., Opdenakker G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog. Brain Res. 2014;214:193–206. doi: 10.1016/B978-0-444-63486-3.00009-8. [DOI] [PubMed] [Google Scholar]
- 13.Lau L.W., Cua R., Keough M.B., Haylock-Jacobs S., Yong V.W. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat. Rev. Neurosci. 2013;14:722–729. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 15.Clark I.M., Swingler T.E., Sampieri C.L., Edwards D.R. The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H., Qi M., Li S., Qi T., Mei H., Huang K., Zheng L., Tong Q. microRNA-9 targets matrix metalloproteinase 14 to inhibit invasion, metastasis, and angiogenesis of neuroblastoma cells. Mol. Cancer Ther. 2012;11:1454–1466. doi: 10.1158/1535-7163.MCT-12-0001. [DOI] [PubMed] [Google Scholar]
- 17.Astarci E., Erson-Bensan A.E., Banerjee S. Matrix metalloprotease 16 expression is downregulated by microRNA-146a in spontaneously differentiating Caco-2 cells. Dev. Growth Differ. 2012;54:216–226. doi: 10.1111/j.1440-169X.2011.01324.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu J., van Mil A., Aguor E.N., Siddiqi S., Vrijsen K., Jaksani S., Metz C., Zhao J., Strijkers G.J., Doevendans P.A., et al. MiR-155 inhibits cell migration of human cardiomyocyte progenitor cells (hCMPCs) via targeting of MMP-16. J. Cell Mol. Med. 2012;16:2379–2386. doi: 10.1111/j.1582-4934.2012.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osaki M., Takeshita F., Sugimoto Y., Kosaka N., Yamamoto Y., Yoshioka Y., Kobayashi E., Yamada T., Kawai A., Inoue T., et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol. Ther. 2011;19:1123–1130. doi: 10.1038/mt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng X., Chopp M., Lu Y., Buller B., Jiang F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329:146–154. doi: 10.1016/j.canlet.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang J.H., Zhou H.C., Zeng C., Yang J., Liu Y., Huang X., Zhang J.P., Guan X.Y., Zhuang S.M. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology. 2011;54:1729–1740. doi: 10.1002/hep.24577. [DOI] [PubMed] [Google Scholar]
- 22.Labrie M., St-Pierre Y. Epigenetic regulation of mmp-9 gene expression. Cell. Mol. Life Sci. 2013;70:3109–3124. doi: 10.1007/s00018-012-1214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudd P.M., Dwek R.A. Glycosylation: heterogeneity and the 3D structure of proteins. Crit. Rev. Biochem. Mol. Biol. 1997;32:1–100. doi: 10.3109/10409239709085144. [DOI] [PubMed] [Google Scholar]
- 24.Dwek R.A. Glycobiology: toward understanding the function of sugars. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 25.Van den Steen P, Rudd P.M., Dwek R.A., Opdenakker G. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 26.Petrescu A.J., Wormald M.R., Dwek R.A. Structural aspects of glycomes with a focus on N-glycosylation and glycoprotein folding. Curr. Opin. Struct. Biol. 2006;16:600–607. doi: 10.1016/j.sbi.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Opdenakker G., Rudd P.M., Ponting C.P., Dwek R.A. Concepts and principles of glycobiology. FASEB J. 1993;7:1330–1337. doi: 10.1096/fasebj.7.14.8224606. [DOI] [PubMed] [Google Scholar]
- 28.Dwek R.A., Edge C.J., Harvey D.J., Wormald M.R., Parekh R.B. Analysis of glycoprotein-associated oligosaccharides. Annu. Rev. Biochem. 1993;62:65–100. doi: 10.1146/annurev.bi.62.070193.000433. [DOI] [PubMed] [Google Scholar]
- 29.Rudd P.M., Mattu T.S., Masure S., Bratt T., Van den Steen P.E., Wormald M.R., Kuster B., Harvey D.J., Borregaard N., Van Damme J., et al. Glycosylation of natural human neutrophil gelatinase B and neutrophil gelatinase B-associated lipocalin. Biochemistry. 1999;38:13937–13950. doi: 10.1021/bi991162e. [DOI] [PubMed] [Google Scholar]
- 30.Van den Steen P.E., Opdenakker G., Wormald M.R., Dwek R.A., Rudd P.M. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim. Biophys. Acta. 2001;1528:61–73. doi: 10.1016/S0304-4165(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 31.Brooks S.A., Carter T.M., Royle L., Harvey D.J., Fry S.A., Kinch C., Dwek R.A., Rudd P.M. Altered glycosylation of proteins in cancer: what is the potential for new anti-tumour strategies. Anticancer Agents Med. Chem. 2008;8:2–21. doi: 10.2174/187152008783330860. [DOI] [PubMed] [Google Scholar]
- 32.Van Wart H.E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baker A.H., Edwards D.R., Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 34.Gomez D.E., Alonso D.F., Yoshiji H., Thorgeirsson U.P. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur. J. Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 35.Triebel S., Blaser J., Reinke H., Tschesche H. A 25 kDa alpha 2-microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 1992;314:386–388. doi: 10.1016/0014-5793(92)81511-J. [DOI] [PubMed] [Google Scholar]
- 36.Kjeldsen L., Johnsen A.H., Sengelov H., Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 37.Vandooren J., Born B., Solomonov I., Zajac E., Saldova R., Senske M., Ugarte-Berzal E., Martens E., Van den Steen P.E., Van Damme J., et al. Circular trimers of gelatinase B/matrix metalloproteinase-9 constitute a distinct population of functional enzyme molecules differentially regulated by tissue inhibitor of metalloproteinases-1. Biochem. J. 2015;465:259–270. doi: 10.1042/BJ20140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bode W., Maskos K. Structural basis of the matrix metalloproteinases and their physiological inhibitors, the tissue inhibitors of metalloproteinases. Biol. Chem. 2003;384:863–872. doi: 10.1515/BC.2003.097. [DOI] [PubMed] [Google Scholar]
- 39.Shipley J.M., Doyle G.A., Fliszar C.J., Ye Q.Z., Johnson L.L., Shapiro S.D., Welgus H.G., Senior R.M. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. J. Biol. Chem. 1996;271:4335–4341. doi: 10.1074/jbc.271.8.4335. [DOI] [PubMed] [Google Scholar]
- 40.Van den Steen P.E., Van Aelst I., Hvidberg V., Piccard H., Fiten P., Jacobsen C., Moestrup S.K., Fry S., Royle L., Wormald M.R., et al. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J. Biol. Chem. 2006;281:18626–18637. doi: 10.1074/jbc.M512308200. [DOI] [PubMed] [Google Scholar]
- 41.Geurts N., Martens E., Van Aelst I., Proost P., Opdenakker G., Van den Steen P.E. Beta-hematin interaction with the hemopexin domain of gelatinase B/MMP-9 provokes autocatalytic processing of the propeptide, thereby priming activation by MMP-3. Biochemistry. 2008;47:2689–2699. doi: 10.1021/bi702260q. [DOI] [PubMed] [Google Scholar]
- 42.Redondo-Munoz J., Ugarte-Berzal E., Garcia-Marco J.A., Hernández del Cerro M., Van den Steen P.E., Opdenakker G., Terol M.J., Garcia-Pardo A. Alpha4beta1 integrin and 190-kDa CD44v constitute a cell surface docking complex for gelatinase B/MMP-9 in chronic leukemic but not in normal B cells. Blood. 2008;112:169–178. doi: 10.1182/blood-2007-08-109249. [DOI] [PubMed] [Google Scholar]
- 43.Redondo-Munoz J., Ugarte-Berzal E., Terol M.J., Van den Steen P.E., Hernandez del Cerro M., Roderfeld M., Roeb E., Opdenakker G., Garcia-Marco J.A., Garcia-Pardo A. Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia b cell survival through its hemopexin domain. Cancer Cell. 2010;17:160–172. doi: 10.1016/j.ccr.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 44.Van den Steen P.E., Dubois B., Nelissen I., Rudd P.M., Dwek R.A., Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit. Rev. Biochem. Mol. Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 45.Kim H., Park H., Montalvo L., Lennarz W.J. Studies on the role of the hydrophobic domain of Ost4p in interactions with other subunits of yeast oligosaccharyl transferase. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1516–1520. doi: 10.1073/pnas.040556797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helenius J., Aebi M. Transmembrane movement of dolichol linked carbohydrates during N-glycoprotein biosynthesis in the endoplasmic reticulum. Semin. Cell Dev. Biol. 2002;13:171–178. doi: 10.1016/S1084-9521(02)00045-9. [DOI] [PubMed] [Google Scholar]
- 47.Jaeken J., Matthijs G. Congenital disorders of glycosylation: a rapidly expanding disease family. Annu. Rev. Genomics Hum. Genet. 2007;8:261–278. doi: 10.1146/annurev.genom.8.080706.092327. [DOI] [PubMed] [Google Scholar]
- 48.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crocker P.R. Siglecs in innate immunity. Curr. Opin. Pharmacol. 2005;5:431–437. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 50.An H.J., Kronewitter S.R., de Leoz M.L., Lebrilla C.B. Glycomics and disease markers. Curr. Opin. Chem. Biol. 2009;13:601–607. doi: 10.1016/j.cbpa.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiro R.G. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43R. [DOI] [PubMed] [Google Scholar]
- 52.Huanna T., Tao Z., Xiangfei W., Longfei A., Yuanyuan X., Jianhua W., Cuifang Z., Manjing J., Wenjing C., Shaochuan Q., et al. GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol. Carcinog. 2015;54:1159–1171. doi: 10.1002/mc.22186. [DOI] [PubMed] [Google Scholar]
- 53.Liu J., Shen L., Yang L., Hu S., Xu L., Wu S. High expression of beta3GnT8 is associated with the metastatic potential of human glioma. Int. J. Mol. Med. 2014;33:1459–1468. doi: 10.3892/ijmm.2014.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steentoft C., Vakhrushev S.Y., Joshi H.J., Kong Y., Vester-Christensen M.B., Schjoldager K.T., Lavrsen K., Dabelsteen S., Pedersen N.B., Marcos-Silva L., et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldberg G.I., Wilhelm S.M., Kronberger A., Bauer E.A., Grant G.A., Eisen A.Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J. Biol. Chem. 1986;261:6600–6605. [PubMed] [Google Scholar]
- 56.Wilhelm S.M., Eisen A.Z., Teter M., Clark S.D., Kronberger A., Goldberg G. Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc. Natl. Acad. Sci. U.S.A. 1986;83:3756–3760. doi: 10.1073/pnas.83.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saarinen J., Welgus H.G., Flizar C.A., Kalkkinen N., Helin J. N-glycan structures of matrix metalloproteinase-1 derived from human fibroblasts and from HT-1080 fibrosarcoma cells. Eur. J. Biochem. 1999;259:829–840. doi: 10.1046/j.1432-1327.1999.00105.x. [DOI] [PubMed] [Google Scholar]
- 58.Grinnell B.W., Hermann R.B., Yan S.B. Human protein C inhibits selectin-mediated cell adhesion: role of unique fucosylated oligosaccharide. Glycobiology. 1994;4:221–225. doi: 10.1093/glycob/4.2.221. [DOI] [PubMed] [Google Scholar]
- 59.Wu C., Guo X., Wang W., Wang Y., Shan Y., Zhang B., Song W., Ma S., Ge J., Deng H., et al. N-Acetylgalactosaminyltransferase-14 as a potential biomarker for breast cancer by immunohistochemistry. BMC Cancer. 2010;10:123. doi: 10.1186/1471-2407-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilhelm S.M., Collier I.E., Kronberger A., Eisen A.Z., Marmer B.L., Grant G.A., Bauer E.A., Goldberg G.I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc. Natl. Acad. Sci. U.S.A. 1987;84:6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Opdenakker G., Van den Steen P.E., Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/S1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 62.Rosenblum G., Van den Steen P.E., Cohen S.R., Grossmann J.G., Frenkel J., Sertchook R., Slack N., Strange R.W., Opdenakker G., Sagi I. Insights into the structure and domain flexibility of full-length pro-matrix metalloproteinase-9/gelatinase B. Structure. 2007;15:1227–1236. doi: 10.1016/j.str.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 63.Mattu T.S., Royle L., Langridge J., Wormald M.R., Van den Steen P.E., Van Damme J., Opdenakker G., Harvey D.J., Dwek R.A., Rudd P.M. O-glycan analysis of natural human neutrophil gelatinase B using a combination of normal phase-HPLC and online tandem mass spectrometry: implications for the domain organization of the enzyme. Biochemistry. 2000;39:15695–15704. doi: 10.1021/bi001367j. [DOI] [PubMed] [Google Scholar]
- 64.Kotra L.P., Zhang L., Fridman R., Orlando R., Mobashery S. N-Glycosylation pattern of the zymogenic form of human matrix metalloproteinase-9. Bioorg. Chem. 2002;30:356–370. doi: 10.1016/S0045-2068(02)00501-1. [DOI] [PubMed] [Google Scholar]
- 65.Duellman T., Burnett J., Yang J. Functional roles of N-linked glycosylation of human matrix metalloproteinase 9. Traffic. 2015;16:1108–1126. doi: 10.1111/tra.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenblum G., Van den Steen P.E., Cohen S.R., Bitler A., Brand D.D., Opdenakker G., Sagi I. Direct visualization of protease action on collagen triple helical structure. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011043. e11043- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fry S.A., Van den Steen P.E., Royle L., Wormald M.R., Leathem A.J., Opdenakker G., McDonnell J.M., Dwek R.A., Rudd P.M. Cancer-associated glycoforms of gelatinase B exhibit a decreased level of binding to galectin-3. Biochemistry. 2006;45:15249–15258. doi: 10.1021/bi061254l. [DOI] [PubMed] [Google Scholar]
- 68.Lang G.A., Yeaman G.R. Autoantibodies in endometriosis sera recognize a Thomsen–Friedenreich-like carbohydrate antigen. J. Autoimmun. 2001;16:151–161. doi: 10.1006/jaut.2000.0465. [DOI] [PubMed] [Google Scholar]
- 69.Yeaman G.R., Collins J.E., Lang G.A. Autoantibody responses to carbohydrate epitopes in endometriosis. Ann. N. Y. Acad. Sci. 2002;955:174–182. doi: 10.1111/j.1749-6632.2002.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 70.Knauper V., Lopez-Otin C., Smith B., Knight G., Murphy G. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 71.Wu Y.I., Munshi H.G., Sen R., Snipas S.J., Salvesen G.S., Fridman R., Stack M.S. Glycosylation broadens the substrate profile of membrane type 1 matrix metalloproteinase. J. Biol. Chem. 2004;279:8278–8289. doi: 10.1074/jbc.M311870200. [DOI] [PubMed] [Google Scholar]
- 72.Wu Y.I., Munshi H.G., Snipas S.J., Salvesen G.S., Fridman R., Stack M.S. Activation-coupled membrane-type 1 matrix metalloproteinase membrane trafficking. Biochem. J. 2007;407:171–177. doi: 10.1042/BJ20070552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ludwig T., Theissen S.M., Morton M.J., Caplan M.J. The cytoplasmic tail dileucine motif LL572 determines the glycosylation pattern of membrane-type 1 matrix metalloproteinase. J. Biol. Chem. 2008;283:35410–35418. doi: 10.1074/jbc.M801816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pei D., Weiss S.J. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- 75.Bassi D.E., Lopez De Cicco R., Mahloogi H., Zucker S., Thomas G., Klein-Szanto A.J. Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10326–10331. doi: 10.1073/pnas.191199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kinoshita T., Sato H., Okada A., Ohuchi E., Imai K., Okada Y., Seiki M. TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J. Biol. Chem. 1998;273:16098–16103. doi: 10.1074/jbc.273.26.16098. [DOI] [PubMed] [Google Scholar]
- 77.Remacle A.G., Chekanov A.V., Golubkov V.S., Savinov A.Y., Rozanov D.V., Strongin A.Y. O-glycosylation regulates autolysis of cellular membrane type-1 matrix metalloproteinase (MT1-MMP) J. Biol. Chem. 2006;281:16897–16905. doi: 10.1074/jbc.M600295200. [DOI] [PubMed] [Google Scholar]
- 78.Osenkowski P., Meroueh S.O., Pavel D., Mobashery S., Fridman R. Mutational and structural analyses of the hinge region of membrane type 1-matrix metalloproteinase and enzyme processing. J. Biol. Chem. 2005;280:26160–26168. doi: 10.1074/jbc.M414379200. [DOI] [PubMed] [Google Scholar]
- 79.Kim S., Huang W., Mottillo E.P., Sohail A., Ham Y.A., Conley-Lacomb M.K., Kim C.J., Tzivion G., Kim H.R., Wang S., et al. Posttranslational regulation of membrane type 1-matrix metalloproteinase (MT1-MMP) in mouse PTEN null prostate cancer cells: Enhanced surface expression and differential O-glycosylation of MT1-MMP. Biochim. Biophys. Acta. 2010;1803:1287–1297. doi: 10.1016/j.bbamcr.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sohail A., Marco M., Zhao H., Shi Q., Merriman S., Mobashery S., Fridman R. Characterization of the dimerization interface of membrane type 4 (MT4)-matrix metalloproteinase. J. Biol. Chem. 2011;286:33178–33189. doi: 10.1074/jbc.M111.253369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pei D., Kang T., Qi H. Cysteine array matrix metalloproteinase (CA-MMP)/MMP-23 is a type II transmembrane matrix metalloproteinase regulated by a single cleavage for both secretion and activation. J. Biol. Chem. 2000;275:33988–33997. doi: 10.1074/jbc.M006493200. [DOI] [PubMed] [Google Scholar]
- 82.Murphy G., Houbrechts A., Cockett M.I., Williamson R.A., O'Shea M., Docherty A.J. The N-terminal domain of tissue inhibitor of metalloproteinases retains metalloproteinase inhibitory activity. Biochemistry. 1991;30:8097–8102. doi: 10.1021/bi00247a001. [DOI] [PubMed] [Google Scholar]
- 83.Willenbrock F., Crabbe T., Slocombe P.M., Sutton C.W., Docherty A.J., Cockett M.I., O'Shea M., Brocklehurst K., Phillips I.R., Murphy G. The activity of the tissue inhibitors of metalloproteinases is regulated by C-terminal domain interactions: a kinetic analysis of the inhibition of gelatinase A. Biochemistry. 1993;32:4330–4337. doi: 10.1021/bi00067a023. [DOI] [PubMed] [Google Scholar]
- 84.Sutton C.W., O'Neill J.A., Cottrell J.S. Site-specific characterization of glycoprotein carbohydrates by exoglycosidase digestion and laser desorption mass spectrometry. Anal. Biochem. 1994;218:34–46. doi: 10.1006/abio.1994.1138. [DOI] [PubMed] [Google Scholar]
- 85.Kim Y.S., Hwang S.Y., Kang H.Y., Sohn H., Oh S., Kim J.Y., Yoo J.S., Kim Y.H., Kim C.H., Jeon J.H., et al. Functional proteomics study reveals that N-Acetylglucosaminyltransferase V reinforces the invasive/metastatic potential of colon cancer through aberrant glycosylation on tissue inhibitor of metalloproteinase-1. Mol. Cell Proteomics. 2008;7:1–14. doi: 10.1074/mcp.M700084-MCP200. [DOI] [PubMed] [Google Scholar]
- 86.Kim H.I., Saldova R., Park J.H., Lee Y.H., Harvey D.J., Wormald M.R., Wynne K., Elia G., Kim H.J., Rudd P.M., et al. The presence of outer arm fucose residues on the N-glycans of tissue inhibitor of metalloproteinases-1 reduces its activity. J. Proteome Res. 2013;12:3547–3560. doi: 10.1021/pr400276r. [DOI] [PubMed] [Google Scholar]
- 87.Apte S.S., Olsen B.R., Murphy G. The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family. J. Biol. Chem. 1995;270:14313–14318. doi: 10.1074/jbc.270.24.14313. [DOI] [PubMed] [Google Scholar]
- 88.Langton K.P., Barker M.D., McKie N. Localization of the functional domains of human tissue inhibitor of metalloproteinases-3 and the effects of a Sorsby's fundus dystrophy mutation. J. Biol. Chem. 1998;273:16778–16781. doi: 10.1074/jbc.273.27.16778. [DOI] [PubMed] [Google Scholar]
- 89.Qi J.H., Dai G., Luthert P., Chaurasia S., Hollyfield J., Weber B.H., Stohr H., Anand-Apte B. S156C mutation in tissue inhibitor of metalloproteinases-3 induces increased angiogenesis. J. Biol. Chem. 2009;284:19927–19936. doi: 10.1074/jbc.M109.013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nabeshima K., Iwasaki H., Koga K., Hojo H., Suzumiya J., Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol. Int. 2006;56:359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 91.Gabison E.E., Hoang-Xuan T., Mauviel A., Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005;87:361–368. doi: 10.1016/j.biochi.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 92.Simizu S., Takagi S., Tamura Y., Osada H. RECK-mediated suppression of tumor cell invasion is regulated by glycosylation in human tumor cell lines. Cancer Res. 2005;65:7455–7461. doi: 10.1158/0008-5472.CAN-04-4446. [DOI] [PubMed] [Google Scholar]
- 93.Sun J., Hemler M.E. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–2281. [PubMed] [Google Scholar]
- 94.Huang W., Luo W. J., Zhu P., Tang J., Yu X.L., Cui H.Y., Wang B., Zhang Y., Jiang J.L., Chen Z.N. Modulation of CD147-induced matrix metalloproteinase activity: role of CD147 N-glycosylation. Biochem. J. 2013;449:437–448. doi: 10.1042/BJ20120343. [DOI] [PubMed] [Google Scholar]
- 95.Papadimitropoulou A., Mamalaki A. The glycosylated IgII extracellular domain of EMMPRIN is implicated in the induction of MMP-2. Mol. Cell. Biochem. 2013;379:107–113. doi: 10.1007/s11010-013-1632-8. [DOI] [PubMed] [Google Scholar]
- 96.Sluijter J.P., Pulskens W.P., Schoneveld A.H., Velema E., Strijder C.F., Moll F., de Vries J.P., Verheijen J., Hanemaaijer R., de Kleijn D.P., et al. Matrix metalloproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke. 2006;37:235–239. doi: 10.1161/01.STR.0000196986.50059.e0. [DOI] [PubMed] [Google Scholar]
- 97.Ranjan A., Kalraiya R.D. Invasive potential of melanoma cells correlates with the expression of MT1-MMP and regulated by modulating its association with motility receptors via N-glycosylation on the receptors. Biomed. Res. Int. 2014;2014:804680. doi: 10.1155/2014/804680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michaelsson E., Malmstrom V., Reis S., Engstrom A., Burkhardt H., Holmdahl R. T cell recognition of carbohydrates on type II collagen. J. Exp. Med. 1994;180:745–749. doi: 10.1084/jem.180.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haurum J.S., Arsequell G., Lellouch A.C., Wong S.Y., Dwek R.A., McMichael A.J., Elliott T. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic T lymphocytes. J. Exp. Med. 1994;180:739–744. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van den Steen P.E., Proost P., Grillet B., Brand D.D., Kang A.H., Van Damme J., Opdenakker G. Cleavage of denatured natural collagen type II by neutrophil gelatinase B reveals enzyme specificity, post-translational modifications in the substrate, and the formation of remnant epitopes in rheumatoid arthritis. FASEB J. 2002;16:379–389. doi: 10.1096/fj.01-0688com. [DOI] [PubMed] [Google Scholar]
- 101.Nelissen I., Martens E., Van den Steen P.E., Proost P., Ronsse I., Opdenakker G. Gelatinase B/matrix metalloproteinase-9 cleaves interferon-beta and is a target for immunotherapy. Brain. 2003;126:1371–1381. doi: 10.1093/brain/awg129. [DOI] [PubMed] [Google Scholar]
- 102.Meighan S.E., Meighan P.C., Rich E.D., Brown R.L., Varnum M.D. Cyclic nucleotide-gated channel subunit glycosylation regulates matrix metalloproteinase-dependent changes in channel gating. Biochemistry. 2013;52:8352–8362. doi: 10.1021/bi400824x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tarrant M.K., Cole P.A. The chemical biology of protein phosphorylation. Annu. Rev. Biochem. 2009;78:797–825. doi: 10.1146/annurev.biochem.78.070907.103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shahbazian M.D., Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 105.Hart G.W., Slawson C., Ramirez-Correa G., Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marino K., Bones J., Kattla J.J., Rudd P.M. A systematic approach to protein glycosylation analysis: a path through the maze. Nat. Chem. Biol. 2010;6:713–723. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 107.Rademacher T.W., Parekh R.B., Dwek R.A. Glycobiology. Annu. Rev. Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- 108.Rudd P.M., Elliott T., Cresswell P., Wilson I.A., Dwek R.A. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 109.Kaneko Y., Nimmerjahn F., Ravetch J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 110.Rudd P.M., Joao H.C., Coghill E., Fiten P., Saunders M.R., Opdenakker G., Dwek R.A. Glycoforms modify the dynamic stability and functional activity of an enzyme. Biochemistry. 1994;33:17–22. doi: 10.1021/bi00167a003. [DOI] [PubMed] [Google Scholar]
- 111.Scott D.W., Patel R.P. Endothelial heterogeneity and adhesion molecules N-glycosylation: implications in leukocyte trafficking in inflammation. Glycobiology. 2013;23:622–633. doi: 10.1093/glycob/cwt014. [DOI] [PubMed] [Google Scholar]
- 112.Opdenakker G., Rudd P.M., Wormald M., Dwek R.A., Van Damme J. Cells regulate the activities of cytokines by glycosylation. FASEB J. 1995;9:453–457. doi: 10.1096/fasebj.9.5.7896019. [DOI] [PubMed] [Google Scholar]
- 113.Garcia-Pardo A., Opdenakker G. Nonproteolytic functions of matrix metalloproteinases in pathology and insights for the development of novel therapeutic inhibitors. Metalloproteinases Med. 2015;2:19–28. doi: 10.2147/MNM.S63629. [DOI] [Google Scholar]
- 114.Dube D.H., Bertozzi C.R. Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 115.Lauc G., Essafi A., Huffman J.E., Hayward C., Knezevic A., Kattla J.J., Polasek O., Gornik O., Vitart V., Abrahams J.L., et al. Genomics meets glycomics-the first GWAS study of human N-Glycome identifies HNF1alpha as a master regulator of plasma protein fucosylation. PLoS Genet. 2010;6:e1001256. doi: 10.1371/journal.pgen.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lauc G., Huffman J.E., Pucic M., Zgaga L., Adamczyk B., Muzinic A., Novokmet M., Polasek O., Gornik O., Kristic J., et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 2013;9:e1003225. doi: 10.1371/journal.pgen.1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saldova R., Huffman J.E., Adamczyk B., Muzinic A., Kattla J.J., Pucic M., Novokmet M., Abrahams J.L., Hayward C., Rudan I., et al. Association of medication with the human plasma N-glycome. J. Proteome Res. 2012;11:1821–1831. doi: 10.1021/pr2010605. [DOI] [PubMed] [Google Scholar]
- 118.Cauwe B., Martens E., Sagaert X., Dillen C., Geurts N., Li S., Mertens J., Thijs G., Van den Steen P.E., Heremans H., et al. Deficiency of gelatinase B/MMP-9 aggravates lpr-induced lymphoproliferation and lupus-like systemic autoimmune disease. J. Autoimmun. 2011;36:239–252. doi: 10.1016/j.jaut.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 119.Weaver A., Goncalves da Silva A., Nuttall R.K., Edwards D.R., Shapiro S.D., Rivest S., Yong V.W. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB J. 2005;19:1668–1670. doi: 10.1096/fj.04-2030fje. [DOI] [PubMed] [Google Scholar]
- 120.Gooyit M., Peng Z., Wolter W.R., Pi H., Ding D., Hesek D., Lee M., Boggess B., Champion M.M., Suckow M.A., et al. A chemical biological strategy to facilitate diabetic wound healing. ACS Chem. Biol. 2014;9:105–110. doi: 10.1021/cb4005468. [DOI] [PMC free article] [PubMed] [Google Scholar]