H. pylori induce ETS2 and Twist1 expression in the infected GCC.
ETS2 and Twist1 transcriptionally activate siah2 in the H. pylori-infected GCCs.
H. pylori-mediated Siah2 induction enhances motility and invasiveness of the infected GCCs.
Keywords: ETS2, gastric cancer, H. pylori, metastasis, Siah2, Twist1
Abstract
Helicobacter pylori infection is one of the most potent factors leading to gastric carcinogenesis. The seven in absentia homologue (Siah2) is an E3 ubiquitin ligase which has been implicated in various cancers but its role in H. pylori-mediated gastric carcinogenesis has not been established. We investigated the involvement of Siah2 in gastric cancer metastasis which was assessed by invasiveness and migration of H. pylori-infected gastric epithelial cancer cells. Cultured gastric cancer cells (GCCs) MKN45, AGS and Kato III showed significantly induced expression of Siah2, increased invasiveness and migration after being challenged with the pathogen. Siah2-expressing stable cells showed increased invasiveness and migration after H. pylori infection. Siah2 was transcriptionally activated by E26 transformation-specific sequence 2 (ETS2)- and Twist-related protein 1 (Twist1) induced in H. pylori-infected gastric epithelial cells. These transcription factors dose-dependently enhanced the aggressiveness of infected GCCs. Our data suggested that H. pylori-infected GCCs gained cell motility and invasiveness through Siah2 induction. As gastric cancer biopsy samples also showed highly induced expression of ETS2, Twist1 and Siah2 compared with noncancerous gastric tissue, we surmise that ETS2- and Twist1-mediated Siah2 up-regulation has potential diagnostic and prognostic significance and could be targeted for therapeutic purpose.
INTRODUCTION
Gastric cancer is one of the most common malignant cancers. It is generally diagnosed at very late stages when the cancer has already metastasized to neighbouring lymph nodes or tissues. This occurs mostly due to the complex initiation and progression mechanisms of the disease as well as the lack of symptoms and detection markers at early stages. Therefore, in spite of a decline in gastric cancer cases in recent years, this disease still remains the second leading cause of cancer-related mortality in the world [1,2]. Helicobacter pylori infection is the prime factor responsible for gastric cancer. Up to 80% of people in certain parts of the world are infected with H. pylori [3]. Host responses towards infection and the possession of a ∼40 kb stretch of genetic element by the pathogen called the cytotoxin-associated gene (cag) pathogenicity island (PAI) play a role in determining the outcome of infection. Like any other solid tumour of epithelial origin, development of the invasive gastric cancer phenotype employs epithelial–mesenchymal transition (EMT) wherein the epithelial cells lose their epithelial characteristics, gain mesenchymal features and show enhanced motility. Aberrant EMT has been closely associated with gastric carcinogenesis. H. pylori induce migration of the primary gastric epithelial cells [4], and enhance motility of the gastric epithelial cancer cell line AGS as well as Madin–Darby canine kidney (MDCK) epithelial cells [5,6]. However, the precise molecular events that contribute in inducing motility and invasiveness of H. pylori-infected gastric cancer cells remain to be determined.
Crucial roles for E3 ubiquitin ligases have been recently identified in modulating cancer progression and metastasis for adenocarcinoma [7]. The really interesting new gene (RING) family E3 ubiquitin ligases regulate metastasis in several cancers [8] and have drawn attention as potential drug targets [9]. The seven in absentia homologue (Siah) proteins belong to the RING family of E3 ubiquitin ligases. Siah proteins impart specificity to proteasomal degradation of target proteins and are required for the ubiquitin-dependent proteolysis of their targets. Siah proteins interact with and regulate the stability of multiple factors involved in oncogenesis including prolyl hydroxylases, β-catenin, NUMB, tumour necrosis factor receptor 2-associated factor and Sprouty [10–14]. Increased Siah2 expression in various cancers signifies its oncogenic role [15–19]. Moreover, enhanced expression of Siah2 in breast, prostate and liver cancer cells is associated with malignancy and cancer invasiveness [15,16]. Wong et al. have shown that loss of Siah2 causes delayed tumour onset and increases the efficacy of chemotherapy in a transgenic model of aggressive breast cancer [20]. Elaborate studies have been performed to identify downstream targets of Siah2 proteins. Recently Siah2 has been reported to regulate tight junction integrity and cell polarity in the hypoxic milieu through the regulation of apoptosis-stimulating proteins of p53 (ASPP) 2 stability [21]. Siah2 mediates ubiquitination and degradation of the CCAAT/enhancer-binding protein δ (C/EBPδ) during breast cancer progression, thus contributing to the transformation of breast tumour cells [22].
Siah2 up-regulation in breast cancer is caused by oestrogen which leads to the proteasomal degradation of the transcriptional co-regulator nuclear receptor corepressor (N-CoR) [23]. Although the exact mechanism is not known, Wnt5a has been found to induce Siah2 expression in colon cancer cells [24]. Hypoxia is another potent inducer of Siah2 [13] and it regulates Siah2 stability by modulating the p38 MAPK and Akt pathways [25,26]. Although independent studies have reported that Siah2 and H. pylori infection can induce stability and accumulation of the hypoxia-inducible factor 1α (HIF1α), a major oncogenic transcription factor induced during hypoxia [13,27], to date no study has identified the effect of H. pylori infection on gastric epithelial Siah2 expression.
Given the crucial role of Siah2 in driving cellular transformation and tumorigenesis in several human cancers, we examined the effect of H. pylori infection on Siah2 expression. We identified that proto-oncogenic transcription factors E26 transformation-specific sequence 2 (ETS2) and Twist-related protein 1 (Twist1) induce siah2 in H. pylori-infected gastric cancer cells (GCCs) and also demonstrated that Siah2 regulates motility and invasiveness of infected GCCs. Our study thus established the role of Siah2 in regulating H. pylori-mediated gastric cancer progression. As human gastric cancer biopsy samples also showed highly-increased expression of Twist1 and ETS2 along with Siah2, these molecules could be tested as novel molecular targets to treat gastric cancer.
EXPERIMENTAL
Cell culture, H. pylori strains, infection and treatments
The human GCCs MKN45, Kato III, AGS along with H. pylori 26695 and 8-1, a cag PAI (+) strain (A.T.C.C.) and a cag PAI (−) strain, respectively, were cultured and maintained as reported previously [28,29]. Another cag PAI (−) strain D154 was received from the archived collection of H. pylori strains at National Institute of Cholera and enteric Diseases, Kolkata, India. Strain 8-1 is an isogenic derivative of the reference strain 26695 lacking the entire cag PAI [28] (DNA isolated from D154 does not produce any cagA or cag PAI amplicon). GCCs were infected with various multiplicity of infection (MOI) of H. pylori strain 26695 for specified periods. Strain 26695 was used for all studies except for comparison studies involving 26695, 8-1 and D154 strains. For inhibitor studies, cells were treated with the proteasome inhibitor MG132 (Sigma–Aldrich) at 50 μM dose for 6 h prior to bacterial infection.
Human gastric mucosal biopsy specimen collection
Gastric biopsy samples from the antral gastric mucosa were collected from patients suffering from gastric cancer and undergoing diagnostic esophagogastroduodenoscopy following a National Institute of Science Education and Research (NISER) Review Board-approved protocol and research was carried out in accordance with the Declaration of Helsinki (2013) of the World Medical Association. Written informed consent was obtained from all patients prior to the study. Note that gastric adenocarcinoma biopsy samples were obtained from patients lacking any previously established case-history. Because the gastric cancer samples were from patients that were urea breath test, rapid urease test as well as tissue-invasion negative, these adenocarcinoma cases could not be linked to H. pylori infection status.
Plasmids and mutagenesis
Twist1 overexpression construct was obtained as a kind gift from Dr Kimitoshi Kohno. ETS2 construct was purchased from Addgene (Addgene plasmid 28128). The Siah2 plasmid was purchased from Origene Technologies (Origene Technologies). The full length human siah2 promoter (NM_005067) was cloned into the pGL3 basic vector (Promega) using restriction sites for KpnI and HindIII. siah2 WT promoter construct was used as a template to generate individual mutations at the ETS2-binding site (EBS) and Twist1-binding site (TBS) using the QuikChange site-directed mutagenesis kit (Agilent Technologies) as per manufacturers’ standard procedure. Sequencing was done to confirm mutations at the EBS and TBS. Primer sequences are shown in Supplementary Figure S1.
Transient transfection of plasmids or siRNAs and generation of stable cell lines
For transient expression of ETS2 and Twist1, 1×106 MKN45 cells were seeded in 6-well cell culture plates 24 h prior to transfection. Cells were transfected with 2 μg of plasmid DNA and 10 μl of Lipofectamine 2000 reagent (Invitrogen, CA, USA). Cells were infected after 36 h of transfection. To generate stable cell lines, MKN45 or AGS cells were seeded in 96-well plates 24 h before transfection and transfection was done as mentioned. Cells were cultured in the presence of 300 μg/ml G418 (Sigma–Aldrich) for 4 weeks. Positive clones were picked using cloning discs (Sigma–Aldrich) and expanded to establish stable cell lines. To knockdown expression of ETS2 and Twist1, 0.2×106 MKN45 cells were seeded in 6-well cell culture plates 24 h prior to transfection. Cells were transfected with 50 nM of siRNA duplexes of ETS2 or Twist1 (Origene) along with 10 μl of Lipofectamine 3000 reagent (Invitrogen). Control duplexes were also transfected. After 60 h of transfection, cells were infected with H. pylori.
In vitro binding assay
5′ biotinylated double-stranded siah2 EBS oligonucleotides [WT (wild-type) or Mut (mutant)] were captured by Streptavidin-coated superparamagnetic beads (Dynabeads M-280 Streptavidin, Dynal, Invitrogen) and binding assays were performed using nuclear lysates as described previously [28]. Bound proteins were dissociated by boiling in 1× Laemmli sample buffer and analysed by Western blotting. Oligonucleotide sequences are shown in Supplementary Figure S1.
Luciferase assay
Activation of the siah2 promoter after H. pylori infection was measured by dual luciferase assays. For this, cells were co-transfected with either the WT or ETS2 and Twist1-Mut siah2 luciferase promoter constructs (cloned in pGL3 basic vector) along with the phRL-TK Renilla luciferase construct (Promega) at a ratio of 50:1 using Lipofectamine 2000 reagent (Invitrogen). For some experiments, cells were co-transfected with the WT siah2 promoter construct along with the WT ETS2 or Twist1 overexpression plasmid and the phRL-TK Renilla luciferase construct at a ratio of 25:25:1 using Lipofectamine 2000 reagent (Invitrogen). At 36 h post-transfection, cells were either left uninfected or were infected with H. pylori for 2 h. Cells were thereafter lysed and luciferase activity was estimated using the Dual-Luciferase Reporter Assay System (Promega) as per manufacturers’ instructions. Quantification of luminescence signal was done using MicroBeta2 LumiJET™ Microplate Counter (PerkinElmer).
MTT assay
pcDNA3.1+ and Siah2 overexpressing stable cells were seeded on 96-well microplates with a seeding density of 5×103 cells per well 24 h before transfection. Cell proliferation was assessed using an MTT cell proliferation kit according to the manufacturer's protocol (HiMedia). The absorbance was measured at 595 nm test and 650 nm reference wavelengths. Data were analysed by t test, and presented as mean±S.E.M., and confirmed by three independent experiments.
Transwell migration and invasion assays
Cell migration and invasion assays were performed using 8-μm pore size Transwell Biocoat control inserts (migration assay) or matrigel-coated inserts, as per manufacture's instruction (Becton Dickinson). In brief, 5×104 AGS cells were seeded on a transwell plate. The chambers were incubated at 37°C, 5% CO2. After incubating for 24 h, cells on the top surface of the transwell were scraped off. Cells were fixed for 30 min with 4% paraformaldehyde, and stained for 30 min with haematoxylin. We counted the number of cells (five high-power fields) that invaded inserts under an inverted microscope (Primo Vert, Carl Zeiss). Individual experiments were repeated thrice.
Soft agar assay
MKN45 cells stably expressing Siah2, ETS2 and Twist1 protein or empty vector were seeded in 24-well plates at a density of 0.1×106 cells per well. After 24 h cells were infected with H. pylori for 6 h followed by 2 h gentamicin treatment to kill extracellular bacteria. Cells were harvested and 1000 cells were mixed with 0.3% top agar and plated on to 0.6% bottom agar in 6 cm cell culture plates. These plates were fed twice weekly and maintained for 3 weeks in humidified incubators containing 5% CO2. At the end of the incubation period, visible colonies on the top agar were directly counted and colony sizes were compared between various treatment groups.
Wound healing assay
We wanted to assess the effect of ETS2, Twist1 or Siah2 expression on wound-healing property of GCCs. As MKN45 cells are partly adherent and partly floating in nature [30], we did not consider these cells suitable for wound-healing assays. Rather, AGS cells being adherent cells were considered more appropriate for this purpose. Various stable cells were seeded in 6-well cell culture plates and were allowed to grow in monolayer till 90% confluency was obtained in complete media. Multiple uniform streaks were made on the monolayer culture with 100 μl pipette tips. Streaked plates were immediately washed with PBS to remove detached cells followed by infection with H. pylori in serum-free media. Cell migration was monitored up to 24 h, and pictures were taken at 0, 6, 12 and 24 h time points using a digital camera attached to an inverted microscope (Primo Vert, Carl Zeiss). Six to eight fields were analysed, and cells filling the wound mark were counted using ImageJ 1.45 software.
To study the effect of Siah2 suppression on wound healing property of GCCs, 0.5×106 AGS cells were seeded in 6-well cell culture plates 24 h prior to transfection of 50 nM of siRNA control or Siah2 duplex and 10 μl of Lipofectamine 3000 reagent. Cells were infected and wound healing assay was performed after 48 h of transfection.
Statistical analysis
Statistical analysis of quantitative data was performed by Student's t tests. Values were given as mean±S.E.M. Statistical significance was determined at *P<0.05.
The online supplementary file contains additional EXPERIMENTAL methods that include immunoblotting, real-time RT (reverse transcription)-PCR, ChIP assay, immunofluorescence and confocal microscopy.
RESULTS
H. pylori induces Siah2 in cultured GCCs
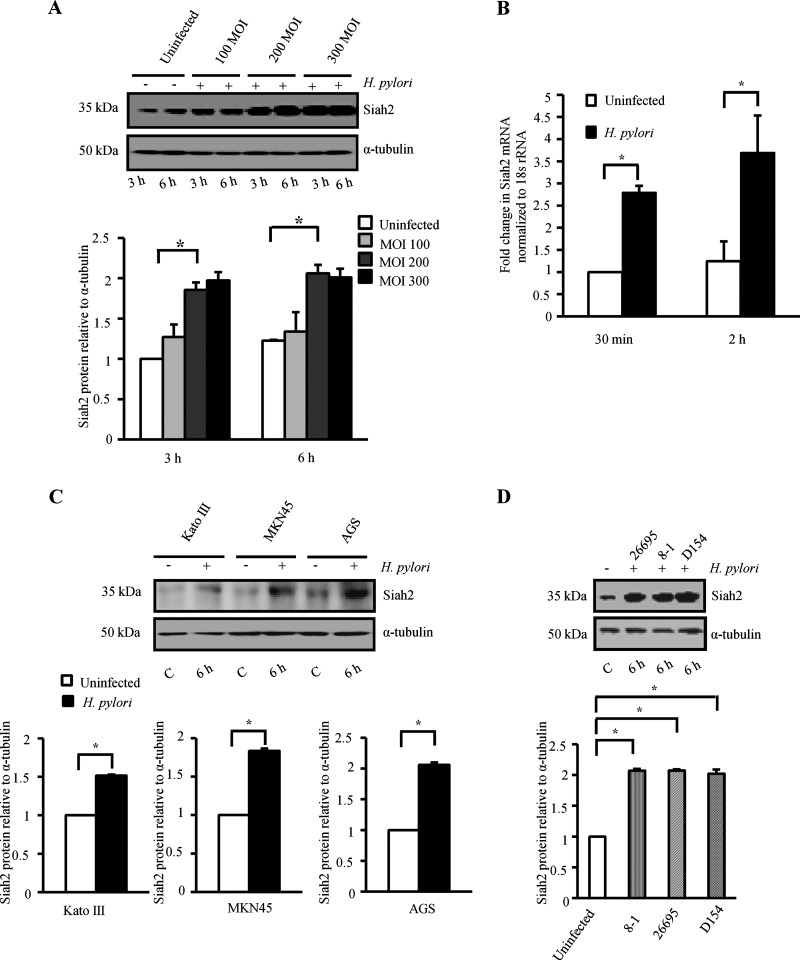
To identify the effect of H. pylori infection on GCCs, MKN45 cells were infected with a cag PAI (+) H. pylori strain 26695 at a MOI of 100, 200 and 300 for 3 and 6 h. A representative Western blot result (n=3) showed significant induction of Siah2 expression by H. pylori at 3 and 6 h post infection (p.i.) with MOI 200 and 300. Results further confirmed that at 6 h p.i., MOI 200 was equivalent to infection with MOI 300 for 6 h with respect to Siah2 induction (Figure 1A). Supplementary Figure S2(A) shows that MOI 200 at 6 h was optimum to induce Siah2 expression. Therefore, all future experiments were performed with MOI 200 with strain 26695 for 6 h to study Siah2 expression unless mentioned otherwise. Fluorescence microscopic images of uninfected cells and infected cells further confirmed H. pylori as a potent inducer of Siah2 (Supplementary Figure S2B). Confocal microscopy revealed that H. pylori substantially induced nuclear accumulation of Siah2 in the infected GCCs (Supplementary Figure S2C). Comparison of basal and p.i. expression of Siah2 at mRNA level (real-time RT-PCR) also showed significant increase in Siah2 expression after H. pylori infection (Figure 1B). As various GCCs could show difference in their protein expression pattern, we sought to determine whether MOI 200 was effective in inducing Siah2 in other GCCs. Western blot of the comparative study revealed that MOI 200 also induced Siah2 in Kato III and AGS cells (Figure 1C). There was an equivalent induction of Siah2 protein when MKN45 cells were infected with either 26695, 8-1 or D154 H. pylori strains indicating that Siah2 induction was not dependent on cag PAI status (Figure 1D).
Figure 1. Siah2 is induced in H. pylori-infected GCCs.
(A) A representative Western blot (n=3) of whole cell lysates prepared from uninfected and infected (3 and 6 h with MOI 100, 200 and 300) MKN45 cells shows Siah2 induction in infected GCCs. α-Tubulin was used as a loading control. A graphical representation of the data confirms that MOI 200 at 6 h is optimal in inducing Siah2. Bars depict normalized data (mean±S.E.M., n=3), *P<0.05. (B) Siah2 expression in MKN45 cells at mRNA level as detected by real-time RT-PCR. Bars depict normalized data (mean±S.E.M., n=3), *P<0.05. (C) A representative Western blot (n=3) shows Siah2 induction in Kato III, MKN45 and AGS cells after being infected with MOI 200 of H. pylori for 6 h. Bars shown below represent normalized data (mean±S.E.M., n=3), *P<0.05. (D) Western blot results (n=3) showing expression of Siah2 in cell lysates prepared from uninfected or infected MKN45 cells. Strains of H. pylori used for the experiment were cag PAI (+) strain 26695, cag PAI (−) strains 8-1 and D154. Bars shown below represent normalized data (mean±S.E.M., n=3), *P<0.05.
ETS2 and Twist1 are induced in H. pylori-infected GCCs
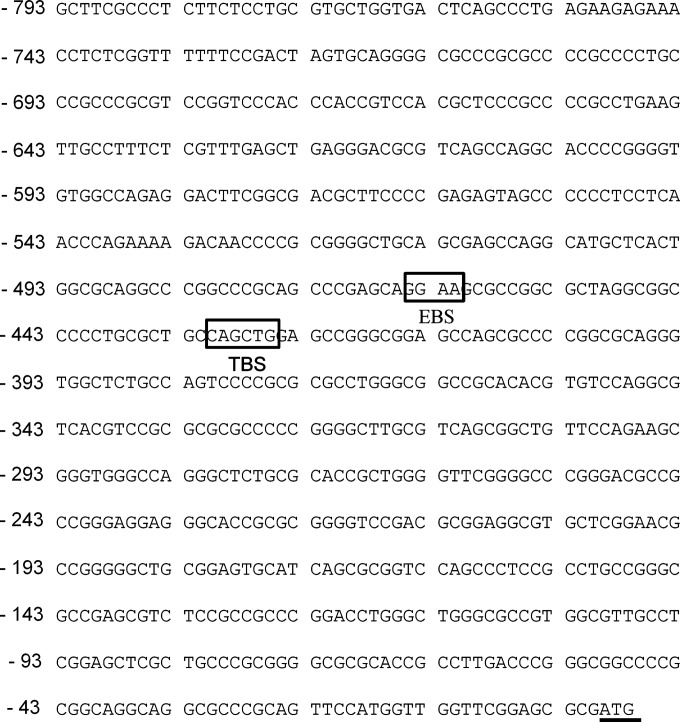
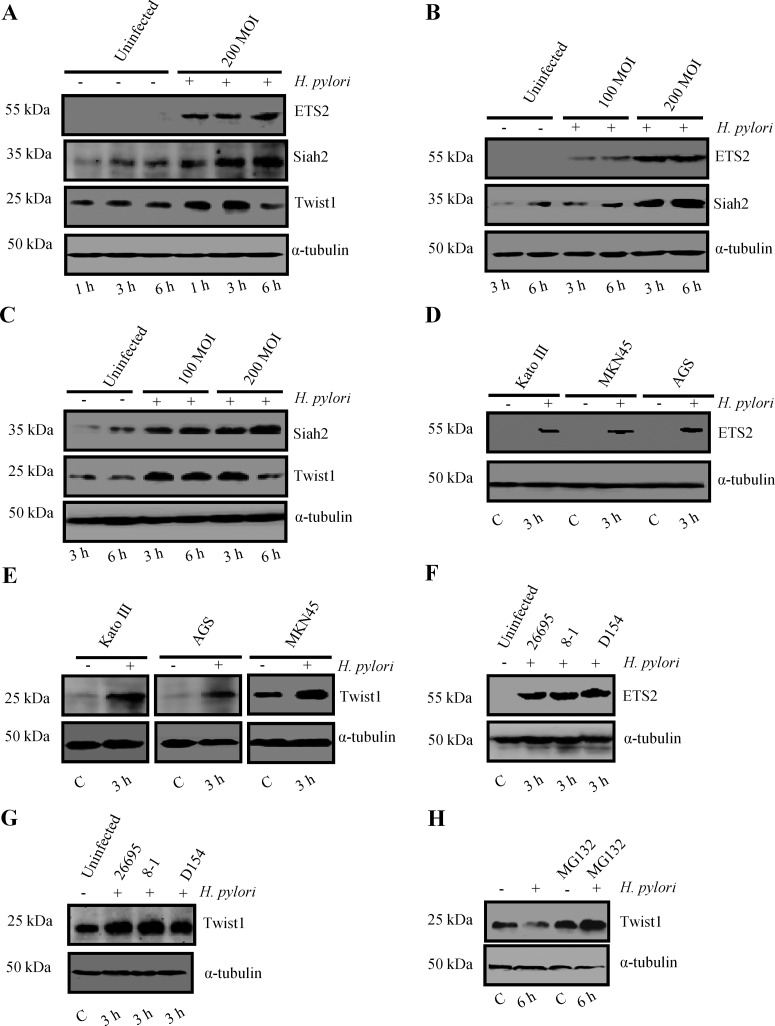
In order to identify transcription factors regulating Siah2 expression, we performed promoter analysis for siah2 using bioinformatics tools such as MatInspector (professional version 6.2.2). Although several transcription factor binding sites were implicated in the siah2 promoter region, ETS2 and Twist1 showed very high probability of binding. The putative EBS (GGAA/T) was between the −465 and −462 bp and TBS (CANNTG) was between the −431 and −426 bp upstream of the siah2 transcription initiation site (Figure 2). We performed Western blot analysis to assess ETS2 expression at protein level in MKN45 cells infected with H. pylori. Although the optimal time and dose for Siah2 expression were determined in Figure 1, we needed to identify the same for ETS2 and Twist1. A time-dependent study showed induced expression of ETS2 as early as 1 h p.i. and that was maintained at the same level even at 6 h p.i. whereas no ETS2 expression was noticed at any time point in uninfected cells (Figure 3A). The same study also revealed that the expression of Twist1 was induced at 1 h p.i. and was maintained at the same level up to 3 h and decreased at 6 h. Dose-kinetics performed at 3 h and 6 h p.i. with MOI 100 and 200 clearly showed that 200 MOI was more effective in inducing ETS2 (Figure 3B) and Twist1 (Figure 3C) expression than MOI 100. We performed Western blotting on cell lysates prepared from other GCCs such as Kato III and AGS along with MKN45 cells at 3 h p.i. (MOI 200) to assess the expression of ETS2 and Twist1. Representative Western blots from independent experiments (n=3) showed marked enhancement in ETS2 (Figure 3D) and Twist1 expression (Figure 3E) in all cell lines. We observed that strain 8-1 and strain D154 were equally effective in inducing ETS2 and Twist1 as strain 26695 (Figures 3F and 3G respectively). As we observed that although Twist1 was induced at 3 h of infection, the level of Twist1 at 6 h p.i. was equal to its basal expression level of either 3 or 6 h, we presumed that proteasomal degradation might have caused Twist1 degradation at 6 h of infection. Pretreatment of MKN45 cells with MG132 prior to H. pylori infection clearly rescued Twist1 protein from degradation indicating that ubiquitination-mediated proteasomal degradation of Twist1 was underway at 6 h p.i. (Figure 3H). Graphical representation of all data is shown in Supplementary Figure S3.
Figure 2. Analysis of human siah2 promoter. The EBS and TBS of the human siah2 promoter are shown. The ATG start codon is underlined.
Figure 3. H. pylori infection induces ETS2, Twist1 and Siah2 expression in GCCs.
(A) Time kinetics of ETS2, Twist1 and Siah2 expression in the infected MKN45 cells (n=3). (B) A representative Western blot shows that MOI 200 is more effective in inducing ETS2 and Siah2 than MOI 100 at both 3 h p.i. and 6 h p.i. (n=3). (C) Twist1 expression pattern at MOI 100 and 200 is compared at 100 and 200 MOI and at 3 and 6 h. (D) Assessment of ETS2 expression in Kato III, MKN45 and AGS cells by Western blot (n=3). (E) Twist1 expression pattern in Kato III, MKN45 and AGS cells (n=3). (F) Representative Western blots from independent experiments (n=3) shows that strain 8-1 and D154 were equally effective as strain 26695 in inducing ETS2 and (G) Twist1 (n=3). (H) Western blot (n=4) showing Twist1 expression in MKN45 cells treated with 50 μM MG132 for 6 h prior to infection with MOI 200 of H. pylori for 6 h.
Antral biopsy samples obtained from consenting patients during gastric endoscopy were used to study expression of Twist1, ETS2 and Siah2 proteins in gastric adenocarcinoma. Fluorescence microscopic analysis of gastric biopsies from adenocarcinoma patients with samples from adjacent non-cancer gastric tissue samples revealed that Siah2 (Supplementary Figure S4A), ETS2 (Supplementary Figure S4B) and Twist1 (Supplementary Figure S4C) were highly increased in adenocarcinoma biopsy samples compared with non-cancer tissues.
ETS2 and Twist1 bind with siah2 promoter in H. pylori-infected GCCs
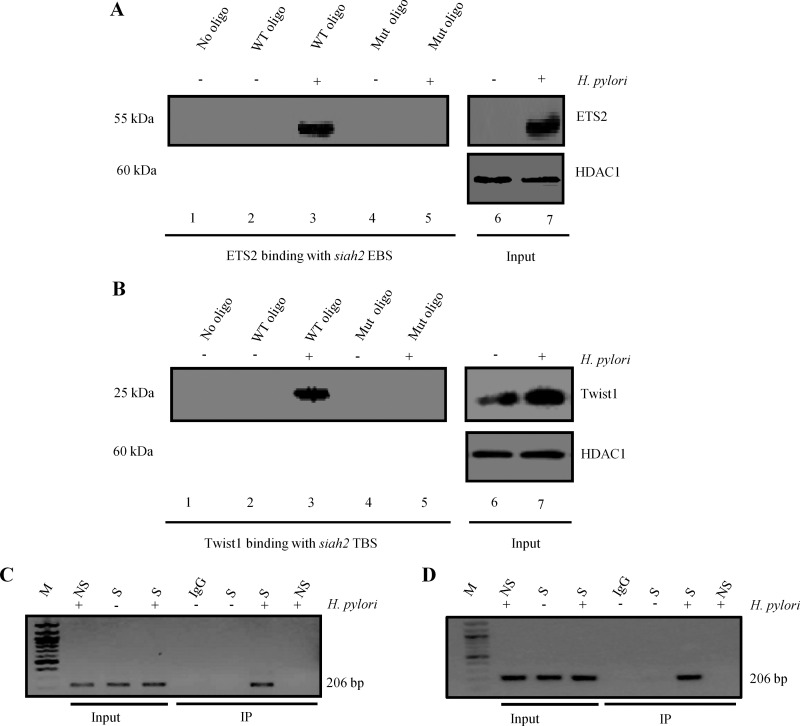
Next we assessed ETS2 binding with the siah2 EBS and TBS in uninfected and infected MKN45 cells. Nuclear extracts were prepared from 3 h H. pylori-infected (MOI 200) MKN45 cells. WT as well as EBS and TBS-Mut siah2 promoter oligonucleotides (Supplementary Figure S1) were synthesized. Magnetic beads were coated with oligonucleotides just prior to incubation with nuclear lysates from infected and uninfected cells. Analysis of bead-bound proteins by Western blot determined binding of ETS2 with the siah2 EBS (Figure 4A) and Twist1 with siah2 TBS only after H. pylori infection (Figure 4B). Input nuclear lysates were also run to assess the expression of ETS2 (Figure 4A) and Twist1 (Figure 4B) in nuclear lysates and histone-deacetylase 1 (HDAC1) was used as a nuclear loading control. Results confirmed that both ETS2 and Twist1 were increased in the nuclear fraction by H. pylori infection. We did not detect any ETS2 expression in the uninfected cells whereas low level of Twist1 expression was observed in the nucleus of uninfected cells.
Figure 4. ETS2 and Twist1 bind to the siah2 promoter in H. pylori-infected GCCs.
(A) Western blot showing H. pylori-induced ETS2 binding with the siah2 promoter (n=3). ETS2 binds to the WT EBS only but not with the EBS-Mut oligo. Input lanes show expression of ETS2 in the nuclear lysates. HDAC1 is the loading control for nuclear lysates. (B) Western blot showing H. pylori-induced Twist1 binding with the siah2 promoter (n=3). Twist1 specifically binds to the WT TBS but not to the TBS-Mut oligo. Input lanes show expression of Twist1 in the nuclear lysates. (C) ChIP analysis of ETS2 immunocomplex for siah2 EBS. (D) ChIP analysis of Twist1 immunocomplex for siah2 TBS. IgG, immunoglobulin G; M, MW marker; NS, non-specific primer, S, specific primer.
In order to detect binding of ETS2 and Twist1 to the siah2 promoter in vivo, MKN45 cells were infected with MOI 200 of strain 26695 for 3 h and nuclear lysates were assessed by ChIP assay using ETS2 (Figure 4C) and Twist1 (Figure 4D) antibodies. The PCR products of DNA in these immunocomplexes corresponding to the siah2 promoter flanking the EBS and TBS (S=specific PCR product), respectively, were not present in the PCR product corresponding to the 5′ far upstream sequence (NS=non-specific PCR product).
ETS2 and Twist1 enhance Siah2 expression in H. pylori-infected GCCs
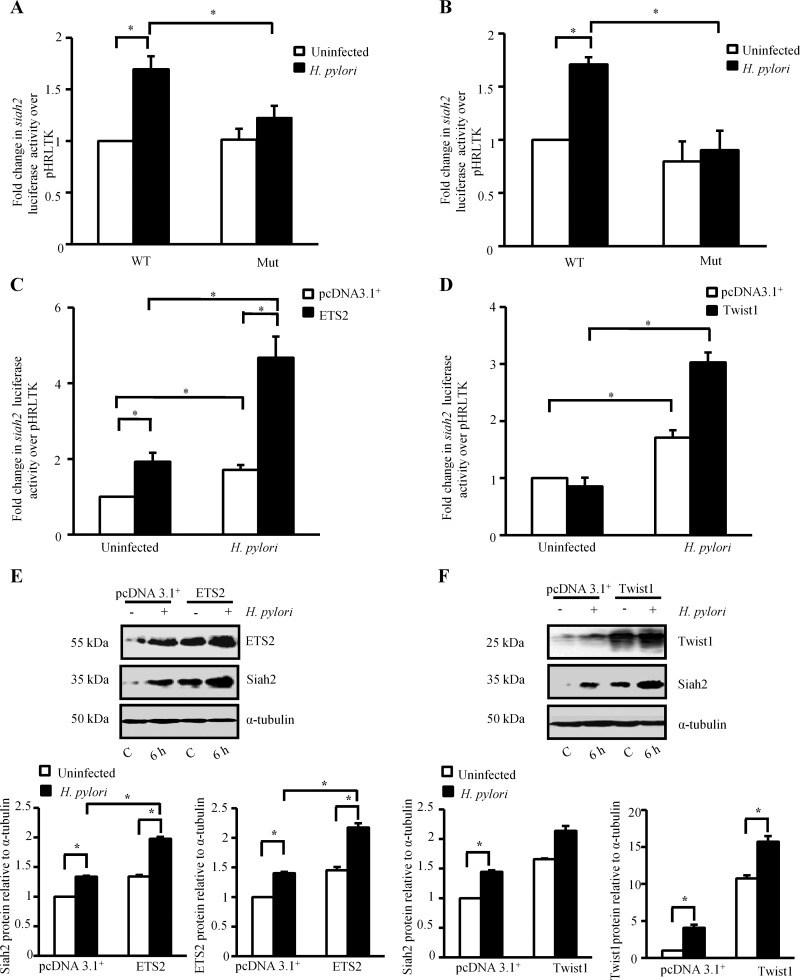
Dual luciferase assays were performed to assess the role of ETS2 and Twist1 in H. pylori infection-induced siah2 transcription. MKN45 cells were first co-transfected with the Renilla luciferase construct phRLTK along with the WT or EBS or TBS-Mut siah2-reporter constructs followed by infection with H. pylori for 2 h. Significant enhancement of siah2 transactivation was observed in the infected MKN45 cells expressing WT siah2 luciferase constructs. The effect was significantly reduced in the EBS Mut (Figure 5A) or TBS Mut-expressing cells (Figure 5B) confirming that ETS2 and Twist1 mediate siah2 transcription in the infected GCCs.
Figure 5. ETS2 and Twist1 enhance siah2 transcription.
(A) Bar graph of dual luciferase activities (mean±S.E.M., n=3) driven by the WT and ETS2-Mut siah2 promoters in MKN45 cells with or without H. pylori infection. *P<0.05. (B) Bar graph of dual luciferase activities (mean±S.E.M., n=3) to show the effect of Twist1 binding with the WT and Twist1-Mut siah2 promoters in presence or absence of H. pylori infection in MKN45 cells. *P<0.05. (C) Dual luciferase assay to show the effect of ectopically expressed ETS2 on the transcriptional activation of WT siah2 promoter (mean±S.E.M., n=3). *P<0.05. (D) Dual luciferase assay showing the effect of ectopically expressed Twist1 on the WT siah2 promoter luciferase activity (mean±S.E.M., n=3). *P<0.05. (E) Western blot analysis showing the effect of overexpression of ETS2 on Siah2 protein expression in H. pylori-infected GCCs. Bars shown below represent normalized data (mean±S.E.M., n=3), *P<0.05. (F) Western blotting shows the effect of Twist1 overexpression on Siah2 expression in H. pylori-infected GCCs. Bars depict normalized data (mean±S.E.M., n=3), *P<0.05.
We next sought to determine the effect of ectopic expression of ETS2 and Twist1 on siah2 transcription. For this, the empty vector or ETS2 overexpression plasmids were transfected along with the WT siah2 promoter construct and the Renilla luciferase construct phRLTK followed by infection with H. pylori for 2 h (Figure 5C). Dual luciferase assay data confirmed that ETS2 significantly enhanced H. pylori-induced siah2 transcription. Twist1 overexpression showed a similar inducing effect of Twist1 on siah2 luciferase activity (Figure 5D). Western blot analysis further established that overexpression of ETS2 and Twist1 mediated induction of Siah2 protein expression in H. pylori-infected GCCs (Figures 5E and 5F respectively). We also noted that although Twist1 was highly induced in uninfected Twist1-expressing cells as compared with the infected pcDNA3.1+-expressing cells, Siah2 expression was lower in the first group which indicated the possibility of a post-translational modification of Twist1 only after infection with H. pylori that might be responsible for its activation.
Siah2 induces migration and invasiveness of H. pylori-infected GCCs
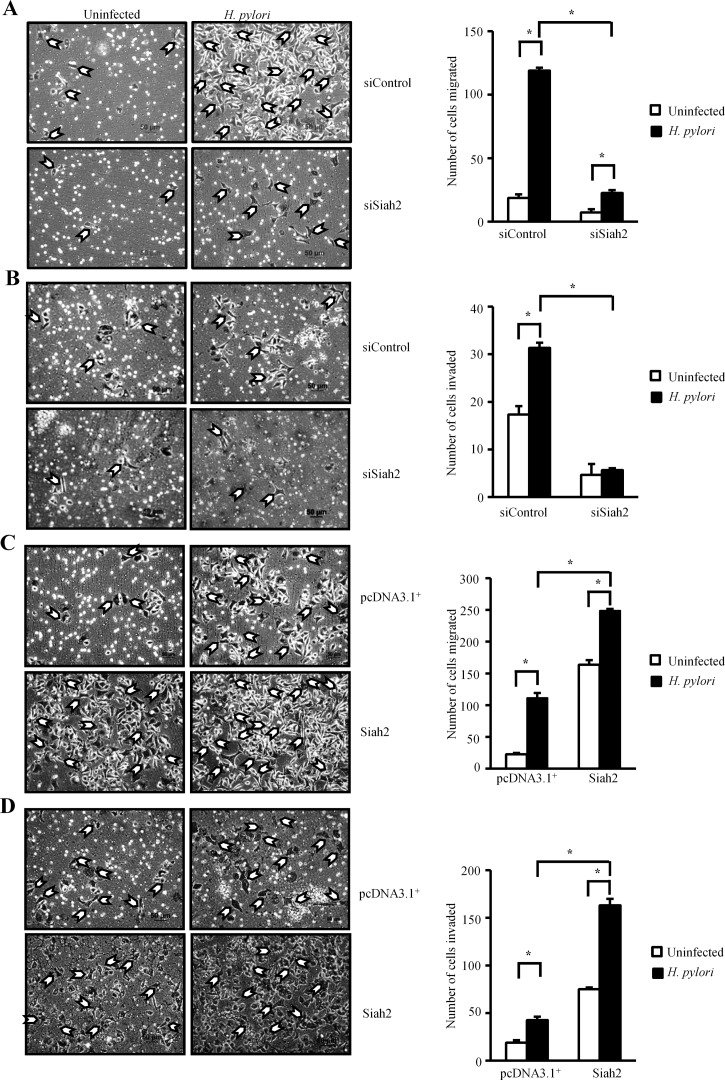
H. pylori induce EMT in infected GCCs. In order to assess the effect of Siah2 overexpression on the migration ability of GCCs, we performed transwell migration and matrigel invasion assays. MKN45 cells are partially adherent and therefore, a more adherent gastric adenocarcinoma cell AGS was used to study the effect of Siah2 expression on cell migration. The inhibitory effects of Siah2 siRNA on H. pylori-induced AGS cell migration and invasion were found to be significant over control siRNA-transfected cells (Figures 6A and 6B respectively). Exogenous overexpression of Siah2 in AGS cells significantly increased H. pylori-mediated cell migration and invasion (Figures 6C and 6D respectively). In addition, we performed wound-healing assays. pcDNA3.1+ or Siah2 stably-transfected AGS cells grown in monolayer were used to prepare wound marks and cells were incubated in the presence or absence of H. pylori. Siah2-expressing H. pylori-infected AGS cells showed significantly higher migration than empty-vector-expressing infected cells (Supplementary Figures S5A and S5B) indicating that Siah2 induced cell migration ability of infected GCCs. The effect of Siah2 overexpression and H. pylori infection on AGS cell proliferation was tested by MTT assay. Siah2 overexpression significantly induced cell proliferation which was further induced by H. pylori, at 24 h of infection (Supplementary Figure S5C). Anchorage-independent growth, an additional indicator of more aggressive cancer cell behaviour, was evaluated by soft-agar colony formation assays. We found a substantial induction in colony formation by H. pylori-infected Siah2 stably-expressing MKN45 cells as compared with the uninfected empty-vector expressing cells (Supplementary Figure S5D). Siah2 protein expression status in the stable MKN45 cells used for the soft-agar assay is shown in the accompanying Western blot image (Supplementary Figure S5D).
Figure 6. Siah2 increases migration and invasion property of H. pylori-infected GCCs.
(A) Transwell migration assay using siControl and siSiah2-transfected AGS cells (n=3).Cells were seeded in serum-free media on to the upper chamber of Transwell. After 24 h of infection (with or without H. pylori), migrated cells at the lower surface of the membrane were stained with haematoxylin and counted. Imaging was done using an inverted microscope. Scale shown=50 μm. Bars represent number of cells migrated from three independent experiments (mean±S.E.M.). *P<0.05. (B) Results of Transwell invasion assay on the Siah2 siRNA and siControl expressed AGS cells (infected with or without H. pylori). Cells were seeded on the upper chamber of the matrigel-precoated Transwell. Cells were allowed to invade through the membrane for 24 h. Invaded cells were counted from three independent experiments. Scale shown=50 μm. Bar graph represents the average number of invaded cells (mean±S.E.M.). *P<0.05. (C) Transwell migration was performed using empty vector and Siah2-overexpressing infected and uninfected AGS cells following procedures mentioned in Figure 6A. Scale shown=50 μm. Bars represent (mean±S.E.M.). *P<0.05. (D) Cell invasiveness was measured using empty vector and Siah2-overexpressing AGS cells with or without infection as explained in Figure 6B. Scale shown=50 μm. Bar graph represents the average number of invaded cells (mean±S.E.M.), *P<0.05.
ETS2- and Twist1-mediated induction of Siah2 migration and wound-healing properties of H. pylori-infected GCCs
Wound healing assay was performed to examine the involvement of ETS2 and Twist1 in H. pylori-mediated enhancement of GCC migration. For this, pcDNA3.1+, ETS2 or Twist1 stably-expressing AGS cells were used. Both ETS2- and Twist1-expressing cells showed significantly higher wound-healing properties following H. pylori infection compared with the pcDNA3.1+-expressing infected cells (Supplementary Figures S6A and S6B respectively). In order to assess the effect of ETS2 and Twist1 in H. pylori-mediated induction of cell invasion, we used stable-transfected cells. Our results confirmed that ETS2 and Twist1 dose-dependently induced colony formation ability of H. pylori-infected cells (Supplementary Figures S7A and S7B respectively). Accompanying Western blot images show ETS2 and Twist1 expression status in stable cells. To further ascertain the role of ETS2 and Twist1 in Siah2 expression we transfected MKN45 cells with ETS2 and Twist1 siRNAs along with control duplex siRNA. Western blotting showed significant reduction in Siah2 expression in ETS2- and Twist1-suppressed and H. pylori-infected cells (Supplementary Figures S8A and S8B respectively). Next, we suppressed Siah2 in MKN45 cells to find out its role in H. pylori-mediated cell migration. Siah2-suppressed cells showed significantly less wound-healing property following H. pylori infection compared with the control duplex-expressing infected cells (Supplementary Figure S8C). Accompanying Western blot results represent Siah2-suppression status in MKN45 cells used for the above experiment.
Taken together, our results consistently proved that ETS2- and Twist1-regulated induction of Siah2 had a significant positive effect on H. pylori-mediated enhancement of GCC migration and invasiveness.
DISCUSSION
Gastric cancer is one of the prime causes of mortality in the developing as well as in the industrialized world. The major contributor in this disease-associated mortality is metastasis which presents either with peritoneal dissemination or haematogenous spread to the liver and lungs. Therefore, understanding the mechanisms regulating gastric cancer progression and metastasis is imperative for the development of effective treatment strategies. We observed Siah2 induction in H. pylori-infected GCCs and identified ETS2 and Twist1 as two novel inducers of Siah2. Further, Siah2 was established as an inducer of motility and invasiveness of infected GCCs. Consistent with our in vitro data, gastric cancer biopsy samples collected from metastatic gastric tumours (stage III) showed marked induction of ETS2, Twist1 and Siah2 expression. Although all tissue samples analysed (see Experimental section) were from urease-negative patients (suggesting absence of H. pylori when biopsies were obtained), it is intriguing to speculate that alterations in Siah2 expression due to initial H. pylori infection may contribute to the development of gastric cancer. Moreover, our observations indicate that Siah2 along with ETS2 and Twist1 may represent promising therapeutic targets for the treatment of gastric cancer.
The metastasis promoting role of Twist1 was reported in various cancers [20,31] including gastric carcinoma [32]. Although, enhancement of lymph node metastasis and distant metastasis were observed with Twist1 induction in GCCs [33], ETS2 had been associated with increased apoptosis or tumour suppression in GCCs [34] and suppression of lung cancer [35]. These results are quite surprising considering the fact that members of the ETS-family of transcription factors are effectors of the RAS/ERK proto-oncogenic pathway [36]. ETS2 was known to be associated with c-Myc induction [37] and angiogenesis in breast cancer cells [38]. Very high expression of ETS2 along with other ETS family proteins was found to be required for the neoplastic transformation of thyroid cancer cells [39], progression of prostate [40] and colon cancer [41]. Although Ras was known to be induced in H. pylori-infected gastric epithelial AGS cells [42], we do not know yet whether it is involved in H. pylori-induced ETS2 expression in GCCs.
The speed of cancer metastasis is accelerated if any or all of the processes that take place during EMT, for example, cell migration and invasion, alteration in cell adhesion, intravasation and extravasation occur with increased rate. The EMT is regulated by a diverse set of growth factors and transcription factors including the HIF1α [43]. Recently Siah proteins were identified as important enhancers of breast cancer [19] and hepatocellular malignancy [15]. Increased metastasis of mouse melanoma [17], prostate cancer [16] and lung cancer [18] has also been associated with enhanced Siah2 expression. Moreover, Siah2 was found responsible for disruption of the tight junction integrity and cell polarity in hypoxic bone-osteosarcoma cells by degrading the tumour suppressor protein apoptosis-stimulating proteins of p53-2 (ASPP2) [21]. For some time now, H. pylori is known to increase cell motility [4,44]. However, Siah2-mediated enhancement of motility in H. pylori-infected GCCs is so far unknown. Our study suggests that Siah2 has a significant role in gastric cancer metastasis. Whether Siah2 is important or not in determining the stage and progression of the disease requires further research.
H. pylori-mediated changes in the GCC morphology and invasiveness, events that play crucial roles in enhancing gastric cancer metastasis, were found to be mediated by defects induced in the apical-junctional complexes; activation of β-catenin and its enhanced nuclear accumulation [45] as well as down-regulation of E-cadherin [46]. Bebb et al. [47] reported H. pylori-mediated disruption of the adherens junction. They found that acute H. pylori infection-mediated disruption of the β-catenin was not dependent on the cag PAI status. We also confirmed that siah2 induction by ETS2 and Twist1 in infected GCCs was independent of the cag PAI. We presume that some cag-independent virulence factors induce ETS2 and Twist1. Other than cag PAI, the blood group antigen-binding adhesion babA and the vacuolating cytotoxin vacA are the two other genes highly associated with gastric cancer risk. Unlike 26695 and 8-1 (both vacA s1/m1 strains), D154 has vacA s2/m2 genotype. Effects of babA and vacA on the epithelial expression of ETS2, Twist1 and Siah2 need to be determined by using respective isogenic and non-isogenic mutants. Nuclear accumulation of Siah2 was recently associated with the majority of hepatic cell carcinomas (HCCs) and was correlated with induced hepatic cell proliferation, tumour progression and distant metastasis [15]. We found very high nuclear accumulation of Siah2 in H. pylori-infected GCCs, which we surmise, could be regulated by posttranslational modifications of Siah2 which are currently being investigated by our group.
GCC responses to H. pylori infection are presented with contradictory effects. For example, H. pylori induce both apoptosis and anti-apoptotic events [29,48]. We observed up-regulated GCC proliferation under the influence of H. pylori in the present study whereas induction of apoptosis was earlier noted [29]. Increased apoptosis induced by H. pylori has been strongly believed to act as a stimulus for continued cell proliferation resulting in neoplastic changes [49,50]. Unfortunately, the mechanism is still not clearly understood. At this point, we find this missing link as a very important area of research and would pursue more studies to understand how exactly H. pylori-mediated apoptosis helps in promoting gastric epithelial cell proliferation and promotes gastric carcinogenesis. Cells undergoing EMT may become less responsive to apoptosis. It is not yet known what role Siah2 plays in GCC apoptosis. It is also not known whether gastric cancer chemoresistance is controlled by Siah2 or not. However, as we identified that H. pylori-mediated Siah2 induction contributed to EMT properties in the infected GCCs, this mechanism could be explored further for therapeutic possibilities. Once thought as very effective anticancer agents, the proteasomal inhibitors showed severe cytotoxicity, inhibition of apoptosis and increased treatment resistance [51,52]. Since, Siah2 could be specifically targeted by RNA interference (RNAi) sparing other ubiquitin ligases including Siah1, untoward side effects during treatment could possibly be avoided.
Collectively, our data represent a novel model of Siah2-mediated induction of cell invasion and motility in H. pylori-infected gastric epithelial cancer cells providing a major conceptual advancement in our understanding of the mechanism of Siah2 induction in H. pylori-infected GCCs. Since Siah2 induces metastasis associated with H. pylori infection, it could be targeted for therapy. The present study also indicates that Siah2, ETS2 and Twist1 might have prognostic significance in H. pylori-mediated gastric cancer.
Abbreviations
- cag
cytotoxin-associated gene
- EBS
ETS2-binding site
- EMT
epithelial–mesenchymal transition
- ETS2
E26 transformation-specific sequence 2
- GCCs
gastric cancer cells
- HDAC1
histone-deacetylase 1
- HIF1α
hypoxia-inducible factor 1α
- MOI
multiplicity of infection
- PAI
pathogenicity island
- real-time RT-PCR
real-time reverse transcription PCR
- RING
really interesting new gene
- Siah
the seven in absentia homologue
- TBS
Twist1-binding site
- Twist1
Twist-related protein 1
AUTHOR CONTRIBUTION
Lopamudra Das performed experiments and analysed data; Shrikant Babanrao Kokate performed wound-healing and soft agar assays; Suvasmita Rath did confocal microscopy; Niranjan Rout and Shivaram Prasad Singh helped with gastric cancer biopsy collection and analysis; Asish Mukhopadhyay provided a crucial reagent; Sheila Crowe gave expert comments; Asima Bhattacharyya conceived the work, designed experiments, analysed the data, supervised the work and wrote the paper.
FUNDING
This work was supported by the Department of Biotechnology, Govt. of India [grant number BT/PR15092/GBD/27/311/2011 (to A.B.)]; the Science and Engineering Research Board, Government of India [grant number SB/SO/BB-0015/2014 (to A.B.)]; the Indian Council of Medical Research fellowship [grant number 3/1/3/JRF-2010/HRD-94 (Roll- 41439) (to S.R.)]; and NISER fellowship, Department of Atomic Energy, Government of India (to L.D. and S.B.K.).
References
- 1.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Tsugane S., Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 3.Bessede E., Staedel C., Acuna Amador L.A., Nguyen P.H., Chambonnier L., Hatakeyama M., Belleannee G., Megraud F., Varon C. Helicobacter pylori generates cells with cancer stem cell properties via epithelial-mesenchymal transition-like changes. Oncogene. 2013;33:4123–4131. doi: 10.1038/onc.2013.380. [DOI] [PubMed] [Google Scholar]
- 4.Krueger S., Hundertmark T., Kuester D., Kalinski T., Peitz U., Roessner A. Helicobacter pylori alters the distribution of ZO-1 and p120ctn in primary human gastric epithelial cells. Pathol. Res. Pract. 2007;203:433–444. doi: 10.1016/j.prp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Amieva M.R., Vogelmann R., Covacci A., Tompkins L.S., Nelson W.J., Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churin Y., Al-Ghoul L., Kepp O., Meyer T.F., Birchmeier W., Naumann M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 2003;161:249–255. doi: 10.1083/jcb.200208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlides S.C., Huang K.T., Reid D.A., Wu L., Blank S.V., Mittal K., Guo L., Rothenberg E., Rueda B., Cardozo T., Gold L.I. Inhibitors of SCF-Skp2/Cks1 E3 ligase block estrogen-induced growth stimulation and degradation of nuclear p27kip1: therapeutic potential for endometrial cancer. Endocrinology. 2013;154:4030–4045. doi: 10.1210/en.2013-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipkowitz S., Weissman A.M. RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat. Rev. Cancer. 2011;11:629–643. doi: 10.1038/nrc3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen M., Schmitt S., Buac D., Dou Q.P. Targeting the ubiquitin-proteasome system for cancer therapy. Expert Opin. Ther. Targets. 2013;17:1091–1108. doi: 10.1517/14728222.2013.815728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habelhah H., Frew I.J., Laine A., Janes P.W., Relaix F., Sassoon D., Bowtell D.D., Ronai Z. Stress-induced decrease in TRAF2 stability is mediated by Siah2. EMBO J. 2002;21:5756–5765. doi: 10.1093/emboj/cdf576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuzawa S.I., Reed J.C. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol. Cell. 2001;7:915–926. doi: 10.1016/S1097-2765(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 12.Nadeau R.J., Toher J.L., Yang X., Kovalenko D., Friesel R. Regulation of Sprouty2 stability by mammalian Seven-in-Absentia homolog 2. J. Cell. Biochem. 2007;100:151–160. doi: 10.1002/jcb.21040. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama K., Frew I.J., Hagensen M., Skals M., Habelhah H., Bhoumik A., Kadoya T., Erdjument-Bromage H., Tempst P., Frappell P.B., et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Susini L., Passer B.J., Amzallag-Elbaz N., Juven-Gershon T., Prieur S., Privat N., Tuynder M., Gendron M.C., Israel A., Amson R., et al. Siah-1 binds and regulates the function of Numb. Proc. Natl. Acad. Sci. U.S.A. 2001;98:15067–15072. doi: 10.1073/pnas.261571998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malz M., Aulmann A., Samarin J., Bissinger M., Longerich T., Schmitt S., Schirmacher P., Breuhahn K. Nuclear accumulation of seven in absentia homologue-2 supports motility and proliferation of liver cancer cells. Int. J. Cancer. 2012;131:2016–2026. doi: 10.1002/ijc.27473. [DOI] [PubMed] [Google Scholar]
- 16.Qi J., Nakayama K., Cardiff R.D., Borowsky A.D., Kaul K., Williams R., Krajewski S., Mercola D., Carpenter P.M., Bowtell D., Ronai Z.A. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. 2010;18:23–38. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi J., Nakayama K., Gaitonde S., Goydos J.S., Krajewski S., Eroshkin A., Bar-Sagi D., Bowtell D., Ronai Z. The ubiquitin ligase Siah2 regulates tumorigenesis and metastasis by HIF-dependent and -independent pathways. Proc. Natl. Acad. Sci. U.S.A. 2008;105:16713–16718. doi: 10.1073/pnas.0804063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed A.U., Schmidt R.L., Park C.H., Reed N.R., Hesse S.E., Thomas C.F., Molina J.R., Deschamps C., Yang P., Aubry M.C., Tang A.H. Effect of disrupting seven-in-absentia homolog 2 function on lung cancer cell growth. J. Natl. Cancer Inst. 2008;100:1606–1629. doi: 10.1093/jnci/djn365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan P., Moller A., Liu M.C., Sceneay J.E., Wong C.S., Waddell N., Huang K.T., Dobrovic A., Millar E.K., O'Toole S.A., et al. The expression of the ubiquitin ligase SIAH2 (seven in absentia homolog 2) is mediated through gene copy number in breast cancer and is associated with a basal-like phenotype and p53 expression. Breast Cancer Res. 2011;13:R19. doi: 10.1186/bcr2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiota M., Zardan A., Takeuchi A., Kumano M., Beraldi E., Naito S., Zoubeidi A., Gleave M.E. Clusterin mediates TGF-beta-induced epithelial-mesenchymal transition and metastasis via Twist1 in prostate cancer cells. Cancer Res. 2012;72:5261–5272. doi: 10.1158/0008-5472.CAN-12-0254. [DOI] [PubMed] [Google Scholar]
- 21.Kim H., Claps G., Moller A., Bowtell D., Lu X., Ronai Z.A. Siah2 regulates tight junction integrity and cell polarity through control of ASPP2 stability. Oncogene. 2014;33:2004–2010. doi: 10.1038/onc.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar T.R., Sharan S., Wang J., Pawar S.A., Cantwell C.A., Johnson P.F., Morrison D.K., Wang J.M., Sterneck E. Identification of a Src tyrosine kinase/SIAH2 E3 ubiquitin ligase pathway that regulates C/EBPdelta expression and contributes to transformation of breast tumor cells. Mol. Cell. Biol. 2012;32:320–332. doi: 10.1128/MCB.05790-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frasor J., Danes J.M., Funk C.C., Katzenellenbogen B.S. Estrogen down-regulation of the corepressor N-CoR: mechanism and implications for estrogen derepression of N-CoR-regulated genes. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13153–13157. doi: 10.1073/pnas.0502782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLeod R.J., Hayes M., Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G403–G411. doi: 10.1152/ajpgi.00119.2007. [DOI] [PubMed] [Google Scholar]
- 25.Jing Y., Liu L.Z., Jiang Y., Zhu Y., Guo N.L., Barnett J., Rojanasakul Y., Agani F., Jiang B.H. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2012;125:10–19. doi: 10.1093/toxsci/kfr256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo C., Dai Y., Kang N., Cui L., He W. Ectopic expression of human MutS homologue 2 on renal carcinoma cells is induced by oxidative stress with interleukin-18 promotion via p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways. J. Biol. Chem. 2012;287:19242–19254. doi: 10.1074/jbc.M112.349936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharyya A., Chattopadhyay R., Hall E.H., Mebrahtu S.T., Ernst P.B., Crowe S.E. Mechanism of hypoxia-inducible factor 1 alpha-mediated Mcl1 regulation in Helicobacter pylori-infected human gastric epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G1177–G1186. doi: 10.1152/ajpgi.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharyya A., Chattopadhyay R., Burnette B.R., Cross J.V., Mitra S., Ernst P.B., Bhakat K.K., Crowe S.E. Acetylation of apurinic/apyrimidinic endonuclease-1 regulates Helicobacter pylori-mediated gastric epithelial cell apoptosis. Gastroenterology. 2009;136:2258–2269. doi: 10.1053/j.gastro.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rath S., Das L., Kokate S.B., Pratheek B.M., Chattopadhyay S., Goswami C., Chattopadhyay R., Crowe S.E., Bhattacharyya A. Regulation of Noxa-mediated apoptosis in Helicobacter pylori-infected gastric epithelial cells. FASEB J. 2015;29:796–806. doi: 10.1096/fj.14-257501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekiguchi M., Suzuki T. 11–gastric tumor cell lines. In: Hay R.K.M., Park J.-G.B., Gazdar A., editors. Atlas of Human Tumor Cell Lines. San Diego: Academic Press; 1994. pp. 287–316. [DOI] [Google Scholar]
- 31.Croset M., Goehrig D., Frackowiak A., Bonnelye E., Ansieau S., Puisieux A., Clezardin P. TWIST1 expression in breast cancer cells facilitates bone metastasis formation. J. Bone Miner. Res. 2014;29:1886–1899. doi: 10.1002/jbmr.2215. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z., Zhang X., Gang H., Li X., Li Z., Wang T., Han J., Luo T., Wen F., Wu X. Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochem. Biophys. Res. Commun. 2007;358:925–930. doi: 10.1016/j.bbrc.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Ru G.Q., Wang H.J., Xu W.J., Zhao Z.S. Upregulation of Twist in gastric carcinoma associated with tumor invasion and poor prognosis. Pathol. Oncol. Res. 2011;17:341–347. doi: 10.1007/s12253-010-9332-0. [DOI] [PubMed] [Google Scholar]
- 34.Liao Y.L., Hu L.Y., Tsai K.W., Wu C.W., Chan W.C., Li S.C., Lai C.H., Ho M.R., Fang W.L., Huang K.H., Lin W.C. Transcriptional regulation of miR-196b by ETS2 in gastric cancer cells. Carcinogenesis. 2012;33:760–769. doi: 10.1093/carcin/bgs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabbout M., Garcia M.M., Fujimoto J., Liu D.D., Woods D., Chow C.W., Mendoza G., Momin A.A., James B.P., Solis L., et al. ETS2 mediated tumor suppressive function and MET oncogene inhibition in human non-small cell lung cancer. Clin. Cancer Res. 2013;19:3383–3395. doi: 10.1158/1078-0432.CCR-13-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabbout M., Dakhlallah D., Sharma S., Bronisz A., Srinivasan R., Piper M., Marsh C.B., Ostrowski M.C. MicroRNA 17–92 cluster mediates ETS1 and ETS2-dependent RAS-oncogenic transformation. PLoS One. 2014;9:e100693. doi: 10.1371/journal.pone.0100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-azawi D., Ilroy M.M., Kelly G., Redmond A.M., Bane F.T., Cocchiglia S., Hill A.D., Young L.S. Ets-2 and p160 proteins collaborate to regulate c-Myc in endocrine resistant breast cancer. Oncogene. 2008;27:3021–3031. doi: 10.1038/sj.onc.1210964. [DOI] [PubMed] [Google Scholar]
- 38.Zabuawala T., Taffany D.A., Sharma S.M., Merchant A., Adair B., Srinivasan R., Rosol T.J., Fernandez S., Huang K., Leone G., Ostrowski M.C. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Res. 2010;70:1323–1333. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Nigris F., Mega T., Berger N., Barone M.V., Santoro M., Viglietto G., Verde P., Fusco A. Induction of ETS-1 and ETS-2 transcription factors is required for thyroid cell transformation. Cancer Res. 2001;61:2267–2275. [PubMed] [Google Scholar]
- 40.Carbone G.M., Napoli S., Valentini A., Cavalli F., Watson D.K., Catapano C.V. Triplex DNA-mediated downregulation of Ets2 expression results in growth inhibition and apoptosis in human prostate cancer cells. Nucleic Acids Res. 2004;32:4358–4367. doi: 10.1093/nar/gkh744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flavin P., Redmond A., McBryan J., Cocchiglia S., Tibbitts P., Fahy-Browne P., Kay E., Treumann A., Perrem K., McIlroy M., et al. RuvBl2 cooperates with Ets2 to transcriptionally regulate hTERT in colon cancer. FEBS Lett. 2011;585:2537–2544. doi: 10.1016/j.febslet.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Cho S.O., Lim J.W., Kim K.H., Kim H. Involvement of Ras and AP-1 in Helicobacter pylori-induced expression of COX-2 and iNOS in gastric epithelial AGS cells. Dig. Dis. Sci. 2010;55:988–996. doi: 10.1007/s10620-009-0828-y. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Moese S., Selbach M., Kwok T., Brinkmann V., Konig W., Meyer T.F., Backert S. Helicobacter pylori induces AGS cell motility and elongation via independent signaling pathways. Infect. Immun. 2004;72:3646–3649. doi: 10.1128/IAI.72.6.3646-3649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogelmann R., Amieva M.R., Falkow S., Nelson W.J. Breaking into the epithelial apical-junctional complex–news from pathogen hackers. Curr. Opin. Cell Biol. 2004;16:86–93. doi: 10.1016/j.ceb.2003.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terres A.M., Pajares J.M., O'Toole D., Ahern S., Kelleher D. H. pylori infection is associated with downregulation of E-cadherin, a molecule involved in epithelial cell adhesion and proliferation control. J. Clin. Pathol. 1998;51:410–412. doi: 10.1136/jcp.51.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bebb J.R., Leach L., Zaitoun A., Hand N., Letley D.P., Thomas R., Atherton J.C. Effects of Helicobacter pylori on the cadherin-catenin complex. J. Clin. Pathol. 2006;59:1261–1266. doi: 10.1136/jcp.2006.036772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanai A., Hirata Y., Mitsuno Y., Maeda S., Shibata W., Akanuma M., Yoshida H., Kawabe T., Omata M. Helicobacter pylori induces antiapoptosis through buclear factor-kappaB activation. J. Infect. Dis. 2003;188:1741–1751. doi: 10.1086/379629. [DOI] [PubMed] [Google Scholar]
- 49.Xia H.H., Talley N.J. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am. J. Gastroenterol. 2001;96:16–26. doi: 10.1016/S0002-9270(00)02240-1. [DOI] [PubMed] [Google Scholar]
- 50.Jang T.J., Kim J.R. Proliferation and apoptosis in gastric antral epithelial cells of patients infected with Helicobacter pylori. J. Gastroenterol. 2000;35:265–271. doi: 10.1007/s005350050344. [DOI] [PubMed] [Google Scholar]
- 51.Kane R.C., Farrell A.T., Sridhara R., Pazdur R. United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin. Cancer Res. 2006;12:2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 52.Sloss C.M., Wang F., Liu R., Xia L., Houston M., Ljungman D., Palladino M.A., Cusack J.C., Jr Proteasome inhibition activates epidermal growth factor receptor (EGFR) and EGFR-independent mitogenic kinase signaling pathways in pancreatic cancer cells. Clin. Cancer Res. 2008;14:5116–5123. doi: 10.1158/1078-0432.CCR-07-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]