Abstract
Data derived principally from peripheral tissues (fat, muscle and liver) show that insulin signals via diverse interconnecting intracellular pathways and that some of the major intersecting points (known as critical nodes) are the IRSs (insulin receptor substrates), PI3K (phosphoinositide kinase)/Akt and MAPK (mitogen-activated protein kinase). Most of these insulin pathways are probably also active in the ovary and their ability to interact with each other and also with follicle-stimulating hormone (FSH) and luteinizing hormone (LH) signalling pathways enables insulin to exert direct modulating influences on ovarian function. The present paper reviews the intracellular actions of insulin and the uptake of glucose by ovarian tissues (granulosa, theca and oocyte) during the oestrous/menstrual cycle of some rodent, primate and ruminant species. Insulin signals through diverse pathways and these are discussed with specific reference to follicular cell types (granulosa, theca and oocyte). The signalling pathways for FSH in granulosa cells and LH in granulosa and theca cells are summarized. The roles of glucose and of insulin-mediated uptake of glucose in folliculogenesis are discussed. It is suggested that glucose in addition to its well-established role of providing energy for cellular function may also have insulin-mediated signalling functions in ovarian cells, involving AMPK (AMP-dependent protein kinase) and/or hexosamine. Potential interactions of insulin signalling with FSH or LH signalling at critical nodes are identified and the available evidence for such interactions in ovarian cells is discussed. Finally the action of the insulin-sensitizing drugs metformin and the thiazolidinedione rosiglitazone on follicular cells is reviewed.
Keywords: Akt, corpus luteum, follicle-stimulating hormone (FSH), granulosa, insulin receptor substrate (IRS), luteinizing hormone (LH), MAPK, metformin, oestradiol, oocyte, progesterone, theca, thiazolidinedione
INTRODUCTION
An effect of diet on human reproduction was first commented on over 2000 years ago by Hippocrates as cited by Franks et al. [1] in an earlier review. Hippocrates noted that hard-working slim servant women were more fertile than their overfed and sedentary Roman employers. In more recent times, during the 19th and 20th Centuries, many authors have observed relationships between nutritional status and/or diet and fertility/infertility in sheep, cattle, rodents and primates. In human medicine, a link between insulin and ovarian pathology was established by demonstrating associations between insulin resistance and polycystic ovarian syndrome (PCOS). PCOS is a set of reproductive and metabolic symptoms associated with an imbalance of reproductive hormones in women. In cattle selected for high milk production, low concentrations of insulin in the post-partum period are associated with reduced fertility [2], and in sheep an increased availability of energy-yielding substrates is associated with increased prolificacy [3]. These and many other observations have stimulated research into the physiological and biochemical basis of these relationships with a view to either improving the fertility of farm animals or curing those forms of human infertility associated with diet and metabolism. The dietary components implicated include protein, lipid and carbohydrate, especially glucose. In the present review we focus on the role of insulin and glucose.
Polycystic ovarian syndrome
In women, PCOS is a common anovulatory syndrome which is associated with a number of metabolic disorders, such as insulin resistance [4] and the metabolic syndrome [5]. Approximately 50% of women with PCOS are insulin-resistant, and approximately 10% of these are severely insulin-resistant [6]. In addition, women with PCOS appear to have multiple lipid abnormalities including elevated levels of non-esterified fatty acids (NEFAs) and cholesterol [7], problems which are also frequently associated with reduced post-partum fertility in the high-yielding dairy cow [8]. In the ovary, PCOS is characterized by increased numbers of pre-antral follicles, arrested follicular maturation and a disruption to the selection of the ovulatory follicle. These are due to either a reduced sensitivity to follicle-stimulating hormone (FSH) or enhanced follicular activity of luteinizing hormone (LH). Patients with PCOS show abnormal secretion of gonadotropin-releasing hormone (GnRH) and excessive LH release that stimulates theca cells to over-produce androgens. The peripheral conversion of circulating androgens in adipose tissue, stroma and skin to oestrogens results in increased oestrogen production in women with PCOS.
Bulimia nervosa and anorexia nervosa
These two human pathologies have secondary reproductive consequences linked to the dietary and metabolic changes associated with the conditions. Bulimia nervosa is a psychiatric disorder, and among its reported side effects are menstrual irregularities and infertility [9]. The infertility is not associated with weight loss as such, and it is probably a consequence of the extremes in glycaemic flux associated with cycles of binge eating and purging and/or periodic starvation on the ovary. Anorexia nervosa is a condition in women suffering starvation-induced chronic malnutrition where the effect of the consequent hypoglycaemia is exerted primarily at a hypothalamic level. It is clearly established in human as well as in animal models that starvation-induced chronic malnutrition reduces the secretion of hypothalamic GnRH and leads to anovulation. With anorexia nervosa, the primary reproductive effect is not ovarian, although direct ovarian effects of hypoglycaemia may be present [10]. The prolonged energy deficit of elite athletes and dancers has the same or very similar reproductive consequences as starvation-induced anorexia.
Cystic ovaries in high producing dairy cattle
The modern dairy cow has been genetically selected for very high milk production and they can produce up to three times the quantity of milk compared with unselected cows. During early lactation, these cows are physically incapable of eating enough to meet the demand for nutrients especially glucose, required to support the extremely high levels of milk production. Consequently the cows are in a state of chronic negative energy balance (NEB) and must mobilize adipose tissue to provide glucose for milk production. This NEB is a risk factor for ovarian dysfunction [11], apparently because of the hormonal and metabolic adaptations that occur in response to NEB. Consequently, these cows have very poor fertility [2] characterized among other things by prolonged post-partum anoestrus, anovulation and the development of cystic ovarian follicles (COFs). The common occurrence of COF in high yielding dairy cows, and the phenotypic [12,13] and genotypic [14] links with the yields of milk, milk fat and milk protein clearly link COFs with the high level of milk production.
The changes in the circulating concentrations of reproductive and metabolic hormones in response to post-partum NEB, disrupts follicular development principally by acting on the hypothalamic–pituitary axis to inhibit the neuro-secretion of GnRH. There is considerable evidence from studies in several species that show that hypoglycaemia and NEB inhibit the reproductive system at a hypothalamic level [15,16]. In most cases, there is a complete cessation of follicular growth beyond the gonadotropin-independent stage of development. The presence of COF in high yielding cows suggests a direct ovarian action of NEB as well [17,18]. A recent study shows that the reduced gene and protein expression of some downstream effectors in the early stages of the insulin signalling pathway may contribute to follicle persistence [19].
Energy-yielding substrates in sheep
In the ewe a short-term increase in the availability of dietary energy during the last few days of the luteal phase is capable of stimulating the final stages of folliculogenesis, leading to an increased ovulation rate and thus an increase in the rate of twinning [20]. This effect is observed initially as an increase in the number of ovulatory follicles without accompanying changes in the circulating concentrations of FSH and LH [21], suggesting an action at an ovarian level. These effects are associated with a short-term increase in the availability of energy-yielding substrates derived from the diet [3], especially glucose [22,23]. Insulin has also been implicated in this physiological response to diet both as a mediator of the uptake of glucose and as insulin [24]. Similarly insulin-like growth factor 1 (IGF-I) has also been implicated; the infusion of glucose into ewes reduced follicular expression of IGF-IR (IGF-I receptor) and IGFBP2 (IGF-binding protein 2) mRNAs [25]. Both insulin and IGF-I increased the sensitivity of follicles to the action of FSH in the terminal stages of growth and development [26]. Several mechanisms of action of insulin and IGF-I at an ovarian level are possible, in particular by the direct activation of their receptors or by an increase in the bioavailability of IGF-I through a nutritionally mediated effect on intra-follicular IGFBPs [27].
GENERAL OVERVIEW OF INTRACELLULAR INSULIN SIGNALLING
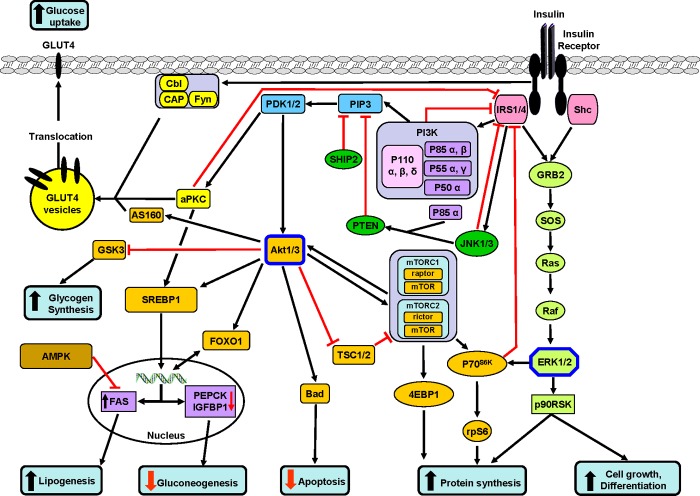
The transduction of the insulin signal has been intensively studied principally using muscle, liver and adipose tissue, and these findings are summarized in Figure 1. Some, if not all, of these pathways appear to be functional in ovarian cells. Insulin exerts its pleiotropic effects after binding to the insulin receptor (IR), a member of the tyrosine kinase receptor family. Upon ligand binding, the IR undergoes conformational changes, which allow auto-phosphorylation of tyrosine residues located on the β subunits. Phosphorylated tyrosine residues serve as docking sites for various cellular substrates. In mammals, at least 11 intracellular substrates of the IR have been identified (reviewed in [28]). These include the family of IRS (insulin receptor substrate) and Shc (Src homology collagen) proteins. Four different IRSs have been reported in various mammals and two additional IRSs have been detected in humans (IRS-5/DOK4 (docking protein 4) and IRS-6/DOK5 (docking protein 5)). These additional IRSs are involved in insulin signalling but are not involved in phosphoinositide kinase (PI3K) activation [29] and both are poor substrates for the IR [30]. The IRSs have different molecular masses (60–180 kDa) and show differential tissue distribution [31]. In mammals, Shc is also an IRS. There are 46, 52 and 66 kDa isoforms that arise from alternative splicing of the primary Shc transcript [32]. The 52 kDa and, to a lesser extent, the 46 kDa Shc isoforms are tyrosine phosphorylated in response to insulin. Most reports suggest that, in mammals, IRS-1 is the major substrate leading to stimulation of glucose transport in muscle and adipose tissue, whereas, in liver, IRS-1 and IRS-2 have complementary roles in insulin signalling and metabolism. In contrast, Shc does not appear to be directly involved in the metabolic signalling of insulin, but has a critical role in insulin-induced mitogenesis [33]. In mammals, tyrosine phosphorylation of IRS and Shc makes these proteins docking sites for various signalling proteins containing an SH2 (Src homology 2) domain. These intracellular interactions lead to the activation of the two main IR signalling pathways: PI3K/Akt and MAPK (mitogen-activated protein kinase) [34,35].
Figure 1. Following insulin binding, the IR auto-phosphorylates and then phosphorylates its substrates including the IRSs and Shc.
Following phosphorylation, specific domains in these substrates interact with other proteins. One of the major complexes interacting with IRSs is PI3K, which is formed of two interacting proteins: a regulatory subunit (p85) and a catalytic subunit (p110). There are several isoforms of both subunits: p85α, p55α, p50α, p85β and p55γ for the regulatory subunit and p110α, β and δ for the catalytic subunit. The binding of the regulatory subunit to IRS forms an active heterodimer which phosphorylates membrane phosphatidylinositol residues in the 3′ position of PIP3 to form PDK1/2 (shown in blue). In turn, PDK1 phosphorylates (on threonine and serine residues) and thereby activates Akt/PKB (a serine/threonine kinase, with three isoforms). Downstream (orange boxes), Akt phosphorylates several kinases or transcription factors (some examples are shown) involved in most of the major metabolic effects of insulin: (i) glycogen synthesis through GSK3, which is inactivated by phosphorylation. The inactivation of GSK3 leads to the de-phosphorylation and activation of GS and hence to an acceleration of glycogen synthesis, (ii) inhibition of gluconeogenesis by turning off the PEPCK gene through inhibition of FOXO1 function (mainly in liver and adipose tissue), (iii) protein synthesis through mTOR and subsequent activation of p70S6K and 4EBP1 (eukaryotic translation initiation factor 4E-binding protein 1), (iv) the Akt pathway also stimulates cell growth and differentiation (not shown in the figure) and (v) exerts anti-apoptotic effects by phosphorylating the anti-apoptotic protein, Bad. The kinase, PI3K also activates aPKCs (atypical PKCs) (yellow boxes), which in concert with Akt (via AS160) exerts different effects according to the tissue: in muscle and adipocytes they initiate the translocation of intracellular GLUT4 on to the membrane and thereby stimulate glucose transport into the cell. Another important and well-documented signalling pathway is the Ras/ERK pathway (green boxes). The binding of Grb2 (growth factor receptor-bound protein 2) and Sos (son of sevenless) to tyrosine-phosphorylated IRS or Shc can also activate the small GTPase Ras cascade, Raf and ultimately ERK1 and ERK2 (green boxes). When activated, these serine kinases simulate protein synthesis through the activation of p90S6K and activate transcription factors and ultimately cell multiplication and differentiation. Some lipid phosphatases including PTEN and SHIP2 act on PIP3 to decrease or extinguish the insulin signal. Some of the insulin-stimulated serine kinases (aPKC, JNKs, p90RSK, ERKs and S6 kinase) may also exert negative effects on insulin sensitivity. Stimulatory connections are shown in black and inhibitory ones are shown in red.
Mammalian PI3K is a heterodimer [36] composed of a catalytic subunit of 110 kDa (p110) and a SH2-domain-containing regulatory subunit of approximately 85 kDa (p85) the two are associated non-covalently. In mammals, PI3K has five isoforms of the regulatory subunit (in three sizes) and three of the catalytic subunit. All four IRSs interact with all the regulatory subunits of PI3K, and thereby activate the catalytic subunit. Once docked to IRS, PI3K phosphorylates the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), thereby producing phosphatidylinositol 3,4,5-trisphosphate (PIP3), which in turn acts as a second messenger to activate PDK1 (phosphoinositide-dependent kinase 1). The biological effects of the PI3K pathway can be negatively regulated at the level of PIP3 by phospholipid phosphatases, including PTEN (phosphatase and tensin homolog and SHIP2 (SH2-domain-containing inositolphosphatase-2, which inactivate PIP3 by de-phosphorylation [37,38]. Activated PDK1 can then phosphorylate Akt/PKB (protein kinase B). Akt comprises three isoforms each encoded by a separate gene and it is a key branching point (critical node) for insulin signalling. Activated Akt directly phosphorylates and inactivates TSC2 (tuberous sclerosis complex 2) and GSK3 (glycogen synthase kinase-3), whereas it directly phosphorylates and activates Bad (Bcl-2-associated death promoter) and the forkhead transcription factor FOXO1 (forkhead box O1). Phosphorylation of FOXO1 results in down-regulation of transcriptional activity and TSC2 is a direct upstream negative regulator of mTORC1 that is involved in the regulation of protein synthesis. Mammalian target of rapamycin (mTOR) is a highly conserved protein kinase that is found in two structurally and functionally distinct protein complexes: mTOR complex-1 (mTORC1) and mTOR complex-2 (mTORC2). In the first, mTOR is associated with raptor (regulatory associated protein of mTOR) and is a key regulator of cell growth, cell proliferation and mRNA translation, whereas in the second, mTOR is associated with rictor (rapamycin-insensitive companion of mTOR) and promotes actin cytoskeletal rearrangement, cell survival and cell cycle progression. The two best characterized downstream targets of mTORC1, 4E-BP1 (eukaryotic initiation factor 4E-binding protein 1) and S6K1 (p70 ribosomal S6 kinase 1), regulate protein synthesis. Phosphorylation of 4E-BP1 by mTORC1 induces the release of eIF4E (eukaryotic translation initiation factor 4E) from 4E-BP1 and the subsequent formation of eIF4F (translation initiation factor 4F) complex. Activation of S6K1 also contributes to increased translation elongation via the phosphorylation of the eEF2 (eukaryotic elongation factor 2). In addition, phosphorylation of S6k1 negatively regulates insulin signalling by phosphorylating IRS-1 on serine residues. The inactivation of GSK3 leads to the de-phosphorylation and activation of glycogen synthase (GS) and hence to an acceleration of glycogen synthesis. The phosphorylation of FOXO1 by Akt in response to insulin leads to the exclusion of FOXO1 from the nucleus and consequently prevents the positive effect of FOXO1 on phosphoenolpyruvate carboxykinase (PEPCK) gene transcription. Akt also exerts anti-apoptotic effects by phosphorylating Bad. In mammals, inhibition of the PI3K/Akt pathway blocks almost all of insulin's metabolic actions, including stimulation of glucose transport, glycogen synthesis and lipid synthesis [39]. Thus, this pathway has a pivotal role in the metabolic actions of insulin.
Thirteen glucose transporter (GLUT)-like proteins have been identified and characterized [40] in mammals. One of them GLUT4, is a member of the facilitative GLUT family, characterized by preferential expression in muscle and adipose tissue, and it is responsible for insulin-stimulated glucose uptake (reviewed in [41]). The insulin-derived signalling pathways regulating the net gain in surface GLUT4 include activation of PI3K, PDK1/2, atypical PKC (protein kinase C) [42], Akt and AS160 (Akt substrate of 160 kDa) and a pathway (Cbl/CAP) that is independent of the PI3K/Akt pathway [43].
In mammals, the MAPK/extracellular-signal-regulated protein kinase 1/2 (ERK1/2) pathway is activated by insulin through IRSs or Shc tyrosine phosphorylation [28]. This pathway is composed of a set of three sequentially acting kinases. The activation of a MAPKKK (MAPK kinase kinase) (Raf) leads to the phosphorylation and activation of a MAPKK (MAPK kinase) called MEK1/2 (MAPK/ERK kinase 1/2), which then stimulates ERK1/2 MAPK activity through dual phosphorylation on threonine and tyrosine residues of ERK1/2 MAPK to regulate the expression of genes including some in the PI3K pathway, controlling cell growth and differentiation [44]. It also positively controls S6K1 phosphorylation [45] and this pathway can be blocked at Shc, by cytokines acting through the JAK (Janus kinase)/STAT (signal transducer and activator of transcription) pathway (Figure 1).
GLUCOSE
From a physiological viewpoint, glucose is an enigmatic molecule. On one hand, it is a critical source of metabolic energy for cellular metabolism and therefore for life itself, and on the other hand it is potentially extremely harmful to protein function. Hypoglycaemia will quickly become life-threatening, whereas prolonged hyperglycaemia will lead to protein malfunction through the process of non-enzymic glycation of proteins and the formation of advanced glycation end-products (AGEs), thus altering their functional properties [46]. Consequently, mammals have evolved homoeostatic mechanisms that maintain blood and tissue concentrations of glucose within very narrow physiological limits. The AGEs have been implicated in reproductive conditions such as ovarian hyperstimulation syndrome and PCOS [47], and glycated proteins have been implicated in the fetal malformations that can accompany diabetic pregnancy [48,49].
Sources of glucose in ruminants and monogastric species
In monogastric species, glucose is derived principally from the diet. Gluconeogenesis does occur, but it is secondary to diet as a source of glucose. In contrast, ruminants absorb very little glucose from the diet. The anaerobic conditions of the rumen reduce most carbohydrates and lipids to a series of volatile fatty acids (VFAs) and these provide over 70% of the energy supply. In the ruminant, although most dietary glucose is reduced in the rumen and absorbed as VFAs (propionic, lactic and butyric acids), some glucose can survive the reducing conditions of the rumen to be absorbed from the small intestine. Although gluconeogenesis occurs in all mammals, ruminants are particularly dependent upon it for the generation of glucose. Propionate is the major VFA produced by rumen fermentation of carbohydrates and 45–60% of glucose in ruminants is synthesized in the liver from propionic acid. Other sources of precursor carbon for synthesis of glucose via gluconeogenesis can be derived from the metabolism of glucogenic amino acids and include most except for leucine.
Uptake of glucose by ovarian tissues and the role of the GLUTs
Glucose is a hydrophilic molecule and cannot permeate the plasma membrane. Consequently, its uptake is mediated by a number of facilitative sugar transporters that exhibit different substrate specificities, kinetic properties and patterns of tissue distribution [50]. Published data show that various GLUTs are present in ovarian tissues, but with considerable species and tissue differences in the pattern of expression. GLUTs 1 and 4 are expressed in follicles from sheep [51] and GLUTs 1, 3 and 4 mRNAs were all expressed in bovine corpora lutea and follicles [52]. In the rat, GLUTs1, 3 and 4 and their mRNAs are expressed in ovaries [53]. In a recent study [54], mRNAs for GLUTs 1, 3, 5, 8 and 13 were constitutively expressed, whereas GLUTs, 4, 6, 9, 10, 11 and 12 were differentially expressed, but GLUTs 2 and 7 were not expressed in human granulosa cells from patients with PCOS. The authors of some of these reports have also demonstrated that the expression of these GLUTs can be regulated by intra-ovarian factors involved in follicular development, maturation and ovulation, such as E2, IGF-I and interleukin-1β [55] as well as the classic regulators of ovarian function, gonadotropins [53].
In the ovary of the rat the mRNA for GLUT3 was increased following an ovulatory stimulus using human chorionic gonadotropin (hCG) [56] and in bovine follicles, highly significant negative correlations were observed between the concentrations of glucose in follicular fluid and the levels of GLUTs1 and 3 mRNAs in granulosa cells [52]. These data imply that the hormonal environment of the follicle can affect glucose uptake and that the ovary has reproductive mechanisms to regulate glucose uptake by ovarian cells.
Role of glucose in the ovary
There is considerable published data reporting that glucose is present in follicular fluid from humans [57], macaques [58], buffalos [59], sheep [60], cattle [61], pigs [62], camels [63] and horses [64], and follicular fluid is the presumed source of glucose for the avascular membrana granulosa. Most authors report that the concentration of glucose in follicular fluid is lower than the concomitant concentration in serum or plasma and furthermore most report significant differences in the concentrations of glucose in follicular fluid of different follicles from the same animal, implying some local (hormonal) regulation of the movement of glucose into or its utilization by follicles. The concentration of glucose in follicular fluid was higher in larger follicles [60,65,66] and highest in pre-ovulatory follicles. Although intra-follicular glucose was increased following an ovulatory stimulus in the macaque, the mural granulosa cells were not able to utilize it, sparing the glucose for the cumulus–oocyte complex [58]. Thus, the intra-follicular concentration of glucose appears to be modulated to some extent by the hormonal environment of the follicle [60,65,66], and it is correlated positively with in vitro fertilization (IVF) outcome [67]. However, the existing data do not reveal a completely clear picture, with numerous contradictory reports describing the relationships between intra-follicular glucose, follicle size and follicle status. Further research is required to establish how the intra-follicular concentration of glucose is regulated during folliculogenesis and its significance to fertility, especially in relation to the hyperinsulinaemia and hyperglycaemia associated with metabolic disorders [68].
Glucose is an important energy substrate for the generation of ATP for the metabolic and physiological functions of the ovary, one of the most dynamic of the endocrine organs. It simultaneously nurtures the cyclic development of several follicles, the maturation and ovulation of the selected follicle(s) and subsequent formation and maintenance of a functional corpus luteum. There is a significant positive difference in the arterio-venous concentration of glucose in the blood flowing to and from the ovary [69], indicating the uptake of glucose by the ovary. In the bovine ovary, the predominant energy substrate appears to be glucose, and the whole ovarian respiratory exchange ratio was calculated as 0.95 [69]. Glucose can be metabolized along multiple pathways, including glycolysis, the pathway of glycogen synthesis, the hexosamine pathway and the pentose phosphate pathway (PPP). Regardless of the pathway, the first step is the metabolism of glucose to glucose 6-phosphate, a step mediated by hexokinase and glucose-6-phosphatase. Glucose 6-phosphate can enter glycolysis through the action of phosphohexose isomerase or PPP via glucose-6-phosphate dehydrogenase. The outcomes of these two pathways are ATP and NADPH and ribose sugars respectively.
Early studies using the rat indicated that LH increased glucose uptake and lactate output, along with increased hexokinase activity [70,71], although relatively little subsequent work has been done on the metabolic fate of ovarian glucose. The exception to this is the cumulus–oocyte complex, where glucose metabolism by the cumulus cells has been shown to provide essential metabolites to the oocyte [72–74]. In sheep granulosa cells, glucose, metabolized under anaerobic conditions to lactate, is the preferred energy substrate to support the gonadotropin-induced differentiation of granulosa cells [75] and theca cells [76] cultured in vitro, and that fructose and pyruvate, but not galactose, are alternative energy substrates despite marked differences in the way that these substrates are metabolized.
The existence of well-characterized energy-sensing mechanisms in cells raises the possibility that glucose, in addition to being a source of energy, may also function as a signalling molecule. The two glucose- or energy- sensing mechanisms that could potentially act as signalling mechanisms in follicular cells are via AMPK (AMP-dependent protein kinase) and the hexosamine pathway. These have been discussed in previous reviews [77,78]. Briefly, the serine/threonine kinase AMPK is an energy sensor that is activated by nutritional stress. It is present in the ovary and its activation leads to alterations in ovarian function. There is inhibition of P4 and E2 production [79] and of FSH-stimulated cell proliferation [80]. Glucose can enter the hexosamine nutrient-sensing pathway which allows glucose to directly mediate gene expression [81,82]. The end-product of this pathway is UDP-N-acetylglucosamine which, in addition to being an important substrate for glycosylation, can also increase the expression of the genes for some growth factors such as fibroblast growth factor 2 (FGF2), transforming growth factor β (TGFβ) [82] and TGFα [83] that have reproductive effects, in particular TGFα suppressed E2 secretion from the ovary in a sheep ovarian auto-transplant model [84]. Glucose inhibited KATP channels in hypothalamic neurons, thus increasing cell excitability [85]. Glucose starvation stimulated AMPK in many cell types, including hypothalamic neurons [86]. In ovarian cells, on one hand, glucose deprivation activates AMPK and induces cell death though modulation of Akt [87], and, on the other hand, the infusion of glucose into sheep reduced the phosphorylation of AMPK in granulosa cell lysates [22,23].
ACTIONS OF INSULIN IN GRANULOSA, THECAL CELLS, LUTEAL CELLS AND OOCYTES
Critical cross-talk nodes
Earlier, it had been suggested that insulin had gonadotrophic actions in the ovary [88]. It now appears much more likely that the gonadotropin-like actions of insulin are mediated via interactions between their respective signalling pathways at critical nodes such as Akt or MAPK [28]. In the follicle, there are several lines of evidence strongly suggesting that insulin–FSH, insulin–LH and FSH–LH cross-talk takes place and that it has biochemical and physiological consequences in the follicle.
Insulin receptors in ovarian cells
The IR is found in granulosa and thecal cells of different species [89,90] and insulin receptor transcripts have been described in human, bovine and rat oocytes [91,92]. The IR has two isoforms, IRA and IRB, which differ by the presence of 12 amino acids at the C-terminus of the α subunit [93]. Both isoforms can hybridize with each other as well as with the closely related IGF-IR (70% homology). The expression of IRA mRNA greater than that of IRB in normal mural granulosa and cumulus cells [94]. In the oocyte, IR is not required for oocyte growth, differentiation and maturation. Indeed, when the IR is ablated in oocytes, the female mice are fertile and exhibit normal oestrous cyclicity, oocyte development and maturation, parturition frequency and litter size [95]. Although GLUTs have been reported in the oocyte [56], it seems that they are non-functional. The oocyte obtains glucose via gap junctions from cumulus cells which take up glucose via GLUT-mediated mechanisms [96].
The in vivo effects of insulin on the ovary
The published in vitro data suggest that insulin affects follicular development in vivo. However, although most of the published in vivo data agree with this broad generalization there are numerous contradictions between data derived from in vitro and in vivo experimental approaches [97]. Data from the two models are not directly comparable, but there should be broad agreement. The main difference between these two experimental methodologies is that in vitro insulin appears to stimulate E2 production by granulosa cells, whereas, in vivo, insulin appears to inhibit the ovarian secretion of E2. Using immature rat ovaries in a perfusion system, Peluso et al. [98] demonstrated that insulin was able to stimulate mitogenic activity and simultaneously to suppress oestradiol secretion. Although the infusion of glucose or the feeding on glucogenic diets, all treatments which increased plasma glucose concentrations, increased the number of follicles and stimulated insulin secretion [20], it reduced the concentration of E2 and the follicular levels of CYP19a1 (cytochrome P450 19a1) [20,22,23]. Using an ovarian auto-transplant sheep model [99] in which the ovary was perfused in vivo with insulin without increasing the peripheral concentration of glucose, thus separating out the actions of insulin and glucose, insulin did not affect follicular steroidogene-sis, and, when glucose was included in the perfusate, follicular steroidogenesis was inhibited [99]. Thus the in vivo effect of insulin on steroidogenesis appears to require the uptake of glucose and is associated with decreased phosphorylation of Akt, ERK and AMPK [22]. Finally, in the post-partum dairy cow, glucogenic diets that stimulated the secretion of insulin enhanced follicular development and advanced the resumption of ovarian cyclicity but, they had detrimental effects on embryo survival [100–102].
The in vitro action of insulin in ovarian cells
In most in vitro systems, insulin stimulates both cell proliferation and steroidogenesis in cultured granulosa and theca calls. Insulin stimulated basal and FSH or LH-induced secretion of P4 and E2 and it was also able to stimulate basal and FSH-induced aromatase activity [103,104]. However, in bovine granulosa cells high doses of insulin did not stimulate aromatase activity [105], thus suggesting that a high concentration of insulin favours the secretion of P4 and androgen secretion over the secretion of E2. Insulin can also directly stimulate granulosa cell proliferation [106]. Finally, insulin increased glucose transport [107], GS activity [108], the intracellular accumulation of free fatty acids [109] and the expression of LH-induced low-density lipoprotein receptors [110]. Insulin-stimulated androgen secretion by human and animal theca cells [111–113] that provides substrates for the synthesis of E2 in granulosa cells [114,115]. In rat thecal cells, insulin-stimulated cell proliferation and the expression of the cell cycle regulatory proteins CDK4 (cyclin-dependent kinase 4), cyclin D3 and PCNA (proliferating-cell nuclear antigen) were all blocked by rapamycin implicating the mTORC1 signalling pathway in these actions of insulin [116]. Landau et al. [100] suggested that insulin may affect oocyte maturation in vivo because there were increased levels of insulin in pre-ovulatory follicles compared with subordinate follicles. Insulin in supra-physiological concentrations had a stimulatory effect on the cleavage and maturation of oocytes in vitro [101], but prolonged hyperinsulinemia in vivo had a negative impact on the developmental competence of oocytes [117,118]. In addition, it was reported that hyperinsulinemia was associated with impaired oocyte quality in cattle [119].
INSULIN SIGNALLING PATHWAYS IN THE OVARY
The various insulin signalling pathways (Figure 1) in ovarian cells can function independently of FSH and LH to, for example, promote glucose transport, cell proliferation, cell differentiation and fatty acid synthesis and to inhibit apoptosis in gonadotropin-independent follicles. In gonadotropin-responsive and gonadotropin-dependent follicles, insulin can also interact with the gonadotropins to modulate hormone secretion and perhaps other differentiated functions. Studies of insulin signalling pathways in ovarian cells have been largely confined to granulosa cells and oocytes, with limited data available for the other cell types in the ovary.
The PI3/Akt pathway
Among the various signalling pathways involved in the regulation of folliculogenesis and oogenesis [120,121], the PI3K/Akt signalling pathway with its many branches appears to be the main non-gonadotropic, regulator of the differentiation, growth and survival of follicles. The observations showing that the deletion of various genes in this pathway can cause premature ovarian failure (POF) and infertility (Tables 1 and 2) points to an essential role in ovarian function.
Table 1. The physiological and biochemical consequences of targeted disruption or overexpression of components of insulin signalling pathways in ovarian cells.
| Component modified | Targeted ovarian cell | Genetic transformation | Physiological and biochemical consequences | Reference |
|---|---|---|---|---|
| IR or IGF-IR | Developing oocytes | Knockout | Fertile and with normal oestrous cycles, oocyte development and maturation, frequency of littering and litter size | [71] |
| IR | Theca-interstitial cells | Knockout | No changes in reproductive development or function in lean mice but when fed on a high-fat diet causing obesity, wild-type (WT) mice were infertile and had increased circulating testosterone levels, whereas knockout mice were fertile and had testosterone concentrations comparable to those found in lean mice | [203] |
| PTEN | Granulosa | Knockout | Did not affect initiation of follicle growth but increased granulosa cell proliferation, extended the lifespan of luteal cells and enhanced ovulation | [148] |
| PTEN | Oocyte (of primordial follicles) | Knockout | Pan-ovarian follicle activation and premature oocyte depletion, primary ovarian insufficiency | [123] |
| PTEN | Oocyte (of primary and developed follicles) | Knockout | Unaltered follicular development, ovulation, oocyte maturation and fertility | [111] |
| PI3K | Immature oocyte | Constitutively active overexpression | Anovulation, enlarged ovaries, increased survival of immature follicles, formation of follicles containing multiple oocytes | [204] |
| TSC1 | Oocyte | Knockout | Primary ovarian insufficiency | [205] |
| TSC1 | Granulosa | Knockout | Infertile with pleiotropic effects on follicle recruitment | [141] |
| TSC2 | Oocyte | Knockout | Over-activation of the entire pool of primordial follicles, primary ovarian insufficiency | [206] |
| PDK1 | Oocyte (of primordial follicles) | Knockout | Gradual loss of ovarian follicles of all classes, but this phenotype can be reversed by simultaneous null mutation of PTEN | [122] |
| rpS6 (the only known ribosomal substrate protein of S6K1) | Oocyte (of primordial follicles) | Knockout | Primary ovarian insufficiency | [122] |
| Raptor (regulatory-associated protein of mTORC1) | Oocyte (of primordial follicles) | Knockout | Follicular development and fertility not affected, loss of mTORC1 signalling in oocytes triggered compensatory activation of the PI3K signalling, thus maintaining normal follicular development and fertility | [207] |
| Rictor (rapamycin-insensitive companion of mTOR, a key component of mTORC2) | Oocyte (of primordial follicles) | Knockout | POF phenotype, massive follicular death, excessive loss of functional ovarian follicles, abnormal gonadal hormone secretion | [208] |
| MAPK, also known as ERK | Granulosa and cumulus cells | Knockout | Failed to ovulate and are completely infertile. Necessary for LH-induced oocyte resumption of meiosis, ovulation and luteinization | [209] |
Table 2. The physiological and biochemical consequences of targeted disruption or overexpression of components of insulin signalling pathways in other (non-ovarian) cells.
Note: knockout of insulin is lethal 2 days after birth [202] and knockout of the IR is lethal 1 week after birth [199].
| Component | Tissue/cell type | Effect on fertility | Ovarian consequences | Reference |
|---|---|---|---|---|
| IR | Brain neurons | Impaired fertility (50%) | Fewer antral follicles and corpora lutea due to reduced release of LH | [210] |
| IRS-1 | All | 70% pregnancy rate | Mildly defective reproductive function | [211] |
| IRS-2 | All | Infertile | Reduced LH levels and gonadotropin number, show reduced gonadotropin-stimulated ovulation and markedly reduced numbers of ovarian follicles and corpora lutea | [212] |
| IRS-2 | All | Infertile | Reduced follicle size, increased numbers of atretic follicles, as well as impaired oocyte growth and antral cavity development, granulosa cell proliferation defective | [213] |
| IRS-2 | CNS and hypothalamus | Fertile | Minimal defects in pituitary and ovarian hormone concentrations, ovarian anatomy and function | [213] |
| IRS-3 | All | Fertile | No effects on fertility or embryonic viability | [214] |
| IRS-4 | All | 50% pregnancy rate | Null females are less nurturing of their pups | [215] |
| FOXO3a | All | A marked age-dependent decline in reproductive fitness and were sterile by 15 weeks of age | Global follicular activation, oocyte death, early depletion of functional follicles and secondary infertility | [216] |
| SHB (3Src homology 2 domain-containing adapter protein B) | All | Fertile. Shb−/− embryos cannot be created on a C57Bl/6 background but only on a mixed genetic background. Heterozygotes (Shb+/−) are viable | Defective meiosis I and early embryo development | [217] |
| p110δ (pik3cd) | All | Sub-fertile | Defects in ovarian follicle maturation | [218] |
| Akt1 | All | Reduced fertility | Abnormal oestrous cycles, POF, no compensation was observed by Akt2 or Akt3 | [219] |
| Akt2 | All | Reduced fertility in aged mice | Aged mice have testosterone concentrations and ovarian cysts with increased numbers of thecal-interstitial cells | [220] |
| Akt2 | All | Normal fertility for young mice | ||
| GSK3 isoforms | Fertile | GSK3α or GSK3β did not induce a lethal phenotype. Individual loss of oocyte but no effect on fertility | [221] | |
| IGFBP1 | Overexpression in liver | Reduced fertility | Impaired fertilization or implantation, interrupted or prolonged pregnancies with fetal and neonatal death | [222] |
The kinase PDK1 is expressed in the oocyte, and appears to be indispensable for the survival of primordial follicles because mice deficient in PDK1 are infertile with accelerated ovarian aging and POF [122]. At a molecular level, the absence of this kinase from oocytes leads to the suppression of Akt/S6K1/rpS6 (ribosomal protein S6) signalling (Table 1). The low activity of rpS6 leads to accelerated loss of primordial follicles; in contrast, its over-activity enhances follicular activation and survival [122,123]. In the mouse ovary, PTEN is specifically expressed by oocytes and contributes to the maintenance of the pool of quiescent primordial follicles [122]. Its deletion causes Akt hyper-activation, thereby inducing the rapid depletion of the primordial follicle pool and POF [123]. It is noteworthy that, whereas the loss of PTEN in oocytes leads to POF due to excessive follicular activation and degeneration, PDK1 deficiency causes POF as a result of abnormal recruitment of primordial follicles [122,123]. Both mutations have been implicated in the onset of POF in humans [122,124].
The role of Akt in the mammalian ovary has been mainly assessed by analysing null mice (Tables 1 and 2). Female mice deficient in Akt1 have reduced fertility, the onset of oestrus cyclicity is delayed by approximately 5 days, increased maternal age at first litter and a reduction in average litter size and a delay in the onset of puberty. The oocytes of primary follicles of Akt1-deficient females are larger than wild-type animals, and sometimes follicles contain multiple oocytes. The consequences of the deletion of Akt2 on fertility are still unclear, but Akt3-deficient females are reported to have normal fertility. In the porcine ovary, Akt1 has been localized in granulosa cells of primordial follicles and in the basal layers of the granulosa cells of pre-antral and antral follicles, but not in atretic follicles or corpora lutea. In the human ovary, Akt1 is expressed in oocytes, in granulosa cells and in the thecal cells of primordial follic-les, in follicles at each growing stage and in the luteal cells [125]. In rodents, Akt1 has been found in both granulosa cells and oocytes [122]. The presence of Akt has been reported in both sheep and cattle [126], and in sheep, the infusion of glucose reduced the level of phosphorylated Akt in sheep granulosa cells [22,23].
The ERK pathway
The MAPK/ERK1/2-dependent pathway appears to be involved in several key processes induced by the LH surge, in pre-ovulatory follicles. These processes include the resumption of meiosis in the oocyte [127–129], the expansion of the cumulus [127], gene expression in the follicle [30,34,35] and the disruption of gap junctions within the follicle [129]. The MAPK/ERK1/2-dependent pathway also has been reported as being involved in the regulation of steroidogenesis in granulosa cells; however, its precise role in this process is controversial. Some studies suggest that activation of MAPK is required for promoting steroidogenesis and steroidogenic gene expression in granulosa cells [130,131], whereas other studies indicate that activation of MAPK3/1 suppresses these [132,133]. Most of these studies use either luteinizing granulosa cells derived from patients undergoing IVF or rat granulosa cells isolated from early antral follicles. Therefore, species-specific variations, as well as other factors, such as the stage of development of the cultured granulosa cells, may account for these discrepancies.
GONADOTROPIN SIGNALLING PATHWAYS IN THE OVARY
Both FSH and LH signalling pathways in ovarian cells have been extensively studied [134–137]. The first reported, and probably the primary pathway, for both gonadotropins is the cAMP/PKA (protein kinase A) pathway. However, subsequently, several other signalling pathways that respond to gonadotropin stimulation have been reported in granulosa, thecal and luteal cells. These include the PI3K/PDK1/Akt pathway, the MAPK pathways and the PKC pathway.
Follicle-stimulating hormone in granulosa cells
Granulosa cells contain FSH receptors and, on ligand binding, adenylate cyclase produces cAMP which then phosphorylates PKA which then phosphorylates multiple downstream substrates along diverse pathways leading to gene transcription. Over 100 FSH-inducible genes have been identified in granulosa cells [134]. Additional FSH signalling pathways that have been identified in granulosa cells include the cAMP-independent pathways PI3K/Akt and PKC that result in cAMP-independent responses to FSH in granulosa cells. The MAPK pathways ERK, JNK (c-Jun N-terminal kinase) and p38MAPK can also be activated in granulosa cells by FSH via cAMP/PKA or independently. This array of FSH signalling networks has considerable overlap with the array of insulin signalling networks providing opportunities for cross-talk between insulin and FSH signalling pathways.
LH in granulosa and thecal cells
Thecal and luteal cells contain LH receptors and, similarly to FSH, ligand binding leads to the production of cAMP and phosphorylation of PKA. The signalling pathways for LH are very similar to those for FSH. Indeed, in the selected ovulatory follicle(s), LH can substitute for FSH to protect the selected follicle during the follicular phase from the damaging influence of the E2-driven decline in the circulating concentrations of FSH [77]. This action of LH providing an alternative to FSH to protect the follicle from atresia is probably mediated by the LH receptor. The principal pathway is the cAMP/PKA/CREB (cAMP-response-element-binding protein) pathway and other known pathways include the PI3K and ERK pathways.
INSULIN OOGENESIS AND FOLLICULOGENESIS
Primordial follicles
The formation of primordial follicles occurs during the latter half of pregnancy in humans and ruminants, but immediately after birth in rodents. The postnatal mouse ovary is mainly populated by primordial follicle each composed of a meiotically arrested primary oocyte surrounded by flattened pre-granulosa cells. Several lines of evidence suggest a close relationship between KIT ligand (KL) and the PI3K/Akt pathway, and that both are necessary for oogenesis and the early stages of folliculogenesis [138]. Because of its chemoattractant action, KL is fundamental in the control of the migration of primordial germ cells (PGCs) into the genital ridges. In purified mouse PGCs isolated 11.5–12.5 days post-coitum, KL stimulates the phosphorylation of Akt by either PI3K or Src kinases. The crucial role of Akt in promoting the survival and proliferation of PGCs has been confirmed by Kimura et al. [139] who found that apoptosis of PGCs was decreased and proliferation increased with the induction of Akt.
The primordial to primary follicle transition is also controlled by KL signalling through induction of the PI3K/Akt pathway. The interaction between KL, which is expressed primarily by granulosa cells and KIT receptor, which is present in oocytes [140], stimulates the recruitment of PI3K from the cytoplasm to the cell membrane through the binding of the SH2 domain of the p85 subunit of PI3K to the phosphorylated tyrosine on KIT [138]. This binding promotes the activation of Akt and the subsequent phosphorylation of downstream target proteins. Activated Akt controls the phosphorylation of TSC2 that, in association with hamartin (TSC1), controls mTOR. In oocytes and granulosa cells, TSC1 is active and helps to regulate the pool of primordial follicles [141]. However, despite this evidence suggesting the importance of Akt for the survival and proliferation of PGCs, the effects of hyperinsulinemia on the formation of PGCs is not known.
Oocytes
The transformation of diploid oogonia into haploid oocytes involves many of the downstream targets of insulin signalling and thus is potentially influenced by insulin. Phosphorylated Akt controls the activity of the serine/threonine kinase, GSK3 and some of the FOXO family of transcription factors (FOXO1, FOXO3a and FOXO4). In mouse oocytes, granulosa cell-derived KL regulates the PI3K/Akt pathway, because the specific PI3Kinhibitor LY294002 inhibits this process. The Akt1-mediated phosphorylation of GSK3α at Ser21 or GSK3β at Ser9 results in the inactivation of GSK3. In the bovine ovary, GSK3β is localized to cytoplasm of granulosa cells and oocytes throughout folliculogenesis. In MII (metaphase-II) oocytes, the kinase is present in a region between the oocyte and the first polar body. The use of specific inhibitors has suggested a role for GSK3β in the regulation of the MI to MII transition, probably participating in the activation of Aurora A kinase [142]. Also mouse oocytes contain GSK3 mRNA and protein, and its pharmacological inhibition causes an abnormal configuration and function of the meiotic spindle, chromatin organization and bivalent chromatin segregation. On the other hand, phosphorylated PKCζ inactivates GSK3β by phosphorylation on Ser9 to help maintain spindle stability during meiotic metaphase arrest [143].
The transcription factors FOXO1, FOXO3a and FOXO4 are also involved in the control of folliculogenesis [144]. One of them, FOXO3a, has been detected in the nucleus of oocytes in primordial and early primary follicles, and its expression is dramatically down-regulated in oocytes of primary and growing follicles [120,145]. Although FOXO1 mRNA is expressed in mouse oocytes [120] and can regulate different functions, it is not expressed in sufficient quantities to substitute for FOXO3a. When oocytes are treated with KL, FOXO3 is phosphorylated and inactivated. In the oocyte, FOXO3a inhibits the production of bone morphogenetic protein (BMP-15) and possibly the activation of SMAD (homolog of Drosophila and C. elgans proteins) pathways that control the proliferation and differentiation of surrounding granulosa cells. It has been suggested that the only role of oocyte PI3K is to regulate FOXO3a activity [146].
Gonadotropin-independent follicles
In the mammalian ovary, FOXO3a controls follicular activation and early follicle development. In fact, it suppresses the initiation of follicular growth and controls the rate of activation of primordial follicles. Knockout experiments in mice (Tables 1 and 2) have demonstrated the importance of FOXO3a but not GSK3 for the growth and development of gonadotropin-independent follicles. The functional consequences of FOXO3a inactivation is a rapid decline in fertility, an increased number of atretic follicle and a depletion of the primordial follicle pool. In these knockout mice, POF and infertility are established by 15 weeks of age [146]. The absence of oocyte-specific FOXO3 induces global activation of primordial follicles, as does deletion of PTEN [123]. However, in the absence of PTEN, PI3K is capable of directly stimulating Akt-dependent phosphorylation and nuclear export of FOXO3a, thereby triggering uncontrolled primordial follicle activation and POF. In contrast, overexpression of FOXO3a leads to female infertility due to impaired follicle development. In fact, in neonatal rat ovaries, the overexpression of FOXO3a and its downstream factors, Bim (B-cell lymphoma-2), FasL (Fas ligand) and the cell cycle inhibitor p27 (CDK inhibitor p27) stimulated apoptosis in naked oocytes. In addition, constitutively active FOXO3a dramatically affects BMP-15, connexin37 and connexin43, thereby inhibiting the growth of oocytes and follicles. Other specific target genes regulated by FOXO3 are p27 and the enzyme galactose-1-phosphate uridyltransferase (GALT), both involved in the control of follicular activation. In particular, FOXO3a enhances p27 expression and prevents its transfer from nucleus to ooplasm, thereby maintaining the growth-inhibitory function of this protein [147].
FSH-responsive and FSH-dependent follicles
Granulosa cells
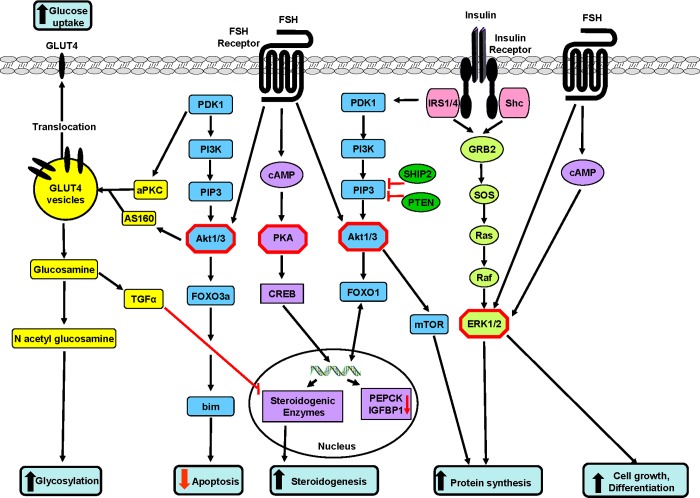
In granulosa cells, FSH regulates ovarian atresia via the PI3K/Akt/FOXO3a pathway (Figure 2). Indeed, in the porcine ovary, FSH inactivated FOXO3a and prevented its binding to the pro-apoptotic Bim promoter [148]. Thus, in granulosa cells, FSH-dependent activation of Akt regulates apoptosis [148] and cell differentiation [149]. In contrast, in cumulus cells, FSH-stimulated phosphorylation of Akt and MAPK participate in the maintenance of cell survival (Figure 2) and the synthesis and retention of hyaluronic acid [150].
Figure 2. Some possible sites of cross-talk (critical nodes) between the insulin and FSH signalling pathways in granulosa cells.
Insulin signalling through Akt1 and FOXO1 can enhance FSH-stimulated steroidogenesis and, similarly, FSH acting through Akt1 can enhance insulin-stimulated glucose transport leading to increased flux through the hexosamine pathway and to the inhibition of aromatase. Thus insulin can interact with FSH to stimulate P4 and inhibit E2 production. Likewise, FSH acting via mTOR and/or ERK can enhance cell proliferation and protein synthesis, cell proliferation and, acting via Akt1 and FOXO3a, it can inhibit apoptosis. The FSH-stimulated FOXO1 pathway appears to have stage -specific effects in follicles; in small follicles granulosa cell proliferation is stimulated, in antral follicles it is steroidogenesis that is stimulated and, at ovulation, atresia is stimulated in non-ovulatory follicles. Stimulatory connections are shown in black and inhibitory ones are shown in red.
The expression and activity of FOXO1 are modulated by FSH and LH through the PI3K/Akt pathway [151] and FOXO1 expression is elevated in granulosa cells of growing follicles. Expression of a constitutively active nuclear form of FOXO1 in granulosa cells reduces cyclin D2, aromatase P450 (CYP19a1), FSH receptor, LH receptor and enzymes of the cholesterol biosynthetic pathway [147]. Following ovulation and luteinization, FOXO1 expression is reduced, and the most intensive FOXO1 staining is seen in the nucleus of granulosa cells of atretic follicles. This finding suggests a causal relationship between follicular atresia and increased nuclear FOXO1. In addition, FOXO1 is negatively regulated by Wnt signalling [152]. Unlike other FOXO members, FOXO4-null mice are viable and have no developmental defects or histological abnormalities suggesting functional redundancy in this subfamily. The PI3K/Akt pathway in the mammalian ovary is subject to regulation by the TGFβ superfamily which includes factors derived from granulosa (activin, BMP-2, -5 and -6) and theca cells (BMP-2, -4 and -7) that are involved in the regulation of granulosa cell proliferation and follicle survival and apoptosis [120]. Recent data support a link between BMP signalling, PI3K/Akt and the small GTPase (Ras)/ERK pathways in the regulation of cell survival. In particular, it has been found that BMP-4 suppresses the apoptosis of granulosa cells by maintaining CAD protein (a ‘fusion’ protein encoding four enzymes of the pyrimidine pathway [152]) in the cytosol instead of the nuclei, where the protein triggers DNA digestion.
Theca cells
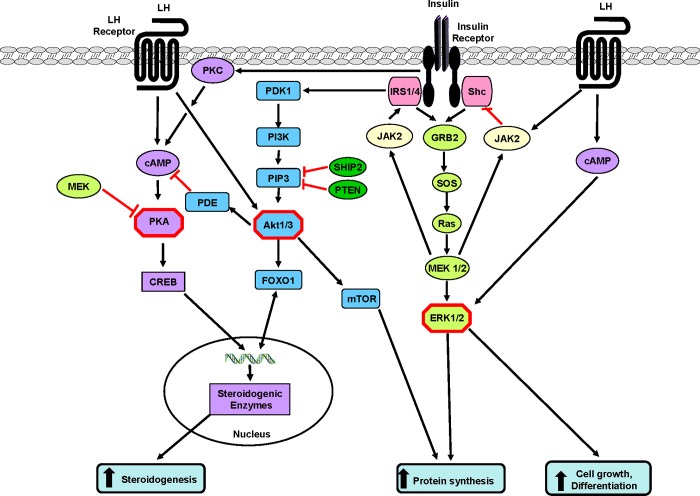
Because LH and insulin act physiologically via distinct intracellular signalling mechanisms, their synergistic enhancement of theca-cell steroidogenesis likely entails important interactions between these two respective pathways (Figure 3). Indeed, it has been shown that insulin significantly increased LH-stimulated cAMP accumulation in cultured porcine theca cells [153]. The insulin-stimulated increase in cAMP is probably induced through PI3K because the activation of adenylate cyclase by insulin was blocked with the use of non-specific inhibitors of PI3K in rat muscle. In addition, LH induced rapid activation of JAK2 in the rat ovary that then activated, through IRS-1, both the PI3K and the MAPK pathways [154]. Simultaneous stimulation with LH and insulin induced higher phosphorylation levels of these proteins compared with each hormone alone. Therefore, important cross-talk exists between the insulin and LH signalling pathways, such that increased activity of one pathway might also increase the responsiveness of the other pathway (Figure 3).
Figure 3. Some possible sites of cross-talk (critical nodes) between the insulin and LH signalling pathways in theca cells.
Insulin signalling through Akt1 can stimulate PDE to inhibit the degradation of cAMP and so enhance LH-stimulated androgen production; similarly, insulin acting via Akt1 and FOXO1 can enhance LH-stimulated steroidogenesis. Acting via mTOR and/or ERK, LH can enhance cell proliferation and protein synthesis and it can inhibit apoptosis. LH acting directly or via cAMP can enhance insulin stimulation of ERK, whereas, at the same time, LH can stimulate JAK2 which inhibits Shc activation of ERK. The balance of these opposing actions can modulate insulin-stimulated cell proliferation and protein synthesis. Stimulatory connections are shown in black and inhibitory ones are shown in red.
A study on normal human theca cells demonstrated that specific blockade of PI3K, but not of MEK, inhibited the stimulating effect of insulin and LH on ovarian cytochrome P450, 17α-hydroxylase/17,20 lyase (CYP C17-lyase) [155]. Another study confirmed these results in theca cells from normal and PCOS women and also constitutively reduced levels of phosphorylated MEK and ERK in PCOS cells. These constitutive defects were correlated with increased androgen production, irrespective of the presence of insulin [156]. Infection with dominant-negative MEK1 increased CYP C17-lyase mRNA, whereas constitutively active MEK1 reduced CYP C17-lyase mRNA. This suggests that alteration of the MAPK pathway can induce androgen hyper-responsiveness to insulin in PCOS ovaries.
Oocyte maturation
In rodents and humans, elevated levels of cAMP are necessary to maintain oocytes in meiotic arrest, and phosphorylated Akt and the mRNA for Akt have been detected in GV (germinal vesicle) stage oocytes [157]. The processes of ovulation, luteinization and oocyte maturation are triggered by the LH surge when this gonadotropin induces some elaborate signalling cascades that activate specific intracellular substrates. The LH surge stimulates PI3K/Akt [158–160]. High activity of Akt is necessary to phosphorylate PDE3A (cAMP-phosphodiesterase 3A) that specifically degrades cAMP [161] during the early stages of oocyte maturation to activate either PDE3A [158] or the downstream mTOR pathway, which phosphorylates factors promoting protein synthesis [162]. Suppression of Akt decreased CDK1 and delayed the resumption of meiosis in mouse oocytes [162].
Following germinal vesicle breakdown, phosphorylated kinases are associated with microtubules of the first meiotic spindle [157,162]. In vitro experiments clearly indicated that inhibition of Akt inhibits MI itself, and, at MII, Akt controls the extrusion of polar body 2 and normal chromosomal alignment on microtubules [159]. This role was confirmed by treatment with LY294002 that decreased phosphorylated Akt-Thr308, altered localization of phosphorylated Akt-Ser473 during oocyte maturation and impaired extrusion of the polar body [159]. In cattle oocytes, Akt was involved in the regulation of meiotic MI–MII transition [160] and furthermore spindle migration and the asymmetric division during meiotic maturation are controlled also by mTOR [163]. Comparison of the levels of mRNA for Akt1 during in vivo maturation showed a significant decrease in Akt1 mRNA from GV to MII and the level of expression can be differentially modulated by FSH [157]. At MII, phosphorylated Akt is still expressed and its role may be to support oocyte survival, at least during the fertilization window. The findings that Akt 1, 2 and 3 mRNA levels are very low in oocytes retrieved from ampullae 29 h post hCG and that protein content was dramatically decreased by 33 h post hCG confirm this hypothesis [164]. These data confirm the importance of PI3K/Akt pathway in oocyte maturation and indicate key roles for FSH and IGF-I, but, apart from being theoretically possible, tell us little of the involvement of insulin in oocyte maturation.
THE EFFECTS OF INSULIN-SENSITIZING DRUGS ON OVARIAN FUNCTION
At an ovarian level, it is thought that hyperinsulinemia has direct and indirect actions on folliculogenesis and intra-ovarian gonadotropin-driven granulosa and thecal cell steroidogenesis [165]. The biguanide metformin and the thiazolidinediones troglitazone (no longer available), pioglitazone and rosiglitazone are all antidiabetic drugs that act by improving the sensitivity of peripheral tissues to insulin, which results in decreased circulating insulin levels [166].
Metformin
Metformin is an oral anti-hyperglycaemic drug that acts as an insulin-sensitizing agent that reduces hyperinsulinaemia and suppresses the excessive ovarian production of androgens in women with PCOS. It improves systemic hyperglycaemia by reducing hepatic glucose production and enhancing peripheral insulin sensitivity [167]. It stimulates the oxidation of fat and reduces the synthesis and storage of fat. The molecular mechanism of this drug is thought to be secondary to its actions on the mitochondrial respiratory chain. Metformin has been identified as a substrate of organic cation transporters (OCTs). Metformin uptake in the liver is mediated by OCT1 [168,169]. Consistent with these data, the knockout of OCT1 reduces metformin uptake in hepatocytes, and humans carrying reduced function polymorphisms of the OCT1 gene display an impaired effect of metformin in lowering blood glucose levels [170]. In the human ovary, a strong expression of OCT1 has been detected suggesting a direct effect of metformin on ovarian cells [171]. One mechanism for these pleiotropic actions is the activation of AMPK, mediated by the proximal kinase, serine/threonine protein kinase 11 (previously termed LKB1) [172]. Although the efficacy of metformin for improving systemic metabolism in PCOS patients has been confirmed, its effect on reproduction remains controversial. Some investigators showed that metformin can improve conception rates following ovulation induction prior to IVF [173,174]. In contrast, other studies demonstrated that metformin has no clinical benefit in terms of ovulation rate or pregnancy outcome [175,176]. In women with PCOS, metformin treatment before or during assisted reproductive technology increased pregnancy rates and decreased the risk of ovarian hyperstimulation syndrome. However, there is no conclusive evidence of a benefit in live birth rates [177].
Although the positive effects of metformin on reproductive parameters such as decreasing hyperandrogenism and inducing ovulation [178] are attributed to its insulin-sensitizing actions, there is accumulating evidence of a direct effect of metformin on ovarian steroidogenesis [179,180]; this is summarized in Table 3. In human granulosa cells, metformin interacted with the IR to activate IRS-1 and -2, leading to an increased insulin-stimulated translocation of GLUT4 to the plasma membrane via PI3K activation of Akt. Metformin directly increased the mRNA expression for IR and IGF-IR [181], whereas it reduced that of aromatase through the MAPK ERK1/2 pathway [182]. These findings may have implications for the treatment of PCOS with metformin [183]. In primary human granulosa, metformin increased visfatin mRNA in a dose-dependent manner after treatment for 24 h, whereas it was reduced after 48 h of incubation [184]. Thus, metformin could increase insulin sensitivity through visfatin, an adipokine that has an important role in regulating insulin sensitivity. During oocyte maturation, the oocyte obtains most of its energy from cumulus cells, which metabolize glucose to produce pyruvate and lactate the preferred substrates of the oocyte [185] and metformin stimulated the production of lactate by human granulosa cells [186]. Lipids are another energy source for oocyte maturation, and metformin increased β-oxidation of fatty acids, which is essential for developmental competence of mouse oocytes [187,188].
Table 3. Effects of insulin sensitizers (metformin, rosiglitazone and pioglitazone) in ovarian cells of different species.
| Insulin sensitizer | Ovarian cells | Species or cell line | Biological effects | Reference |
|---|---|---|---|---|
| Metformin | Granulosa | Human | Inhibition of basal and insulin-stimulated E2 and aromatase via an ERK-mediated pathway | [182] |
| Metformin | Granulosa | Human | Enhancement of insulin-stimulated lactate production at sub-optimal concentrations of insulin | [186] |
| Metformin | Granulosa | HGL5 (human) | Improvement of basal cell viability and Akt phosphorylation | [223] |
| Metformin | Granulosa | Rat | Reduction in progesterone and oestradiol production | [224] |
| Metformin | Granulosa | Cattle | Inhibition of steroidogenesis and MAPK ERK1/2 phosphorylation through AMPK activation | [79] |
| Inhibition of IGF-I-stimulated cell growth, protein synthesis, MAPK ERK1/2 and p90RSK phosphorylation through an AMPK-dependent mechanism | [225] | |||
| Metformin | Granulosa | Pig | Improved activation of insulin signalling | [226] |
| Metformin | Theca-interstitial | Rat | Inhibition of proliferation via an AMPK-dependent mechanism | [227] |
| Metformin | Theca | Human | Inhibition of androgen production | [179] |
| Metformin | Cumulus oocyte complex | Cattle | Blockage of meiotic progression at the germinal vesicle stage through an activation of AMPK and inhibition of MAPK ERK1/2 | [79] |
| Metformin | Follicle | Knockout | Gradual loss of ovarian follicles of all classes, but this phenotype can be reversed by simultaneous null mutation of PTEN | [122] |
| Rosiglitazone | Granulosa cells | Sheep | Inhibited granulosa cell proliferation and increased the secretion of P4 in vitro | [194] |
| Rosiglitazone | Granulosa lutein cells | Human | Stimulated StAR but had no effect on steroidogenic enzymes E2 or P4 | [228] |
| Rosiglitazone | Mixed granulosa, theca and stroma cells | Human | Stimulated P4 and IGFBP-1, inhibited E2 and testosterone, abolished insulin-induced stimulation of testosterone and insulin-dependent stimulation of E2 in the presence of FSH and enhanced insulin-induced inhibition of IGFBP-1 | [199] |
| Rosiglitazone | Ovarian cells | Pig | Increased P4 secretion and PPARγ expression and 3βHSD activity. Decreased androstendione and testosterone by reducing the expression and activity of CYP17-lyase and 17βHSD (17β-hydroxysteroid dehydrogenase), but no change in E2 secretion and CYP19a1 | [229] |
| Rosiglitazone | Oocytes | Mouse | Stimulated AMPK and enhanced resumption of meiosis in oocytes | [230] |
| Rosiglitazone and pioglitazone | Diabetic women with PCOS | Improved insulin resistance and hyperandrogenism by an unknown mechanism | [231] | |
| Pioglitazone | NCI-H295R cells | Human cell line | Inhibited androgen production by regulating expression of CYP17 and 3βHSD (3β-hydroxysteroid dehydrogenase) | [231] |
Rosiglitazone
Contrary to metformin that primarily reduces hepatic glucose output, the thiazolidinedione rosiglitazone increases insulin sensitivity. Thiazolidinediones are a class of oral insulin-sensitizing agents that exert their action as ligands of peroxisome-proliferator-activated receptor (PPARγ). The PPARs are a family of transcriptional nuclear factors with three isoforms (α, β and γ) belonging to the nuclear receptor superfamily of transcription factors [189]. Upon stimulation, PPARγ heterodimerizes with the retinoid X receptor (RXR) to bind to ‘PPAR-responsive’ elements, and thereby activating the transcription of specific genes. The activation of PPARγ regulates the synthesis of steroid hormones in granulosa cells and the disruption of PPARγ in the ovary leads to female sub-fertility [190,191]. However the cardiovascular safety of rosiglitazone is questionable [192] and therefore its use in human medicine is limited.
Expression of PPARγ has been reported in granulosa cells from rats [193], mice [191], sheep [194], humans [195], pigs [196] and cattle [197], and in human oocytes [198]. In follicles, PPARγ is detected in primary, secondary and pre-ovulatory follicles and its expression increases as follicles develop, but after the LH surge, PPARγ mRNA expression is reduced [193]. Insulin and the thiazolidinediones can independently stimulate the expression of PPARγ, IR, IRS-1 and StAR (steroidogenic acute regulatory protein) in human ovarian cells. Thus, PPARγ, IR with its signalling pathways and StAR constitute, in humans, a novel ovarian regulatory system with complex interactions among its components [199,200]. Moreover, PPARγ has been defined as a critical regulator of ovulation because granulosa cell-specific deletion of PPARγ results in defective follicular rupture in mice [190]. In sheep granulosa cells, rosiglitazone inhibited cellular proliferation and increased the secretion of progesterone in vitro [194]. In contrast, it had no effect on LH, FSH, prolactin and growth hormone secretion by cultured ovine pituitary cells. Overall, these data suggest that PPARγ ligands stimulate follicular differentiation in vivo most likely through a direct action on granulosa cells rather than by modulating pituitary hormone secretion [194]. The ovarian actions of rosiglitazone are summarized in Table 3.
Various endogenous factors, such as eicosanoids, fatty acids and prostaglandins metabolites, known to regulate ovarian function, are natural ligands of PPARγ (for a review, see [193]). Among these, the lipoxygenase-derived and the epoxygenase-derived metabolites of AA (arachidonic acid) enhanced the transcription of StAR. It has been demonstrated that thiazolidinediones, synthetic ligands of PPARγ, modulate steroid production in reproductive tissues. For example, the activation of PPARγ by thiazolidinediones in porcine theca cells [196], in rat [201] and ovine granulosa [194] cells, and in bovine luteal cells [197] leads to increased progesterone production. Somewhat different effects are observed in cultured human granulosa cells where activators of PPARγ decreased the activity of aromatase by blocking nuclear factor-κB (NF-κB) signalling [202]. Previously, the expression of StAR was increased in response to thiazolidinediones in both a mixed human ovarian tissue culture containing granulosa, theca and stroma cells, as well as purified human granulosa cells [199]. Furthermore, in mice, PPARγ is regulated by P4 in the pre-ovulatory mouse follicles [190]. In addition, PPARγ may be indirectly involved in oocyte maturation via the granulosa cells. Indeed, disruption of PPARγ gene in the ovary using Cre-LoxP recombination technology led to female sub-fertility [191].
CONCLUSIONS
The action of insulin and insulin-mediated uptake of glucose on ovarian cells is of importance for animal production (dairy cattle, small ruminants and pigs) as well as to some fields of human reproductive medicine. It is evident from the available literature that the function of the ovary is subject to some form of local or direct modulation by the insulin and insulin/glucose systems. Although the gonadotropins, LH and FSH, are the primary regulators of terminal folliculogenesis, early folliculogenesis can proceed in the absence of the gonadotropins. Furthermore, in the later gonadotropin-dependent stages of development, this process can be modulated by numerous external influences, insulin being one of them. Likewise, the converse is also possible, that is, the gonadotropins can also modulate the action of insulin in follicular cells. Thus in granulosa cells, insulin signalling through Akt1 and FOXO1 can enhance FSH-stimulated steroidogenesis, and similarly, FSH acting through Akt1 can enhance insulin-stimulated glucose transport leading to increased flux through the hexosamine pathway and to the inhibition of aromatase. Likewise, FSH acting via mTOR and/or ERK can enhance cell proliferation and protein synthesis, and FSH acting via Akt1 and FOXO3a can inhibit apoptosis.
In theca cells, insulin signalling through Akt1 can stimulate PDE to inhibit the degradation of cAMP and so enhance LH-stimulated androgen production, similarly insulin acting via Akt1 and FOXO1 can enhance LH-stimulated steroidogenesis. Acting via mTOR and/or ERK, LH can enhance cell proliferation and protein synthesis and can inhibit apoptosis. LH acting directly or via cAMP can enhance insulin stimulation of ERK, while, at the same time, LH can stimulate JAK2 which inhibits Shc activation of ERK. The balance of these opposing actions of LH could modulate insulin-stimulated cell proliferation and protein synthesis in ovarian cells.
Folliculogenesis is a dynamic process, and the effects of the gonadotropins on folliculogenesis are stage-dependent and this stage-dependence is likely to also apply to the actions of insulin and to the efficacy of insulin-sensitizing treatments. For example, the FSH-stimulated FOXO1 pathway appears to have stage-specific effects in follicles: in small follicles, granulosa cell proliferation is stimulated; in medium-sized antral follicles, it is steroidogenesis that is stimulated; whereas, at ovulation, atresia is stimulated in the atretic non-ovulatory follicles. However, the FOXO1 pathway is but one example and the picture is far from complete, and we neither know nor understand all of the regulatory mechanism involved in the stage-specific effects of insulin during the continuum of folliculogenesis. Clearly, further research is indicated to understand more fully the mechanisms of action of insulin and the role of glucose in the follicle.
Acknowledgments
We thank Dr Hannah Brown of the University of Adelaide for her helpful comments.
Abbreviations
- AGE
advanced glycation end-product
- AMPK
AMP-dependent protein kinase
- AS160
Akt substrate of 160 kDa
- Bad
Bcl-2-associated death promoter
- BMP
bone morphogenetic protein
- CDK
cyclin-dependent kinase
- COF
cystic ovarian follicle
- CYP
cytochrome P450
- 4E-BP1
eukaryotic initiation factor 4E-binding protein 1
- ERK
extracellular-signal-regulated protein kinase
- FOXO
forkhead box O
- FSH
follicle-stimulating hormone
- GLUT
glucose transporter
- GnRH
gonadotropin-releasing hormone
- GS
glycogen synthase
- GSK3
glycogen synthase kinase-3
- GV
germinal vesicle
- hCG
human chorionic gonadotropin
- IGF
insulin-like growth factor
- IGFBP
IGF-binding protein
- IGF-IR
IGF-I receptor
- IR
insulin receptor
- IRS
insulin receptor substrate
- IVF
in vitro fertilization
- JAK2
Janus kinase 2
- JNK
c-Jun N-terminal kinase
- KL
KIT ligand
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- MII
metaphase-II
- mTOR
mammalian target of rapamycin
- mTORC
mTOR complex
- NEB
negative energy balance
- OCT
organic cation transporter
- PCOS
polycystic ovarian syndrome
- PDE3A
cAMP-phosphodiesterase 3A
- PDK
phosphoinositide-dependent kinase
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC
primordial germ cell
- PKA
protein kinase A
- PKB
protein kinase B
- PKC
protein kinase C
- PI3K
phosphoinositide kinase
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- POF
premature ovarian failure
- PPAR
peroxisome-proliferator-activated receptor
- PPP
pentose phosphate pathway
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- rpS6
ribosomal protein S6
- SH2
Src homology 2
- Shc
Src homology collagen
- SHIP2
SH2-domain-containing inositol phosphatase-2
- S6K1
p70 ribosomal S6 kinase 1
- StAR
steroidogenic acute regulatory protein
- TGF
transforming growth factor
- TSC2
tuberous sclerosis complex 2, VFA, volatile fatty acid
FUNDING
This work was supported by the Région Centre [grant numbers DURAREP; 2008 00030333 and DURAREP 2; 2011 00064290]; the European Union Framework 6 funding programme [grant number MEXC-CT-2006-042499]; and the European Union Marie Curie Chair of Excellence [grant number MEXC-CT-2006-042499 (to R.J.S.)].
References
- 1.Franks S., Robinson S., Willis D.S. Nutrition, insulin and polycystic ovary syndrome. Rev. Reprod. 1996;1:47–53. doi: 10.1530/ror.0.0010047. [DOI] [PubMed] [Google Scholar]
- 2.Wathes D.C., Fenwick M., Cheng Z., Bourne N., Llewellyn S., Morris D.G., Kenny D., Murphy J., Fitzpatrick R. Influence of negative energy balance on cyclicity and fertility in the high producing dairy cow. Theriogenology. 2007;68(Suppl. 1):S232–S241. doi: 10.1016/j.theriogenology.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Teleni E., Rowe J.B., Croker K.P., Murray P.J., King W.R. Lupins and energy-yielding nutrients in ewes. II. Responses in ovulation rate in ewes to increased availability of glucose, acetate and amino acids. Reprod. Fertil. Dev. 1989;1:117–125. doi: 10.1071/RD9890117. [DOI] [PubMed] [Google Scholar]
- 4.Gambineri A., Pelusi C., Vicennati V., Pagotto U., Pasquali R. Obesity and the polycystic ovary syndrome. Int. J. Obes. Relat. Metab. Disord. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 5.Carmina E. Metabolic syndrome in polycystic ovary syndrome. Minerva Ginecol. 2006;58:109–114. [PubMed] [Google Scholar]
- 6.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr. Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 7.Wild R.A., Rizzo M., Clifton S., Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil. Steril. 2011;95:1073–1079. doi: 10.1016/j.fertnstert.2010.12.027. e1071–e1011. [DOI] [PubMed] [Google Scholar]
- 8.Grummer R.R. Etiology of lipid-related metabolic disorders in periparturient dairy cows. J. Dairy Sci. 1993;76:3882–3896. doi: 10.3168/jds.S0022-0302(93)77729-2. [DOI] [PubMed] [Google Scholar]
- 9.Poyastro Pinheiro A., Thornton L.M., Plotonicov K.H., Tozzi F., Klump K.L., Berrettini W.H., Brandt H., Crawford S., Crow S., Fichter M.M., et al. Patterns of menstrual disturbance in eating disorders. Int. J. Eat. Disord. 2007;40:424–434. doi: 10.1002/eat.20388. [DOI] [PubMed] [Google Scholar]
- 10.Chen M.D., O'Byrne K.T., Chiappini S.E., Hotchkiss J., Knobil E. Hypoglycemic ‘stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology. 1992;56:666–673. doi: 10.1159/000126291. [DOI] [PubMed] [Google Scholar]
- 11.Leroy J.L., Opsomer G., Van Soom A., Goovaerts I.G., Bols P.E. Reduced fertility in high-yielding dairy cows: are the oocyte and embryo in danger? Part I. The importance of negative energy balance and altered corpus luteum function to the reduction of oocyte and embryo quality in high-yielding dairy cows. Reprod. Domest. Anim. 2008;43:612–622. doi: 10.1111/j.1439-0531.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 12.Ortega H.H., Marelli B.E., Rey F., Amweg A.N., Diaz P.U., Stangaferro M.L., Salvetti N.R. Molecular aspects of bovine cystic ovarian disease pathogenesis. Reproduction. 2015;149:R251–R264. doi: 10.1530/REP-14-0618. [DOI] [PubMed] [Google Scholar]
- 13.Vanholder T., Opsomer G., de Kruif A. Aetiology and pathogenesis of cystic ovarian follicles in dairy cattle: a review. Reprod. Nutr. Dev. 2006;46:105–119. doi: 10.1051/rnd:2006003. [DOI] [PubMed] [Google Scholar]
- 14.Hooijer G.A., Lubbers R.B., Ducro B.J., van Arendonk J.A., Kaal-Lansbergen L.M., van der Lende T. Genetic parameters for cystic ovarian disease in Dutch black and white dairy cattle. J. Dairy Sci. 2001;84:286–291. doi: 10.3168/jds.S0022-0302(01)74478-5. [DOI] [PubMed] [Google Scholar]
- 15.Furman M., Wade G.N. Animal models in the study of nutritional infertility. Curr. Opin. Endocrinol. Diabetes Obes. 2007;14:475–481. doi: 10.1097/MED.0b013e3282f1cb7e. [DOI] [PubMed] [Google Scholar]
- 16.Katz M.G., Vollenhoven B. The reproductive endocrine consequences of anorexia nervosa. BJOG. 2000;107:707–713. doi: 10.1111/j.1471-0528.2000.tb13329.x. [DOI] [PubMed] [Google Scholar]
- 17.Lucy M.C. Mechanisms linking nutrition and reproduction in postpartum cows. Reprod. Suppl. 2003;61:415–427. [PubMed] [Google Scholar]
- 18.Diskin M.G., Mackey D.R., Roche J.F., Sreenan J.M. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim. Reprod. Sci. 2003;78:345–370. doi: 10.1016/S0378-4320(03)00099-X. [DOI] [PubMed] [Google Scholar]
- 19.Hein G.J., Panzani C.G., Rodriguez F.M., Salvetti N.R., Diaz P.U., Gareis N.C., Benitez G.A., Ortega H.H., Rey F. Impaired insulin signaling pathway in ovarian follicles of cows with cystic ovarian disease. Anim. Reprod. Sci. 2015;156:64–74. doi: 10.1016/j.anireprosci.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Scaramuzzi R.J., Campbell B.K., Downing J.A., Kendall N.R., Khalid M., Munoz-Gutierrez M., Somchit A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006;46:339–354. doi: 10.1051/rnd:2006016. [DOI] [PubMed] [Google Scholar]
- 21.Downing J.A., Scaramuzzi R.J. Nutrient effects on ovulation rate, ovarian function and the secretion of gonadotrophic and metabolic hormones in sheep. J. Reprod. Fertil. Suppl. 1991;43:209–227. [PubMed] [Google Scholar]
- 22.Scaramuzzi R.J., Zouaidi N., Menassol J.B., Dupont J. The effects of intravenous, glucose versus saline on ovarian follicles and their levels of some mediators of insulin signalling. Reprod. Biol. Endocrinol. 2015;13:6. doi: 10.1186/1477-7827-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallet C., Dupont J., Campbell B.K., Monniaux D., Guillaume D., Scaramuzzi R.J. The infusion of glucose in ewes during the luteal phase increases the number of follicles but reduces oestradiol production and some correlates of metabolic function in the large follicles. Anim. Reprod. Sci. 2011;127:154–163. doi: 10.1016/j.anireprosci.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Scaramuzzi R.J., Brown H.M., Dupont J. Nutritional and metabolic mechanisms in the ovary and their role in mediating the effects of diet on folliculogenesis: a perspective. Reprod. Domest. Anim. 2010;45(Suppl. 3):32–41. doi: 10.1111/j.1439-0531.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Gutierrez M., Blache D., Martin G.B., Scaramuzzi R.J. Ovarian follicular expression of mRNA encoding the type I IGF receptor and IGF-binding protein-2 in sheep following five days of nutritional supplementation with glucose, glucosamine or lupins. Reproduction. 2004;128:747–756. doi: 10.1530/rep.1.00439. [DOI] [PubMed] [Google Scholar]
- 26.Mazerbourg S., Bondy C.A., Zhou J., Monget P. The insulin-like growth factor system: a key determinant role in the growth and selection of ovarian follicles? A comparative species study. Reprod. Domest. Anim. 2003;38:247–258. doi: 10.1046/j.1439-0531.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong D.G., Gong J.G., Webb R. Interactions between nutrition and ovarian activity in cattle: physiological, cellular and molecular mechanisms. Reprod. Suppl. 2003;61:403–414. [PubMed] [Google Scholar]
- 28.Taniguchi C.M., Emanuelli B., Kahn C.R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 29.Cai D., Dhe-Paganon S., Melendez P.A., Lee J., Shoelson S.E. Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J. Biol. Chem. 2003;278:25323–25330. doi: 10.1074/jbc.M212430200. [DOI] [PubMed] [Google Scholar]
- 30.Versteyhe S., Blanquart C., Hampe C., Mahmood S., Christeff N., De Meyts P., Gray S.G., Issad T. Insulin receptor substrates-5 and -6 are poor substrates for the insulin receptor. Mol. Med. Rep. 2010;3:189–193. doi: 10.3892/mmr_00000239. [DOI] [PubMed] [Google Scholar]
- 31.Sun X.J., Pons S., Wang L.M., Zhang Y., Yenush L., Burks D., Myers M.G., Jr, Glasheen E., Copeland N.G., Jenkins N.A., et al. The IRS-2 gene on murine chromosome 8 encodes a unique signaling adapter for insulin and cytokine action. Mol. Endocrinol. 1997;11:251–262. doi: 10.1210/mend.11.2.9885. [DOI] [PubMed] [Google Scholar]
- 32.Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Pawson T., Pelicci P.G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-L. [DOI] [PubMed] [Google Scholar]
- 33.Sasaoka T., Kobayashi M. The functional significance of Shc in insulin signaling as a substrate of the insulin receptor. Endocr. J. 2000;47:373–381. doi: 10.1507/endocrj.47.373. [DOI] [PubMed] [Google Scholar]
- 34.Diamanti-Kandarakis E., Papavassiliou A.G. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol. Med. 2006;12:324–332. doi: 10.1016/j.molmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Myers M.G., Jr, White M.F. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993;42:643–650. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- 36.Ghigo A., Morello F., Perino A., Hirsch E. Phosphoinositide 3-kinases in health and disease. Subcell. Biochem. 2012;58:183–213. doi: 10.1007/978-94-007-3012-0. [DOI] [PubMed] [Google Scholar]
- 37.Wijesekara N., Konrad D., Eweida M., Jefferies C., Liadis N., Giacca A., Crackower M., Suzuki A., Mak T.W., Kahn C.R., et al. Muscle-specific Pten deletion protects against insulin resistance and diabetes. Mol. Cell. Biol. 2005;25:1135–1145. doi: 10.1128/MCB.25.3.1135-1145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleeman M.W., Wortley K.E., Lai K.M., Gowen L.C., Kintner J., Kline W.O., Garcia K., Stitt T.N., Yancopoulos G.D., Wiegand S.J., Glass D.J. Absence of the lipid phosphatase SHIP2 confers resistance to dietary obesity. Nat. Med. 2005;11:199–205. doi: 10.1038/nm1178. [DOI] [PubMed] [Google Scholar]
- 39.Cheatham B., Vlahos C.J., Cheatham L., Wang L., Blenis J., Kahn C.R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. 1994;14:4902–4911. doi: 10.1128/MCB.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joost H.G., Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Mol. Membr. Biol. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- 41.Shisheva A. Phosphoinositides in insulin action on GLUT4 dynamics: not just PtdIns(3,4,5)P3. Am. J. Physiol. Endocrinol. Metab. 2008;295:E536–E544. doi: 10.1152/ajpendo.90353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farese R.V., Sajan M.P., Yang H., Li P., Mastorides S., Gower W.R., Jr, Nimal S., Choi C.S., Kim S., Shulman G.I., et al. Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J. Clin. Invest. 2007;117:2289–2301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumann C.A., Ribon V., Kanzaki M., Thurmond D.C., Mora S., Shigematsu S., Bickel P.E., Pessin J.E., Saltiel A.R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- 44.Avruch J. Insulin signal transduction through protein kinase cascades. Mol. Cell. Biochem. 1998;182:31–48. doi: 10.1023/A:1006823109415. [DOI] [PubMed] [Google Scholar]
- 45.Duchene S., Audouin E., Berri C., Dupont J., Tesseraud S. Tissue-specific regulation of S6K1 by insulin in chickens divergently selected for growth. Gen. Comp. Endocrinol. 2008;156:190–198. doi: 10.1016/j.ygcen.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Wautier J.L., Schmidt A.M. Protein glycation: a firm link to endothelial cell dysfunction. Circ. Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- 47.Merhi Z., McGee E.A., Buyuk E. Role of advanced glycation end-products in obesity-related ovarian dysfunction. Minerva Endocrinol. 2014;39:167–174. [PubMed] [Google Scholar]
- 48.Guosheng L., Hongmei S., Chuan N., Haiying L., Xiaopeng Z., Xianqiong L. The relationship of serum AGE levels in diabetic mothers with adverse fetal outcome. J. Perinatol. 2009;29:483–488. doi: 10.1038/jp.2009.12. [DOI] [PubMed] [Google Scholar]
- 49.Li R., Yang P., Chen X., Wang L. Maternal serum AGEs levels in pregnancies associated with neural tube defects. Int. J. Dev. Neurosci. 2014;33:57–61. doi: 10.1016/j.ijdevneu.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Wood I.S., Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br. J. Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 51.Williams S.A., Blache D., Martin G.B., Foot R., Blackberry M.A., Scaramuzzi R.J. Effect of nutritional supplementation on quantities of glucose transporters 1 and 4 in sheep granulosa and theca cells. Reproduction. 2001;122:947–956. doi: 10.1530/rep.0.1220947. [DOI] [PubMed] [Google Scholar]
- 52.Nishimoto H., Matsutani R., Yamamoto S., Takahashi T., Hayashi K.G., Miyamoto A., Hamano S., Tetsuka M. Gene expression of glucose transporter (GLUT) 1, 3 and 4 in bovine follicle and corpus luteum. J. Endocrinol. 2006;188:111–119. doi: 10.1677/joe.1.06210. [DOI] [PubMed] [Google Scholar]
- 53.Kodaman P.H., Behrman H.R. Hormone-regulated and glucose-sensitive transport of dehydroascorbic acid in immature rat granulosa cells. Endocrinology. 1999;140:3659–3665. doi: 10.1210/endo.140.8.6938. [DOI] [PubMed] [Google Scholar]
- 54.Kim E., Seok H.H., Lee S.Y., Lee D.R., Moon J., Yoon T.K., Lee W.S., Lee K.A. Correlation between expression of glucose transporters in granulosa cells and oocyte quality in women with polycystic ovary syndrome. Endocrinol. Metab. (Seoul) 2014;29:40–47. doi: 10.3803/EnM.2014.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kol S., Ben-Shlomo I., Ruutiainen K., Ando M., Davies-Hill T.M., Rohan R.M., Simpson I.A., Adashi E.Y. The midcycle increase in ovarian glucose uptake is associated with enhanced expression of glucose transporter 3. Possible role for interleukin-1, a putative intermediary in the ovulatory process. J. Clin. Invest. 1997;99:2274–2283. doi: 10.1172/JCI119403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang C., Niu W., Wang Z., Wang X., Xia G. The effect of gonadotropin on glucose transport and apoptosis in rat ovary. PLoS One. 2012;7:e42406. doi: 10.1371/journal.pone.0042406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jozwik M., Teng C., Battaglia F.C. Concentrations of monosaccharides and their amino and alcohol derivatives in human preovulatory follicular fluid. Mol. Hum. Reprod. 2007;13:791–796. doi: 10.1093/molehr/gam060. [DOI] [PubMed] [Google Scholar]
- 58.Brogan R.S., MacGibeny M., Mix S., Thompson C., Puttabyatappa M., VandeVoort C.A., Chaffin C.L. Dynamics of intra-follicular glucose during luteinization of macaque ovarian follicles. Mol. Cell. Endocrinol. 2011;332:189–195. doi: 10.1016/j.mce.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan F.A., Das G.K., Pande M., Mir R.A., Shankar U. Changes in biochemical composition of follicular fluid during reproductive acyclicity in water buffalo (Bubalus bubalis) Anim. Reprod. Sci. 2011;127:38–42. doi: 10.1016/j.anireprosci.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 60.Nandi S., Girish Kumar V., Manjunatha B.M., Ramesh H.S., Gupta P.S. Follicular fluid concentrations of glucose, lactate and pyruvate in buffalo and sheep, and their effects on cultured oocytes, granulosa and cumulus cells. Theriogenology. 2008;69:186–196. doi: 10.1016/j.theriogenology.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 61.Nishimoto H., Hamano S., Hill G.A., Miyamoto A., Tetsuka M. Classification of bovine follicles based on the concentrations of steroids, glucose and lactate in follicular fluid and the status of accompanying follicles. J. Reprod. Dev. 2009;55:219–224. doi: 10.1262/jrd.20114. [DOI] [PubMed] [Google Scholar]
- 62.Bertoldo M.J., Nadal-Desbarats L., Gerard N., Dubois A., Holyoake P.K., Grupen C.G. Differences in the metabolomic signatures of porcine follicular fluid collected from environments associated with good and poor oocyte quality. Reproduction. 2013;146:221–231. doi: 10.1530/REP-13-0142. [DOI] [PubMed] [Google Scholar]
- 63.El-Bahr S.M., Ghoneim I.M., Waheed M.M. Biochemical and hormonal analysis of follicular fluid and serum of female dromedary camels (Camelus dromedarius) with different sized ovarian follicles. Anim. Reprod. Sci. 2015;159:98–103. doi: 10.1016/j.anireprosci.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Salazar-Ortiz J., Monget P., Guillaume D. The influence of nutrition on the insulin-like growth factor system and the concentrations of growth hormone, glucose, insulin, gonadotropins and progesterone in ovarian follicular fluid and plasma from adult female horses (Equus caballus) Reprod. Biol. Endocrinol. 2014;12:72. doi: 10.1186/1477-7827-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nandi S., Kumar V.G., Manjunatha B.M., Gupta P.S. Biochemical composition of ovine follicular fluid in relation to follicle size. Dev. Growth Differ. 2007;49:61–66. doi: 10.1111/j.1440-169X.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 66.Shabankareh H.K., Kor N.M., Hajarian H. The influence of the corpus luteum on metabolites composition of follicular fluid from different sized follicles and their relationship to serum concentrations in dairy cows. Anim. Reprod. Sci. 2013;140:109–114. doi: 10.1016/j.anireprosci.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 67.Anifandis G.M., Dafopoulos K., Messini C.I., Chalvatzas N., Liakos N., Pournaras S., Messinis I.E. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod. Biol. Endocrinol. 2010;8:91. doi: 10.1186/1477-7827-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foong S.C., Abbott D.H., Zschunke M.A., Lesnick T.G., Phy J.L., Dumesic D.A. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J. Clin. Endocrinol. Metab. 2006;91:2327–2333. doi: 10.1210/jc.2005-2142. [DOI] [PubMed] [Google Scholar]
- 69.Rabiee A.R., Lean I.J., Gooden J.M., Miller B.G., Scaramuzzi R.J. An evaluation of transovarian uptake of metabolites using arterio-venous difference methods in dairy cattle. Anim. Reprod. Sci. 1997;48:9–25. doi: 10.1016/S0378-4320(97)00032-8. [DOI] [PubMed] [Google Scholar]
- 70.Seamark R.F., Amato F., Hendrickson S., Moor R.M. Oxygen uptake, glucose utilization, lactate release and adenine nucleotide content of sheep ovarian follicles in culture: effect of human chorionic gonadotrophin. Aust. J. Biol. Sci. 1976;29:557–563. doi: 10.1071/bi9760557. [DOI] [PubMed] [Google Scholar]
- 71.Armstrong D.T., Greep R.O. Effect of gonadotrophic hormones on glucose metabolism by luteinized rat ovaries. Endocrinology. 1962;70:701–710. doi: 10.1210/endo-70-5-701. [DOI] [PubMed] [Google Scholar]
- 72.Downs S.M., Humpherson P.G., Leese H.J. Pyruvate utilization by mouse oocytes is influenced by meiotic status and the cumulus oophorus. Mol. Reprod. Dev. 2002;62:113–123. doi: 10.1002/mrd.10067. [DOI] [PubMed] [Google Scholar]
- 73.Sutton-McDowall M.L., Gilchrist R.B., Thompson J.G. Cumulus expansion and glucose utilisation by bovine cumulus–oocyte complexes during in vitro maturation: the influence of glucosamine and follicle-stimulating hormone. Reproduction. 2004;128:313–319. doi: 10.1530/rep.1.00225. [DOI] [PubMed] [Google Scholar]
- 74.Ratchford A.M., Esguerra C.R., Moley K.H. Decreased oocyte-granulosa cell gap junction communication and connexin expression in a type 1 diabetic mouse model. Mol. Endocrinol. 2008;22:2643–2654. doi: 10.1210/me.2007-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell B.K., Onions V., Kendall N.R., Guo L., Scaramuzzi R.J. The effect of monosaccharide sugars and pyruvate on the differentiation and metabolism of sheep granulosa cells in vitro. Reproduction. 2010;140:541–550. doi: 10.1530/REP-10-0146. [DOI] [PubMed] [Google Scholar]
- 76.Campbell B.K., Kendall N.R., Onions V., Guo L., Scaramuzzi R.J. Effect of monosaccharide sugars on LH-induced differentiation and sugar transport facilitator (SLC2A) expression in sheep theca cells in vitro. Reprod. Fertil. Dev. 2014;26:453–461. doi: 10.1071/RD12064. [DOI] [PubMed] [Google Scholar]
- 77.Scaramuzzi R.J., Baird D.T., Campbell B.K., Driancourt M.A., Dupont J., Fortune J.E., Gilchrist R.B., Martin G.B., McNatty K.P., McNeilly A.S., et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011;23:444–467. doi: 10.1071/RD09161. [DOI] [PubMed] [Google Scholar]
- 78.Dupont J., Reverchon M., Bertoldo M.J., Froment P. Nutritional signals and reproduction. Mol. Cell. Endocrinol. 2014;382:527–537. doi: 10.1016/j.mce.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 79.Tosca L., Chabrolle C., Uzbekova S., Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK) Biol. Reprod. 2007;76:368–378. doi: 10.1095/biolreprod.106.055749. [DOI] [PubMed] [Google Scholar]
- 80.Kayampilly P.P., Menon K.M. Follicle-stimulating hormone inhibits adenosine 5′-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology. 2009;150:929–935. doi: 10.1210/en.2008-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang T.T., Wu X.H., Zhang S.L., Chan J.S. Effect of glucose on the expression of the angiotensinogen gene in opossum kidney cells. Kidney Int. 1998;53:312–319. doi: 10.1046/j.1523-1755.1998.00775.x. [DOI] [PubMed] [Google Scholar]
- 82.McClain D.A., Paterson A.J., Roos M.D., Wei X., Kudlow J.E. Glucose and glucosamine regulate growth factor gene expression in vascular smooth muscle cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8150–8154. doi: 10.1073/pnas.89.17.8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J., Liu R., Hawkins M., Barzilai N., Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 84.Campbell B.K., Gordon B.M., Scaramuzzi R.J. The effect of ovarian arterial infusion of transforming growth factor alpha on ovarian follicle populations and ovarian hormone secretion in ewes with an autotransplanted ovary. J. Endocrinol. 1994;143:13–24. doi: 10.1677/joe.0.1430013. [DOI] [PubMed] [Google Scholar]
- 85.Lam T.K., Gutierrez-Juarez R., Pocai A., Bhanot S., Tso P., Schwartz G.J., Rossetti L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat. Med. 2007;13:171–180. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- 86.Kahn B.B., Alquier T., Carling D., Hardie D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 87.Priebe A., Tan L., Wahl H., Kueck A., He G., Kwok R., Opipari A., Liu J.R. Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol. Oncol. 2011;122:389–395. doi: 10.1016/j.ygyno.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 88.Poretsky L., Kalin M.F. The gonadotropic function of insulin. Endocr. Rev. 1987;8:132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- 89.Samoto T., Maruo T., Ladines-Llave C.A., Matsuo H., Deguchi J., Barnea E.R., Mochizuki M. Insulin receptor expression in follicular and stromal compartments of the human ovary over the course of follicular growth, regression and atresia. Endocr. J. 1993;40:715–726. doi: 10.1507/endocrj.40.715. [DOI] [PubMed] [Google Scholar]
- 90.el-Roeiy A., Chen X., Roberts V.J., Shimasakai S., Ling N., LeRoith D., Roberts C.T., Jr, Yen S.S. Expression of the genes encoding the insulin-like growth factors (IGF-I and II), the IGF and insulin receptors, and IGF-binding proteins-1-6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J. Clin. Endocrinol. Metab. 1994;78:1488–1496. doi: 10.1210/jcem.78.6.7515389. [DOI] [PubMed] [Google Scholar]
- 91.Lighten A.D., Hardy K., Winston R.M., Moore G.E. Expression of mRNA for the insulin-like growth factors and their receptors in human preimplantation embryos. Mol. Reprod. Dev. 1997;47:134–139. doi: 10.1002/(SICI)1098-2795(199706)47:2<134::AID-MRD2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 92.Schultz G.A., Hogan A., Watson A.J., Smith R.M., Heyner S. Insulin, insulin-like growth factors and glucose transporters: temporal patterns of gene expression in early murine and bovine embryos. Reprod. Fertil. Dev. 1992;4:361–371. doi: 10.1071/RD9920361. [DOI] [PubMed] [Google Scholar]
- 93.Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J. Mol. Endocrinol. 2011;47:R1–R10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- 94.Phy J.L., Conover C.A., Abbott D.H., Zschunke M.A., Walker D.L., Session D.R., Tummon I.S., Thornhill A.R., Lesnick T.G., Dumesic D.A. Insulin and messenger ribonucleic acid expression of insulin receptor isoforms in ovarian follicles from nonhirsute ovulatory women and polycystic ovary syndrome patients. J. Clin. Endocrinol. Metab. 2004;89:3561–3566. doi: 10.1210/jc.2003-031888. [DOI] [PubMed] [Google Scholar]
- 95.Pitetti J.L., Torre D., Conne B., Papaioannou M.D., Cederroth C.R., Xuan S., Kahn R., Parada L.F., Vassalli J.D., Efstratiadis A., Nef S. Insulin receptor and IGF1R are not required for oocyte growth, differentiation, and maturation in mice. Sex Dev. 2009;3:264–272. doi: 10.1159/000252813. [DOI] [PubMed] [Google Scholar]
- 96.Wang Q., Chi M.M., Moley K.H. Live imaging reveals the link between decreased glucose uptake in ovarian cumulus cells and impaired oocyte quality in female diabetic mice. Endocrinology. 2012;153:1984–1989. doi: 10.1210/en.2011-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poretsky L., Cataldo N.A., Rosenwaks Z., Giudice L.C. The insulin-related ovarian regulatory system in health and disease. Endocr. Rev. 1999;20:535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 98.Peluso J.J., Delidow B.C., Lynch J., White B.A. Follicle-stimulating hormone and insulin regulation of 17 beta-estradiol secretion and granulosa cell proliferation within immature rat ovaries maintained in perifusion culture. Endocrinology. 1991;128:191–196. doi: 10.1210/endo-128-1-191. [DOI] [PubMed] [Google Scholar]
- 99.Downing J.A., Joss J., Scaramuzzi R.J. The effect of a direct arterial infusion of insulin and glucose on the ovarian secretion rates of androstenedione and oestradiol in ewes with an autotransplanted ovary. J. Endocrinol. 1999;163:531–541. doi: 10.1677/joe.0.1630531. [DOI] [PubMed] [Google Scholar]
- 100.Landau S., Braw-Tal R., Kaim M., Bor A., Bruckental I. Preovulatory follicular status and diet affect the insulin and glucose content of follicles in high-yielding dairy cows. Anim. Reprod. Sci. 2000;64:181–197. doi: 10.1016/S0378-4320(00)00212-8. [DOI] [PubMed] [Google Scholar]
- 101.Gong J.G., Lee W.J., Garnsworthy P.C., Webb R. Effect of dietary-induced increases in circulating insulin concentrations during the early postpartum period on reproductive function in dairy cows. Reproduction. 2002;123:419–427. doi: 10.1530/rep.0.1230419. [DOI] [PubMed] [Google Scholar]
- 102.Garnsworthy P.C., Fouladi-Nashta A.A., Mann G.E., Sinclair K.D., Webb R. Effect of dietary-induced changes in plasma insulin concentrations during the early post partum period on pregnancy rate in dairy cows. Reproduction. 2009;137:759–768. doi: 10.1530/REP-08-0488. [DOI] [PubMed] [Google Scholar]
- 103.Campbell B.K., Scaramuzzi R.J., Webb R. Control of antral follicle development and selection in sheep and cattle. J. Reprod. Fertil. Suppl. 1995;49:335–350. [PubMed] [Google Scholar]
- 104.Maruthini D., Harris S.E., Barth J.H., Balen A.H., Campbell B.K., Picton H.M. The effect of metformin treatment in vivo on acute and long-term energy metabolism and progesterone production in vitro by granulosa cells from women with polycystic ovary syndrome. Hum. Reprod. 2014;29:2302–2316. doi: 10.1093/humrep/deu187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhatia B., Price C.A. Insulin alters the effects of follicle stimulating hormone on aromatase in bovine granulosa cells in vitro. Steroids. 2001;66:511–519. doi: 10.1016/S0039-128X(00)00218-X. [DOI] [PubMed] [Google Scholar]
- 106.Gutierrez C.G., Campbell B.K., Webb R. Development of a long-term bovine granulosa cell culture system: induction and maintenance of estradiol production, response to follicle-stimulating hormone, and morphological characteristics. Biol. Reprod. 1997;56:608–616. doi: 10.1095/biolreprod56.3.608. [DOI] [PubMed] [Google Scholar]
- 107.Diamanti-Kandarakis E., Chatzigeorgiou A., Papageorgiou E., Koundouras D., Koutsilieris M. Advanced glycation end-products and insulin signaling in granulosa cells. Exp. Biol. Med. (Maywood) 2015 doi: 10.1177/1535370215584937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Y., Qiao J., Yan L.Y., Huang S., Zhao P.L., Yan J. Selective impairment in glycogen synthase kinase-3 and mitogen-activated protein kinase phosphorylation: comparisons with the hyperandrogenic and the hyperinsulinemic rats. Fertil. Steril. 2009;92:1447–1455. doi: 10.1016/j.fertnstert.2008.08.073. [DOI] [PubMed] [Google Scholar]
- 109.Richardson M.C., Cameron I.T., Simonis C.D., Das M.C., Hodge T.E., Zhang J., Byrne C.D. Insulin and human chorionic gonadotropin cause a shift in the balance of sterol regulatory element-binding protein (SREBP) isoforms toward the SREBP-1c isoform in cultures of human granulosa cells. J. Clin. Endocrinol. Metab. 2005;90:3738–3746. doi: 10.1210/jc.2004-2057. [DOI] [PubMed] [Google Scholar]
- 110.Sekar N., Garmey J.C., Veldhuis J.D. Mechanisms underlying the steroidogenic synergy of insulin and luteinizing hormone in porcine granulosa cells: joint amplification of pivotal sterol-regulatory genes encoding the low-density lipoprotein (LDL) receptor, steroidogenic acute regulatory (stAR) protein and cytochrome P450 side-chain cleavage (P450scc) enzyme. Mol. Cell. Endocrinol. 2000;159:25–35. doi: 10.1016/S0303-7207(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 111.Dunaif A., Graf M. Insulin administration alters gonadal steroid metabolism independent of changes in gonadotropin secretion in insulin-resistant women with the polycystic ovary syndrome. J. Clin. Invest. 1989;83:23–29. doi: 10.1172/JCI113864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bergh C., Carlsson B., Olsson J.H., Selleskog U., Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil. Steril. 1993;59:323–331. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- 113.Nestler J.E., Jakubowicz D.J., de Vargas A.F., Brik C., Quintero N., Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J. Clin. Endocrinol. Metab. 1998;83:2001–2005. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 114.Magoffin D.A. Ovarian theca cell. Int. J. Biochem. Cell Biol. 2005;37:1344–1349. doi: 10.1016/j.biocel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 115.Young J.M., McNeilly A.S. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- 116.Palaniappan M., Menon B., Menon K.M. Stimulatory effect of insulin on theca-interstitial cell proliferation and cell cycle regulatory proteins through MTORC1 dependent pathway. Mol. Cell. Endocrinol. 2013;366:81–89. doi: 10.1016/j.mce.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eppig J.J., O'Brien M.J. Comparison of preimplantation developmental competence after mouse oocyte growth and development in vitro and in vivo. Theriogenology. 1998;49:415–422. doi: 10.1016/S0093-691X(97)00413-5. [DOI] [PubMed] [Google Scholar]
- 118.Dumesic D.A., Schramm R.D., Peterson E., Paprocki A.M., Zhou R., Abbott D.H. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J. Clin. Endocrinol. Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- 119.Adamiak S.J., Mackie K., Watt R.G., Webb R., Sinclair K.D. Impact of nutrition on oocyte quality: cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol. Reprod. 2005;73:918–926. doi: 10.1095/biolreprod.105.041483. [DOI] [PubMed] [Google Scholar]
- 120.Edson M.A., Nagaraja A.K., Matzuk M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Canipari R., Cellini V., Cecconi S. The ovary feels fine when paracrine and autocrine networks cooperate with gonadotropins in the regulation of folliculogenesis. Curr. Pharm. Des. 2012;18:245–255. doi: 10.2174/138161212799040411. [DOI] [PubMed] [Google Scholar]
- 122.Reddy P., Adhikari D., Zheng W., Liang S., Hamalainen T., Tohonen V., Ogawa W., Noda T., Volarevic S., Huhtaniemi I., Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- 123.Reddy P., Liu L., Adhikari D., Jagarlamudi K., Rajareddy S., Shen Y., Du C., Tang W., Hamalainen T., Peng S.L., et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 124.Shimizu Y., Kimura F., Takebayashi K., Fujiwara M., Takakura K., Takahashi K. Mutational analysis of the PTEN gene in women with premature ovarian failure. Acta Obstet. Gynecol. Scand. 2009;88:824–825. doi: 10.1080/00016340902971458. [DOI] [PubMed] [Google Scholar]
- 125.Goto M., Iwase A., Ando H., Kurotsuchi S., Harata T., Kikkawa F. PTEN and Akt expression during growth of human ovarian follicles. J. Assist. Reprod. Genet. 2007;24:541–546. doi: 10.1007/s10815-007-9156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Evans A.C., Martin F. Kinase pathways in dominant and subordinate ovarian follicles during the first wave of follicular development in sheep. Anim. Reprod. Sci. 2000;64:221–231. doi: 10.1016/S0378-4320(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 127.Su Y.Q., Wigglesworth K., Pendola F.L., O'Brien M.J., Eppig J.J. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- 128.Su Y.Q., Denegre J.M., Wigglesworth K., Pendola F.L., O'Brien M.J., Eppig J.J. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev. Biol. 2003;263:126–138. doi: 10.1016/S0012-1606(03)00437-8. [DOI] [PubMed] [Google Scholar]
- 129.Sela-Abramovich S., Chorev E., Galiani D., Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–1244. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- 130.Dewi D.A., Abayasekara D.R., Wheeler-Jones C.P. Requirement for ERK1/2 activation in the regulation of progesterone production in human granulosa-lutein cells is stimulus specific. Endocrinology. 2002;143:877–888. doi: 10.1210/endo.143.3.8677. [DOI] [PubMed] [Google Scholar]
- 131.Tosca L., Froment P., Solnais P., Ferre P., Foufelle F., Dupont J. Adenosine 5′-monophosphate-activated protein kinase regulates progesterone secretion in rat granulosa cells. Endocrinology. 2005;146:4500–4513. doi: 10.1210/en.2005-0301. [DOI] [PubMed] [Google Scholar]
- 132.Moore R.K., Otsuka F., Shimasaki S. Role of ERK1/2 in the differential synthesis of progesterone and estradiol by granulosa cells. Biochem. Biophys. Res. Commun. 2001;289:796–800. doi: 10.1006/bbrc.2001.6052. [DOI] [PubMed] [Google Scholar]
- 133.Seger R., Hanoch T., Rosenberg R., Dantes A., Merz W.E., Strauss, 3rd J.F., Amsterdam A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J. Biol. Chem. 2001;276:13957–13964. doi: 10.1074/jbc.M003766200. [DOI] [PubMed] [Google Scholar]
- 134.Hunzicker-Dunn M., Maizels E.T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell. Signal. 2006;18:1351–1359. doi: 10.1016/j.cellsig.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Richards J.S. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol. Endocrinol. 2001;15:209–218. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- 136.Richards J.S., Fitzpatrick S.L., Clemens J.W., Morris J.K., Alliston T., Sirois J. Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog. Horm. Res. 1995;50:223–254. doi: 10.1016/b978-0-12-571150-0.50014-7. [DOI] [PubMed] [Google Scholar]
- 137.Richards J.S., Russell D.L., Ochsner S., Hsieh M., Doyle K.H., Falender A.E., Lo Y.K., Sharma S.C. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog. Horm. Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- 138.Liu K. Stem cell factor (SCF)-kit mediated phosphatidylinositol 3 (PI3) kinase signaling during mammalian oocyte growth and early follicular development. Front. Biosci. 2006;11:126–135. doi: 10.2741/1785. [DOI] [PubMed] [Google Scholar]
- 139.Kimura T., Tomooka M., Yamano N., Murayama K., Matoba S., Umehara H., Kanai Y., Nakano T. Akt signaling promotes derivation of embryonic germ cells from primordial germ cells. Development. 2008;135:869–879. doi: 10.1242/dev.013474. [DOI] [PubMed] [Google Scholar]
- 140.Cecconi S., Ciccarelli C., Barberi M., Macchiarelli G., Canipari R. Granulosa cell–oocyte interactions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004;115(Suppl. 1):S19–S22. doi: 10.1016/j.ejogrb.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 141.Tanaka Y., Park J.H., Tanwar P.S., Kaneko-Tarui T., Mittal S., Lee H.J., Teixeira J.M. Deletion of tuberous sclerosis 1 in somatic cells of the murine reproductive tract causes female infertility. Endocrinology. 2012;153:404–416. doi: 10.1210/en.2011-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uzbekova S., Salhab M., Perreau C., Mermillod P., Dupont J. Glycogen synthase kinase 3B in bovine oocytes and granulosa cells: possible involvement in meiosis during in vitro maturation. Reproduction. 2009;138:235–246. doi: 10.1530/REP-09-0136. [DOI] [PubMed] [Google Scholar]
- 143.Baluch D.P., Capco D.G. GSK3 beta mediates acentromeric spindle stabilization by activated PKC zeta. Dev. Biol. 2008;317:46–58. doi: 10.1016/j.ydbio.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 144.Uhlenhaut N.H., Treier M. Forkhead transcription factors in ovarian function. Reproduction. 2011;142:489–495. doi: 10.1530/REP-11-0092. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y., Lehmann M. FOXO-independent suppression of programmed cell death by the PI3K/Akt signaling pathway in Drosophila. Dev. Genes Evol. 2006;216:531–535. doi: 10.1007/s00427-006-0063-x. [DOI] [PubMed] [Google Scholar]
- 146.John G.B., Gallardo T.D., Shirley L.J., Castrillon D.H. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev. Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Richards J.S., Pangas S.A. The ovary: basic biology and clinical implications. J. Clin. Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fan H.Y., Liu Z., Cahill N., Richards J.S. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol. Endocrinol. 2008;22:2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wayne C.M., Fan H.Y., Cheng X., Richards J.S. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol. Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 150.Nemcova L., Nagyova E., Petlach M., Tomanek M., Prochazka R. Molecular mechanisms of insulin-like growth factor 1 promoted synthesis and retention of hyaluronic acid in porcine oocyte-cumulus complexes. Biol. Reprod. 2007;76:1016–1024. doi: 10.1095/biolreprod.106.057927. [DOI] [PubMed] [Google Scholar]
- 151.Liu Z., Rudd M.D., Hernandez-Gonzalez I., Gonzalez-Robayna I., Fan H.Y., Zeleznik A.J., Richards J.S. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol. Endocrinol. 2009;23:649–661. doi: 10.1210/me.2008-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fan H.Y., O'Connor A., Shitanaka M., Shimada M., Liu Z., Richards J.S. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol. Endocrinol. 2010;24:1529–1542. doi: 10.1210/me.2010-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang G., Garmey J.C., Veldhuis J.D. Interactive stimulation by luteinizing hormone and insulin of the steroidogenic acute regulatory (StAR) protein and 17alpha-hydroxylase/17,20-lyase (CYP17) genes in porcine theca cells. Endocrinology. 2000;141:2735–2742. doi: 10.1210/endo.141.8.7595. [DOI] [PubMed] [Google Scholar]
- 154.Carvalho C.R., Carvalheira J.B., Lima M.H., Zimmerman S.F., Caperuto L.C., Amanso A., Gasparetti A.L., Meneghetti V., Zimmerman L.F., Velloso L.A., Saad M.J. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- 155.Huang X., Jin J., Shen S., Xia Y., Xu P., Zou X., Wang H., Yi L., Wang Y., Gao Q. Modulation of expression of 17-hydroxylase/17,20 lyase (CYP17) and P450 aromatase (CYP19) by inhibition of MEK1 in a human ovarian granulosa-like tumor cell line. Gynecol. Endocrinol. 2016;32:201–205. doi: 10.3109/09513590.2015.1106470. [DOI] [PubMed] [Google Scholar]
- 156.Nelson-Degrave V.L., Wickenheisser J.K., Hendricks K.L., Asano T., Fujishiro M., Legro R.S., Kimball S.R., Strauss, 3rd J.F., McAllister J.M. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol. Endocrinol. 2005;19:379–390. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- 157.Cecconi S., Rossi G., Santilli A., Stefano L.D., Hoshino Y., Sato E., Palmerini M.G., Macchiarelli G. Akt expression in mouse oocytes matured in vivo and in vitro. Reprod. Biomed. Online. 2010;20:35–41. doi: 10.1016/j.rbmo.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 158.Han S.J., Vaccari S., Nedachi T., Andersen C.B., Kovacina K.S., Roth R.A., Conti M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hoshino Y., Sato E. Protein kinase B (PKB/Akt) is required for the completion of meiosis in mouse oocytes. Dev. Biol. 2008;314:215–223. doi: 10.1016/j.ydbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 160.Tomek W., Smiljakovic T. Activation of Akt (protein kinase B) stimulates metaphase I to metaphase II transition in bovine oocytes. Reproduction. 2005;130:423–430. doi: 10.1530/rep.1.00754. [DOI] [PubMed] [Google Scholar]
- 161.Zhang M., Xia G. Hormonal control of mammalian oocyte meiosis at diplotene stage. Cell. Mol. Life Sci. 2011;69:1279–1288. doi: 10.1007/s00018-011-0867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kalous J., Kubelka M., Solc P., Susor A., Motlik J. Akt (protein kinase B) is implicated in meiotic maturation of porcine oocytes. Reproduction. 2009;138:645–654. doi: 10.1530/REP-08-0461. [DOI] [PubMed] [Google Scholar]
- 163.Lee S.E., Sun S.C., Choi H.Y., Uhm S.J., Kim N.H. mTOR is required for asymmetric division through small GTPases in mouse oocytes. Mol. Reprod. Dev. 2012;79:356–366. doi: 10.1002/mrd.22035. [DOI] [PubMed] [Google Scholar]
- 164.Cecconi S., Mauro A., Cellini V., Patacchiola F. The role of Akt signalling in the mammalian ovary. Int. J. Dev. Biol. 2012;56:809–817. doi: 10.1387/ijdb.120146sc. [DOI] [PubMed] [Google Scholar]
- 165.Franks S., Stark J., Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update. 2008;14:367–378. doi: 10.1093/humupd/dmn015. [DOI] [PubMed] [Google Scholar]
- 166.Yki-Jarvinen H. Thiazolidinediones. N. Engl. J. Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 167.Kirpichnikov D., McFarlane S.I., Sowers J.R. Metformin: an update. Ann. Intern. Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 168.Wang D.S., Jonker J.W., Kato Y., Kusuhara H., Schinkel A.H., Sugiyama Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002;302:510–515. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
- 169.Chen L., Shu Y., Liang X., Chen E.C., Yee S.W., Zur A.A., Li S., Xu L., Keshari K.R., Lin M.J., et al. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc. Natl. Acad. Sci. U.S.A. 2014;111:9983–9988. doi: 10.1073/pnas.1314939111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shu Y., Sheardown S.A., Brown C., Owen R.P., Zhang S., Castro R.A., Ianculescu A.G., Yue L., Lo J.C., Burchard E.G., et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Invest. 2007;117:1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jung N., Lehmann C., Rubbert A., Knispel M., Hartmann P., van Lunzen J., Stellbrink H.J., Faetkenheuer G., Taubert D. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab. Dispos. 2008;36:1616–1623. doi: 10.1124/dmd.108.020826. [DOI] [PubMed] [Google Scholar]
- 172.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lord J.M., Flight I.H., Norman R.J. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951–953. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Velazquez E.M., Mendoza S., Hamer T., Sosa F., Glueck C.J. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism. 1994;43:647–654. doi: 10.1016/0026-0495(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 175.Creanga A.A., Bradley H.M., McCormick C., Witkop C.T. Use of metformin in polycystic ovary syndrome: a meta-analysis. Obstet. Gynecol. 2008;111:959–968. doi: 10.1097/AOG.0b013e31816a4ed4. [DOI] [PubMed] [Google Scholar]
- 176.Legro R.S., Barnhart H.X., Schlaff W.D., Carr B.R., Diamond M.P., Carson S.A., Steinkampf M.P., Coutifaris C., McGovern P.G., Cataldo N.A., et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 177.Tso L.O., Costello M.F., Albuquerque L.E., Andriolo R.B., Marjoribanks J., Macedo C.R. Metformin treatment before and during in vitro fertilization or intracytoplasmic sperm injection in women with polycystic ovary syndrome: summary of a Cochrane review. Fertil. Steril. 2015;104:542–544. doi: 10.1016/j.fertnstert.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 178.Dunaif A. Drug insight: insulin-sensitizing drugs in the treatment of polycystic ovary syndrome–a reappraisal. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:272–283. doi: 10.1038/ncpendmet0787. [DOI] [PubMed] [Google Scholar]
- 179.Attia G.R., Rainey W.E., Carr B.R. Metformin directly inhibits androgen production in human thecal cells. Fertil. Steril. 2001;76:517–524. doi: 10.1016/S0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 180.Palomba S., Falbo A., Giallauria F., Russo T., Tolino A., Zullo F., Colao A., Orio F. Effects of metformin with or without supplementation with folate on homocysteine levels and vascular endothelium of women with polycystic ovary syndrome. Diabetes Care. 2010;33:246–251. doi: 10.2337/dc09-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fuhrmeister I.P., Branchini G., Pimentel A.M., Ferreira G.D., Capp E., Brum I.S., von Eye Corleta H. Human granulosa cells: insulin and insulin-like growth factor-1 receptors and aromatase expression modulation by metformin. Gynecol. Obstet. Invest. 2008;77:156–162. doi: 10.1159/000358829. [DOI] [PubMed] [Google Scholar]
- 182.Rice S., Pellatt L., Ramanathan K., Whitehead S.A., Mason H.D. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology. 2009;150:4794–4801. doi: 10.1210/en.2009-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kai Y., Kawano Y., Yamamoto H., Narahara H. A possible role for AMP-activated protein kinase activated by metformin and AICAR in human granulosa cells. Reprod. Biol. Endocrinol. 2015;13:27. doi: 10.1186/s12958-015-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Reverchon M., Cornuau M., Cloix L., Rame C., Guerif F., Royere D., Dupont J. Visfatin is expressed in human granulosa cells: regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013;19:313–326. doi: 10.1093/molehr/gat002. [DOI] [PubMed] [Google Scholar]
- 185.Sutton-McDowall M.L., Gilchrist R.B., Thompson J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139:685–695. doi: 10.1530/REP-09-0345. [DOI] [PubMed] [Google Scholar]
- 186.Richardson M.C., Ingamells S., Simonis C.D., Cameron I.T., Sreekumar R., Vijendren A., Sellahewa L., Coakley S., Byrne C.D. Stimulation of lactate production in human granulosa cells by metformin and potential involvement of adenosine 5′ monophosphate-activated protein kinase. J. Clin. Endocrinol. Metab. 2009;94:670–677. doi: 10.1210/jc.2008-2025. [DOI] [PubMed] [Google Scholar]
- 187.Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M., Gomes A.P., Ward T.M., Minor R.K., Blouin M.J., et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dunning K.R., Cashman K., Russell D.L., Thompson J.G., Norman R.J., Robker R.L. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development. Biol. Reprod. 2010;83:909–918. doi: 10.1095/biolreprod.110.084145. [DOI] [PubMed] [Google Scholar]
- 189.Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M., Kliewer S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 190.Kim J., Sato M., Li Q., Lydon J.P., Demayo F.J., Bagchi I.C., Bagchi M.K. Peroxisome proliferator-activated receptor gamma is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol. Cell. Biol. 2008;28:1770–1782. doi: 10.1128/MCB.01556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cui Y., Miyoshi K., Claudio E., Siebenlist U.K., Gonzalez F.J., Flaws J., Wagner K.U., Hennighausen L. Loss of the peroxisome proliferation-activated receptor gamma (PPARgamma) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. J. Biol. Chem. 2002;277:17830–17835. doi: 10.1074/jbc.M200186200. [DOI] [PubMed] [Google Scholar]
- 192.Nissen S.E., Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch. Intern. Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 193.Komar C.M. Peroxisome proliferator-activated receptors (PPARs) and ovarian function–implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod. Biol. Endocrinol. 2005;3:41. doi: 10.1186/1477-7827-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Froment P., Fabre S., Dupont J., Pisselet C., Chesneau D., Staels B., Monget P. Expression and functional role of peroxisome proliferator-activated receptor-gamma in ovarian folliculogenesis in the sheep. Biol. Reprod. 2003;69:1665–1674. doi: 10.1095/biolreprod.103.017244. [DOI] [PubMed] [Google Scholar]
- 195.Lambe K.G., Tugwood J.D. A human peroxisome-proliferator-activated receptor-gamma is activated by inducers of adipogenesis, including thiazolidinedione drugs. Eur. J. Biochem. 1996;239:1–7. doi: 10.1111/j.1432-1033.1996.0001u.x. [DOI] [PubMed] [Google Scholar]
- 196.Schoppee P.D., Garmey J.C., Veldhuis J.D. Putative activation of the peroxisome proliferator-activated receptor gamma impairs androgen and enhances progesterone biosynthesis in primary cultures of porcine theca cells. Biol. Reprod. 2002;66:190–198. doi: 10.1095/biolreprod66.1.190. [DOI] [PubMed] [Google Scholar]
- 197.Lohrke B., Viergutz T., Shahi S.K., Pohland R., Wollenhaupt K., Goldammer T., Walzel H., Kanitz W. Detection and functional characterisation of the transcription factor peroxisome proliferator-activated receptor gamma in lutein cells. J. Endocrinol. 1998;159:429–439. doi: 10.1677/joe.0.1590429. [DOI] [PubMed] [Google Scholar]
- 198.Wood J.R., Dumesic D.A., Abbott D.H., Strauss, 3rd J.F. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J. Clin. Endocrinol. Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 199.Seto-Young D., Avtanski D., Strizhevsky M., Parikh G., Patel P., Kaplun J., Holcomb K., Rosenwaks Z., Poretsky L. Interactions among peroxisome proliferator activated receptor-gamma, insulin signaling pathways, and steroidogenic acute regulatory protein in human ovarian cells. J. Clin. Endocrinol. Metab. 2007;92:2232–2239. doi: 10.1210/jc.2006-1935. [DOI] [PubMed] [Google Scholar]
- 200.Seto-Young D., Paliou M., Schlosser J., Avtanski D., Park A., Patel P., Holcomb K., Chang P., Poretsky L. Direct thiazolidinedione action in the human ovary: insulin-independent and insulin-sensitizing effects on steroidogenesis and insulin-like growth factor binding protein-1 production. J. Clin. Endocrinol. Metab. 2005;90:6099–6105. doi: 10.1210/jc.2005-0469. [DOI] [PubMed] [Google Scholar]
- 201.Komar C.M., Braissant O., Wahli W., Curry T.E., Jr Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology. 2001;142:4831–4838. doi: 10.1210/endo.142.11.8429. [DOI] [PubMed] [Google Scholar]
- 202.Fan W., Yanase T., Morinaga H., Mu Y.M., Nomura M., Okabe T., Goto K., Harada N., Nawata H. Activation of peroxisome proliferator-activated receptor-gamma and retinoid X receptor inhibits aromatase transcription via nuclear factor-kappaB. Endocrinology. 2005;146:85–92. doi: 10.1210/en.2004-1046. [DOI] [PubMed] [Google Scholar]
- 203.Wu S., Divall S., Nwaopara A., Radovick S., Wondisford F., Ko C., Wolfe A. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2014;63:1270–1282. doi: 10.2337/db13-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim S.Y., Ebbert K., Cordeiro M.H., Romero M., Zhu J., Serna V.A., Whelan K.A., Woodruff T.K., Kurita T. Cell autonomous phosphoinositide 3-kinase activation in oocytes disrupts normal ovarian function through promoting survival and overgrowth of ovarian follicles. Endocrinology. 2015;156:1464–1476. doi: 10.1210/en.2014-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Adhikari D., Zheng W., Shen Y., Gorre N., Hamalainen T., Cooney A.J., Huhtaniemi I., Lan Z.J., Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum. Mol. Genet. 2010;19:397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Adhikari D., Flohr G., Gorre N., Shen Y., Yang H., Lundin E., Lan Z., Gambello M.J., Liu K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol. Hum. Reprod. 2009;15:765–770. doi: 10.1093/molehr/gap092. [DOI] [PubMed] [Google Scholar]
- 207.Gorre N., Adhikari D., Lindkvist R., Brannstrom M., Liu K., Shen Y. mTORC1 Signaling in oocytes is dispensable for the survival of primordial follicles and for female fertility. PLoS One. 2014;9:e110491. doi: 10.1371/journal.pone.0110491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen Z., Kang X., Wang L., Dong H., Wang C., Xiong Z., Zhao W., Jia C., Lin J., Zhang W., et al. Rictor/mTORC2 pathway in oocytes regulates folliculogenesis, and its inactivation causes premature ovarian failure. J. Biol. Chem. 2015;290:6387–6396. doi: 10.1074/jbc.M114.605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fan H.Y., Liu Z., Shimada M., Sterneck E., Johnson P.F., Hedrick S.M., Richards J.S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bruning J.C., Gautam D., Burks D.J., Gillette J., Schubert M., Orban P.C., Klein R., Krone W., Muller-Wieland D., Kahn C.R. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 211.Tamemoto H., Kadowaki T., Tobe K., Yagi T., Sakura H., Hayakawa T., Terauchi Y., Ueki K., Kaburagi Y., Satoh S., et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 212.Burks D.J., Font de Mora J., Schubert M., Withers D.J., Myers M.G., Towery H.H., Altamuro S.L., Flint C.L., White M.F. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407:377–382. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- 213.Neganova I., Al-Qassab H., Heffron H., Selman C., Choudhury A.I., Lingard S.J., Diakonov I., Patterson M., Ghatei M., Bloom S.R., et al. Role of central nervous system and ovarian insulin receptor substrate 2 signaling in female reproductive function in the mouse. Biol. Reprod. 2007;76:1045–1053. doi: 10.1095/biolreprod.106.059360. [DOI] [PubMed] [Google Scholar]
- 214.Liu S.C., Wang Q., Lienhard G.E., Keller S.R. Insulin receptor substrate 3 is not essential for growth or glucose homeostasis. J. Biol. Chem. 1999;274:18093–18099. doi: 10.1074/jbc.274.25.18093. [DOI] [PubMed] [Google Scholar]
- 215.Fantin V.R., Wang Q., Lienhard G.E., Keller S.R. Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2000;278:E127–E133. doi: 10.1152/ajpendo.2000.278.1.E127. [DOI] [PubMed] [Google Scholar]
- 216.Castrillon D.H., Miao L., Kollipara R., Horner J.W., DePinho R.A. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 217.Calounova G., Livera G., Zhang X.Q., Liu K., Gosden R.G., Welsh M. The Src homology 2 domain-containing adapter protein B (SHB) regulates mouse oocyte maturation. PLoS One. 2010;5:e11155. doi: 10.1371/journal.pone.0011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li Q., He H., Zhang Y.L., Li X.M., Guo X., Huo R., Bi Y., Li J., Fan H.Y., Sha J. Phosphoinositide 3-kinase p110delta mediates estrogen- and FSH-stimulated ovarian follicle growth. Mol. Endocrinol. 2013;27:1468–1482. doi: 10.1210/me.2013-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brown C., LaRocca J., Pietruska J., Ota M., Anderson L., Smith S.D., Weston P., Rasoulpour T., Hixon M.L. Subfertility caused by altered follicular development and oocyte growth in female mice lacking PKB alpha/Akt1. Biol. Reprod. 2010;82:246–256. doi: 10.1095/biolreprod.109.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Restuccia D.F., Hynx D., Hemmings B.A. Loss of PKBbeta/Akt2 predisposes mice to ovarian cyst formation and increases the severity of polycystic ovary formation in vivo. Dis. Model Mech. 2012;5:403–411. doi: 10.1242/dmm.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Monteiro da Rocha A., Ding J., Slawny N., Wolf A.M., Smith G.D. Loss of glycogen synthase kinase 3 isoforms during murine oocyte growth induces offspring cardiac dysfunction. Biol. Reprod. 2015;92:127. doi: 10.1095/biolreprod.115.128181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gay E., Seurin D., Babajko S., Doublier S., Cazillis M., Binoux M. Liver-specific expression of human insulin-like growth factor binding protein-1 in transgenic mice: repercussions on reproduction, ante- and perinatal mortality and postnatal growth. Endocrinology. 1997;138:2937–2947. doi: 10.1210/endo.138.7.5282. [DOI] [PubMed] [Google Scholar]
- 223.Sonntag B., Gotte M., Wulfing P., Schuring A.N., Kiesel L., Greb R.R. Metformin alters insulin signaling and viability of human granulosa cells. Fertil. Steril. 2005;84(Suppl. 2):1173–1179. doi: 10.1016/j.fertnstert.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 224.Tosca L., Solnais P., Ferre P., Foufelle F., Dupont J. Metformin-induced stimulation of adenosine 5′ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol. Reprod. 2006;75:342–351. doi: 10.1095/biolreprod.106.050831. [DOI] [PubMed] [Google Scholar]
- 225.Tosca L., Rame C., Chabrolle C., Tesseraud S., Dupont J. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction. 2010;139:409–418. doi: 10.1530/REP-09-0351. [DOI] [PubMed] [Google Scholar]
- 226.Lee M.S., Kim S.H., Kim D.S., Min K.S., Yoon J.T. Metformin enhances the action of insulin on porcine granulosa-lutein cells in vitro. Anim. Reprod. Sci. 2012;136:100–107. doi: 10.1016/j.anireprosci.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 227.Will M.A., Palaniappan M., Peegel H., Kayampilly P., Menon K.M. Metformin: direct inhibition of rat ovarian theca-interstitial cell proliferation. Fertil. Steril. 2012;98:207–214. doi: 10.1016/j.fertnstert.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen Q., Sun X., Chen J., Cheng L., Wang J., Wang Y., Sun Z. Direct rosiglitazone action on steroidogenesis and proinflammatory factor production in human granulosa-lutein cells. Reprod. Biol. Endocrinol. 2009;7:147. doi: 10.1186/1477-7827-7-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rak-Mardyla A., Karpeta A. Rosiglitazone stimulates peroxisome proliferator-activated receptor gamma expression and directly affects in vitro steroidogenesis in porcine ovarian follicles. Theriogenology. 2014;82:1–9. doi: 10.1016/j.theriogenology.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 230.Chen J., Hudson E., Chi M.M., Chang A.S., Moley K.H., Hardie D.G., Downs S.M. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev. Biol. 2006;291:227–238. doi: 10.1016/j.ydbio.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 231.Kempna P., Hofer G., Mullis P.E., Fluck C.E. Pioglitazone inhibits androgen production in NCI-H295R cells by regulating gene expression of CYP17 and HSD3B2. Mol. Pharmacol. 2007;71:787–798. doi: 10.1124/mol.106.028902. [DOI] [PubMed] [Google Scholar]