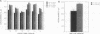Abstract
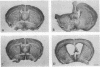
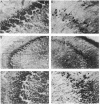
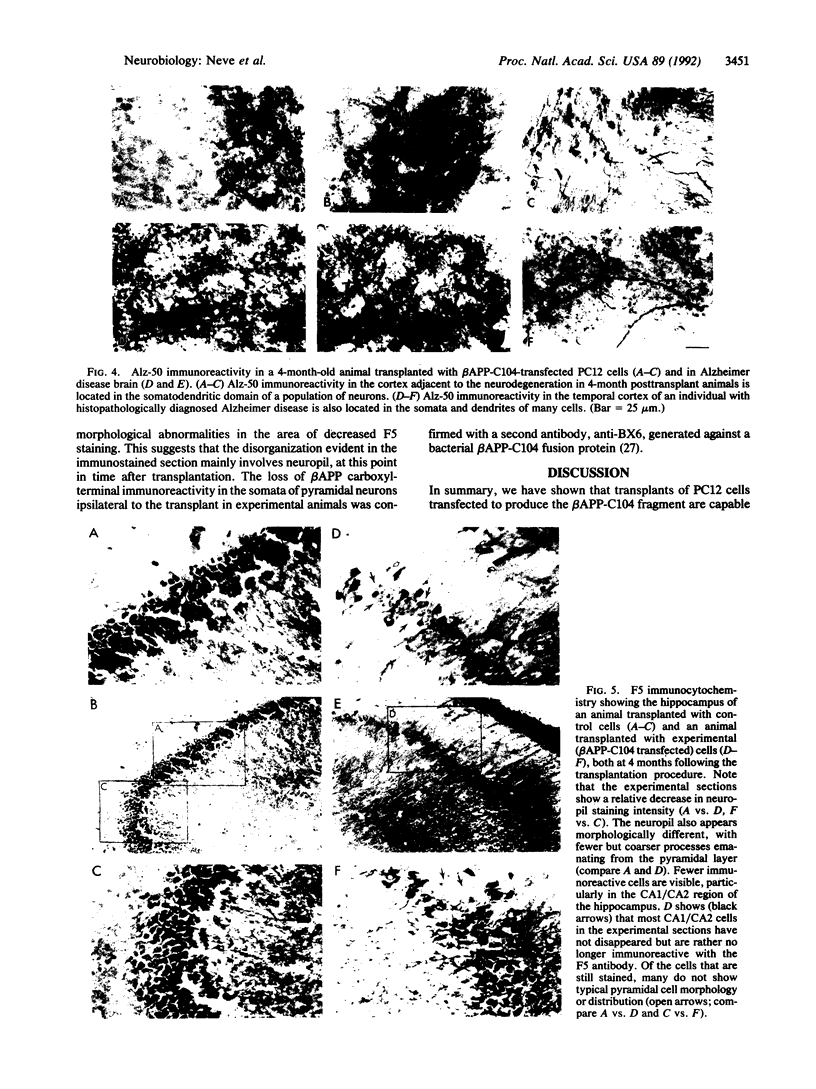
PC12 cells transfected with retroviral recombinants expressing the carboxyl-terminal 104 amino acids of the Alzheimer amyloid protein precursor (beta APP-C104) or PC12 cells transfected with the retroviral vector (DO) alone were transplanted into the brains of newborn mice. At 20 days after grafting, transplants could be detected in all of the mouse brains examined. At 4 months after transplantation, experimental animals exhibited significant cortical atrophy. Some also revealed immunoreactivity with Alz-50, an antibody that detects an Alzheimer disease-related protein, in the somatodendritic domain of neurons in the cortex surrounding the transplants. In addition, disorganization of the neuropil in the CA2/3 region of the hippocampus ipsilateral to the transplant was revealed by staining with an antibody to the carboxyl-terminal end of the amyloid protein precursor. A decrease in cell body immunoreactivity for this portion of the amyloid protein precursor was also detected with this antibody. Together, these results suggest that the carboxyl-terminal fragment of beta APP may cause specific neuropathology and neurodegeneration in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benowitz L. I., Rodriguez W., Paskevich P., Mufson E. J., Schenk D., Neve R. L. The amyloid precursor protein is concentrated in neuronal lysosomes in normal and Alzheimer disease subjects. Exp Neurol. 1989 Dec;106(3):237–250. doi: 10.1016/0014-4886(89)90156-8. [DOI] [PubMed] [Google Scholar]
- Dyrks T., Weidemann A., Multhaup G., Salbaum J. M., Lemaire H. G., Kang J., Müller-Hill B., Masters C. L., Beyreuther K. Identification, transmembrane orientation and biogenesis of the amyloid A4 precursor of Alzheimer's disease. EMBO J. 1988 Apr;7(4):949–957. doi: 10.1002/j.1460-2075.1988.tb02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Forloni G., Hohmann C., Coyle J. T. Developmental expression of somatostatin in mouse brain. I. Immunocytochemical studies. Brain Res Dev Brain Res. 1990 Apr 1;53(1):6–25. doi: 10.1016/0165-3806(90)90120-n. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Alzheimer's disease. The commonest form of amyloidosis. Arch Pathol Lab Med. 1983 Jun;107(6):281–282. [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Hohmann C. F., Brooks A. R., Coyle J. T. Neonatal lesions of the basal forebrain cholinergic neurons result in abnormal cortical development. Brain Res. 1988 Aug 1;470(2):253–264. doi: 10.1016/0165-3806(88)90244-1. [DOI] [PubMed] [Google Scholar]
- Höhmann C. F., Capone G., Oster-Granite M. L., Coyle J. T. Transplantation of brain tissue from murine trisomy 16 into euploid hosts: effects of gene imbalance on brain development. Prog Brain Res. 1990;82:203–214. doi: 10.1016/s0079-6123(08)62606-0. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., Kosik K. S. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol. 1987 Nov;22(5):639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Davies P., Yen S. H. Alz 50, a monoclonal antibody to Alzheimer's disease antigen, cross-reacts with tau proteins from bovine and normal human brain. J Biol Chem. 1988 Jun 15;263(17):7943–7947. [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lund R. D., Rao K., Kunz H. W., Gill T. J., 3rd Instability of neural xenografts placed in neonatal rat brains. Transplantation. 1988 Aug;46(2):216–223. doi: 10.1097/00007890-198808000-00006. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Terakado K., Usami M., Yoshikawa K. Formation of amyloid-like fibrils in COS cells overexpressing part of the Alzheimer amyloid protein precursor. Nature. 1990 Oct 11;347(6293):566–569. doi: 10.1038/347566a0. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto A., Correas I., Montejo de Garcini E., Avila J. A modified form of microtubule-associated tau protein is the main component of paired helical filaments. Biochem Biophys Res Commun. 1988 Jul 29;154(2):660–667. doi: 10.1016/0006-291x(88)90190-8. [DOI] [PubMed] [Google Scholar]
- Nukina N., Kosik K. S., Selkoe D. J. The monoclonal antibody, Alz 50, recognizes tau proteins in Alzheimer's disease brain. Neurosci Lett. 1988 May 3;87(3):240–246. doi: 10.1016/0304-3940(88)90455-7. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Roth M., Tomlinson B. E., Blessed G. Correlation between scores for dementia and counts of 'senile plaques' in cerebral grey matter of elderly subjects. Nature. 1966 Jan 1;209(5018):109–110. doi: 10.1038/209109a0. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Peck A., DeTeresa R., Schechter R., Horoupian D. S. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981 Aug;10(2):184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Uéda K., Masliah E., Saitoh T., Bakalis S. L., Scoble H., Kosik K. S. Alz-50 recognizes a phosphorylated epitope of tau protein. J Neurosci. 1990 Oct;10(10):3295–3304. doi: 10.1523/JNEUROSCI.10-10-03295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner H., Brundin P. Immunological aspects of grafting in the mammalian central nervous system. A review and speculative synthesis. Brain Res. 1988 Nov;472(3):287–324. doi: 10.1016/0165-0173(88)90010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D., Quon D., Wang Y., Cordell B. Identification and characterization of C-terminal fragments of the beta-amyloid precursor produced in cell culture. EMBO J. 1990 Jul;9(7):2079–2084. doi: 10.1002/j.1460-2075.1990.tb07375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]