Abstract
“Sickle cell anemia: tracking down a mutation” is a full-day, inquiry-based, biology experience for high school students enrolled in genetics or advanced biology courses. In the experience, students use restriction endonuclease digestion, cellulose acetate gel electrophoresis, and microscopy to discover which of three putative patients have the sickle cell genotype/phenotype using DNA and blood samples from wild-type and transgenic mice that carry a sickle cell mutation. The inquiry-based, problem-solving approach facilitates the students' understanding of the basic concepts of genetics and cellular and molecular biology and provides experience with contemporary tools of biotechnology. It also leads to students' appreciation of the causes and consequences of this genetic disease, which is relatively common in individuals of African descent, and increases their understanding of the first principles of genetics. This protocol provides optimal learning when led by well-trained facilitators (including the classroom teacher) and carried out in small groups (6:1 student-to-teacher ratio). This high-quality experience can be offered to a large number of students at a relatively low cost, and it is especially effective in collaboration with a local science museum and/or university. Over the past 15 yr, >12,000 students have completed this inquiry-based learning experience and demonstrated a consistent, substantial increase in their understanding of the disease and genetics in general.
Keywords: sickle cell, restriction digest, cellulose acetate, genetics, inquiry-based learning
genetic variation and natural selection are known to lead to both benefits and deleterious effects on organisms. In this activity, students explore a genetic disease from the level of DNA to protein to cell to phenotype using contemporary molecular biology tools and techniques such as restriction endonucleases, DNA agarose electrophoresis, cellulose acetate electrophoresis, and microscopy. Combined with classroom discussion, this intensive experience provides students with an opportunity to integrate science content knowledge via a hands-on experience. This laboratory is one of 4 day-long laboratory experiences in molecular biology that are part of The University of Alabama at Birmingham Center for Community OutReach Development's GENEius program hosted at McWane Science Center. As an out-of-class/in-research laboratory experience, students use cutting-edge biotechnology tools, gain an understanding of the concepts underlying the phenotypic expression of a genetic disease, and have an opportunity to integrate their content knowledge with hands-on experience. Furthermore, the laboratory is modifiable, so that it can be offered in kit form and conducted in a school laboratory.
Background
The United States has a great need for a science workforce for the 21st century, especially in the area of biomedical sciences (12a, 12b); however, relatively few students are strongly engaged in advanced science courses in precollege. This lack of engagement is especially acute among minority students (9, 12a, 12b, 12c). Thus, many colleges are collaborating with precollege education to discover ways to connect these students with engaging experiences that teach the first principles of science in a way that excites them. One of the challenges in secondary science education is finding interactive lesson plans that teach important principles that are cost and time effective and engage students in the learning process. This sickle cell anemia laboratory has proven to be an engaging physiology lesson plan that fulfills all of these goals, especially for students of African descent, almost all of whom know someone who suffers from sickle cell anemia.
Sickle cell anemia is a conundrum, a disease that protects from one disease (malaria) but eventually kills the “saved” patient. Malaria is one of the most common infectious diseases worldwide, with ∼500,000 new cases each year (14). Most malaria infections occur in the equatorial regions of the world (primarily in Africa and Asia), but it is rare in the United States, with only ∼1,000 new cases per year. Worldwide, malaria causes severe symptoms in many patients and leads to ∼1 death every 30 s (4). Plasmodium parasites (primarily P. falciparum but also P. vivax, P. ovale, P. malariae, and P. knowlesi) cause malaria, which is transmitted to people by female Anopheles mosquitoes (the infectious vector, which is the only mosquito species that can transmit the disease). The mosquito initially becomes infected by obtaining a blood meal containing microscopic malaria parasites from an infected person. When the mosquito take its next blood meal (∼1 wk later), the parasites are injected via the mosquito's saliva into another person. These parasites multiply within red blood cells, causing symptoms that include anemia, lightheadedness, shortness of breath, tachycardia, fever, chills, nausea, and, potentially, coma and death. Malaria transmission can be reduced by preventing mosquito bites using physical or chemical barriers or by controlling mosquitoes via insecticide spraying and by draining standing water where mosquitoes lay their eggs. Pharmaceutical treatments can decrease the severity and frequency of malaria symptoms, but they do not prevent or cure the disease. In contrast, new genetic treatments may provide methods to prevent or even “cure” the disease, a topic that may provide an excellent opportunity for extending the day-long sickle cell experience.
Hemoglobin A is the most common form of human hemoglobin. Sickle cell disease is a genetic disorder in which red blood cells contain hemoglobin S (S represents sickle). In contrast to hemoglobin A, hemoglobin S shortens the life of red blood cells (from ∼120 days to no more than 20 days, normally ∼16 days) and causes the cells to become rigid and crescent shaped rather than round. The stiff, sickle-shaped red blood cells block small blood vessels, leading to reduced blood flow and, ultimately, to damage of the tissue and organs normally perfused by that vessel. Individuals with the sickle cell trait produce both normal and abnormal hemoglobin. These individuals are heterozygous for the sickle cell gene but do not carry as severe clinical symptoms as homozygous individuals with the sickle cell gene.
In contrast to its adverse effects on cardiovascular health, sickle cell disease has beneficial effects relative to another disease, malaria, an infection that significantly lowers cytoplasm pH (increasing acidity), causing hemoglobin to release oxygen and thereby slowing the transit of red blood cells through capillaries. Sickling of the red blood cells disrupts the proliferation of the Plasmodium parasites, in part by depleting cellular K+, which is required for the parasite to grow. This results in the rapid death of Plasmodium parasites. Even if not all parasites die, sickling increases malaria resistance by reducing the rate of Plasmodium proliferation, thus providing the immune system with time to mount a significant response to the parasite, greatly reducing the adverse consequences of the infection (1, 13).
Sickle cell disease is an autosomal recessive disorder. Approximately 8.3% of African-Americans are heterozygous for the sickle cell gene (known as the sickle cell trait), but they do not develop anemia or clinical signs of the disease. In contrast, ∼0.1% of African-American newborns are homozygous for the sickle cell gene, leading to a high rate of morbidity and mortality (5). A single base or point mutation in the gene encoding hemoglobin leads to sickle cell disease. This affects the primary red blood cell protein that is necessary for the transport of oxygen from the lungs to tissues. The hemoglobin molecule is a tetramer composed of two α-subunits and two β-subunits. A single DNA base change, from adenine to thymine, leads to the substitution of valine for glutamic acid in the sixth position on the β-subunits (globin chain), thus altering the net charge and conformation of the sickle hemoglobin protein, causing deoxygenated hemoglobin to form insoluble polymers. The polymerization of sickle cell hemoglobin is responsible for the characteristic distorted shape of sickle cells and the severe symptoms of the disease (10).
While sickle cell disease protects against malaria, it did not develop as a direct response to malaria; it occurred by chance. People living in areas where malaria epidemics were common were at an increased risk of malaria, and with little or no medical care, the severe fever, enlarged spleen, and anemia led to rapid death, especially among children. The relatively small number of individuals with homozygous sickle cell disease (SS) typically died early in life due to complications of sickle cell anemia. In contrast, 80 times more individuals were heterozygous carriers of the disease (AS), and they were protected from malaria and, therefore, could pass the genes on to their children.
Learning Objectives
After finishing the activity and associated discussions, students should be able to:
1. Describe the basic genetic cause(s) of sickle cell disease, a disease that is relatively common.
2. Define the consequences of carrying a single or double copy of a recessive gene.
3. Develop an understanding of how a small genetic change results in a major change in the phenotype of an individual.
4. Use biology, chemistry, and physics concepts to assess the cause of a common disease.
5. Assess the importance of natural selection in leading to gene changes that benefit the organism in some environments but not in other environments.
Activity Level
This activity is suitable for high school and early college students who have a basic understanding of genetics. Because electrophoresis, restriction digest, and pipetting techniques are novel for the target audience, additional time is needed to introduce these concepts. Teachers may find it helpful to carry out a micropipetting practice exercise before the laboratory experience.
Prerequisite Student Knowledge or Skills
Before engaging in this activity, students should have a basic understanding of the first principles of molecular biology, such as the central dogma. Before the laboratory, a pipetting practice exercise and a basic understanding of the use of a microscope will prove helpful.
Time Required
The preassessment and lecture take ∼40 min. All components of the student laboratory experience take 4–5 h.
METHODS
Equipment and Supplies
The protocol requires filter purified water, 10× buffer E, restriction enzyme Bsu36I, BSA, and the appropriate enzyme buffer. Reagents can be purchased from Promega (www.promega.com). Cellulose acetate paper can be purchased from Sigma-Aldrich (ww.sigmaaldrich.com). SuperZ applicator and sample well plates can be ordered from Helena Laboratories (http://www.helena.com/catalog/sampleapplication.htm). 6× DNA loading dye can be prepared using 50% glycerol and 0.4% bromophenol blue. 50× Tris-acetate-EDTA (TAE) and Tris-EDTA-borate (TEB) buffer can be diluted to l× concentrations; 1.5% agarose gels for electrophoresis can be prepared by mixing agarose and l× TAE buffer and heating it in a microwave oven to melt the agarose. Ethidium bromide [EtBr; Fischer Scientific (www.fischersci.com)] stock solution (10 mg/ml) is added to melted agarose upon cooling (final: 0.01 mg/ml). The agarose-ethidium mix is poured into an agarose gel well tray. Finally, DNA, whole blood, and hemolysates can be obtained from transgenic mice by contacting Dr. Tim Townes (ttownes@uab.edu).
Instructions
The protocol for “Sickle cell anemia: tracking down a mutation” has three main steps (see Supplemental Material, appendix 1): DNA restriction digest, protein analysis, and microscopy.1
DNA restriction digest.
Students working in small groups are provided with mock patient samples labeled X, Y, and Z, representing AA, AS, or SS genotypes; students are blinded to the identity of the samples. Each student team assigns members to be the control and experimental samples. The control student labels their microcentrifuge tubes by writing the letter of their patient and “u,” meaning uncut DNA, e.g., Xu is uncut X. The experimental group, which will add the restriction enzyme Bsu36I, writes their patient letter and “c,” meaning that DNA is cut with the restriction enzyme, e.g., Xc is cut X. The restriction digest solution is mixed into Xc, and an agarose gel is prepared. To begin digestion, 20 μl filter purified water, 4 μl of 10× buffer E, 4 μl BSA, 10 μl unknown DNA, and 2 μl Bsu36I should be added to a microcentrifuge tube. The tube is placed in a microcentrifuge and pulsed for 10 s to mix the reagents. The tubes are incubated in a 37°C water bath for 2 h to allow digestion of the DNA, and gel electrophoresis is then performed. To view digested DNA by agarose gel electrophoresis, agarose gel electrophoresis of the digested DNA is carried out as previously described (12). Four microliters of 6× loading dye are added to 20 μl of the restriction digest, and this is loaded onto a 1.5% agarose gel prepared using 1× TAE buffer. Because the agarose gel contains high concentrations of EtBr, a suspected carcinogen, protective gloves should be worn and extreme caution should be taken when handling the gel once electrophoresis is completed. The gel is place on an ultraviolet light source for visualization of the DNA bands, and photomicrographic images can be taken of the gels to enable students to analyze the results and draw conclusions.
Hemoglobin protein analysis by cellulose acetate electrophoresis.
TEB buffer (75 ml) is poured in each side of the electrophoresis chamber, leaving the middle chamber empty, and 8.0 μl of each hemolysate sample is placed into the wells on the sample well plate. Hemolysates are blotted onto celluloses acetate paper and run at 200 V for 40 min. The gels are stained with amido black and decolorize with destaining solution (47.5% methanol and 5% acetic acid). Protocols for buffers, stains, and reagents can be found at Lab-Manual.com (11).
Microscopic observation of red blood cells.
Students should prepare a wet mount using 1 or 2 μl of transgenic (AA, AS, or SS) mouse blood (diluted 1:1 with PBS) onto the center of the slide. A coverslip is placed on top of the blood. A tissue is then placed on top of the coverslip and pressed gently with a thumb. Using a ×100 oil-immersion lens, students can observe the blood smear throughout the day to observe any changes in cell shape, size, or structure.
Troubleshooting
Because students work in small groups with a facilitator, experimental results tend to be predictable; however, students' lack of experience and the multistep protocols can result in less than optimal results. Common examples include the digested DNA sample appearing as a smear. This may result from the gel being punctured during loading or nuclease contamination. Another commonly obtained result is the unexpected digestion of samples that should not be cut, which is like due to cross contamination during the student's pipetting of the enzyme.
Safety Considerations
When using a microwave to liquefy gels, students should wear insulated gloves and let the gel cool (∼65°C) before adding EtBr. Only the instructors should handle EtBr while using appropriate precautions. Students should wear eye protection and gloves when visualizing the agarose gels under ultraviolet light. The instructor may choose to use alternative dye for EtBr, e.g., alternative gel systems, such as the MiniOne System (http://theminione.com/), which would allow students to track their DNA bands migration, in real time, without the use of EtBr or other hazardous dyes. Upon completion of the protocol, gels and any other contaminated materials should be properly disposed as hazardous waste.
RESULTS
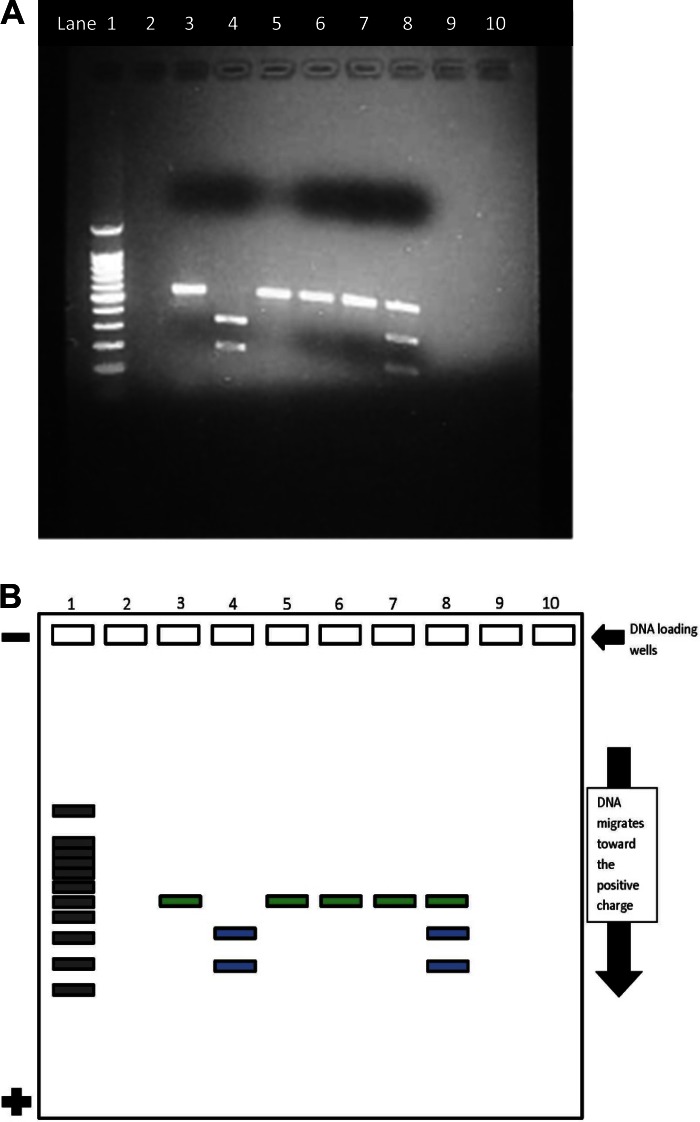
DNA digestion and electrophoresis demonstrate the phenotype of the DNA of patients X, Y, and Z, thereby allowing students to determine the correct genotypes for each mock patient sample. Typical results of the gel electrophoresis for each “patient's” DNA, both control (sample X uncut) and experimental (sample X cut), are shown in Fig. 1. Students should be able to determine the correct genotype because the restriction enzyme Bsu36I cuts normal DNA at the specific sequence CCT̂NAGG, resulting in 331- and 200-bp fragments of the 531-bp gene. Normal (A) DNA is cut once by the restriction enzyme, but the mutated sickle (S) DNA is not cut. Thus, students will find that the sample of patient X has two bands at 331 and 200 bp and that patient X has a normal homozygous AA genotype. Samples from patient Y yield three bands at 331, 200, and 531 bp, suggesting that both normal and sickle DNA are present. Students will conclude that patient Y is a heterozygous carrier of sickle cell anemia (genotype AS). A single ∼531-bp band will be apparent in the samples from patient Z, because the DNA was not cut by the enzyme, indicating a homozygous genotype (SS). The success rate for this experiment is high, ∼80%, but the experiments that fail can be equally valuable since they can lead to a discussion focused on exploring why the failure occurred, thus potentially stimulating even deeper learning.
Fig. 1.
A: atypical agarose gel photo micrographic image of digested DNA from normal, homozygous, and heterozygous sickle cell samples. See B for further details. Lane 1, 100-bp DNA marker; lane 2, empty; lane 3, sample X uncut; lane 4, sample X cut; lane 5, sample Y uncut; lane 6, sample Y cut; lane 7, sample Z uncut; lane 8, sample Z cut. B: a demonstration mockup of ideal results of the sickle cell agarose gel electrophoresis in the experience. The green fill demonstrates the slow migration of the uncut DNA. In control (unaffected) individuals, the DNA is cut by the restriction enzyme (Bsu36I) and splits into two bands (blue). The lack of an enzyme site in the homozygous sickle cell patient blocks the ability of Bsu36I to cut the DNA, and, therefore, only one green band appears. In heterozygous carriers, both the uncut and cut band appear, indicating that the individual has both unaffected (green) and affected (blue) DNA. Lane 1, 100-bp DNA marker, lane 2, empty; lane 3, sample X uncut; lane 4, sample X cut; lane 5, sample Y uncut; lane 6, sample Y cut; lane 7, sample Z uncut; lane 8, sample Z cut.
For the protein analysis portion of the study, students use patient hemolysate samples (samples A–C) to determine the form of hemoglobin in these “patients.” Cellulose acetate electrophoresis of lysates from red blood cells demonstrates that the glutamate to valine substitution, which occurs in sickle cell, changes the electrostatic charge of hemoglobin. Glutamate has a carboxyl (COOH) group that gives the normal β-globin protein a negative charge. In contrast, valine has an isopropyl [CH(CH3)2] group, which carries a neutral charge. This difference can be detected on the cellulose acetate, since the protein containing the glutamate residues will travel farther toward the positive pole of the gel than the protein containing the valine residues. The neutral or less negatively charged hemoglobin S does not migrate a significant distance away from the origin. Sample A will result in a single band close to the positive end, indicating that the sample contains normal β-globin protein and that the patient's genotype is AA. Sample B will have two protein bands, indicating that the patient has an AS genotype and is a carrier of the sickle cell gene. Sample C will present a single band that is relatively close to the negative pole (compared with sample A), indicating that this patient is homozygous for sickle cell disease (SS).
Misconceptions
Misconception 1.
Students often believe that sickle cell is a communicable disease, because it has some relation to malaria. They learn in this exercise that sickle cell disease is a genetic disease, inherited from parents, and that is not transmitted from one organism to another.
Misconception 2.
Students also often believe that all sickle cell patients die in childhood from organ failure, infection, and other complications. Participating in the sickle cell experience includes becoming informed about new treatments that allow sickle cell patients to live into their 50s and beyond (7).
Misconception 3.
Students are often unaware that there are effective treatments and cures for genetic diseases like sickle cell disease. They learn about new genetic approaches, combined with bone marrow transplantations, that have led to new opportunities to treat and even potentially cure sickle cell disease (8).
Misconception 4.
Many think that because they are not African-American, they do not need to worry about this disease. Sickle cell disease tends to preferentially affect certain ethnicities, because being a carrier of unusual hemoglobin may help protect against malaria in childhood. Thus, in places where malaria has been widespread, the genes became more “advantageous.” These areas include the Mediterranean region, sub-Saharan Africa, the Caribbean, the Middle East, and Asia. Today, both migration and mixed parentage mean that we live in increasingly diverse communities, and, thus, almost anyone could be affected.
Misconception 5.
Some students think that if a person is a carrier, their status is not something they need to know, especially as a young person, e.g., because it complicates the way of childbearing. In this disease, like others, it is important for parents and other relatives to be open with each other and their children about their health status, including genetic disorders. Being a carrier of unusual hemoglobin does not require any treatment, but understanding the risks will help individuals make informed choices.
Misconception 6.
Other students have the misconception that being a carrier means the individual is fully protected from malaria, a great advantage in regions that have high malaria rates. Being a carrier of the sickle cell gene protects somewhat against malaria, particularly during childhood; however, people who are carriers can still contract malaria. Furthermore, the advantage comes at a considerable potential cost for their children.
Assessment of Student Understanding
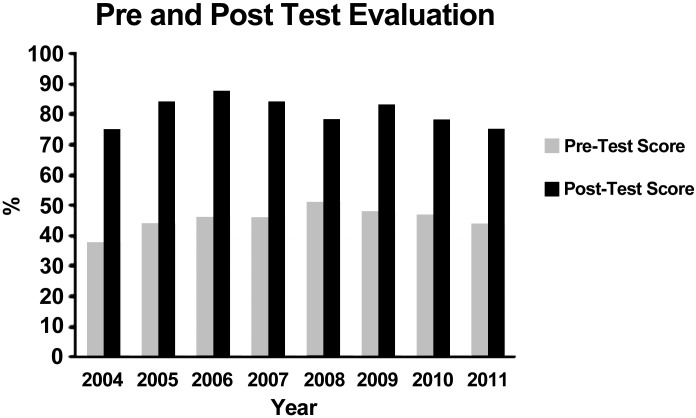
Pre- and posttests, using a clicker system (www.einstruction.com), are used to assess students' learning of the concepts underlying the sickle cell disease experience. The pretest is designed to assess students' prior knowledge of genetics, biotechnology, and the central dogma of molecular biology (see Fig. 2 and Supplemental Material, appendix 2). After the preassessment, one of the GENEius program facilitators presents a 15-min PowerPoint introduction to sickle cell disease. This presentation includes a review of the genetic and molecular basis for sickle cell disease and clinical features, prevalence, diagnosis, and treatment of sickle cell disease. The introduction also includes a description of the laboratory techniques that the students will carry out in the laboratory as well as descriptions/representative examples of predicted results and an interpretation and analysis of the results. PowerPoint (see Supplemental Material, appendix 3) is used for the presentation since it easily provides animations, internet movies, and video clips, which capture and maintain student attention.
Fig. 2.
Pre- and posttest scores (percent correct) for high school students taking this module of GENEius during the 2004–2011 school years (n = 18,128). See the test instrument in the Supplemental Material appendix 2.
After the introduction, each group of students (maximum of 6 students) is assigned a facilitator (typically an undergraduate, graduate, or postdoctoral trainee; however, peer students who have previously conducted the laboratory can also be outstanding facilitators, especially if the project is done in a school setting). The facilitators assist the students in conducting the experimental procedure and help them to understand the scientific concepts underlying the experience.
At the end of the experiments, students are given a posttest (Supplemental Material, appendix 2) to evaluate their understanding and knowledge gained from the laboratory experience. Students respond to basic questions regarding the experiment and the biotechnology used. After the posttest, students engage in a competitive and interactive “game” in which questions are asked about all aspects of the experience. Students enjoy the game, which challenges their knowledge and understanding of concepts related to genetics in general and more specifically to the techniques with which they have just gained experience. For this activity, students are divided into groups of six, with each group having a spokesperson that provides the answer that the group decides on. Scores are kept, and, at the conclusion of the game, the group with the most points wins. In the case of a tie, students must name and draw the expected results for the two types of electrophoresis used in the laboratory experiment.
Inquiry Applications
Inquiry application 1.
While this activity could be carried out in a cookbook fashion, the experience is enhanced by the teacher's encouraging students to carry out discovery-based learning and move beyond the experiment. It is possible that some groups' experiments will fail to yield the intended results. From these failures, students are encouraged to learn the basis for the failure, exercising their troubleshooting and critical thinking skills. From these “failures,” they may realize that the deeper interrogation, used to account for the failure, may lead to a more thorough understanding of a technique, a biological process, and a disease.
Inquiry application 2.
Interest generated by the experience will likely lead to the students' desire to learn more about sickle cell. Teachers may encourage students to research journal articles about sickle cell disease, thereby realizing the potential of recently developed therapies to alter the course of the disease. Their literature exploration should be discussed within working groups that eventually report their findings to the entire class, thus promoting team and presentation skills.
Wider Educational Applications
There are many concepts central to the sickle cell disease experience that may be extended and transferred to other related topics, for example:
1. Students may be encouraged to compare and contrast the consequences of genetic mutations in sickle cell disease with those from other common genetic diseases.
2. Students may be encouraged to consider if a genetic variant always carries some benefit to the organism bearing it. For instance, is there a benefit to having one copy of the cystic fibrosis gene or having Huntington's disease? Many other examples may be probed.
3. How might eye color or mitochondrial differences convey advantages to some bearing those genetic traits?
4. Students may also explore the ethical considerations related to how and when genetic information should be shared with individuals, in particular, with children. What is the consequence of not conveying this information to children, and why is it beneficial to learn more about one's genetic makeup?
5. The progress in genetic treatments for sickle cell disease is moving very rapidly and, in many ways, more rapidly than in other areas of genetic disease prevention. This is largely due to the availability of animal models of the disease; students may be reminded that they used blood obtained from animal models in their experiments. This may lead to a discussion of the use of animals in research, especially for genetic diseases and other biomedical studies.
GRANTS
This work was funded in part by National Institutes of Health Science Education Partnership Award Grants RR-022745 and OD-016490 and a grant from the United States Department of Education through the Alabama Commission on Higher Education.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.J., M.W., D.R., and J.M.W. conception and design of research; K.J., M.W., S.H., and D.R. performed experiments; K.J. analyzed data; K.J. interpreted results of experiments; K.J. prepared figures; K.J., S.H., and J.M.W. drafted manuscript; K.J., M.W., D.R., and J.M.W. edited and revised manuscript; K.J., M.W., S.H., and J.M.W. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the McWane Science Center for the generous support for, and collaboration in, these outreach activities.
Footnotes
Supplemental Material for this article is available at the Advances in Physiology Education website.
REFERENCES
- 1.Adam MR, George D, Douglas S, Fotis CK. Plasmodium activates the innate immune response of Anopheles gambiae mosquitoes. EMBO J 16: 6114–6119, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention. Malaria (online). http://www.cdc.gov/malaria/ [23 December 2015].
- 5.Centers for Disease Control and Prevention. Sickle Cell Disease (online). http://www.cdc.gov/ncbddd/sicklecell/index.html [23 December 2015].
- 7.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117–171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravitz L, Pincock S. Sickle-cell disease. Nature 515: S1–S1, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Hrabowski FA, Maton KI. Beating the Odds: Raising Academically Successful African American Males. New York: Oxford Univ. Press, 1998. [Google Scholar]
- 10.Keidan AJ, Sowter MC, Johnson CS, Noguch CT, Girling AJ, Stevens SME, Stuart J. Effect of polymerization tendency on haematological, rheological and clinical parameters in sickle cell anaemia. Br J Haematol 71: 551–557, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Lab-Manual.com. Lab-Manual.com homepage (online). http://www.lab-manual.com [23 December 2015].
- 12.Maniatis N, Collins A, Gibson J, Zhang W, Tapper W, Morton NE. Positional cloning by linkage disequilibrium. Am J Hum Genet 74: 846–55, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.National Academy of Sciences, National Academy of Engineering, Institute of Medicine of the National Academies. Rising Above the Gathering Storm: Energizing and Employing America for a Brighter Economic Future (online). http://www.nsf.gov/attachments/105652/public/NAS-Gathering-Storm-11463.pdf [23 December 2015].
- 12b.National Academy of Sciences, National Academy of Engineering, Institute of Medicine of the National Academies. Rising Above the Gathering Storm, Revisited: Rapidly Approaching Category 5 (online). https://www.unavco.org/education/advancing-geodetic-skills/short-courses/2014/field-education/Rise_Above_Gathering_Storm_Revisited_2010.pdf [23 December 2015]. [PubMed]
- 12c.Nature Immunology Editorial. Encouraging minority scientists. Nat Immunol 10: 927 2009. [DOI] [PubMed] [Google Scholar]
- 13.Tripet F, Aboagye-Antwi F, Hurd H. Ecological immunology of mosquito-malaria interactions. Trends Parasitol 24: 219–227, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. World Malaria Report (online). http://www.who.int/malaria/publications/world_malaria_report_2013/en/ [23 December 2015].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.