Abstract
High-capacity running (HCR) rats are protected against the early (i.e., ∼11 wk postsurgery) development of ovariectomy (OVX)-induced insulin resistance (IR) compared with low-capacity running (LCR) rats. The purpose of this study was to utilize the hyperinsulinemic euglycemic clamp to determine whether 1) HCR rats remain protected from OVX-induced IR when the time following OVX is extended to 27 wk and 2) tissue-specific glucose uptake differences are responsible for the protection in HCR rats under sedentary conditions. Female HCR and LCR rats (n = 40; aged ∼22 wk) randomly received either OVX or sham (SHM) surgeries and then underwent the clamp 27 wk following surgeries. [3-3H]glucose was used to determine glucose clearance, whereas 2-[14C]deoxyglucose (2-DG) was used to assess glucose uptake in skeletal muscle, brown adipose tissue (BAT), subcutaneous white adipose tissue (WAT), and visceral WAT. OVX decreased the glucose infusion rate and glucose clearance in both lines, but HCR had better insulin sensitivity than LCR (P < 0.05). In both lines, OVX significantly reduced glucose uptake in soleus and gastrocnemius muscles; however, HCR showed ∼40% greater gastrocnemius glucose uptake compared with LCR (P < 0.05). HCR also exhibited greater glucose uptake in BAT and visceral WAT compared with LCR (P < 0.05), yet these tissues were not affected by OVX in either line. In conclusion, OVX impairs insulin sensitivity in both HCR and LCR rats, likely driven by impairments in insulin-mediated skeletal muscle glucose uptake. HCR rats have greater skeletal muscle, BAT, and WAT insulin-mediated glucose uptake, which may aid in protection against OVX-associated insulin resistance.
Keywords: ovariectomy, insulin resistance, aerobic fitness, glucose uptake, brown adipose tissue
women are now spending almost half of their lives in menopause, characterized in part by ovarian hormone deficiency. This ovarian insufficiency leads to metabolic dysregulation, ultimately increasing risk for the development of type 2 diabetes (T2D) (3) and other cardiometabolic diseases to which postmenopausal women are particularly susceptible (5). The underlying mechanism(s) of menopause-associated changes in body composition and metabolic function is unclear; however, studies suggest that reduced physical activity (11), rather than increased food intake (10, 30), plays a critical role. Reduced physical activity decreases aerobic fitness, and impaired aerobic fitness is a strong risk factor for all-cause mortality (23). Conversely, exercise training or increased physical activity attenuates the prevalence of metabolic syndrome and T2D in postmenopausal women (12, 23). Ovariectomy (OVX) in rodents, a model of ovarian hormone depletion, is a good model of human menopause, as it provides insight into some of the menopause-associated changes such as increased adiposity and insulin resistance (IR) (36, 48). At least one rodent study (17) reported that exercise training mitigates OVX-induced metabolic disorders, but the impact of inherent aerobic capacity per se on attenuating OVX-induced metabolic dysfunction is still unclear.
Koch and Britton (22) developed a rat model of contrasting aerobic fitness [high-capacity running (HCR) and low-capacity running (LCR) rats] determined by endurance treadmill running. HCR rats display ∼30% greater aerobic fitness measured by V̇o2 max and ∼3.5-fold longer run time to exhaustion than the LCR rats. These contrasting phenotypes are evident even in the sedentary condition, so this model allows the investigation of inherent aerobic fitness independent of structured exercise training. Evidence based on investigation of the HCR/LCR model suggests a protective role of high aerobic capacity on a variety of metabolic outcomes (27, 29, 31, 38, 45, 47, 50). Female HCR rats showed greater mitochondrial aerobic capacity, including fatty acid oxidation in skeletal muscle (29, 38), and greater inherent insulin sensitivity compared with LCR rats (50). The greater basal skeletal muscle oxidative capacity in HCR appeared to prevent or attenuate metabolic dysfunction, including IR associated with consumption of a high-fat diet (29, 31). Recently, we demonstrated that HCR rats are protected from early development (i.e., ∼11 wk postsurgery) of OVX-induced IR assessed by homeostatic model assessment of insulin resistance (HOMA-IR) (47) and found that, unlike HCR rats, LCR rats had an exaggerated insulin response to a glucose tolerance test. In this study, we determined whether HCR rats remain protected from OVX-induced IR when the time following OVX is extended to 27 wk and how these rat lines differ in tissue-specific insulin-mediated glucose uptake across tissues in both OVX and ovary-intact [i.e., sham (SHM)] conditions.
It is known that ovary-intact female HCR rats display 30–50% greater insulin-induced glucose uptake and oxidation in skeletal muscle compared with LCR rats (35). Indeed, striking skeletal muscle phenotypic differences have been observed between HCR and LCR rats (29, 35, 38). Additionally, white (WAT) and brown adipose tissues (BAT) are gaining appreciation as also being important tissues in whole body glucose regulation (26) and may influence changes in glucose metabolism associated with both loss of ovarian hormones (39) and low aerobic capacity (44). No studies have investigated whether HCR rats possess greater insulin-mediated glucose metabolism in WAT and BAT compared with LCR rats and/or whether OVX affects this intrinsically enhanced phenotype in WAT and BAT.
In the present study, we employed the “gold standard” hyperinsulinemic euglycemic clamp technique to determine whether rats selectively bred for high aerobic capacity are protected from OVX-induced reduction in whole body insulin sensitivity and glucose clearance, and if so, what specific tissues may be driving this protection. To this end, insulin-mediated tissue-specific glucose uptake was measured and compared among groups using 2-[14C]deoxyglucose (2-DG) radiolabeled isotope during a clamp procedure. We hypothesized that protection against OVX-induced IR in HCR rats would be attributed largely to greater insulin-stimulated skeletal muscle glucose uptake and also in part to enhanced glucose uptake in WAT and BAT.
METHODS
Animals.
Previous research has described the HCR/LCR rat model well (27, 29, 31, 45). Forty female HCR and LCR rats from generation 33 were shipped to the University of Missouri and housed singly under standard humidity and temperature on a 12:12-h light-dark cycle. All animals were fed standard rodent chow (Harlan Teklad Rodent Diet 8604) and water ad libitum throughout the study. Animal procedures were approved by Institutional Animal Care and Use Committee at the University of Missouri-Columbia.
Experimental design.
At ∼22 wk of age, HCR and LCR rats were randomized to receive OVX or SHM surgeries, generating the following groups: HCRSHM, HCROVX, LCRSHM, and LCROVX (n = 10/group). All rats underwent a hyperinsulinemic euglycemic clamp procedure 27 wk following OVX or SHM surgery. Three to five days following surgical catheterization of arterial and venous lines, rats were fasted for 5 h, and a hyperinsulinemic euglycemic clamp was initiated to assess whole body insulin sensitivity. During the clamp, [3-3H]glucose radiolabeled isotope was used to determine glucose clearance and glycolytic rate, whereas 2-DG was used to examine the tissue-specific assessment of glucose uptake. Following the clamp, skeletal muscle (soleus and gastrocnemius), BAT, subcutaneous (SC), and visceral WAT [i.e., retroperitoneal (RP), perigonadal (PG), and omental (OMEN)] were collected and stored at −80°C until they were analyzed. Body weight was measured pre-, mid-, and post-surgery.
OVX and SHM surgeries.
OVX and SHM surgeries were conducted as described previously (18). Briefly, while rats were anesthetized with inhaled 2% isoflurane, a one-inch incision at midline of the dorsal surface was made, followed by bilateral incisions through the muscle layer. The whole ovarian bursa was removed for OVX procedure, whereas it was externalized and located back inside the body for the SHM procedure. The skin incision was closed using wound clips, and acetaminophen (500 mg/kg) was given.
Catheterization surgeries.
Catheterization surgeries were performed as described previously (1). The right jugular vein and the left common carotid artery were catheterized under anesthetization with inhaled 2% isoflurane. The free ends of the catheter lines tunneled subcutaneously to the back of the neck and were exteriorized and sealed with steel plugs. The clamp experiments were performed when the body weights of the rats reached within 5% of presurgery weight (3–5 days). To maintain the proper function of the catheter lines during these recovery days, the lines were flushed with heparinized saline twice a day.
Hyperinsulinemic euglycemic clamps.
Following an ∼5-h fast, hyperinsulinemic euglycemic clamps were conducted in conscious rats as described previously (1). Following collection of a baseline blood sample, a priming bolus (20 μCi) of 3H-radiolabeled isotope was infused, followed by a constant infusion (0.2 μCi/min) of 3H for 2 h. A second baseline of blood sample was collected. A priming bolus of insulin (20 mU/kg) was given, followed by a constant infusion of insulin (4 mU·kg−1·min−1) and [3-3H]glucose (0.4 μCi/min) for 2 h. Glucose infusion rate (glucose concentration: 50 g/100 ml) was adjusted for a 2-h period to maintain euglycemia during the clamp. Blood samples were taken every 10 min for the last 40 min out of 2 h (80- to 120-min time points) to assess glucose infusion rate, glucose clearance, and glycolytic rate. At the end of the clamp a bolus of 2-DG (39 μCi) was infused, and 25 min following the bolus, targeted tissues were promptly harvested and frozen for assessment of the tissue-specific assessment of glucose uptake. Rates of whole body glucose clearance were determined as the ratio of the [3H]-specific activity of plasma glucose to the net glucose levels during the final 40 min. Glycolytic rate was determined by 3H2O accumulation, the difference in plasma [3H] radioactivity between nondried and dried samples during the final 40 min.
Tissue-specific assessment of glucose uptake.
Glucose uptakes in skeletal muscle (i.e., soleus and gastrocnemius), BAT, subcutaneous WAT, and visceral WAT were determined via the assessment of 2-DG. Briefly, ∼60 mg of powdered tissues was homogenized in 1.5 ml of 0.5% perchloric acid and centrifuged for 20 min at 2,000 g at 4°C. The supernatant was neutralized at 7.5 pH, mixed with 125 μl each of 0.3 N BaOH and 0.3 N ZnSO4, and analyzed by liquid scintillation counter (Beckman Coulter, Brea, CA). Glucose uptake was calculated as disintegrations/min of [14C] radioactivity per milligram of tissue per specific activity of the tracers.
Tracer incorporation into skeletal muscle glycogen.
Incorporation of [3-3H]glucose-radiolabeled isotopes into skeletal muscle glycogen was measured to determine glucose retention into glycogen. Approximately 30 mg of powdered skeletal muscle tissue was solubilized in 500 μl of 1 N NaOH for 1 h at 37°C (vortex every 15 min). Following mixed carrier glycogen (33 μl of 60 mg/ml) and 1.2 ml of 75% ethanol to homogenates, the glycogen was precipitated overnight at 4°C. Samples were centrifuged for 10 min (10,000 g, 4°C), and the supernatant was discarded. The glycogen pellets were dried at the bottom of each tube, resuspended in 600 μl of H2O, and analyzed by liquid scintillation counter (Beckman Coulter). Glucose retention into glycogen was calculated as disintegrations/min of [3H] radioactivity per milligram of tissue per specific activity of the tracers.
Statistical analysis.
Statistical differences were analyzed using a 2 (HCR vs. LCR) × 2 (SHM vs. OVX) ANOVA. We determined whether differences occurred between line (HCR vs. LCR) and treatment (SHM vs. OVX) and whether treatment × line interactions existed. Bivariate Spearman's correlations were performed to determine the tissue-specific association between the whole body glucose clearance and glucose uptake in HCR and LCR rats. All data were analyzed using SPSS 22.0. In all cases, P < 0.05 was considered statistically significant, and data are reported as means ± SE.
RESULTS
Animal characteristics and body composition.
Prior to OVX, body weight was ∼18% greater in LCR compared with HCR (line main effect, P < 0.001; Fig. 1A), and both HCR and LCR experienced OVX-induced weight gain (treatment main effect, P = 0.003; Fig. 1B). LCR rats were heavier than HCR rats at the end of the study (line main effect, P < 0.001; Fig. 1B). The body weight differences paralleled differences in visceral (i.e., RP, PG, and OMEN) and SC WAT weight (Table 1) such that both lines increased WAT percentage following OVX (treatment main effect, P = 0.012), and LCR had greater adiposity than HCR rats (line main effect, P < 0.001) (Fig. 1C).
Fig. 1.
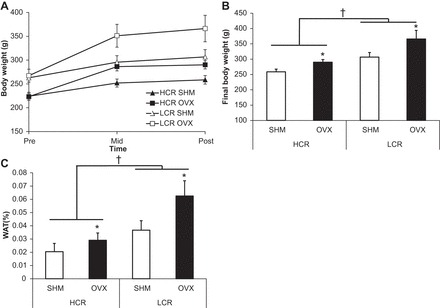
Body weight and fat percentage. A: body weight gain curves pre-, mid-, and postexperiment. B: final body weight. C: white adipose tissue (WAT) weight. Values are means ± SE (n = 7–10/group). †P < 0.05, line main effect, high-capacity running (HCR) vs. low-capacity running (LCR) rats; *P < 0.05, treatment main effect, sham (SHM) vs. ovariectomy (OVX).
Table 1.
Tissue weights
| Tissue, g | HCR SHM | OVX | LCR SHM | OVX | Two-Way ANOVA Statistics |
|---|---|---|---|---|---|
| Visceral WAT | |||||
| RP | 1.12 ± 0.30 | 2.24 ± 0.38 | 2.58 ± 0.48 | 5.68 ± 1.37 | Line, P = 0.001; treatment, P = 0.005 |
| PG | 1.87 ± 0.52 | 2.15 ± 0.52 | 3.26 ± 0.71 | 5.16 ± 1.10 | Line, P = 0.003 |
| OMEN | 0.72 ± 0.15 | 1.24 ± 0.27 | 1.37 ± 0.29 | 2.39 ± 0.61 | Line, P = 0.014; treatment, P = 0.034 |
| SC | 1.46 ± 0.27 | 2.47 ± 0.36 | 3.65 ± 0.71 | 7.72 ± 1.32 | Line, P < 0.001; treatment, P < 0.001; line × treatment, P = 0.038 |
| Liver | 7.88 ± 0.37 | 7.90 ± 0.37 | 8.61 ± 0.47 | 8.75 ± 0.69 | NS |
| Heart | 0.948 ± 0.046 | 0.933 ± 0.047 | 0.873 ± 0.025 | 0.913 ± 0.040 | NS |
| Uterus | 0.483 ± 0.037 | 0.074 ± 0.013 | 0.528 ± 0.043 | 0.129 ± 0.028 | Treatment, P < 0.001 |
Values are means ± SE; n = 8–10/group. HCR, high-capacity running; OVX, ovariectomy; LCR, low-capacity running; SHM, sham; WAT, white adipose tissue; RP, retroperitoneal; PG, perigonadal; OMEN, omental; SC, subcutaneous; NS, not significant. Body composition was analyzed. Two-way ANOVAs for line (i.e., HCR vs. LCR), treatment (i.e., SHM vs. OVX), and line × diet interaction effects were determined.
Insulin sensitivity and glycolytic rate.
No significant differences in glucose levels were found between groups at any time points for the last 40 min during the clamp, which was indicative of a maintained euglycemic state. The HCR rats were more insulin sensitive, as indicated by a greater glucose infusion rate and glucose clearance compared with LCR rats (line main effect, P < 0.001 and P = 0.037, respectively), yet OVX decreased glucose infusion rate and glucose clearance in both lines (treatment main effect, P = 0.014 and P = 0.005, respectively; Fig. 2, B and C). Glycolytic rate during the hyperinsulinemic euglycemic clamp was greater in HCR compared with LCR rats (line main effect, P = 0.002; Fig. 2D). OVX also tended to decrease glycolytic rate in both lines (treatment main effect, P = 0.053; Fig. 2D).
Fig. 2.
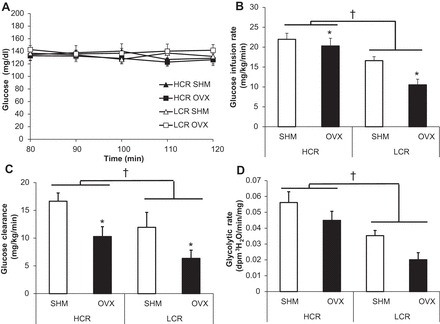
Insulin sensitivity and glucose clearance. A: glucose levels for the last 40 min during the clamp (80- to 120-min time points). B: glucose infusion rate. C: glucose clearance. D: glycolytic rate. Values are means ± SE (n = 6–8/group). †P < 0.05, line main effect, HCR vs. LCR rats; *P < 0.05, treatment main effect, SHM vs. OVX.
Tissue-specific assessment of glucose uptake.
Glucose uptake in slow-twitch predominant soleus muscle tended to be greater in HCR compared with LCR rats (line main effect, P = 0.072), whereas in both lines OVX significantly reduced glucose uptake in this muscle type (treatment main effect, P = 0.023; Fig. 3). Compared with LCR rats, HCR rats showed significantly greater glucose uptake in the fast-twitch predominant gastrocnemius muscle (line main effect, P = 0.002), and as in the case with soleus, OVX reduced gastrocnemius glucose uptake in both lines (treatment main effect, P < 0.001; Fig. 3). In examining adipose tissues, HCR rats exhibited greater glucose uptake in BAT and visceral WAT than LCR rats (1.7- and 2-fold greater uptake, respectively; line main effects, P = 0.005 and P = 0.003, respectively; Fig. 3), and there was a similar trend in subcutaneous WAT (line main effect, P = 0.062). Unlike with skeletal muscle, OVX had no effect on insulin-stimulated glucose uptake in any of the adipose tissue depots.
Fig. 3.
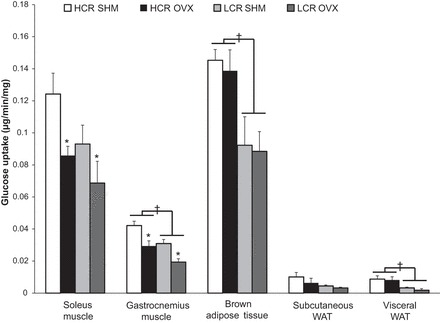
Tissue-specific glucose uptake. Glucose uptake in skeletal muscle (i.e., soleus and gastrocnemius), brown adipose tissue, and WAT (subcutaneous and visceral WAT). Values are means ± SE (n = 5–8/group). †P < 0.05, line main effect, HCR vs. LCR rats; *P < 0.05, treatment main effect, SHM vs. OVX.
Tracer incorporation into glycogen.
Compared with LCR rats, HCR rats had greater [3H]glucose retention in glycogen in both soleus and gastrocnemius muscles (line main effect, P = 0.014 and P = 0.003, respectively), and OVX reduced [3H]glucose retention in glycogen in soleus and gastrocnemius in both lines (treatment main effect, P = 0.013 and P = 0.016, respectively; Fig. 4A).
Fig. 4.
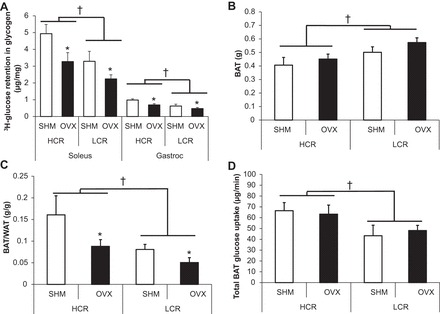
Glucose retention as skeletal muscle glycogen and contribution of brown adipose tissue (BAT) glucose uptake. A: [3H]glucose retention in glycogen. B: BAT mass. C: BAT/WAT ratio. D: total BAT glucose uptake. Values are means ± SE (n = 5–8/group). †P < 0.05, line main effect, HCR vs. LCR rats; *P < 0.05, treatment main effect, SHM vs. OVX.
BAT glucose uptake.
BAT mass was greater in LCR than in HCR rats (line main effect, P = 0.01; Fig. 4B). The BAT/WAT ratio was higher in HCR than LCR, and OVX reduced it in both lines (line main effect, P = 0.01; treatment main effect, P = 0.023; Fig. 4C). The total amount of BAT glucose uptake was greater in HCR than in LCR (line main effect, P = 0.044; Fig. 4D).
Tissue-specific relationship between glucose uptake and whole body glucose clearance.
Whole body glucose clearance was strongly associated with glucose uptake by skeletal muscles, BAT, and subcutaneous WAT (soleus: r = 0.441, P = 0.02; gastrocnemius: r = 0.680, P < 0.001, BAT: r = 0.490, P = 0.014; SC WAT: r = 0.569, P = 0.005; Fig. 5). In comparing HCR and LCR rats in terms of how specific tissues contributed to whole body glucose clearance, adipose tissue glucose uptake was the strongest correlation to systemic glucose clearance in LCR rats [r = 0.68 and r = 0.61 for WAT and BAT, respectively (P < 0.02 in each case), compared with r = 0.65 (P < 0.01) and r = 0.34 (P = 0.12) for WAT and BAT, respectively, among HCR]. Among HCR rats, the strongest correlation to systemic glucose clearance was skeletal muscle [r = 0.72 and r = 0.56 for gastrocnemious and soleus muscles, respectively (P < 0.01 for both)], whereas those correlations among LCR were r = 0.59 (P = 0.02) and r = 0.21 (P = 0.07), respectively.
Fig. 5.
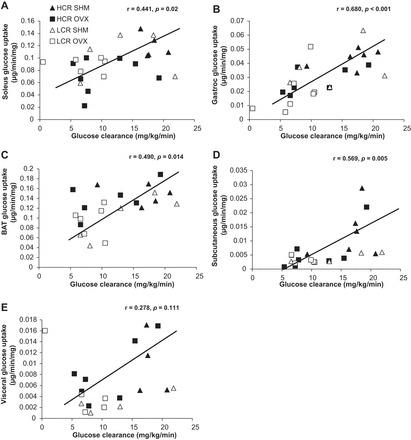
Relationships between tissue-specific glucose uptake and whole body glucose clearance. A: association between soleus glucose uptake and whole body glucose clearance. B: association between gastrocnemius glucose uptake and whole body glucose clearance. C: association between BAT glucose uptake and whole body glucose clearance. D: association between subcutaneous glucose uptake and whole body glucose clearance. E: association between visceral glucose uptake and whole body glucose clearance. Values are means ± SE (n = 5–8/group).
DISCUSSION
The purpose of this study was to determine 1) whether high aerobic capacity, independent of exercise training, protects against the development of OVX-induced reduction in whole body insulin sensitivity and glucose clearance during hyperinsulinemic euglycemic clamp conditions and 2) what specific tissues may be driving this protection. By ∼7 mo following OVX, both high-fit HCR and low-fit LCR rats developed IR indicated by reduced glucose infusion rate and glucose clearance during the clamp procedure. However, HCR rats remained more insulin sensitive than LCR rats in both SHM and OVX conditions. Given the increasing body of evidence utilizing the HCR/LCR rat model to illustrate the robust protection imparted by high aerobic fitness on a wide variety of metabolic outcomes, the fact that the HCR rat is not resilient to OVX-associated IR is quite remarkable. Moreover, the OVX-associated impairment in glucose clearance observed in both rat lines appeared to be due specifically to impairment in skeletal muscle insulin-mediated glucose uptake rather than impairments in BAT or WAT. This was especially surprising since a key distinguishing feature between HCR and LCR rats is their divergence in skeletal muscle metabolic phenotype (31). That this enhanced skeletal muscle phenotype of the HCR rat was not sufficient to protect against OVX-mediated impairments highlights the strong physiological effect of OVX on skeletal muscle insulin sensitivity.
Skeletal muscle is thought to dispose of up to ∼80% of the glucose load during hyperinsulinemic clamp conditions (8); thus skeletal muscle insulin sensitivity and oxidative metabolism play an important role in maintaining euglycemia during these conditions (20). Moreover, decreased skeletal muscle mitochondrial content and function associated with low aerobic capacity is thought to cause IR (21, 34). Our group and others using the HCR/LCR model have reported that HCR rats have greater mitochondrial aerobic capacity, increased mitochondrial content in skeletal muscle (29, 38, 47), and 30–50% greater insulin-mediated glucose uptake and oxidation in skeletal muscle (35). Despite that, both the HCR and LCR rats studied here similarly reduced insulin-mediated skeletal muscle glucose uptake in response to OVX, indicated in part by reduced insulin-mediated [14C]glucose uptake in soleus muscle (HCR: ∼30%; LCR: ∼26% decrease compared with each SHM group). The evidence in [3H]glucose retention in skeletal muscle glycogen confirmed this (HCR: ∼35%; LCR: ∼32% decrease compared with each SHM group), which paralleled the OVX-induced impairments in whole body glucose clearance (HCR: ∼37%; LCR: ∼45% decrease compared with each SHM group). Interestingly, Bergeron et al. (2) showed that OVX rats did not develop systemic IR 6 wk following surgery, yet they did develop impairments in skeletal muscle insulin signaling. This may suggest that OVX-mediated impairment in skeletal muscle insulin sensitivity is an early result of OVX and perhaps an initiating factor for systemic IR.
An important question is whether lack of ovarian hormones directly or indirectly impairs insulin signaling. Importantly, OVX reduces spontaneous physical activity (SPA) (46), even in HCR rats that we previously demonstrated to show an ∼20% reduction in SPA following OVX, consistent with the reduction observed in LCR rats (47). Although we did not measure SPA in this particular set of animals, it is possible that an OVX-mediated drop in SPA is mechanistically responsible for the OVX-mediated reduction in skeletal muscle insulin sensitivity noted here. It might be that the emergence of IR in the HCR rat after several months is the result of the cumulative effect of reduced SPA over this extended time frame. This hypothesis needs to be addressed in future studies. Bergeron et al. (2) did not assess the OVX-mediated reduction in physical activity but did find that voluntary wheel running rescued the muscle IR observed is sedentary OVX rats. Future work should elucidate whether OVX-mediated impairments in insulin sensitivity are fully or partially explained by an OVX-associated reduction in SPA. Alternatively, the OVX-mediated skeletal muscle impairments may have been attributed directly to ovarian hormone loss (e.g., loss of estrogen signaling in skeletal muscle), which has been associated with reduced skeletal muscle mitochondrial aerobic capacity (6). Others have shown that OVX in rats with normal intrinsic aerobic capacity led to an ∼50% attenuation in skeletal muscle insulin-stimulated [14C]glucose uptake and incorporation into glycogen (24), and a study investigating skeletal muscle ex vivo of OVX female rodents showed an ∼30% reduction in insulin-mediated [14C]glucose uptake compared with that of SHM rodents (33).
Rivas et al. (35) hypothesized that skeletal muscle mitochondrial content differences in HCR/LCR rats are fiber type specific, reporting greater mitochondrial content in HCR compared with LCRbrats, but only in fast-twitch predominant white muscle (i.e., extensor digitorum longus), whereas slow-twitch predominant soleus was not different between lines. Although we did not measure skeletal muscle mitochondrial aerobic capacity or content in the present study, we found that glucose uptake into both muscle types tended to be greater in HCR compared with LCR rats, although the line difference for soleus did not reach statistical significance (P = 0.072), whereas that in gastrocnemious did. Soleus displayed an approximately threefold greater insulin-stimulated glucose uptake than gastrocnemius muscle in both HCR and LCR rats. Red skeletal muscle (e.g., slow-twitch predominant soleus) is thought to be more insulin sensitive than white skeletal muscle (e.g., fast-twitch predominant gastrocnemius) (16) due to its higher oxidative capacity and mitochondrial density. That OVX affected both muscle types similarly may suggest that the effects of OVX on skeletal muscle insulin sensitivity are not fiber type specific. More research is certainly needed to determine the precise mechanisms by which OVX affects skeletal muscle metabolic function.
Similarly to postmenopausal women, OVX rodents increase adiposity along with their development of IR (42, 51). Moreover, adipose tissue is influenced heavily by female sex hormones, and we have demonstrated previously that OVX leads to adipose tissue inflammation, which precedes the development of systemic IR in that model (46). Female ovary-intact HCR rats are protected against diet induced IR (29, 31), which is thought to be driven by their increased skeletal muscle substrate oxidation (29, 38) and mitochondrial content (47), as mentioned above. Our group demonstrated recently that HCR rats are also protected from OVX-induced increases in adiposity and systemic IR assessed by fasting glucose and HOMA-IR, unlike LCR rats, which did experience impairments in those variables (47). Thus, we wanted to compare adipose tissue and skeletal muscle insulin sensitivity following OVX in HCR and LCR rats. Contrary to our original hypothesis, HCR rats are not resilient to developing OVX-induced IR and/or OVX-associated changes in body composition over the long term (i.e., 27 wk). We found that both HCR and LCR rats ultimately develop OVX-associated increased adiposity and IR, as assessed by the reduced glucose infusion rate during a glucose clamp. In our previous study (47), HCR rats exhibited a gradual (albeit non-significant) increase over time in body weight during the 11-wk period but were protected from the overt metabolic manifestations of OVX observed in LCR. Perhaps the attenuation of weight gain and adiposity in HCR delays, but does not prevent, the development of OVX-associated metabolic syndrome; increased adiposity does often precede the development of metabolic syndrome (13). The reason why HCR rats were not fully protected from OVX-induced IR despite their enhanced aerobic capacity and skeletal muscle mitochondrial content and function compared with LCR rats is not clear but may involve the fact that HCR rats had significantly better adipose tissue insulin sensitivity, at least under clamp conditions, than LCR rats across both brown and white depots.
Importantly, HCR rats in the present study did have >30% greater insulin sensitivity and glucose clearance compared with LCR rats in both ovarian conditions, supporting that high intrinsic fitness does at least lessen the metabolically damaging effects of OVX. Although OVX did not affect adipose tissue insulin sensitivity in either line, the markedly greater glucose uptake in both BAT and WAT of HCR rats may have “buffered” their OVX-associated systemic IR. Adipose tissue metabolism, which is gaining more and more appreciation as being vital to systemic metabolism (14), is disrupted in obesity and IR (15). Impaired adipose tissue mitochondrial regulation may contribute to the metabolic dysfunction of adipose tissue (7) supported by, for example, reduced mitochondrial gene expression in adipose tissues from individuals with T2D (49) and insulin-resistant rodents (37).
BAT is a unique adipose depot in part because of its strikingly greater mitochondrial density compared with WAT depots. For the last several years, BAT has been studied intensively due to its possibility of being an anti-obesity target, as one study (32) calculated that ∼50 g of BAT can burn ∼125 kcal/day in humans. Emerging evidence also implicates BAT as an important contributor to systemic insulin sensitivity in both rodents (43) and humans (41). Thus, we assessed the unique roles of intrinsic fitness and/or OVX on insulin sensitivity of BAT. We observed that although BAT mass was less in HCR rats, the total amount of BAT glucose uptake in response to insulin (Fig. 4D) was actually much greater compared with LCR rats and was very strongly associated with their greater whole body glucose clearance (Fig. 5C). Thus, the greater insulin-mediated BAT glucose uptake associated with high aerobic capacity reported here may help explain the enhanced whole body glucose clearance observed in HCR rats. Given the fact that, among the tissues observed, BAT showed the greatest capacity of glucose uptake per tissue weight (even greater than skeletal muscle; Fig. 3), its contribution to whole body glucose homeostasis should be emphasized. Interestingly, one recent human study demonstrated a positive relationship between physical activity and BAT activity as assessed via PET scans (9). Although we did not assess BAT activity per se, greater insulin-mediated glucose uptake in this tissue may indicate higher activity; nevertheless, the effects of intrinsic fitness on BAT activity need to be tested more precisely in future studies. Since research has reported that OVX alters BAT mitochondrial oxidative capacity assessed by uncoupling protein 1 and peroxisome proliferator-activated receptor-γ coactivator-1 protein expression (28, 47), which is associated with changes in insulin sensitivity (25), we were interested in investigating the effect of OVX on BAT insulin sensitivity. Although we found no OVX effect on BAT mass and/or glucose uptake, the BAT/WAT ratio was reduced following OVX in both lines. In our first study with the shorter followup time period, the relative amount of BAT based on this ratio was reduced following OVX only in LCR rats and correlated significantly with resting energy expenditure (47). New evidence suggests that increased adiposity is associated with the “whitening” of BAT (40). The increase in adiposity observed here even in the HCR rats may have been associated with some whitening of BAT, resulting in an increase in mass. Because we did not assess BAT whitening in the present study, that hypothesis could not be tested but should be addressed in future studies.
A limitation of this study is that HCR rats are habitually more physically active than their LCR counterparts in their cages, preventing us from being able to separate the effects of aerobic capacity from those attributed to higher SPA. Indeed, there is a close relationship between physical activity level, aerobic capacity, and metabolic function, making investigation of each independent factor extremely difficult. However, since we demonstrated previously that both HCR and LCR rats are equally affected by OVX in that they both experience an ∼20% reduction in SPA, one would expect OVX to reduce BAT and WAT insulin sensitivity in both lines. This was not the case, as neither HCR nor LCR rats were affected by OVX in terms of any changes in BAT or WAT insulin sensitivity. The fact that skeletal muscle insulin sensitivity was affected similarly with OVX in HCR and LCR rats may suggest that this effect may have been attributed to the OVX-associated reduction in SPA. Another limitation was the fact that liver insulin sensitivity was not measured; the possibility exists that the differences in systemic insulin sensitivity between HCR and LCR rats were due to differences at the level of the liver. In fact, the liver is known to play a significant role in glucose disposal under clamp conditions (19). Importantly, it has been shown that estradiol can prevent IR in OVX mice specifically by improving hepatic as well as skeletal muscle IR (4). How fitness may affect liver insulin sensitivity in OVX rodents is an important question to be addressed in future studies.
In summary, both HCR and LCR rats experience OVX-induced reductions in whole body and skeletal muscle insulin sensitivity, although in both SHM and OVX conditions, HCR rats display greater insulin sensitivity compared with their respective LCR controls, demonstrating greater skeletal muscle, BAT, and WAT insulin-stimulated glucose uptake. The systemic IR induced by OVX in both lines appeared to be due to a deficit in skeletal muscle insulin sensitivity associated with OVX but not adipose tissue. In conclusion, in the absence of exercise training, rats selectively bred for high running capacity experience greater tissue-specific glucose uptake compared with LCR rats. This intrinsically higher insulin-mediated glucose uptake in skeletal muscle, BAT, and WAT may help buffer OVX-induced systemic IR in HCR rats.
GRANTS
This work was also supported by a University of Missouri Research Council grant (V. Vieira-Potter) and National Institutes of Health Grants R01-DK-088940 (J. P. Thyfault), R24-OD-010950 (L. G. Koch and S. L. Britton), RO1-DK-077200 (S. L. Britton), R01-GM-104194 (S. L. Britton), K01-HL-125503 (J. Padilla), and VHA-CDA2 1 IK2 BX001299 (R. S. Rector).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.-M.P. and V.J.V.-P. conception and design of research; Y.-M.P., T.M.Z., S.L.B., and L.G.K. performed experiments; Y.-M.P., M.E.M., and V.J.V.-P. analyzed data; Y.-M.P., R.S.R., J.P.T., M.E.M., J.A.K., and V.J.V.-P. interpreted results of experiments; Y.-M.P. prepared figures; Y.-M.P. drafted manuscript; Y.-M.P., R.S.R., J.P.T., T.M.Z., J.P., R.J.W., J.A.K., and V.J.V.-P. edited and revised manuscript; Y.-M.P., R.S.R., J.P.T., T.M.Z., J.P., R.J.W., G.M.M., M.E.M., S.L.B., L.G.K., F.W.B., J.A.K., and V.J.V.-P. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
REFERENCES
- 1.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bergeron R, Mentor JS, Côté I, Ngo Sock ÉT, Rabasa-Lhoret R, Lavoie JM. Loss of ovarian estrogens causes only mild deterioration of glucose homeostasis in female ZDF rats preventable by voluntary running exercise. Horm Metab Res 46: 774–781, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Brand JS, van der Schouw YT, Onland-Moret NC, Sharp SJ, Ong KK, Khaw KT, Ardanaz E, Amiano P, Boeing H, Chirlaque MD, Clavel-Chapelon F, Crowe FL, de Lauzon-Guillain B, Duell EJ, Fagherazzi G, Franks PW, Grioni S, Groop LC, Kaaks R, Key TJ, Nilsson PM, Overvad K, Palli D, Panico S, Quirós JR, Rolandsson O, Sacerdote C, Sánchez MJ, Slimani N, Teucher B, Tjonneland A, Tumino R, van der A DL, Feskens EJ, Langenberg C, Forouhi NG, Riboli E, Wareham NJ; InterAct Consortium. Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care 36: 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camporez JP, Jornayvaz FR, Lee HY, Kanda S, Guigni BA, Kahn M, Samuel VT, Carvalho CR, Petersen KF, Jurczak MJ, Shulman GI. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology 154: 1021–1028, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404–2411, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Cavalcanti-de-Albuquerque JP, Salvador IC, Martins EL, Jardim-Messeder D, Werneck-de-Castro JP, Galina A, Carvalho DP. Role of estrogen on skeletal muscle mitochondrial function in ovariectomized rats: a time course study in different fiber types. J Appl Physiol (1985) 116: 779–789, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Dankel SN, Staalesen V, Bjørndal B, Berge RK, Mellgren G, Burri L. Tissue-specific effects of bariatric surgery including mitochondrial function. J Obes 2011: 435245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76: 149–155, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinas PC, Nikaki A, Jamurtas AZ, Prassopoulos V, Efthymiadou R, Koutedakis Y, Georgoulias P, Flouris AD. Association between habitual physical activity and brown adipose tissue activity in individuals undergoing PET-CT scan. Clin Endocrinol 82: 147–154, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Duval K, Prud'homme D, Rabasa-Lhoret R, Strychar I, Brochu M, Lavoie JM, Doucet E. Effects of the menopausal transition on dietary intake and appetite: a MONET Group Study. Eur J Clin Nutr 68: 271–276, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duval K, Prud'homme D, Rabasa-Lhoret R, Strychar I, Brochu M, Lavoie JM, Doucet E. Effects of the menopausal transition on energy expenditure: a MONET Group Study. Eur J Clin Nutr 67: 407–411, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earnest CP, Johannsen NM, Swift DL, Lavie CJ, Blair SN, Church TS. Dose effect of cardiorespiratory exercise on metabolic syndrome in postmenopausal women. Am J Cardiol 111: 1805–1811, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farooq W, Farwa U, Khan FR. The metabolic syndrome and inflammation: role of insulin resistance and increased adiposity. Oman Med J 30: 100–103, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: a key to lean phenotype. Biochim Biophys Acta 1831: 986–1003, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Frayn KN, Humphreys SM. Metabolic characteristics of human subcutaneous abdominal adipose tissue after overnight fast. Am J Physiol Endocrinol Metab 302: E468–E475, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James DE, Zorzano A, Boni-Schnetzler M, Nemenoff RA, Powers A, Pilch PF, Ruderman NB. Intrinsic differences of insulin receptor kinase activity in red and white muscle. J Biol Chem 261: 14939–14944, 1986. [PubMed] [Google Scholar]
- 17.Jeong S, Yoon M. Swimming's prevention of ovariectomy-induced obesity through activation of skeletal-muscle PPARalpha. Int J Sport Nutr Exerc Metab 22: 1–10, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol 22: 226–237, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley D, Mitrakou A, Marsh H, Schwenk F, Benn J, Sonnenberg G, Arcangeli M, Aoki T, Sorensen J, Berger M, Sonksen P, Gerich J. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. J Clin Invest 81: 1563–1571, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest 115: 1699–1702, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Koch LG, Britton SL. Development of animal models to test the fundamental basis of gene-environment interactions. Obesity 16, Suppl 3: S28–S32, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai S, Holmäng A, Björntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand 149: 91–97, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 4: R1–R15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morley TS, Xia JY, Scherer PE. Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements. Nat Commun 6: 7906, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris EM, Jackman MR, Johnson GC, Liu TW, Lopez JL, Kearney ML, Fletcher JA, Meers GM, Koch LG, Britton SL, Rector RS, Ibdah JA, MacLean PS, Thyfault JP. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab 307: E355–E364, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadal-Casellas A, Proenza AM, Llado I, Gianotti M. Effects of ovariectomy and 17-beta estradiol replacement on rat brown adipose tissue mitochondrial function. Steroids 76: 1051–1056, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, Koch LG, Britton SL, Ibdah JA, Thyfault JP. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab 35: 151–162, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nigro M, Santos AT, Barthem CS, Louzada RA, Fortunato RS, Ketzer LA, Carvalho DP, de Meis L. A change in liver metabolism but not in brown adipose tissue thermogenesis is an early event in ovariectomy-induced obesity in rats. Endocrinology 155: 2881–2891, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab 293: E31–E41, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122: 545–552, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puah JA, Bailey CJ. Effect of ovarian hormones on glucose metabolism in mouse soleus muscle. Endocrinology 117: 1336–1340, 1985. [DOI] [PubMed] [Google Scholar]
- 34.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB 3rd, Hawley JA. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R835–R843, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161–2168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 56: 1751–1760, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Seifert EL, Bastianelli M, Aguer C, Moffat C, Estey C, Koch LG, Britton SL, Harper ME. Intrinsic aerobic capacity correlates with greater inherent mitochondrial oxidative and H2O2 emission capacities without major shifts in myosin heavy chain isoform. J Appl Physiol (1985) 113: 1624–1634, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen M, Kumar SP, Shi H. Estradiol regulates insulin signaling and inflammation in adipose tissue. Horm Mol Biol Clin Investig 17: 99–107, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, Walsh K. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest 124: 2099–2112, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125: 478–486, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spangenburg EE, Wohlers LM, Valencia AP. Metabolic dysfunction under reduced estrogen levels: looking to exercise for prevention. Exerc Sport Sci Rev 40: 195–203, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, Tseng YH, Goodyear LJ. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 64: 2002–2014, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephenson EJ, Lessard SJ, Rivas DA, Watt MJ, Yaspelkis BB 3rd, Koch LG, Britton SL, Hawley JA. Exercise training enhances white adipose tissue metabolism in rats selectively bred for low- or high-endurance running capacity. Am J Physiol Endocrinol Metab 305: E429–E438, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, Obin MS, Greenberg AS. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology 153: 4266–4277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira-Potter VJ, Padilla J, Park YM, Welly RJ, Scroggins RJ, Britton SL, Koch LG, Jenkins NT, Crissey JM, Zidon T, Morris EM, Meers GM, Thyfault JP. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 308: R530–R542, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walton C, Godsland IF, Proudler AJ, Wynn V, Stevenson JC. The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. Eur J Clin Invest 23: 466–473, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Wang XC, Zhang ZY, Mou B, Hu RM. Impaired mitochondrial oxidative phosphorylation in multiple insulin-sensitive tissues of humans with type 2 diabetes mellitus. J Int Med Res 38: 769–781, 2010. [PubMed] [Google Scholar]
- 50.Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Yonezawa R, Wada T, Matsumoto N, Morita M, Sawakawa K, Ishii Y, Sasahara M, Tsuneki H, Saito S, Sasaoka T. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. Am J Physiol Endocrinol Metab 303: E445–E456, 2012. [DOI] [PubMed] [Google Scholar]


