Abstract
We review the evolving findings from studies that examine the relationship between the structural and functional properties of skeletal muscle's vasculature and muscle metabolism. Unique aspects of the organization of the muscle microvasculature are highlighted. We discuss the role of vasomotion at the microscopic level and of flowmotion at the tissue level as modulators of perfusion distribution in muscle. We then consider in some detail how insulin and exercise each modulate muscle perfusion at both the microvascular and whole tissue level. The central role of the vascular endothelial cell in modulating both perfusion and transendothelial insulin and nutrient transport is also reviewed. The relationship between muscle metabolic insulin resistance and the vascular action of insulin in muscle continues to indicate an important role for the microvasculature as a target for insulin action and that impairing insulin's microvascular action significantly affects body glucose metabolism.
Keywords: muscle microvasculature, vasomotion, flowmotion, endothelium, insulin resistance
muscle's microvasculature is the final interface through which circulating nutrients, hormones, gases, and electrolytes must pass in journeying to and from the systemic circulation. It has evolved multiple structural and functional adaptations to flexibly and efficiently fulfill its role in optimizing muscle function. Here, we examine how the minute-to-minute metabolic activity of skeletal muscle is coupled to the function of muscle's vasculature. We will discuss how bulk blood flow and flow distribution can regulate nutrient delivery to muscle microvasculature as well as how transendothelial transport processes can modulate the exchange of hormones and metabolites between plasma and the myocytes. We focus on acute regulatory relationships and defer discussion of chronic vascular or myocyte adaptation to environmental, nutritional, and most pathological processes.
Muscle blood flow has been measured in numerous classical limb balance and metabolic tracer studies of the acute effects of fasting, feeding, exercise, and hormonal manipulation on muscle nutrient exchange. These methods, however, treat muscle's vasculature as a “black box.” Here, we consider muscle microvasculature's specialized architecture and how that architecture might impact nutrient fluxes into and out of the muscle. We will discuss the role of vasomotion and flowmotion in the regulation of microvascular perfusion. We recognize that factors acting on the endothelial cell (EC), the vascular smooth muscle cell (SMC), or pericytes may affect muscle perfusion. We will consider in more detail the effect insulin and exercise have on the endothelium and directly or indirectly on the vascular SMC to influence nutrient delivery.
Beyond muscle perfusion, we will consider the transit/transport of fatty acids, glucose, and insulin across the vascular endothelium of muscle, as these processes offer another pathway whereby the vasculature may regulate myocyte metabolic function. In aggregate, this review will delineate how vascular regulation of blood flow, flow distribution, and endothelial function coordinate to optimally serve myocyte function.
Skeletal Muscle Architecture
Pioneering work by Spalteholz (105) and Krogh (63, 64) has demonstrated muscle's highly organized vasculature. Arterioles branch off primary arteries, down to terminal arterioles, oriented perpendicular to muscle fibers, and supplying them at regular intervals (∼1 mm). Each terminal arteriole supplies 15–20 capillaries running parallel to fibers, with many anastomoses, to form a rich network around muscle fibers. Each terminal arteriole with its capillaries forms the smallest unit of control for capillary perfusion (referred to as a microvascular unit) (34). Venules are arrayed as arterioles and found between two terminal arterioles. Lymph vessels originate within muscle and can ascend with arterioles or venules (see Fig. 1) (48, 103).
Fig. 1.
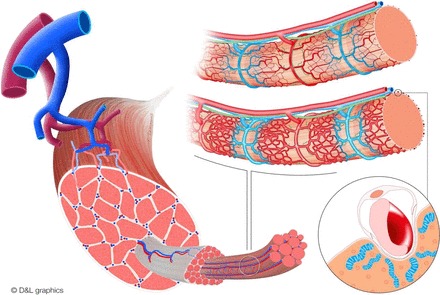
Schematic representation of muscle's vascular architecture. Left: arterioles branch off primary arteries down to terminal arterioles; these are oriented perpendicular to muscle fibers. Top right: 2 muscle fibers and their microvascular units, with terminal arterioles (red) feeding multiple capillaries (red to blue) as the smallest unit of control and lymph vessels (green) ascending along arterioles and venules (blue). In resting muscle (top fiber), the microvasculature is intermittently and not equally perfused. Insulin and exercise can each stimulate muscle (bottom fiber) and induce vasodilation at the level of the terminal arterioles and enhance perfusion. Bottom right: magnification of a capillary embedded in a sarcolemma “channel,” thereby expanding the contact area between myocytes and endothelial cells. Myocyte mitochondria cluster along the embedded capillary, thereby reducing the distance oxygen and nutrients need to diffuse. Illustration provided by MediCorporate bv/D & L Graphics (www.dlgraphics.nl).
The proximity between muscle fibers and capillaries is underscored by recent findings showing capillaries embedded in grooves indenting the sarcolemma and expanding the contact area between myocytes and ECs. Moreover, muscle fiber mitochondria appear to cluster along these grooves, thereby reducing the distance oxygen and fatty acids need to diffuse (39). Recent elegant imaging studies have also shown that muscle's “mitochondrial reticulum” is further specialized with respiratory complexes that generate membrane potential (e.g., complexes III and IV) enriched in the perivascular region of the mitochondrial matrix, whereas components using the proton-motive force for ATP synthesis are distributed more within the fiber. This arrangement minimizes oxygen diffusion distances and may allow conductive distribution of proton-motive force (38).
Although arterioles and venules are organized similarly in most mammalian skeletal muscles, some differences in microvascular architecture exist between aerobic and anaerobic muscles. Aerobic muscles typically have more capillaries, larger capillary length per fiber, and more capillary branches per fiber (65), and microvessels may be embedded deeper in sarcolemma and have more perivascular mitochondria (39). These are all adaptations that could facilitate gas and nutrient exchange. Muscle's vascular architecture down to the level of terminal arterioles allows blood flow and nutrient exchange to be regulated at several levels. Total skeletal muscle blood flow can increase with rising arterial pressure and maintained muscle resistance artery tone or by vasodilation of resistance arterioles with maintained pressure. Terminal arterioles can increase the endothelial surface area by increasing the fraction of perfused capillaries through vasodilation and SMC-driven vasomotion, thus enhancing nutrient exchange.
Muscle Perfusion and Nutrient Flux With Exercise and Insulin
Perfusion delivers oxygen, free fatty acids (FFA), glucose, triglycerides, and amino acids to and removes metabolites (amino acids, lactate, carbon dioxide, and ammonia) from muscle. Muscle is a major site of carbohydrate, protein, and fat storage during feeding and a major supplier of nutrients during periods of deprivation. The regulation of fuel fluxes in and out of muscle has been studied intensely in vivo in animals and humans using the limb balance method, which is at times supplemented with metabolite tracer measurements. Here, we will consider the relationship between muscle blood flow and nutrient supply to muscle in response to exercise or insulin stimulation, which has been studied intensively. In particular, we will emphasize data from in vivo studies in humans. We will consider both intense and very modest stimulation to provide the reader with a sense of the hierarchical relationship between muscle perfusion and metabolic function.
Total limb flow can be assessed in different ways (e.g., plethysmography, dye or thermal dilution, and Doppler ultrasound). The advantages and limitations of these methods have been reviewed elsewhere (12). Although flow measurement methods have been compared with one another, none can be considered to be the gold standard to measure skeletal muscle blood flow (12, 57, 113). Of note is that each measures blood flow directed to all tissues of the limb, not just muscle. In overnight-fasted humans, resting limb blood flow averages ∼2–5 ml·min−1·100 ml−1 of limb (84, 91, 94) in both men and women (52), using the arm or leg (33, 44, 111). Because resting skin and subcutaneous adipose blood flow (on a per 100 ml of tissue basis) are similar to that of muscle (15, 47, 71), these resting limb flow values are assumed to reflect average blood flow to muscle, i.e., the methods treat the limb as a homogenous tissue represented by muscle.
Vasomotion, the rhythmic vessel diameter oscillations first described by Jones (54) in the bat wing in 1852, is more prevalent in small arteries and arterioles than in conduit arteries (1). These oscillations modify blood flow and are one contribution to periodic flow fluctuations known as flowmotion (1, 98). In addition to vasomotion, heart rate, respiration, and autonomic factors influence flowmotion. The several components of flowmotion are distinguished by their frequencies; heart rate and respiratory contributions arise at ∼1 and ∼0.3 Hz, respectively, whereas low-frequency signals associated with vasomotion arise at ∼0.1 Hz for myogenic activity, ∼0.04 Hz for neurogenic activity, and ∼0.01 Hz for endothelial activity (17, 67, 107).
Vasomotion originates from an oscillator component in the SMCs and synchronizing mechanisms, which are modified by ECs (1). Upon increased metabolic needs, signals originating from the microvasculature travel upstream to larger arterioles and arteries, possibly via gap junctions in the SMCs (6), thereby influencing vessel diameter. In addition, vasomotor responses in arterioles can be triggered by stimulation of individual capillaries, and changes in membrane potential appear to be responsible for these observations (6, 14, 104). Moreover, NO can regulate EC-to-SMC signaling at myoendothelial junctions, and reduced NO production attenuates the duration of conducted vasodilation (6, 29). These findings suggest that muscle microcirculation modulates upstream vasodilation and vasomotion.
In vivo, vasomotion may regulate microvascular flow distribution to optimize delivery of nutrients and regulate local hydraulic resistance (88). In addition to regulating blood flow distribution, vasomotion of terminal arterioles also affects lymph flow, thus influencing water transport in muscle interstitium and tissue homeostasis (103). Vasomotion, by continuously altering flow delivery to vessels over time, increases the number of ECs exposed to plasma for nutrient or hormone exchange (46) without total flow or cardiac output being changed. When perfusion demand exceeds flowmotion's compensatory capacity, blood flow control shifts upstream, enabling increases in total flow (1, 100).
Studying muscle vasomotion requires invasive direct blood vessel visualization (e.g., intravital microscopy) (13, 25, 40, 49), and it is affected by anesthetic agents and a host of experimental variables (25). Its invasive nature limits the study of vasomotion in humans. By contrast, cutaneous flowmotion can be studied using laser-Doppler flowmetry (LDF) and has become popular in clinical studies (56, 81, 87, 90). To quantify flowmotion, Fourier or wavelet analyses methods are used to determine the relative contribution of each frequency to the observed LDF signal (17, 25, 87). A drawback of LDF measurements is that they are affected by nearby vessels and blood pressure fluctuations, and therefore, they may not necessarily reflect only the rhythmic activities of blood vessels themselves (1, 35, 41). Several disorders influence vasomotion and flowmotion; hemorrhage increases vasomotion, whereas obesity and diabetes reduce flowmotion and vasomotion (1, 87). Taken together, vasomotion provides a local mechanism for vascular adaptation to altered metabolic needs, and its regulation impacts muscle perfusion.
Exercise and Muscle Perfusion
Myocyte and related whole body metabolic changes with acute and chronic exercise were reviewed recently (30). Oxygen and nutrient delivery to muscle during intense exercise and the accompanying blood flow changes have been studied extensively. The increased bulk blood flow during exercise arises both from local factors [e.g., potassium, adenosine, phosphate, lactate, hydrogen ion, nitric oxide (NO), and prostaglandins] released by the muscle and by neurovascular changes that relax muscle vasculature. With very intense whole body exercise, blood flow and oxygen delivery to the tissue become limiting secondary to limitations of respiratory and cardiac function (3, 83). By contrast, with intense exercise of isolated muscle groups, neither cardiorespiratory function nor hemodynamic factors limit muscle performance. Thus, blood flow to the human quadriceps can increase >50-fold during brief, intense exercise (3). Increasing the arterial oxygen content does not further increase quadriceps O2 extraction or peak work rate by either trained or untrained muscle (85). Muscle's remarkable flow reserve can meet even extreme muscle O2 demands. In addition to oxygen, glucose and fatty acids are important fuels during exercise. Carbohydrates (both circulating glucose and muscle glycogen) are the preferred fuel for intense, brief-duration muscle exercise. With intense muscle exercise, glucose extraction can increase more than sixfold and leg blood flow 10-fold, whereas plasma glucose changes little (59). Interestingly, whereas glucose concentrations in resting muscle cytosol are extremely low, intense exercise dramatically increases glucose both in muscle interstitium (76) and within muscle cells in humans (59) and rodents (43). The >30-fold increase in leg glucose uptake seen with intense exercise indicates that both the delivery of glucose to muscle microvasculature and transcapillary glucose transfer have capacity sufficient to meet glucose metabolic needs.
Vascular responses to light exercise (operationally, we consider ≤25% max as “light”) have been studied much less, and their link to muscle performance is less clear. However, studies of light exercise provide insight into the hierarchical regulation of muscle perfusion. With light exercise, vasomotion within the terminal arterioles appears to cease, and the number of perfused capillary increases without significant changes in total muscle blood flow (45, 46, 51). Honig et al. (46), by flash-freezing and sectioning muscle, showed that low-frequency electrical stimulation was nearly as effective as near-tetanic stimulation for recruiting hypoperfused microvasculature in canine gracilis muscle. Further increasing contractile activity increases total blood flow, as regulation shifts upstream to progressively larger arterioles (100). Factors driving microvascular perfusion changes include venous Po2, which, by enhancing venular EC NO production and erythrocyte NO release (95, 100, 106, 112), promotes dilation of nearby arterioles. Hemodynamic forces, i.e., shear stress-induced NO production, further modulate hyperemic responses (100). These are aided by prostaglandins and endothelium-derived hyperpolarizing factors to increase perfusion with exercise (83). It appears that inhibition of one system (e.g., NO synthase) can be compensated by the remaining factors, since skeletal muscle blood flow is preserved in such circumstances (18). If, however, two pathways are inhibited simultaneously, the increase in skeletal muscle blood flow is reduced (82, 83). These effectors act as sympatholytics to counteract the exercise-induced vasoconstriction of distal arterioles and feed arteries by sympathetic nerves (83).
Insulin and Muscle Perfusion
The effects of insulin on limb metabolism have been studied extensively using limb balance methods combined with euglycemic insulin infusion. Andres et al. (4) first reported insulin-stimulated forearm glucose uptake and noted an accompanying increase in forearm blood flow. Baron (7) and Laakso et al. (70) first reported that insulin-induced muscle glucose uptake paralleled insulin's ability to increase total leg blood flow. In subsequent work, obesity, type 1 and type 2 diabetes, and increased plasma free fatty acid concentrations (9, 69, 70, 109) each impaired insulin-stimulated limb blood flow and glucose uptake in parallel. These studies suggested that 1) insulin stimulates limb blood flow in both humans and animals; 2) during a high-dose insulin clamp, increased limb flow correlates with muscle glucose uptake; 3) acute and chronic metabolic insulin resistance is associated with impaired insulin-stimulated increases of limb blood flow; and 4) insulin enhances limb blood flow by increasing nitric oxide production (70, 78, 108, 109). This led investigators to suggest that impaired arteriolar relaxation in response to insulin contributes significantly to metabolic dysfunction in insulin-resistant muscle.
Insulin's vasodilatory effects arise from its binding to the insulin receptor on ECs. This stimulates the phosphatidylinositol 3-kinase/Akt pathway to activate endothelial NO synthase (eNOS) (53, 124) and induces NO-mediated vasodilation (20). Insulin also stimulates the MAPK pathway and enhances production of the vasoconstrictor endothelin-1 (78, 86, 108). In health, insulin's vasodilatory effect dominates. With endothelial dysfunction, as occurs with obesity and type 2 diabetes, the PI3K pathway appears to be selectively inhibited by low-grade inflammation, FFA, oxidative stress, and reduced perivascular adiponectin release (55, 61, 75, 79, 123). In these circumstances, a reduced vasodilatory response or even paradoxical vasoconstriction can be seen with hyperinsulinemia (23, 55).
Insulin at high physiological concentrations relaxes resistance arterioles and increases limb blood flow in humans. In addition, there is convincing evidence that this response is blunted in insulin-resistant individuals with obesity (68) and type 1 (8) or type 2 diabetes (10). This effect of insulin on blood flow is most evident when insulin is infused continuously at a rate of 3 mU·kg−1·min−1 to achieve marked steady-state hyperinsulinemia for ≥2 h. It has been more difficult to reproduce this effect infusing insulin at a lower rate or for shorter duration (121). This has led to questioning of the physiological importance of this response. In addition, in insulin-resistant subjects, pharmacologically increasing blood flow often does not improve insulin's metabolic action measured as limb glucose uptake (89). However, in the latter circumstance, it remains likely that while perfusion is improved insulin resistance in the myocyte persists, and short-term correction of perfusion is insufficient to overcome that metabolic defect.
Over the last 15 years, research on insulin's action on muscle perfusion has added focus on the microvasculature. There, analogous to the effect of low-frequency stimulation reported by Honig et al. (45, 46), relaxation of terminal arterioles could recruit hypoperfused capillaries and expand the EC surface available for nutrient exchange (11) without changing total flow. However, there were no noninvasive methods available to quantify the microvascular volume perfused in intact muscle in animals or humans. In 1997, a technique was introduced to estimate the endothelial surface available for nutrient exchange based on the single-pass conversion of 1-methylxanthine (1-MX) to 1-methylurate (1-MU) by xanthine oxidase, which is present on capillary endothelium. Increased endothelial exposure to substrate (i.e., capillary recruitment) would increase 1-MX to 1-MU conversion (92, 93). Later, contrast-enhanced ultrasound (CEUS) was used to investigate microvascular perfused volume regulation (24, 28). Using either method, we found that in healthy animals and humans, physiological doses of insulin and carbohydrate-containing meals each increased microvascular perfusion volume even in the absence of increases in total limb blood flow (23, 24, 115). Using CEUS, we found that, from the start of an euglycemic insulin clamp, microvascular blood volume increases within 15–30 min, and this microvascular change precedes and correlates with the rise in forearm glucose uptake (31, 32, 116). Moreover, chronic (obesity) and acutely induced (lipid infusion) insulin resistance are associated with an impaired microvascular response to insulin or meal ingestion (23, 50, 60, 74). Using CEUS (51, 115), we also confirmed the original finding by Honig et al. (45) that light exercise increased microvascular perfused volume ∼3-fold with minimal increases in total limb blood flow (see Fig. 2). However, with more intense exercise, both microvascular blood volume and total limb blood flow increase (51, 101, 115). Interestingly, cardiac muscle behaves similarly to skeletal muscle during hyperinsulinemia (73) and feeding (99), and its increases in microvascular blood volume are blunted by insulin resistance as well (72).
Fig. 2.
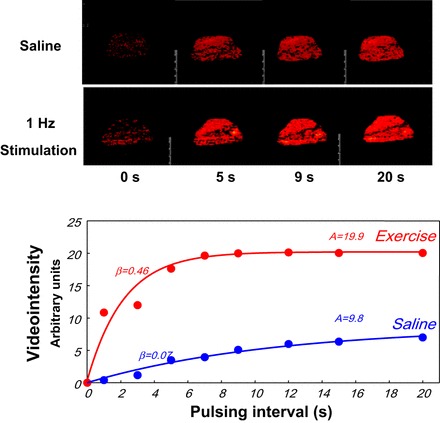
Top: contrast-enhanced ultrasound images of rat thigh muscle as a function of time after a high-energy ultrasound pulse with the muscle at rest or given a 1-Hz electrical stimulation. Bottom: greater plateau video intensity (red) corresponding to a larger number of microbubbles in the imaged volume of the stimulated muscle.
Insulin is not the only hormone that can “recruit” muscle microvasculature. Both glucagon-like peptide-1 (GLP-1) (21, 110) and adiponectin (125), like insulin, increase microvascular volume, albeit each acting by different biochemical pathways. By contrast, epinephrine can increase muscle blood flow without increasing perfused microvascular volume (22). The responses to physiological hyperinsulinemia and low-intensity exercise suggest a hierarchical response by muscle vasculature whereby microvascular units respond to these stimuli, whereas with more intense stimulation (high physiological insulin concentrations or heavy exercise) more proximal arterial elements also respond, leading to the flow increases seen in those settings. Whether this graded response involves antidromic signals through the vascular network or again is a response to multiple locally generated metabolic signals is not known.
Nutrient and Hormone Transfer Across the Endothelium
Here we very briefly review evolving evidence that the vascular endothelium in tissues like muscle and adipose may actively mediate the transendothelial transport (TET) of substrates (e.g., glucose or FFA) and hormones, including insulin. As would be expected, such a role for the endothelium is of consequence principally in tissues with a continuous endothelium, like muscle and adipose, but less consequential in liver and other splanchnic tissues (2).
We consider first the endothelium's role in FFA transport, as FFAs are a major muscle fuel. It has long been known that lipoprotein lipase (LPL) is secreted by skeletal myocytes and adipocytes but resides on the luminal surface of the vascular EC in muscle and fat, where it cleaves fatty acid monomers from triglycerides bound to circulating lipoproteins (97). This process can be regulated by insulin, which increases LPL expression. Recently, it was found that the EC possesses a receptor for LPL [glycosylphosphotidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1)] (26) and that when LPL binds to GPIHBP1 a vesicular transport system mediates LPL's TET to the luminal membrane, where it encounters circulating triglycerides (27). Mutations in either GPIHBP1 or LPL that interfere with this pathway can cause severe hypertriglyceridemia in humans (122).
In addition to this pathway for LPL transport that provides triglyceride-derived fatty acids to the EC, the EC possesses membrane-associated fatty acid transport proteins (FATPs) as well as fatty acid-binding proteins (FABPs). The former take up FFA derived either from triglyceride hydrolysis or circulating as FFA associated with albumin or other plasma proteins. FATP3 and FATP4 are expressed by ECs along with the scavenger receptor CD36. Each appears to be involved in movement of fatty acids across the EC luminal plasma membrane. Expression of both FATP3 and FATP4 is increased by VEGFβ secreted by myocytes and adipocytes acting via VEGFR1. Muscle expression of VEGFβ is increased by the transcriptional coactivator PGC-1α, which is a central player in the regulation of mitochondrial oxidative metabolism within the myocyte. In the context of increasing muscle oxidative capacity, muscle signaling to adjacent ECs to enhance FFA uptake may be part of an adaptive response. Expression of CD36 by ECs is upregulated by peroxisome proliferator-activated receptor-γ, which enhances fat mass and adipogenesis. Interestingly, inhibition of VEGFβ in diabetic animal models decreases muscle ectopic fat and improves glucose metabolism (42). By contrast, VEGFA knockout in muscle increases susceptibility to high-fat diet induced insulin resistance, which is due at least in part to decreased microvascular perfusion (16). The transport of FFA across the ECs is presumably bidirectional, particularly in adipose tissue, although this has not been clarified in detail.
In contrast to the complex regulatory system involved in the movement of FFA across the EC, glucose transport from the vascular lumen into and across the endothelium is mediated by the constitutive activity of the GLUT1 transporter. The system has a high capacity in the absence of exogenous insulin, as indicated by the marked increases in glucose transport that occur with exercise (see above). The GLUT1 transporter is expressed in both vascular smooth muscle and endothelial cells. In the former, the transporter activity is downregulated by high ambient glucose concentrations (58). This adaptive response is absent in aortic ECs. The function of this transporter is important to the metabolic activity of the EC, which receives a significant fraction of its energy from glycolytic ATP production (77). The specific transport capacity in brain microvascular ECs has been studied extensively, as ECs perform a critical function in assuring adequate glucose delivery to the central nervous system. Cardiac muscle microvascular glucose transport capacity has been investigated and utilizes the same high-capacity GLUT1 transporter reported for other endothelia (77). To our knowledge, GLUT1 transporter function specific to skeletal muscle microvasculature has not been studied. As noted previously, the observation that muscle interstitial and myocyte glucose concentrations rise during intense exercise suggests that glucose transport through the vascular endothelium is not limiting even under conditions of high substrate demand. Indeed, the high capacity of the system may in part be responsible for damage that occurs to the microvascular endothelium as a result of hyperglycemia in the diabetic state (19). The EC does not appear to defend itself from high levels of intracellular glucose, which via several pathways can generate reactive oxygen species (19, 37). When this is unchecked, as occurs in persistent hyperglycemic states, vascular injury occurs.
The EC in tissues with a continuous endothelium also constitutes a barrier to insulin movement into the tissue. Indeed, estimates of muscle interstitial insulin concentrations using either lymphatic sampling (120) or microdialysis (102) suggest that insulin TET could be rate limiting for insulin's action on muscle. This led us to examine the cellular pathway of insulin TET. Most ECs express insulin, IGF-I, and the insulin/IGF-I hybrid receptor (62). In aortic ECs and preadipocytes (36), the insulin receptor associates with a specialized lipid raft domain, i.e., caveolae. Caveolae can be internalized and mediate TET of a variety of proteins (e.g., albumin in pulmonary microvascular ECs) (80). In a series of experiments, we found that caveolae are required for insulin uptake by and transport across aortic ECs (118). In these ECs, preserved insulin signaling through phosphatidylinositol 3-kinase to Akt and eNOS is necessary for insulin TET (119). Interestingly, inflammatory cytokines or several days of high-fat diet diminish EC insulin transport (119). If muscle microvascular ECs behave similarly, then impaired EC insulin signaling and TET could contribute to the impaired muscle insulin action of insulin resistance. As we had seen for microvascular recruitment, for insulin TET, NO appears to be a critical regulatory factor. When phosphatidylinositol 3-kinase or NO synthase is inhibited, insulin transport declines. Giving sodium nitroprusside (3–30 μM) can restore insulin uptake (117) in aortic ECs. Surprisingly, an EC-specific insulin receptor knockout mouse (with Cre-recombinase driven by Tie-2) did not display any metabolic phenotype (114). However, more recently, another EC-specific insulin receptor knockout (with Cre-recombinase driven by a vascular endothhelial-cadherin promoter) displayed glucose intolerance and delayed insulin action on insulin target tissues (Konishi M, Sakaguchi M, Cai W, Rask-Madsen C, and Kahn CR, unpublished observations). Likewise, the EC-specific IRS-2 knockout mouse is glucose intolerant and has impaired insulin-induced microvascular perfusion and diminished muscle insulin delivery (66). These genetic models underscore the role of EC insulin signaling to whole body metabolic functioning.
Recent work using adipose microvascular ECs has suggested a similar vesicle-mediated insulin TET process, and initial studies implicate clathrin-coated vesicles, not caveolae (5). However, there is a lack of data clarifying whether insulin regulates that transport or whether a transendothelial insulin concentration gradient is present in adipose tissue and whether insulin TET limits insulin action in adipose tissue (96).
In summary, a hierarchical, graded control of muscle perfusion arises from cooperative interactions among the factors released locally by myocytes, by SMCs, or by ECs along with systemically and neurally delivered signals. Very modest contractile activity or physiological hyperinsulinemia promptly increases microvascular perfusion volume even in the absence of changes in limb blood flow. In both cases, EC exchange surface for nutrient or hormone delivery expands without requiring increased cardiac work. Greater contractile activity or hyperinsulinemia triggers signals that may ascend the vascular network and provoke relaxation of progressively larger vessels, increasing total flow to the expanded microvascular network. Beyond insulin and exercise, other humoral factors (e.g., GLP-1, angiotensin II, and adiponectin) similarly enhance muscle microvascular perfusion. However, the role of these factors in normal muscle physiology is still being unraveled. Evolving evidence indicates that (at least for insulin and triglycerides/FFA), beyond altered perfusion, the microvasculature, particularly the EC, actively regulates the delivery of insulin and FFA to the muscle via regulation of specialized transport mechanisms. Thus, the EC of muscle vasculature is increasingly recognized as a key regulator of normal muscle physiology and metabolic health. Much remains to be learned regarding the normal regulation of the integrated functioning of the skeletal muscle myocytes and its vasculature and how this regulation is altered by disease or dysfunction.
GRANTS
This work was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-057878 and DK-073059) and American Diabetes Association (11-BS6 to E. J. Barrett) and by the Foundation “De Drie Lichten” in The Netherlands to Y. H. A. M. Kusters.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.H.K. and E.J.B. conception and design of research; Y.H.K. and E.J.B. analyzed data; Y.H.K. interpreted results of experiments; Y.H.K. and E.J.B. prepared figures; Y.H.K. and E.J.B. drafted manuscript; Y.H.K. and E.J.B. approved final version of manuscript.
REFERENCES
- 1.Aalkjaer C, Boedtkjer D, Matchkov V. Vasomotion - what is currently thought? Acta Physiol (Oxf) 202: 253–269, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100: 158–173, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andres E, Baltzan MA, Cader G, Zierler KL. Effect of insulin on carbohydrate metabolism and on potassium in the forearm of man. J Clin Invest 41: 108–115, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azizi PM, Zyla RE, Guan S, Wang C, Liu J, Bolz SS, Heit B, Klip A, Lee WL. Clathrin-dependent entry and vesicle-mediated exocytosis define insulin transcytosis across microvascular endothelial cells. Mol Biol Cell 26: 740–750, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 202: 271–284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron A. Hemodynamic actions of insulin. Am J Physiol Endocrinol Metab 267: E187–E202, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab 73: 637–643, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab 73: 637–643, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Baron AD, Laakso M, Brechtel G, Edelman SV. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J Clin Invest 87: 1186–1194, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett EJ, Eringa EC. The vascular contribution to insulin resistance: promise, proof, and pitfalls. Diabetes 61: 3063–3065, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett EJ, Rattigan S. Muscle perfusion: its measurement and role in metabolic regulation. Diabetes 61: 2661–2668, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett IS, Crane GJ, Neild TO, Segal SS. Electrophysiological basis of arteriolar vasomotion in vivo. J Vasc Res 37: 568–575, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Beach JM, McGahren ED, Duling BR. Capillaries and arterioles are electrically coupled in hamster cheek pouch. Am J Physiol Heart Circ Physiol 275: H1489–H1496, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Blaak E, van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH. Total forearm blood flow as an indicator of skeletal muscle blood flow: effect of subcutaneous adipose tissue blood flow. Clin Sci (Lond) 87: 559–566, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 62: 5672–5580, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bracic M, Stefanovska A. Wavelet-based analysis of human blood-flow dynamics. Bull Math Biol 60: 919–935, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Bradley SJ, Kingwell BA, McConell GK. Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes 48: 1815–1821, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Panza JA. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 100: 820–825, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 61: 888–896, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark AD, Barrett EJ, Rattigan S, Wallis MG, Clark MG. Insulin stimulates laser Doppler signal by rat muscle in vivo consistent with nutritive flow recruitment. Clin Sci (Lond) 100: 283–290, 2001. [PubMed] [Google Scholar]
- 23.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Coggins MP, Lindner J, Rattigan S, Fasy E, Jahn L, Kaul S, Barrett EJ. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50: 2682–2690, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol Heart Circ Physiol 246: H508–H517, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Davies BS, Beigneux AP, Barnes RH 2nd, Tu Y, Gin P, Weinstein MM, Nobumori C, Nyrén R, Goldberg I, Olivecrona G, Bensadoun A, Young SG, Fong LG. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab 12: 42–52, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies BS, Goulbourne CN, Barnes RH 2nd, Turlo KA, Gin P, Vaughan S, Vaux DJ, Bensadoun A, Beigneux AP, Fong LG, Young SG. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. J Lipid Res 53: 2690–2697, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 282: E714–E720, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol 579: 175–186, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Eggleston EM, Jahn LA, Barrett EJ. Early microvascular recruitment modulates subsequent insulin-mediated skeletal muscle glucose metabolism during lipid infusion. Diabetes Care 36: 104–110, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 56: 2958–2963, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Eklund B, Kaijser L, Knutsson E. Blood flow in resting (contralateral) arm and leg during isometric contraction. J Physiol 240: 111–124, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emerson GG, Segal SS. Alignment of microvascular units along skeletal muscle fibers of hamster retractor. J Appl Physiol (1985) 82: 42–48, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Erni D, Sigurdsson GH, Banic A, Wheatley AM. Regular slow wave flowmotion in skeletal muscle is not determined by nitric oxide and endothelin. Microvasc Res 58: 167–176, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Foti M, Porcheron G, Fournier M, Maeder C, Carpentier JL. The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 104: 1242–1247, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523: 617–620, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glancy B, Hsu LY, Dao L, Bakalar M, French S, Chess DJ, Taylor JL, Picard M, Aponte A, Daniels MP. In vivo microscopy reveals extensive embedding of capillaries within the sarcolemma of skeletal muscle fibers. Microcirculation 21: 131–147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustafsson H, Mulvany MJ, Nilsson H. Rhythmic contractions of isolated small arteries from rat: influence of the endothelium. Acta Physiol Scand 148: 153–163, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Hafner HM, Brauer K, Eichner M, Koch I, Heinle H, Rocken M, Strolin A. Wavelet analysis of skin perfusion in healthy volunteers. Microcirculation 14: 137–144, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Hagberg CE, Mehlem A, Falkevall A, Muhl L, Fam BC, Ortsäter H, Scotney P, Nyqvist D, Samén E, Lu L, Stone-Elander S, Proietto J, Andrikopoulos S, Sjöholm A, Nash A, Eriksson U. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 490: 426–430, 2012. [DOI] [PubMed] [Google Scholar]
- 43.Halseth AE, Bracy DP, Wasserman DH. Limitations to exercise- and maximal insulin-stimulated muscle glucose uptake. J Appl Physiol 85: 2305–2313, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Helge JW, Klein DK, Andersen TM, van Hall G, Calbet J, Boushel R, Saltin B. Interleukin-6 release is higher across arm than leg muscles during whole-body exercise. Exp Physiol 96: 590–598, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Honig CR, Odoroff CL, Frierson JL. Active and passive capillary control in red muscle at rest and in exercise. Am J Physiol Heart Circ Physiol 243: H196–H206, 1982. [DOI] [PubMed] [Google Scholar]
- 46.Honig CR, Odoroff CL, Frierson JL. Capillary recruitment in exercise: rate, extent, uniformity, and relation to blood flow. Am J Physiol Heart Circ Physiol 238: H31–H42, 1980. [DOI] [PubMed] [Google Scholar]
- 47.Houben AJ, Slaaf DW, Huvers FC, de Leeuw PW, Nieuwenhuijzen Kruseman AC, Schaper NC. Diurnal variations in total forearm and skin microcirculatory blood flow in man. Scand J Clin Lab Invest 54: 161–168, 1994. [DOI] [PubMed] [Google Scholar]
- 48.Huxley VH, Scallan J. Lymphatic fluid: exchange mechanisms and regulation. J Physiol 589: 2935–2943, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res 5: 309–312, 1973. [DOI] [PubMed] [Google Scholar]
- 50.Inyard AC, Chong DG, Klibanov AL, Barrett EJ. Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes 58: 2457–2463, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 56: 2194–2000, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Jensen MD, Nguyen TT, Hernández Mijares A, Johnson CM, Murray MJ. Effects of gender on resting leg blood flow: implications for measurement of regional substrate oxidation. J Appl Physiol 84: 141–145, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 104: 447–457, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones TW. Discovery that the veins of the bat's wing (which are furnished with valves) are endowed with rythmical contractility, and that the onward flow of blood is accelerated by each contraction. Philos Trans Royal Soc London 142: 131–136, 1852. [Google Scholar]
- 55.Jonk AM, Houben AJ, de Jongh RT, Serné EH, Schaper NC, Stehouwer CD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda) 22: 252–260, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Jonk AM, Houben AJ, Schaper NC, de Leeuw PW, Serné EH, Smulders YM, Stehouwer CD. Meal-related increases in microvascular vasomotion are impaired in obese individuals: a potential mechanism in the pathogenesis of obesity-related insulin resistance. Diabetes Care 34, Suppl 2: S342–S348, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jorfeldt L, Rutberg H. Comparison of dye-dilution and plethysmographic blood flow measurements: an evaluation of the influence of invasive techniques on blood flow and on arterial and femoral venous substrate variables in man. Clin Sci (Lond) 79: 81–87, 1990. [DOI] [PubMed] [Google Scholar]
- 58.Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 42: 80–89, 1993. [DOI] [PubMed] [Google Scholar]
- 59.Katz A, Broberg S, Sahlin K, Wahren J. Leg glucose uptake during maximal dynamic exercise in humans. Am J Physiol Endocrinol Metab 251: E65–E70, 1986. [DOI] [PubMed] [Google Scholar]
- 60.Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 32: 1672–1677, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko SH, Cao W, Liu Z. Hypertension management and microvascular insulin resistance in diabetes. Curr Hypertens Rep 12: 243–251, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kondo T, Vicent D, Suzuma K, Yanagisawa M, King GL, Holzenberger M, Kahn CR. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J Clin Invest 111: 1835–1842, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol 52: 409–415, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol 52: 457–474, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kubinova L, Janacek J, Ribaric S, Cebasek V, Erzen I. Three-dimensional study of the capillary supply of skeletal muscle fibres using confocal microscopy. J Muscle Res Cell Motil 22: 217–227, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K, Kadowaki T. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011. [DOI] [PubMed] [Google Scholar]
- 67.Kvernmo HD, Stefanovska A, Kirkeboen KA, Kvernebo K. Oscillations in the human cutaneous blood perfusion signal modified by endothelium-dependent and endothelium-independent vasodilators. Microvasc Res 57: 298–309, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. J Clin Invest 85: 1844–1852, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes 41: 1076–1083, 1992. [DOI] [PubMed] [Google Scholar]
- 70.Laakso M, Edelman SV, Olefsky JM, Brechtel G, Wallace P, Baron AD. Kinetics of in vivo muscle insulin-mediated glucose uptake in human obesity. Diabetes 39: 965–974, 1990. [DOI] [PubMed] [Google Scholar]
- 71.Linde B, Hjemdahl P, Freyschuss U, Juhlin-Dannfelt A. Adipose tissue and skeletal muscle blood flow during mental stress. Am J Physiol Endocrinol Metab 256: E12–E18, 1989. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab 96: 438–446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 293: E1250–E1255, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 94: 3543–3549, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes 56: 728–734, 2007. [DOI] [PubMed] [Google Scholar]
- 76.MacLean DA, Bangsbo J, Saltin B. Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. J Appl Physiol 87: 1483–1490, 1999. [DOI] [PubMed] [Google Scholar]
- 77.Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83: 183–252, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest 123: 1003–1004, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet B, Richter EA, Smulders YM, van Hinsbergh VW, Serné EH, Eringa EC. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes 62: 590–598, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol 285: L1179–L1183, 2003. [DOI] [PubMed] [Google Scholar]
- 81.Montero D, Walther G, Perez-Martin A, Santamaria C, Roche E, Mercier C, Vinet A. Decreased microvascular myogenic response to insulin in severely obese adolescents. Clin Hemorheol Microcirc 57: 23–32, 2014. [DOI] [PubMed] [Google Scholar]
- 82.Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mortensen SP, Saltin B. Regulation of the skeletal muscle blood flow in humans. Exp Physiol 99: 1552–1558, 2014. [DOI] [PubMed] [Google Scholar]
- 84.Mossberg KA, Mullani N, Gould KL, Taegtmeyer H. Skeletal muscle blood flow in vivo: detection with rubidium-82 and effects of glucose, insulin, and exercise. J Nucl Med 28: 1155–1163, 1987. [PubMed] [Google Scholar]
- 85.Mourtzakis M, González-Alonso J, Graham TE, Saltin B. Hemodynamics and O2 uptake during maximal knee extensor exercise in untrained and trained human quadriceps muscle: effects of hyperoxia. J Appl Physiol 97: 1796–1802, 2004. [DOI] [PubMed] [Google Scholar]
- 86.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. [DOI] [PubMed] [Google Scholar]
- 87.Muris DM, Houben AJ, Kroon AA, Henry RM, van der Kallen CJ, Sep SJ, Koster A, Dagnelie PC, Schram MT, Stehouwer CD. Age, waist circumference, and blood pressure are associated with skin microvascular flow motion: the Maastricht Study. J Hypertens 32: 2439–2449; discussion 2449, 2014. [DOI] [PubMed] [Google Scholar]
- 88.Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction: an emerging pathway in the pathogenesis of obesity-related insulin resistance. Rev Endocr Metab Disord 14: 29–38, 2013. [DOI] [PubMed] [Google Scholar]
- 89.Natali A, Quinones Galvan A, Pecori N, Sanna G, Toschi E, Ferrannini E. Vasodilation with sodium nitroprusside does not improve insulin action in essential hypertension. Hypertension 31: 632–636, 1998. [DOI] [PubMed] [Google Scholar]
- 90.Nilsson GE, Tenland T, Oberg PA. Evaluation of a laser Doppler flowmeter for measurement of tissue blood flow. IEEE Trans Biomed Eng 27: 597–604, 1980. [DOI] [PubMed] [Google Scholar]
- 91.Nuutila P, Raitakari M, Laine H, Kirvelä O, Takala T, Utriainen T, Mäkimattila S, Pitkänen OP, Ruotsalainen U, Iida H, Knuuti J, Yki-Järvinen H. Role of blood flow in regulating insulin-stimulated glucose uptake in humans. Studies using bradykinin, [15O]water, and [18F]fluoro-deoxy-glucose and positron emission tomography. J Clin Invest 97: 1741–1747, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rattigan S, Clark MG, Barrett EJ. Acute insulin resistance in rat skeletal muscle in vivo induced by vasoconstriction. Diabetes 48: 564–569, 1999. [DOI] [PubMed] [Google Scholar]
- 93.Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes 46: 1381–1388, 1997. [DOI] [PubMed] [Google Scholar]
- 94.Rudroff T, Weissman JA, Bucci M, Seppanen M, Kaskinoro K, Heinonen I, Kalliokoski KK. Positron emission tomography detects greater blood flow and less blood flow heterogeneity in the exercising skeletal muscles of old compared with young men during fatiguing contractions. J Physiol 592: 337–349, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saito Y, Eraslan A, Hester RL. Importance of venular flow in control of arteriolar diameter in hamster cremaster muscle. Am J Physiol Heart Circ Physiol 265: H1294–H1300, 1993. [DOI] [PubMed] [Google Scholar]
- 96.Sandqvist M, Strindberg L, Schmelz M, Lonnroth P, Jansson PA. Impaired delivery of insulin to adipose tissue and skeletal muscle in obese women with postprandial hyperglycemia. J Clin Endocrinol Metab 96: E1320–E1324, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Saxena U, Klein MG, Goldberg IJ. Transport of lipoprotein lipase across endothelial cells. Proc Natl Acad Sci USA 88: 2254–2258, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmidt JA, Borgström P, Firestone GP, von Wichert P, Intaglietta M, Fronek A. Periodic hemodynamics (flow motion) in peripheral arterial occlusive disease. J Vasc Surg 18: 207–215, 1993. [DOI] [PubMed] [Google Scholar]
- 99.Scognamiglio R, Negut C, De Kreutzenberg SV, Tiengo A, Avogaro A. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation 112: 179–184, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005. [DOI] [PubMed] [Google Scholar]
- 101.Sjøberg KA, Rattigan S, Hiscock N, Richter EA, Kiens B. A new method to study changes in microvascular blood volume in muscle and adipose tissue: real-time imaging in humans and rat. Am J Physiol Heart Circ Physiol 301: H450–H458, 2011. [DOI] [PubMed] [Google Scholar]
- 102.Sjöstrand M, Holmäng A, Lönnroth P. Measurement of interstitial insulin in human muscle. Am J Physiol Endocrinol Metab 276: E151–E154, 1999. [DOI] [PubMed] [Google Scholar]
- 103.Skalak TC, Schmid-Schonbein GW, Zweifach BW. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc Res 28: 95–112, 1984. [DOI] [PubMed] [Google Scholar]
- 104.Song H, Tyml K. Evidence for sensing and integration of biological signals by the capillary network. Am J Physiol Heart Circ Physiol 265: H1235–H1242, 1993. [DOI] [PubMed] [Google Scholar]
- 105.Spalteholz W. Die Vertheilung der Blutgelfasse im Muskel. Mat-phys KI sachs Ges Wiss 14: 509, 1888. [Google Scholar]
- 106.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997. [DOI] [PubMed] [Google Scholar]
- 107.Stefanovska A, Bracic M, Kvernmo HD. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans Biomed Eng 46: 1230–1239, 1999. [DOI] [PubMed] [Google Scholar]
- 108.Steinberg HO, Brechtel G, Johnson A, Fineberg F, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94: 1172–1179, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 49: 1231–1238, 2000. [DOI] [PubMed] [Google Scholar]
- 110.Subaran SC, Sauder MA, Chai W, Jahn LA, Fowler DE, Aylor KW, Basu A, Liu Z. GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin Sci (Lond) 127: 163–170, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taylor JA, Joyner MJ, Chase PB, Seals DR. Differential control of forearm and calf vascular resistance during one-leg exercise. J Appl Physiol (1985) 67: 1791–1800, 1989. [DOI] [PubMed] [Google Scholar]
- 112.Tigno XT, Ley K, Pries AR, Gaehtgens P. Venulo-arteriolar communication and propagated response. A possible mechanism for local control of blood flow. Pflugers Arch 414: 450–456, 1989. [DOI] [PubMed] [Google Scholar]
- 113.Tschakovsky ME, Shoemaker JK, Hughson RL. Beat-by-beat forearm blood flow with Doppler ultrasound and strain-gauge plethysmography. J Appl Physiol 79: 713–719, 1995. [DOI] [PubMed] [Google Scholar]
- 114.Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest 111: 1373–1380, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290: E1191–E1197, 2006. [DOI] [PubMed] [Google Scholar]
- 116.Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 51: 42–48, 2002. [DOI] [PubMed] [Google Scholar]
- 117.Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes 62: 4030–4042, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang H, Wang AX, Barrett EJ. Caveolin-1 is required for vascular endothelial insulin uptake. Am J Physiol Endocrinol Metab 300: E134–E144, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang H, Wang AX, Liu Z, Barrett EJ. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes 57: 540–547, 2008. [DOI] [PubMed] [Google Scholar]
- 120.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 84: 1620–1628, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yki-Jarvinen H, Utriainen T. Insulin-induced vasodilatation: physiology or pharmacology? Diabetologia 41: 369–379, 1998. [DOI] [PubMed] [Google Scholar]
- 122.Young SG, Davies BS, Voss CV, Gin P, Weinstein MM, Tontonoz P, Reue K, Bensadoun A, Fong LG, Beigneux AP. GPIHBP1, an endothelial cell transporter for lipoprotein lipase. J Lipid Res 52: 1869–1884, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365: 1817–1820, 2005. [DOI] [PubMed] [Google Scholar]
- 124.Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98: 894–898, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao L, Chai W, Fu Z, Dong Z, Aylor KW, Barrett EJ, Cao W, Liu Z. Globular adiponectin enhances muscle insulin action via microvascular recruitment and increased insulin delivery. Circ Res 112: 1263–1271, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


