Abstract
Roux-en-Y gastric bypass (RYGB) causes profound weight loss and remission of diabetes by influencing metabolic physiology, yet the mechanisms behind these clinical improvements remain undefined. After RYGB, levels of glucagon-like peptide-1 (GLP-1), a hormone that enhances insulin secretion and promotes satiation, are substantially elevated. Because GLP-1 signals in both the periphery and the brain to influence energy balance and glucose regulation, we aimed to determine the relative requirements of these systems to weight loss and improved glucose tolerance following RYGB surgery in mice. By pharmacologically blocking peripheral or central GLP-1R signaling, we examined whether GLP-1 action is necessary for the metabolic improvements observed after RYGB. Diet-induced obese mice underwent RYGB or sham operation and were implanted with osmotic pumps delivering the GLP-1R antagonist exendin-(9–39) (2 pmol·kg−1·min−1 peripherally; 0.5 pmol·kg−1·min−1 centrally) for up to 10 wk. Blockade of peripheral GLP-1R signaling partially reversed the improvement in glucose tolerance after RYGB. In contrast, fasting glucose and insulin sensitivity, as well as body weight, were unaffected by GLP-1R antagonism. Central GLP-1R signaling did not appear to be required for any of the metabolic improvements seen after this operation. Collectively, these results suggest a detectable but only modest role for GLP-1 in mediating the effects of RYGB and that this role is limited to its well-described action on glucose regulation.
Keywords: glucagon-like peptide-1, Roux-en-Y gastric bypass, glucose tolerance, obesity, central regulation
the high prevalence of obesity and its related comorbidities, including type 2 diabetes mellitus (T2DM), underscores the need for effective weight loss treatments. Conventional therapies to treat obesity, including behavioral modification relating to diet and exercise, often yield only short-term benefits, whereas gastrointestinal weight loss operations such as Roux-en-Y gastric bypass (RYGB) have been effective in producing long-term weight loss and remission of diabetes in most patients (2). RYGB operations convey these metabolic improvements by influencing the physiology of energy balance and glucose regulation that is disordered in obesity and diabetes, respectively. The rapid and profound improvement in glucose regulation following RYGB suggests the stimulation of weight-independent effects of the surgery, although the precise mechanisms of these effects have only begun to be elucidated (21, 25).
Physiologically, RYGB initiates changes in the secretion and circulation of numerous hormones, including peptide YY (PYY), insulin, amylin, and glucagon-like peptide-1 (GLP-1) in directions that suggest their contribution to improved glucose regulation after surgery (16, 28). GLP-1 is an incretin hormone that is synthesized and secreted from enteroendocrine L cells in the intestine and promotes insulin release from pancreatic β-cells in response to elevated glucose levels. Dramatic rises in postprandial GLP-1 levels, as well as modest increases in fasting levels, have been widely demonstrated in both human patients and animal models of this operation (13, 14, 17, 28).
Within the central nervous system, a distinct population of neurons in the brainstem nucleus of the solitary tract produces GLP-1, where it acts as a neuropeptide. GLP-1 neurons project to hindbrain and forebrain areas, with the highest density of fibers terminating in parts of the hypothalamus known to control the regulation of energy balance and glucose homeostasis (15, 19). Exogenous administration of GLP-1 directly into the hypothalamus and brainstem in rats has been shown to decrease food intake and body weight (8, 20, 27, 29, 31), whereas selective loss of GLP-1 function in the rodent brain leads to increased fat deposition and hyperphagia (1, 23). Central GLP-1 signaling can also influence glucose homeostasis and enhance glucose-stimulated insulin secretion (1, 11, 12, 27).
Given the known role of GLP-1 in regulating energy balance and glucose homeostasis and the large increase in circulating postprandial GLP-1 levels, we sought to determine the distinct requirements of peripheral and central GLP-1R action to the surgical effects of RYGB. Signaling through central mechanisms, such as the melanocortin and leptin regulatory pathways, has been shown to be important in generating the beneficial physiological response to RYGB (6, 7), and still, not all weight regulatory systems are engaged after RYGB (3). With regard to glucose regulation, studies in rats and humans have demonstrated an attenuation of the improved glucose tolerance that was seen after RYGB when GLP-1R signaling in the periphery was blocked (4, 10, 26).
Because endocrine and neural GLP-1 action appears to act in mutually reinforcing directions with regard to energy balance and glucose regulation, in the present study we used a pharmacological approach in diet-induced obese (DIO) mice to determine the distinct requirements of peripheral and central GLP-1 receptor (GLP-1R) signaling to weight loss and improved metabolic regulation after RYGB. We hypothesized that blockade of GLP-1R would impair glucose tolerance and weight loss improvements in RYGB mice to a greater extent than effects observed from antagonist administration in sham-operated mice. We observed that peripheral GLP-1 action is selectively required for improvement in glucose tolerance after RYGB but that neither central nor peripheral GLP-1R signaling is necessary for the effects on body weight, food intake, or improvement in insulin signaling observed after this operation. Thus, much of the effect of RYGB on metabolic regulation and energy balance appears to be mediated by other signaling mechanisms.
MATERIALS AND METHODS
Animals.
DIO C57BL/6J (Jackson Laboratories) male mice 20–26 wk old were housed individually in a barrier animal facility with a 12-h light-dark cycle (lights on at 0700) and an ambient temperature of 19–22°C and 40–60% humidity. Mice were maintained on a high-fat diet (HFD) that contained 60% calories from fat, 20% from protein, and 20% from carbohydrates (D12492 High-Fat Diet; Research Diets, New Brunswick, NJ). Age- and weight-matched mice were used in one of the following three experiments: 1) pharmacological blockade of peripheral GLP-1 action, 2) acute pharmacological blockade of GLP-1 action in the central nervous system (CNS), or 3) chronic pharmacological blockade of GLP-1 action in the CNS. The Massachusetts General Hospital Subcommittee on Research Animal Care approved all experiments.
Gastrointestinal surgical procedures.
DIO mice were fasted overnight and anesthetized and maintained on isoflurane inhalation (1–4%) throughout the surgical procedure (Viking Medical, Medford Lakes, NJ) (13, 18). A standard sterile technique was used. After laparotomy, the stomach was isolated by transection of the gastrohepatic and gastrosplenic ligaments. To create the biliopancreatic limb, the jejunum was transected 2–3 cm distal to the ligament of Treitz (4–8 cm from the pylorus), and the bowel was reconstructed in a Roux-en-Y configuration, with ∼15% of the small intestine included in the biliopancreatic limb and an equal portion in the Roux limb. To create a gastric pouch, a vascular clip was used, followed by creation of a gastro-jejunal anastomosis. The laparotomy was then repaired in two layers. Mice used for the chronic intracerebroventricular (icv) experiments received a slightly modified operation; instead of applying a surgical clip, the gastric pouch and distal stomach were created by double-suturing and transecting the stomach. In all studies, the sham operation included gastrostomy with repair. After surgery, mice were kept on a warming pad until full recovery from anesthesia. They were provided with a liquid diet (40% Vital AF 1.2 Cal; Abbott Laboratories, Abbott Park, IL) ad libitum during the 2 wk immediately following surgery and were gradually weaned back onto the HFD during this time.
Intracerebroventricular surgery.
Three weeks after the abdominal surgery, mice were implanted with an icv cannula targeting the lateral ventricle. Animals were anesthetized and maintained on isoflurane inhalation (1–4%). The head of the mouse was then fixed in a stereotaxic apparatus (Harvard Apparatus, Holliston, MA), and a midline sagittal incision (∼2 cm) was made with a scalpel blade. A tiny hole was drilled in the skull to accommodate the cannula. A 30-gauge guide cannula (Alzet, Cupertino, CA, or Plastics One, Roanoke, VA) was implanted to target the lateral ventricle using the following coordinates relative to Bregma: 0.5 mm posterior, 1.0 mm lateral, and 2.0 mm ventral. Cyanoacrylate glue and Cerebond skull adhesive (Plastics One) were applied to fix the cannula to the skull.
For the acute icv delivery experiments, the guide cannula was closed with an obturator. For the chronic icv delivery experiments, osmotic pumps (Model 2004; Alzet) were implanted subcutaneously on the back of the mouse. The osmotic pump was then connected to the icv cannula with a vinyl catheter tube. During all surgical procedures, mice were given buprenorphine (0.05 mg/kg im) for pain. At euthanization, all mice were given an injection of 2% methylene blue to verify appropriate cannula placement in the lateral ventricle.
Pharmacological blockade of peripheral GLP-1 action.
Age- and weight-matched mice were randomly allocated into the following surgical groups: sham-vehicle (n = 5), sham-exendin (9–39) (Ex-9) (n = 5), RYGB-vehicle (n = 5), and RYGB-Ex-9 (n = 5). A weight-matched sham (WMS) group (n = 5) received the sham operation and was then calorically restricted to match the final weight of the RYGB-vehicle group. Two weeks after the abdominal operations, an osmotic pump (Alzet Model 2006; Alza, Palo Alto, CA) was subcutaneously implanted in the back of the mice filled with either Ex-9 (Bachem, Torrance, CA) infused at a rate of 2 pmol·kg−1·min−1 or vehicle control (distilled water) solution (30). After 5 wk, mice were anesthetized with isoflurane inhalation, and the implanted osmotic pump was replaced with a new pump filled with the same treatment. Body weight was measured weekly until postoperative week 8. Ad libitum cumulative food intake was measured from postoperative week 2 until postoperative week 7. Cumulative caloric intake (kcal) was calculated using the following formula: total caloric intake (kcal) = cumulative food intake of HFD (g) × 5.24 (kcal/g).
Acute pharmacological blockade of central GLP-1 signaling.
Sham (n = 9) and RYGB-operated (n = 10) mice were given icv injections of either the vehicle artificial cerebrospinal fluid (ACSF; 2 μl) or Ex-9 (8 μg/2 μl) in ACSF 20 min prior to an oral glucose tolerance test. Injections were given over 3 min using a 10-μl Hamilton syringe, during which time mice were allowed to roam freely in their cages. No injection-related symptoms were observed in either group. All mice received treatments in a counterbalanced manner.
Chronic pharmacological blockade of central GLP-1 action.
Mice were allocated to the following surgical groups: sham-ACSF (n = 5), sham-Ex-9 (n = 7), RYGB-ACSF (n = 10), or RYGB-Ex-9 (n = 9). After osmotic pump implantation for delivery of Ex-9 (0.5 pmol·kg−1·min−1) or ACSF, body weight was measured weekly for 4 wk. The doses of Ex-9 used are those previously demonstrated by several groups to block central GLP-1R, demonstrating the role of central GLP-1 signaling in influencing insulin secretion and hepatic glycogen storage in response to a gastric glucose infusion (12) as well as its role in inhibiting endogenous glucose production under euglycemic clamped conditions (24). After 4 wk, mice were anesthetized with isoflurane inhalation, and the implanted osmotic pump was replaced with a new pump filled with the same treatment. Glucose tolerance testing and evaluation of glucose-stimulated insulin release were performed after 6–8 wk of drug delivery. Body composition was determined at 8 wk of drug delivery by magnetic resonance imaging (Bruker Minispec; Bruker, The Woodlands, TX). Animals were placed in a clear plastic cylinder (50 mm in diameter) and kept immobile by insertion of a plunger. The tube was then lowered into the instrument for the duration of the scan (<2 min).
Glucose tolerance tests.
Oral and intraperitoneal glucose tolerance tests (1 g/kg body wt) were performed on mice fasted overnight, and blood glucose was measured by placing a drop of tail blood on a glucometer (Alpha TRAK; Abbot Animal Health, Abbot Park, IL). For acute central GLP-1 blockade experiments, mice were injected icv 20 min prior to the oral gavage of glucose. In the peripheral and central chronic blockade experiments the oral glucose tolerance procedure was repeated on a separate day, and blood was collected from the tail vein at 0, 10, 15, and 30 min. Plasma insulin concentrations were measured using a mouse ultrasensitive insulin ELISA (ALPCO, Salem, NH). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as described previously (5).
Statistical analysis.
Data are presented as means ± SE. Glucose tolerance and glucose-stimulated insulin secretion were analyzed by the area under the curve (AUC) analysis calculated using the trapezoidal rule. Data were compared using ANOVA, followed by Bonferroni post hoc tests between selected groups. The statistical analysis was performed using Graphpad Prism software (Graphpad Software, La Jolla, CA).
RESULTS
Peripheral GLP-1R signaling contributes to glucose tolerance improvement after RYGB.
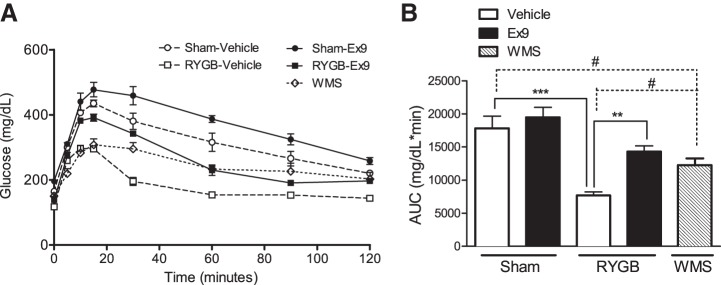
After surgery, RYGB-treated mice exhibited a significant improvement in oral glucose tolerance, with an accelerated resolution phase of glucose excursion and a 57% reduction in the AUC compared with sham-operated mice (Fig. 1, A and B). Notably, mice that received sham operations but were calorically restricted to match the body weight of RYGB-vehicle mice (WMS) displayed a glucose tolerance phenotype that was intermediate between sham and RYGB mice receiving vehicle treatment, indicating both weight-dependent and -independent effects of the surgery on glucose tolerance (Fig. 1, A and B). When GLP-1R action was blocked in sham mice by chronic infusion of the GLP-1R antagonist Ex-9, glucose excursion after an oral glucose load was not significantly different from that in sham animals receiving the vehicle infusion. In RYGB mice, however, Ex-9 caused an 86% increase in the AUC compared with vehicle-treated mice, which corresponds to a 65% reversal of the improvement in oral glucose tolerance after RYGB (Fig. 1B).
Fig. 1.
Peripheral glucagon-like peptide-1 receptor (GLP-1R) signaling contributes to the improvements in glucose tolerance after Roux-en-Y gastric bypass (RYGB). A: blockade of GLP-1R by chronic exendin-(9–39) (Ex-9) infusion (2 pmol·kg−1·min−1) impaired oral glucose tolerance to a greater extent in RYGB vs. sham mice. B: area under the curve (AUC) calculations for glucose excursion curves after oral glucose administration are shown. Values represent means ± SE. **P < 0.01 and ***P < 0.001, significant differences in comparisons of Sham and RYGB groups treated with Vehicle and Ex-9; #P < 0.05, significant differences in comparisons of weight-matched sham (WMS) with sham and RYGB vehicle-treated groups.
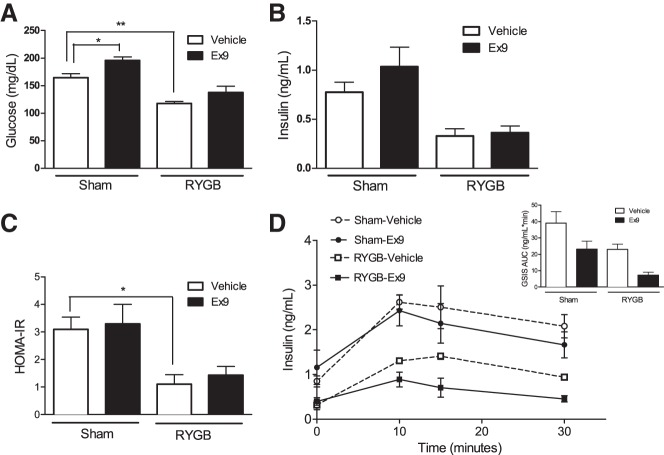
Peripheral GLP-1R signaling is not required for improvements in fasting blood glucose or fasting insulin concentrations after RYGB.
RYGB-vehicle mice exhibited fasting blood glucose concentrations that were 30% lower than sham mice treated with vehicle (Fig. 2A). Blockade of GLP-1R by Ex-9 infusion did not reverse the improvement in fasting glucose concentrations in RYGB mice (Fig. 2A). Similarly, Ex-9 treatment did not influence insulin levels after RYGB (Fig. 2B). We used HOMA-IR to estimate the contribution of altered insulin signaling to the partial reversal of glucose tolerance improvement after Ex-9 infusion in the RYGB-operated mice. Although this analysis suggested an improvement in insulin sensitivity after RYGB, it showed no difference in the insulin sensitivity between RYGB and sham mice treated with Ex-9 (Fig. 2C). In addition, although Ex-9 tended to inhibit insulin secretion in both surgical groups, the decreases did not reach statistical significance (Fig. 2D).
Fig. 2.
Peripheral GLP-1R signaling is not required for improvements in fasting blood glucose or fasting insulin concentrations after RYGB. Fasting blood glucose (A), insulin concentrations (B), homeostasis model assessment of insulin resistance (HOMA-IR; C), and glucose-stimulated insulin secretion (D) were not influenced by chronic Ex-9 infusion in sham or RYGB mice. Values represent means ± SE. *P < 0.05; **P < 0.01.
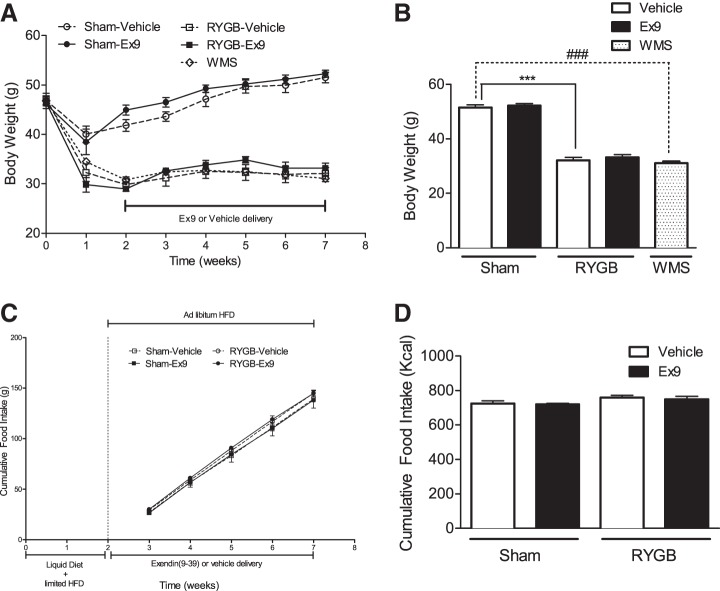
Peripheral GLP-1R signaling is not required for weight loss or altered food intake after RYGB.
Because of its increased levels after RYGB and its anorexigenic effect on food intake, GLP-1 has been proposed to play a role in RYGB-mediated weight loss. At postoperative week 7, RYGB mice weighed on average 37% less than sham control mice (Fig. 3B). Blockade of peripheral GLP-1R action with Ex-9 did not reverse the weight loss effect of RYGB or influence the weekly body weight gain in sham mice (Fig. 3, A and B). By design, WMS mice had a body weight similar to RYGB-vehicle mice. Cumulative food intake in RYGB-operated mice was similar to the intake observed in sham-operated mice (Fig. 3, C and D). Chronic GLP-1R blockade by Ex-9 infusion did not alter food intake in either RYGB- or sham-operated mice compared with their respective vehicle-infused controls (Fig. 3, C and D).
Fig. 3.
Peripheral GLP-1R signaling is not required for energy balance phenotypes after RYGB. Weight progression over 7 wk (A) and final weight at postoperative week 7 (B) were not altered by blockade of GLP-1R signaling by chronic infusion of Ex-9 in RYGB or sham mice. Cumulative food intake (C and D) over 5 consecutive wk in sham and RYGB mice was also not altered by Ex-9 infusion. Values represent means ± SE. ***P < 0.001, significant differences in comparisons of sham and RYGB groups treated with vehicle and Ex-9; ###P < 0.001, significant differences in comparisons of weight-matched sham (WMS) with sham and RYGB vehicle-treated groups.
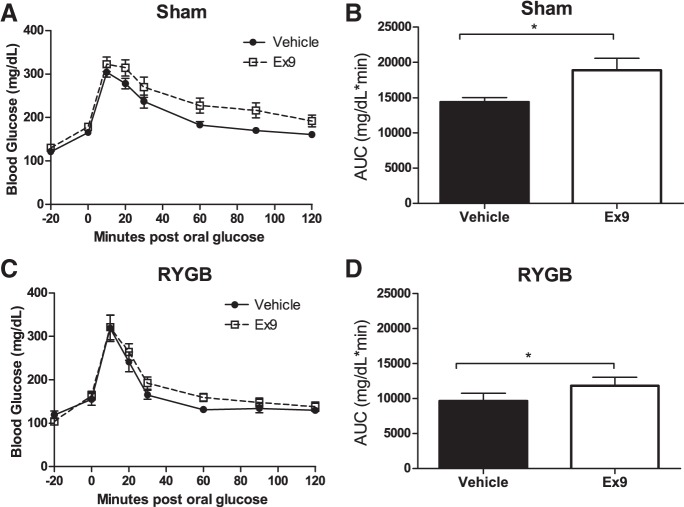
Acute blockade of central GLP-1R signaling impairs glucose tolerance to a similar extent in RYGB and sham mice.
As expected, RYGB mice treated with the vehicle demonstrated significantly improved glucose tolerance compared with sham mice treated with vehicle (AUC: RYGB-vehicle 9,653 ± 1,106 vs. sham-vehicle 14,396 ± 644, P < 0.01; Fig. 4, B and D). In the sham mice, Ex-9 pretreatment into the brain impaired glucose tolerance compared with vehicle-treated control sham mice, confirming the known role of central GLP-1R signaling in normal glucose tolerance (Fig. 4A). In RYGB mice, blocking central GLP-1R also impaired glucose tolerance (Fig. 4C). However, the degree of impairment in RYGB mice (a 22% increase in AUC of glucose excursion) was no different than in the sham-operated conditions (a 31% increase, P = 0.77). If increased central GLP-1R signaling was responsible for the improved glucose tolerance after RYGB, we would predict that the effect of Ex-9 to impair glucose tolerance would be greater in RYGB-treated animals than in controls. Because this effect was no different (and, in fact, less in value) than in sham controls, these data suggest that the RYGB-mediated improvement in glucose tolerance does not require central GLP-1R signaling.
Fig. 4.
Acute blockade of central GLP-1 signaling impairs glucose tolerance to a similar extent in RYGB and sham mice. Intracerebroventricular (icv) pretreatment (20 min) with Ex-9 (8 μg/2 μl) impaired oral glucose tolerance in sham (A) and RYGB (C) mice. Area under the curve (AUC) calculations for glucose excursion curves in sham (B) and RYGB (D) mice are shown. Values are means ± SE. *P < 0.05.
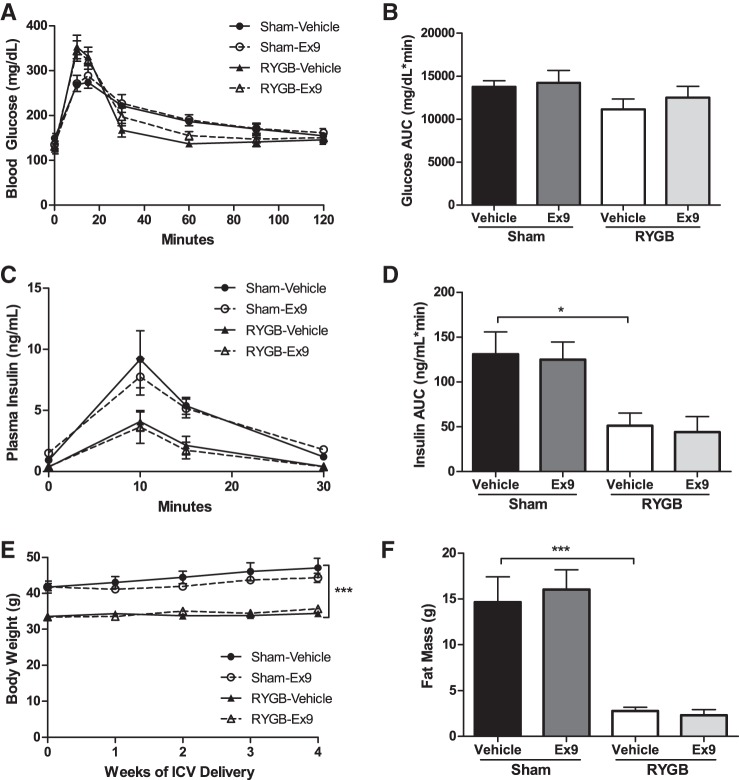
Chronic blockade of central GLP-1R signaling does not attenuate the improved glucose tolerance after RYGB.
To exclude the possibility that the effect of Ex-9 after RYGB may require longer-term exposure, we sought to determine whether chronic blockade of central GLP-1R might have an impact on glucose tolerance. Six weeks of continuous Ex-9 delivery neither prevented nor reversed the improvement in glucose tolerance seen after RYGB (Fig. 5, A and B) and did not influence glucose-stimulated insulin secretion in the RYGB-treated mice (Fig. 5, C and D). These results thus provide further evidence that central GLP-1R signaling is not necessary to meditate glucose regulation after RYGB.
Fig. 5.
Chronic blockade of central GLP-1 signaling does not attenuate the improved glucose tolerance or weight loss after RYGB. Chronic blockade of central GLP-1R (0.5 pmol·kg−1·min−1) did not influence oral glucose tolerance (A and B) or oral glucose-stimulated insulin secretion (C and D). Weekly body weight over 4 wk (E) and fat mass after 8 wk (F) of chronic infusion was not altered by Ex-9. Values are means ± SE. *P < 0.05; ***P < 0.001.
Chronic blockade of central GLP-1R signaling does not impair weight loss after RYGB.
Body weight and fat mass in sham and RYGB mice receiving chronic Ex-9 infusion were no different from those mice treated with vehicle control (Fig. 5, E and F). Moreover, food intake measured during the 5th wk of icv infusion was not altered by central treatment (data not shown).
DISCUSSION
The considerable postprandial rise in the incretin hormone GLP-1 after RYGB and the known effects of this peptide on energy balance and glucose homeostasis has raised the possibility that GLP-1 may be responsible for surgery-associated weight loss and improvement in glucose regulation. Here, we demonstrate that blockade of peripheral GLP-1R signaling partially reverses the improvement in oral glucose tolerance induced by RYGB, a result supported by studies performed in rats and human patients (4, 9, 26, 32). In contrast, however, we found that neither peripheral nor central GLP-1R signaling is required for the weight loss observed after RYGB.
The finding that increased peripheral GLP-1R action after RYGB contributes to improved oral glucose tolerance is consistent with similar observations after other gastrointestinal metabolic operations. Peripheral GLP-1R antagonism with Ex-9 after an enterogastroanastomosis operation in mice, in which the midjejunum is brought up and connected to the stomach, excluding the duodenum and proximal jejunum from nutrient flow in a manner similar to RYGB in human patients, degraded oral glucose tolerance and impaired glucose-stimulated insulin secretion without affecting insulin sensitivity or weight loss (30). Ex-9 also reversed the improvement in glucose tolerance observed in Goto-Kakizaki rats that underwent a duodenal-jejunal exclusion operation (10). Although the enterogastroanastomosis and duodenal-jejunal exclusion procedures do not include the gastric reduction component of the RYGB, they mimic the change in pattern of nutrient and biliopancreatic delivery into the jejunum induced by RYGB. Given the similar effects of these three operations on glucose homeostasis and their similar responses to Ex-9, these observations suggest that the rerouting of the intestine is largely responsible for the GLP-1-mediated improvement in glucose tolerance. However, it is important to note that Ex-9 also blocked the improvement in meal tolerance and insulin secretion after vertical sleeve gastrectomy, an operation that manipulates the stomach only (4). This result suggests that there could be multiple gastrointestinal mechanisms in RYGB that signal and lead to GLP-1 action on improved oral glucose tolerance.
In line with the notion that there are likely multiple mechanisms to improved surgical outcomes, recent publications unexpectedly found that mice with a global deletion of the GLP-1R responded to RYGB and vertical sleeve gastrectomy operations in manners similar to wild-type mice, raising doubt about the requirement for GLP-1R signaling in bariatric surgery outcomes (22, 33, 34). Although it is likely that the mice deficient in GLP-1R from conception developed mechanisms to compensate for the GLP-1R absence, thereby producing the improved surgical outcomes, these studies nonetheless reveal the existence of GLP-1-independent mechanisms capable of generating the metabolic improvements of surgery. Thus, these studies underscore the notion that although GLP-1 action contributes to RYGB metabolic outcomes, it is not a necessity.
Although we can conclude that GLP-1 does not appear to be essential for the metabolic effects of RYGB, we cannot exclude the possibility that RYGB-treated mice would require a higher dose of antagonist to combat the higher postsurgical levels of GLP-1. We consider this explanation unlikely, however, as a recent study in rats that examined a supramaximal dose of Ex-9 similarly demonstrated the absence of a requirement for central GLP-1R signaling for weight loss after RYGB (34). Furthermore, the demonstration that GLP-1R-deficient mouse models are still able to mount weight loss phenotypes following RYGB further corroborates our conclusions that GLP-1R signaling is not required for RYGB weight loss (22, 34). Nonetheless, we cannot exclude the possibility that GLP-1 may have secondary effects on RYGB outcomes, perhaps to potentiate the effects of another regulatory factor when both are present. This potential modulating action may not be apparent by the blockade of GLP-1R alone.
Despite the rapid and substantial rise in GLP-1 after RYGB, the requirement for GLP-1 action in mediating RYGB effects appears limited to its peripheral action on glucose regulation. Because GLP-1R signaling in any site is not required for surgically induced weight loss, it suggests that other mechanisms, likely working in parallel to GLP-1, are required for these effects of surgery. Identification of these mechanisms is likely to provide more effective targets for nonsurgical therapies that more directly mimic the benefits of RYGB for the treatment of obesity and diabetes.
GRANTS
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-095567 (J. S. Carmody), DK-088661, and DK-090956 (L. M. Kaplan) and a research grant from Ethicon.
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
J.S.C., R.M., and L.M.K. conception and design of research; J.S.C., R.M., and H.Y. performed experiments; J.S.C. and R.M. analyzed data; J.S.C., R.M., and L.M.K. interpreted results of experiments; J.S.C. and R.M. prepared figures; J.S.C. and R.M. drafted manuscript; J.S.C. and L.M.K. edited and revised manuscript; L.M.K. approved final version of manuscript.
REFERENCES
- 1.Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122: 248.e5–256.e5, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Carmody JS, Ahmad NN, Machineni S, Lajoie S, Kaplan LM. Weight Loss After RYGB Is Independent of and Complementary to Serotonin 2C Receptor Signaling in Male Mice. Endocrinology 156: 3183–3191, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gersin KS, Keller JE, Stefanidis D, Simms CS, Abraham DD, Deal SE, Kuwada TS, Heniford BT. Duodenal- jejunal bypass sleeve: a totally endoscopic device for the treatment of morbid obesity. Surg Innov 14: 275–278, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Hao Z, Munzberg H, Rezai-Zadeh K, Keenan M, Coulon D, Lu H, Berthoud HR, Ye J. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int J Obes (Lond) 39: 798–805, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatoum IJ, Stylopoulos N, Vanhoose AM, Boyd KL, Yin DP, Ellacott KL, Ma LL, Blaszczyk K, Keogh JM, Cone RD, Farooqi IS, Kaplan LM. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab 97: E1023–E1031, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jørgensen NB, Dirksen C, Bojsen-Møller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 62: 3044–3052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kindel TL, Yoder SM, Seeley RJ, D'Alessio DA, Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg 13: 1762–1772, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, Colom A, Uldry M, Rastrelli S, Sabatier E, Godet N, Waget A, Penicaud L, Valet P, Burcelin R. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 149: 4768–4777, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115: 3554–3563, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucharczyk J, Nestoridi E, Kvas S, Andrews R, Stylopoulos N. Probing the mechanisms of the metabolic effects of weight loss surgery in humans using a novel mouse model system. J Surg Res 179: e91–e98, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93: 2479–2485, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270, 1997. [DOI] [PubMed] [Google Scholar]
- 16.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243: 108–114, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol 301: R1057–R1066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 5: 178ra41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockie SH. Glucagon-like peptide-1 receptor in the brain: role in neuroendocrine control of energy metabolism and treatment target for obesity. J Neuroendocrinol 25: 597–604, 2013. [DOI] [PubMed] [Google Scholar]
- 20.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol Regul Integr Comp Physiol 274: R23–R29, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366: 1577–1585, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 3: 191–201, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nogueiras R, Pérez-Tilve D, Veyrat-Durebex C, Morgan DA, Varela L, Haynes WG, Patterson JT, Disse E, Pfluger PT, López M, Woods SC, DiMarchi R, Diéguez C, Rahmouni K, Rohner-Jeanrenaud F, Tschöp MH. Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J Neurosci 29: 5916–5925, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parlevliet ET, de Leeuw van Weenen JE, Romijn JA, Pijl H. GLP-1 treatment reduces endogenous insulin resistance via activation of central GLP-1 receptors in mice fed a high-fat diet. Am J Physiol Endocrinol Metab 299: E318–E324, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341: 406–410, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 60: 2308–2314, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57: 2046–2054, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151: 1588–1597, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Møller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol Regul Integr Comp Physiol 271: R848–R856, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, Andreelli F. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 8: 201–211, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Vetter ML, Wadden TA, Teff KL, Khan ZF, Carvajal R, Ritter S, Moore RH, Chittams JL, Iagnocco A, Murayama K, Korus G, Williams NN, Rickels MR. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes 64: 434–446, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 62: 2380–2385, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Münzberg H, Morrison CD, Drucker DJ, Berthoud HR. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol 306: R352–R362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]