Distinct neurohumoral profiles were detected in blood collected during head-up tilt in children with postural orthostatic tachycardia syndrome, orthostatic hypotension, and syncope, revealing different compensatory mechanisms. This first comprehensive neurohumoral profile in children with orthostatic intolerance provides a basis for future strategic treatment options in children.
Keywords: orthostatic intolerance, head-upright tilt test, postural orthostatic tachycardia syndrome, orthostatic hypotension, arginine vasopressin
Abstract
Studies of adults with orthostatic intolerance (OI) have revealed altered neurohumoral responses to orthostasis, which provide mechanistic insights into the dysregulation of blood pressure control. Similar studies in children with OI providing a thorough neurohumoral profile are lacking. The objective of the present study was to determine the cardiovascular and neurohumoral profile in adolescent subjects presenting with OI. Subjects at 10–18 yr of age were prospectively recruited if they exhibited two or more traditional OI symptoms and were referred for head-up tilt (HUT) testing. Circulating catecholamines, vasopressin, aldosterone, renin, and angiotensins were measured in the supine position and after 15 min of 70° tilt. Heart rate and blood pressure were continuously measured. Of the 48 patients, 30 patients had an abnormal tilt. Subjects with an abnormal tilt had lower systolic, diastolic, and mean arterial blood pressures during tilt, significantly higher levels of vasopressin during HUT, and relatively higher catecholamines and ANG II during HUT than subjects with a normal tilt. Distinct neurohumoral profiles were observed when OI subjects were placed into the following groups defined by the hemodynamic response: postural orthostatic tachycardia syndrome (POTS), orthostatic hypotension (OH), syncope, and POTS/syncope. Key characteristics included higher HUT-induced norepinephrine in POTS subjects, higher vasopressin in OH and syncope subjects, and higher supine and HUT aldosterone in OH subjects. In conclusion, children with OI and an abnormal response to tilt exhibit distinct neurohumoral profiles associated with the type of the hemodynamic response during orthostatic challenge. Elevated arginine vasopressin levels in syncope and OH groups are likely an exaggerated response to decreased blood flow not compensated by higher norepinephrine levels, as observed in POTS subjects. These different compensatory mechanisms support the role of measuring neurohumoral profiles toward the goal of selecting more focused and mechanistic-based treatment options for pediatric patients with OI.
NEW & NOTEWORTHY
Distinct neurohumoral profiles were detected in blood collected during head-up tilt in children with postural orthostatic tachycardia syndrome, orthostatic hypotension, and syncope, revealing different compensatory mechanisms. This first comprehensive neurohumoral profile in children with orthostatic intolerance provides a basis for future strategic treatment options in children.
orthostatic intolerance (OI) affects 500,000 Americans, rendering them unable to maintain adequate blood perfusion during an upright position (42). When standing, patients often experience lightheadedness, sweating, and nausea (22, 42). An abnormal cardiovascular response to head-up tilt (HUT) orthostatic challenge can be broadly grouped into the following: postural orthostatic tachycardia syndrome (POTS), orthostatic hypotension (OH), and syncope. Management and treatment of OI is typically dependent on the specific cardiovascular diagnosis, with most treatments having variable effectiveness in adult and adolescent populations (4, 8, 13, 30, 39). The pathophysiological mechanisms responsible for these cardiovascular changes are not well defined as presentation and symptoms are often heterogenous (14). This heterogeneity leads to the empirical treatment of symptoms without a evidence-based approach related to their underlying cause (3, 6).
In response to HUT, a reduction in blood volume or pressure at the level of the heart or carotid arteries is associated with reflex increases in catecholamines, arginine vasopressin (AVP), renin, ANG II, and aldosterone (Aldo) (8a). Although there are many additional nonreflex regulators that influence these systems, previous studies in adults with OI have revealed exaggerated neurohumoral responses to upright posture, including elevated catecholamines (16, 24), ANG II (34, 35), and AVP (11, 25, 37, 54). Neurohumoral responses also differ among OI subtypes, underscoring the possibility of differential mechanisms related to specific hemodynamic profiles. Adults with POTS have been observed to have elevated levels of plasma norepinephrine (NE) and epinephrine (Epi) at the time of orthostatic challenge, suggesting disproportionally higher sympathetic activation likely compensating for low blood pressure (BP) (16, 34, 58). Elevated supine ANG II is also associated with POTS subjects with hypovolemia (49). In adults with OH, greater supine AVP and endothelin-1 concentrations have shown to be predictive of decreases in systolic BP (SBP) during HUT (37). Increases in AVP and Epi during HUT have also been observed in both adult OH and vasovagal syncope subjects (23, 32, 37). OI is also observed in pediatric patients (12) but is particularly difficult to diagnose and treat as it is underrecognized, and testing, such as HUT, can be invasive for children. Characterization of the neurohumoral response to orthostatic challenge in children with OI has not been well described, but dysregulation of these systems likely plays a role in its pathogenesis (12, 13).
We hypothesized that children with OI undergoing HUT would demonstrate distinct neurohumoral responses in association with specific patterns of changes in BP and heart rate (HR). We further suggest that specific cardiovascular responses in OI subtypes such as POTS, OH, and syncope are associated with unique neurohumoral profiles. To test this, we measured circulating levels of Epi, NE, AVP, Aldo, renin, and angiotensins [ANG-(1–7) and ANG II] both in the supine position and during HUT and compared these findings with the hemodynamic response in pediatric subjects with OI.
METHODS
Patients
Forty-eight male and female subjects at 10–18 yr of age were recruited from the pediatric clinic at Wake Forest Baptist Medical Center if they experienced two or more symptoms consistent with OI, in which HUT was deemed necessary, for at least 2 mo before testing. These OI symptoms included nausea, dizziness, lightheadedness, abdominal pain/bloating, syncope, excessive sweating, or chronic fatigue. Patients were excluded if a metabolic, mechanical, or mucosal inflammatory finding was identified as the cause for their symptoms. All subjects were required to undergo a 45-min HUT test during which blood was drawn to measure Epi, NE, ANG II, ANG-(1–7), Aldo, renin, and AVP after 15 min in the supine position and after 15 min of HUT. The Institutional Review Board of Wake Forest Baptist Medical Center approved this study. Parents and children provided written consent/assent before study participation.
HUT
Each subject was positioned supine on the tilt table for 15 min before HUT followed by an immediate upright tilt from 0 to 70° for up to 45 min. Continuous measures of BP and HR were obtained using noninvasive finger arterial pressure measurements and analyzed by BIOPAC acquisition software (Santa Barbara, CA). An average of BPs and HRs in the last 5 min of the supine period were used for baseline measures. Average HUT BPs and HRs were calculated in the 2 min before blood samples were collected (after 15 min of HUT) to compare with baseline supine BP and HR. After 45 min, the tilt table was brought back to the supine position for an additional 15 min. The test was terminated early for excessive gastrointestinal symptoms (nausea or abdominal pain), syncope, cardiac arrhythmia, or at the request of the patient for severe discomfort.
Patients were diagnosed as having an abnormal HUT [HUT(+)] if they met criteria for POTS, OH, syncope, or POTS/syncope (P/S). Otherwise, patients were defined as having a normal HUT [HUT(−)]. POTS was defined as either a HR of >120 beats/min or a ≥40-beats/min increase in HR from mean supine HR in the first 10 min of HUT and sustained for at least 2 min (46). OH was defined as a 25-mmHg decrease in SBP from mean supine SBP in the first 10 min of HUT sustained for a minimum of 2 min without an associated increase in HR (14). Syncope was defined as a temporary loss of consciousness during 45-min HUT. Subjects were classified as P/S if they met criteria for POTS as defined above followed by syncope at any time during the 45-min HUT.
Measurements of Blood Hormones
Measures of Aldo, renin, and angiotensins were carried out in the Clinical Laboratory Improvement Amendment-certified Hypertension Core Assay Laboratory at Wake Forest. Measures of catecholamines (Epi, NE, and dopamine) and AVP were carried out in the Pathology laboratory at Wake Forest Baptist Health. Radioimmunoassays (RIAs) previously described for human studies were used to measure three angiotensin peptides [ANG I, ANG II, and ANG-(1–7)] (27, 28). ANG I was measured using a modified version of the Peninsula assay, ANG II was measured using ALPCO (Windham, NH), and ANG-(1–7) was measured using an in-house antibody. AVP was measured using an ALPCO Diagnostics RIA (Windham, NH). Serum Aldo and plasma renin were measured as previously described (34). Epi, NE, and dopamine were measured after whole plasma extraction by HPLC. Dopamine was not detectable in plasma of any of the subjects.
Statistical Analysis
For continuous variables, means and SEs were calculated; for categorical variables, counts and percentages were calculated. Two-way ANOVA tests were conducted to examine the effect of HUT on HUT(+) versus HUT(−) groups as well as HUT(−), OH, POTS, syncope, and P/S groups. When appropriate, post hoc t-tests and one-way ANOVAs were conducted to identify specific effects of HUT on hemodynamic and neurohumoral variables between and among groups. Unpaired t-tests were performed to compare HUT(+) and HUT(−) groups followed by a Welch's correction if there were unequal variances. Mann-Whitney tests were conducted when data sets were not normally distributed. Paired t-tests were performed to compare supine with HUT measures within groups, and Wilcoxin's correction tests were used when data sets were not normally distributed. One-way ANOVAs followed by Tukey's post hoc multiple-comparisons tests were performed to define differences between HUT(−), POTS, OH, syncope, and P/S groups. Kruskal-Wallis tests were performed when group data were not normally distributed. Pearson's correlation test was performed to compare neurohumoral and hemodynamic variables. Spearman's correlation test was performed when data sets were not normally distributed. All statistical analyses were performed using GraphPad Prism 5.0. Results with P < 0.05 were considered statistically significant.
RESULTS
Forty-eight patients (36 female subjects and 12 male subjects) presenting with OI symptoms for at least 2 mo prior underwent HUT testing. The median age (±SE) was 15.2 ± 0.3 yr. Eighteen patients were HUT(−) and 30 patients were HUT(+) over the 45-min HUT. The 30 HUT(+) subjects met criteria for one of the four groups [POTS (n = 7), OH (n = 5), syncope (n = 8), or P/S (n = 10)]. There were no differences in age or weight between groups. The body mass index was significantly higher in HUT(−) subjects than HUT(+) subjects. As expected, HUT(−) subjects were able to sustain HUT significantly longer than HUT(+) subjects. Syncope and P/S subjects sustained significantly less time on HUT compared with HUT(−) subjects, and POTS subjects were able to stay on HUT longer than syncope subjects (Table 1).
Table 1.
Patient demographics
| Groups |
Subgroups |
|||||||
|---|---|---|---|---|---|---|---|---|
| HUT(−) | HUT(+) | P Value | POTS | OH | Syncope | P/S | P Value | |
| n | 18 | 30 | 7 | 5 | 8 | 10 | ||
| Sex, male/female | 3/15 | 9/21 | 3/4 | 1/4 | 1/7 | 4/6 | ||
| Age, yr | 15 ± 0.4 | 15 ± 0.4 | NS | 14.7 ± 0.5 | 14.4 ± 1.3 | 15.3 ± 1.0 | 16 ± 0.6 | NS |
| Weight, kg | 63 ± 3.9 | 57.9 ± 2.6 | NS | 60.4 ± 7.9 | 57.8 ± 6.1 | 52.6 ± 4.3 | 60.6 ± 3.6 | NS |
| Body mass index | 24.7 ± 1.5 | 21.2 ± 0.7 | 0.03* | 21.2 ± 1.8 | 22.1 ± 2.1 | 20.8 ± 1.2 | 21.1 ± 0.9 | NS |
| Time on HUT | 45.6 ± 0.2a | 27.4 ± 2.4b | <0.0001 | 41.4 ± 3.3c | 31.8 ± 6.8d | 17.7 ± 2.6e | 23.2 ± 2.9f | <0.0001 |
| <0.0001a vs. e | ||||||||
| <0.01a vs. f | ||||||||
| <0.05c vs. e | ||||||||
All values are expressed as exact numbers (n) or means ± SE. Subjects with a normal response to head-up tilt (HUT) [HUT(−) subjects] had a negative HUT, and subjects with an abnormal response to HUT [HUT(+) subjects] had a positive HUT. Unpaired t-tests were used to assess differences between HUT(−) and HUT(+) subjects. One-way ANOVAs were used to assess differences between HUT(+) subgroups. There were no differences in age or weight between groups. HUT(+) subjects had a lower body mass index than HUT(−) subjects. HUT(+) subjects were unable to sustain HUT for the full 45-min HUT. Syncope subjects sustained the least amount of time on HUT, which was significantly less time than HUT(−) and postural orthostatic tachycardia syndrome (POTS) subjects. POTS/syncope (P/S) subjects also sustained less time on HUT compared with HUT(−) subjects.
OH, orthostatic hypotension; NS, not significant.
HUT(−),
HUT(+),
POTS,
OH,
syncope, and
P/S.
HUT(+) Versus HUT(−) Subjects
Hemodynamic measurements.
There were no significant differences between HUT(+) and HUT(−) subjects for BP and HR in the supine position (Fig. 1). During tilt, HUT(+) subjects had significantly lower SBP and diastolic BP (DBP) compared with HUT(−) subjects [interaction, P < 0.05; HUT(+) vs. HUT(−), P < 0.0005; Fig. 1, A and B]. Similar patterns were observed for mean arterial pressure (MAP). Additionally, HUT(−) subjects had a significant increase in DBP upon HUT compared with supine (P < 0.05). No other significant BP changes were observed from supine to HUT in either group. Both groups had a significant increase of HR from supine to HUT, but there were no differences at supine or HUT between groups (Fig. 1C).
Fig. 1.
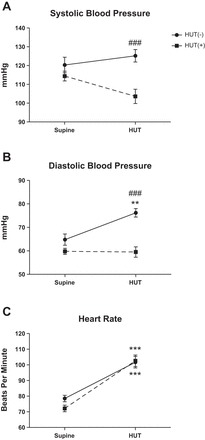
Orthostatic changes in systolic blood pressure (SBP; A), diastolic blood pressure (DBP; B), and heart rate (HR; C) upon head-up tilt (HUT) testing in children. SBP and DBP were significantly higher in subjects with a normal response to HUT [HUT(−) subjects (n = 18)] than in subjects with an abnormal response to HUT [HUT(+) subjects (n = 30)] during HUT. HUT(−) subjects had a significant increase in DBP and HR from the supine position to HUT. HUT(+) subjects had a significant increase in HR from the supine position to HUT but no change in DBP. Supine position vs. HUT: **P < 0.01 and ***P < 0.001; HUT(−) vs. HUT(+) subjects: ###P < 0.001.
Neurohumoral measurements.
There were no significant differences between HUT(−) and HUT(+) subjects for neurohumoral measures in the supine position (Fig. 2). Plasma levels of NE (Fig. 2A), Epi (Fig. 2 B), renin (Fig. 2D), and ANG II (Fig. 2F) significantly increased from supine to HUT for both HUT(−) and HUT(+) groups, but there were no differences in these hormones between groups during HUT. Plasma AVP concentrations increased in both groups from supine to HUT [P < 0.05 for HUT(−) and P < 0.001 for HUT(+)] but were higher in HUT(+) versus HUT(−) subjects during HUT (P < 0.05; interaction, P = 0.08; Fig. 2C). There were no differences in Aldo or ANG-(1–7) levels between or among groups (Fig. 2, E and G).
Fig. 2.
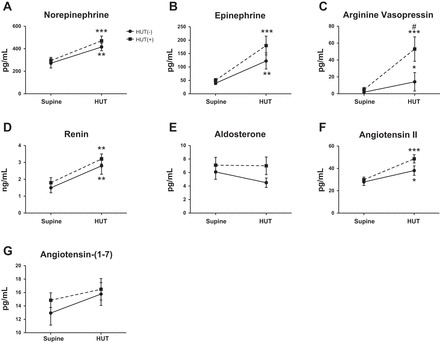
Orthostatic changes in norepinephrine (NE; A), epinephrine (Epi; B), arginine vasopressin (AVP; C), renin (D), aldosterone (Aldo; E), ANG II (F), and ANG-(1–7) (G) in children. There were no differences between groups in the supine position or with HUT. However, both HUT(−) (n = 18) and HUT(+) (n = 30) groups exhibited significant increases in NE, Epi, AVP, renin, and ANG II from the supine position to HUT. HUT(+) subjects had significantly higher AVP than HUT(−) subjects during HUT. There were no changes in Aldo or ANG-(1–7) levels observed from the supine position to HUT. Supine position vs. HUT: *P < 0.05, **P < 0.01, and ***P < 0.001; HUT(−) vs. HUT(+) subjects: #P < 0.05.
Correlative Assessment of Neurohumoral Biomarkers and Hemodynamics
Based on our findings, we applied regression analysis to further elucidate the association between these neurohumoral biomarkers and hemodynamics at supine and in response to HUT considering all 48 subjects and HUT(+) and HUT(−) groups alone (Table 2). Supine NE had a negative correlation with Epi and ANG-(1–7) in both supine and HUT positions and positive correlation with supine AVP. As expected, NE during HUT had a positive relationship with both supine and HUT HR. These relationships were also observed in HUT(+) subjects but not in HUT(−) subjects. HUT levels of NE correlated in a positive manner with supine AVP and negatively with HUT ANG-(1–7). The negative relationship between HUT NE and HUT ANG-(1–7) was also observed in HUT(−) subjects. Supine Epi correlated directly with supine HR, whereas HUT Epi had an inverse relationship with MAP. HUT Epi also directly correlated with ANG-(1–7) in both supine and HUT positions considering all 48 subjects and HUT(+) subjects alone (Table 2).
Table 2.
Correlations between neurohumoral biomarkers and hemodynamics in the supine position and in response to HUT
| All 48 Subjects |
HUT(−) Subjects |
HUT(+) Subjects |
||||
|---|---|---|---|---|---|---|
| Supine position | HUT | Supine position | HUT | Supine position | HUT | |
| Norepinephrine | ||||||
| HR-supine | 0.39† | 0.39* | ||||
| HR-HUT | 0.56‡ | 0.59‡ | ||||
| Epi-supine | −0.30* | |||||
| Epi-HUT | −0.39† | |||||
| AVP-supine | 0.41† | 0.33* | ||||
| ANG-(1–7)-supine | −0.43† | |||||
| ANG-(1–7)-HUT | −0.42† | −0.34* | −0.58† | |||
| Epi | ||||||
| MAP-HUT | −0.42† | |||||
| HR-supine | 0.35† | |||||
| AVP-HUT | 0.38† | |||||
| Ang-(1–7)-supine | 0.30* | 0.43* | ||||
| Ang-(1–7)-HUT | 0.33* | 0.51† | ||||
| AVP | ||||||
| MAP-supine | 0.43† | 0.43† | ||||
| MAP-HUT | −0.46‡ | −0.46‡ | ||||
| ANG II | ||||||
| HR-supine | 0.34* | |||||
| Renin-supine | 0.47‡ | 0.41‡ | 0.57* | 0.61† | ||
| Renin-HUT | 0.45† | 0.44† | 0.51† | |||
| Aldo-supine | 0.36† | 0.41† | 0.39* | |||
| Aldo-HUT | 0.35* | 0.49† | 0.52* | 0.49† | ||
| Renin | ||||||
| HR-supine | 0.31* | |||||
| Aldo-supine | 0.57‡ | 0.35† | 0.58‡ | 0.4* | ||
| Aldo-HUT | 0.52† | 0.32* | 0.52* | 0.50† | ||
HR, heart rate; Epi, epinephrine; AVP, arginine vasopressin; MAP, mean arterial pressure; Aldo, aldosterone.
Pearson's correlation test was used for normally distributed data sets. Spearman's correlation test was used for data sets that were not normally distributed.
P < 0.05;
P ≤ 0.01;
P ≤ 0.001.
In addition to relationships with NE and Epi, AVP associated with MAP in the supine postion and during HUT. In the supine position, AVP showed a positive correlation with MAP. In contrast, during HUT, a significant negative correlation between MAP and AVP during HUT was observed. Both of these correlations were also observed in HUT(+) subjects but not in HUT(−) subjects. ANG II in the supine position correlated directly with supine HR. Significant positive relationships were also observed between ANG II and renin, ANG II and Aldo, and renin and Aldo in supine and HUT positions (Table 2).
HUT(+) Subgroups Versus HUT(−) Subjects
Hemodynamic measurements.
To explore differences among patients with specific OI subgroup diagnoses during HUT, we analyzed hemodynamic and neurohumoral changes in POTS, OH, syncope, and P/S subjects compared with HUT(−) subjects. There was a significant interaction for SBP during HUT, indicating differences among groups at baseline and in response to tilt. Specifically, subjects with OH (P < 0.0001), POTS (P < 0.01), and syncope (P < 0.01) had significantly lower SBP during HUT compared with HUT(−) subjects, and OH subjects had significantly lower SBP than P/S subjects (P < 0.01). The only subgroup to have a significant effect from supine to HUT for SBP was the syncope group, which had a decrease in SBP with HUT (P < 0.05; Fig. 3A). There was also a significant interaction for DBP (P = 0.04) and MAP (P = 0.009) from supine to HUT, in which all subgroups had significantly lower DBP and MAP than HUT(−) patients (P < 0.0001; Fig. 3B). HUT(−) subjects had significantly higher SBP, DBP, and MAP during HUT compared with all other groups, and OH subjects had significantly lower SBP and MAP than POTS, syncope, and P/S subjects (P < 0.0001; Fig. 3). HUT(−), POTS, syncope, and P/S subjects had a significant increase in HR from supine to HUT. Consistent with the clinical definition of POTS, the POTS group had significantly higher HR during HUT than all other subgroups (P < 0.01) and a greater increase in HR from supine to HUT (P < 0.0001). Additionally, OH subjects had significantly lower HR than HUT(−) and P/S subjects during HUT (P < 0.05; Fig. 3C).
Fig. 3.
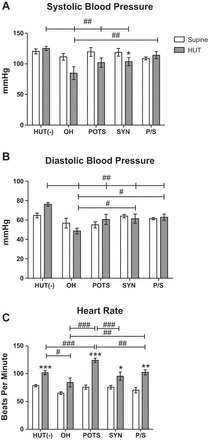
Orthostatic changes in SBP (A), DBP (B), and HR (C) in children from the following five diagnostic HUT subgroups: HUT(−), orthostatic hypotension (OH), postural orthostatic tachycardia syndrome (POTS), syncope (Syn), and POTS/syncope (P/S). HUT(−) subjects (n = 18) had significantly higher SBP than all other groups during HUT. OH subjects (n = 5) had significantly lower SBP than P/S subjects (n = 10) during HUT. The pattern of response in SBP was similar to that in DBP. HUT(−), POTS (n = 7), Syn (n = 8), and P/S groups had elevated HR from the supine position to HUT. POTS subjects had significantly higher HR and a more exaggerated increase from the supine position to HUT. Supine position vs. HUT: *P < 0.05, **P < 0.01, and ***P < 0.001; HUT(−) vs. HUT(+) subgroups: #P < 0.05, ##P < 0.01, and ###P < 0.001.
Catecholamine measurements.
In the supine position, there were no significant differences among groups. Subjects with POTS had significantly higher levels of NE than HUT(−) subjects during HUT (P < 0.001). POTS subjects had exaggerated increases in NE (P < 0.001) and Epi (P < 0.05) from supine to HUT and higher plasma levels of NE compared with all other abnormal HUT subgroups (P < 0.01). P/S subjects were the only other group to have a significant change from supine to HUT in these variables, with a significant increase in NE with HUT (P < 0.05). OH (P = 0.1), syncope (P = 0.2), and P/S (P = 0.07) groups showed a trending increase in Epi levels from supine to HUT. HUT(−) subjects had a significant increase from supine to HUT in NE and Epi (Fig. 4, A and B).
Fig. 4.
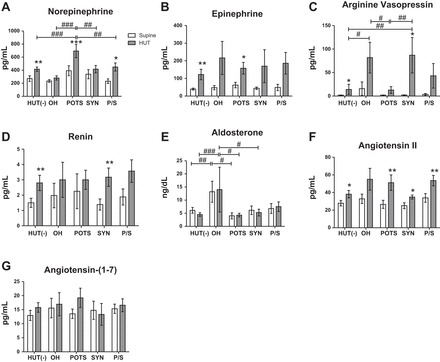
Orthostatic changes in NE (A), Epi (B), AVP (C), renin (D), Aldo (E), ANG II (F), and ANG-(1–7) (G) in children in five diagnostic HUT subgroups. There were no differences between groups at baseline for all neurohumoral measures except Aldo. HUT(−) subjects (n = 18) had a significant increase in NE, Epi, AVP, ANG II, and renin from the supine position to HUT. POTS subjects (n = 7) had significantly higher NE than all other groups during HUT and a significant increase in Epi upon HUT. OH (n = 5) and Syn (n = 8) groups had exaggerated increases in AVP upon standing and significantly higher AVP during HUT compared with HUT(−) and POTS groups. Increased renin was observed in all groups from the supine position to HUT. Aldo was significantly higher in the supine position and during HUT for OH subjects. ANG II increased upon standing in all groups, and P/S subjects (n = 10) had significantly higher ANG II than HUT(−) and Syn groups. There were no differences between or among groups for ANG-(1–7). Supine position vs. HUT: *P < 0.05, **P < 0.01, and ***P < 0.001; HUT(−) vs. HUT(+) subjects: #P < 0.05, ##P < 0.01, and ###P < 0.001.
Vasopressin measurements.
At baseline, there were no differences among groups in plasma AVP concentrations. OH (P < 0.05) and syncope (P < 0.01) subjects had significantly higher AVP during HUT than POTS and HUT(−) subjects. Syncope subjects also had a significant increase in AVP from supine to HUT (P < 0.001), and OH subjects had a variably high but not statistically significant increase in AVP (Fig. 4C). These two subgroups did not show a significant decrease in BP during HUT, but both had lower BP during HUT compared with the HUT(−) group, and the OH subgroup had lower pressure than most other groups during HUT.
Renin-angiotensin-Aldo system measurements.
There were no differences among groups for renin in the supine postion or during HUT. An increase in renin from the supine position to HUT was observed in HUT(−) and syncope subjects (P < 0.01; Fig. 4D). There was no change in serum Aldo from the supine position to HUT in any group. OH subjects had higher Aldo measurements than HUT(−) and POTS subjects in the supine position and HUT(−), POTS, and syncope subjects during HUT (Fig. 4E).
For ANG II, there were no differences in supine values among subgroups. HUT(−), POTS, syncope, and P/S groups had a significant increase from the supine postion to HUT. There was a trend for group differences (P = 0.054), likely driven by the higher ANG II during HUT in P/S subjects relative to HUT(−) and syncope subjects (Fig. 4F). There were no changes in ANG-(1–7) elicited by HUT and no differences among subgroups (Fig. 4G).
DISCUSSION
The objective of our study was to better characterize the neurohumoral phenotype and its relationship with hemodynamic changes to HUT in children with OI (14, 46). The neurohumoral profile included measurements of circulating catecholamines (Epi and NE), the renin-angiotensin-aldosterone system axis, and AVP to provide a comprehensive panel for subjects with OI to test the effects of orthostatic challenge. Our methodology was unique in that the neurohumoral profile was measured during HUT as opposed to previous studies in adults in which sample collection was taken in the supine position alone, after completion of a 30-min test, or after an evoked HUT response (11, 19, 20, 34, 37). Our approach aimed to more accurately reflect real-time neurohumoral changes as they related to cardiovascular symptoms including HR and BP changes, especially for neurohumoral factors that are less stable and metabolized quickly (1).
As we have previously reported (12, 13), pediatric subjects presenting with various OI symptoms frequently demonstrated an abnormal HUT, raising the possibility of a cardiovascular basis for their OI, including gastrointestinal symptoms. The majority (62.5%) of patients with reported orthostatic dizziness were HUT(+), consistent with findings in adults (36) in which autonomic dysfunction was associated with a high rate of symptoms of lightheadedness, dizziness, fatigue, nausea, and/or abdominal pain. While the majority of HUT(+) subjects in our study had POTS (17 of 30 OI subjects), consistent with other studies (14, 43, 46, 49, 53), over half of the POTS patients experienced syncope later in the HUT (10). Subjects with P/S or syncope sustained significantly less time on HUT compared with HUT(−) subjects. POTS subjects were able to endure the HUT for a longer time compared with syncope subjects. While there was no statistical significance, P/S subjects were only able to sustain half as much time on HUT compared with POTS subjects (Table 1). This is most likely due to P/S subjects experiencing syncope after 10 min of HUT compared with POTS subjects who were able to sustain the 45-min HUT.
When all HUT(+) subgroups were analyzed collectively, an increase in hormone secretion from the supine position to HUT included AVP, NE, Epi, renin, and ANG II. Although ANG-(1–7) has been reported to influence AVP and NE release and baroreflex function in animal studies (7, 17, 44, 57), there were no differences in ANG-(1–7) between groups. A negative relationship was observed between ANG-(1–7) and NE, consistent with evidence of a neuromodulatory action of ANG-(1–7) on the release of NE (7, 17, 48, 57). Overall, only AVP was markedly elevated between HUT(+) and HUT(−) patients. These findings, taken in the context of decreased BP upon HUT in OI, suggests that AVP release is in response to a reduction in blood volume and perfusion of heart and atrial vessels as well as carotid arteries. Whether the hormone is effectively contributing to an increase in vasoconstriction and volume expansion (5, 25, 54), albeit insufficient to maintain normal arterial pressure, is not clear.
To determine whether the hemodynamic pattern presented by specific OI phenotypes was associated with specific neurohumoral profiles, HUT(+) patients were divided into four diagnostic subgroups. Adult POTS patients compensate for a reduction in blood perfusion during orthostatic challenge by increasing HR to prevent a decrease in BP (38, 42, 43), while different compensatory mechanisms and neurohumoral patterns occur relative to adult OH and syncope subjects (3, 14, 23, 32, 55). Our findings in children were similar to adults in that POTS subjects showed an elevation in NE compared with HUT(−) subjects, consistent with a hyperadrenergic state (16, 34, 38). There was little to no change during HUT in renin or Aldo. The lack of an Aldo response in POTS subjects is consistent with other studies in which an increase in Aldo secretion was absent despite increases in ANG II during HUT (34, 38, 49). Plasma ANG II increased significantly from the supine position to HUT in POTS subjects; however, ANG II levels were not statistically different from those of HUT(−) subjects. There are several theories for the ANG II and Aldo responses, including hypovolemia (49), impaired adrenal ANG II type 1 receptors (56), reduced angiotensin-converting enzyme 2 activity (50), or altered Na+ loading (47, 49). Interestingly, AVP was not significantly increased in the POTS subgroup during HUT. Both of these observations may reflect the fact that BP and perhaps blood flow, at least in POTS subjects, is maintained during HUT via the increased HR in these subjects.
Furthermore, in the P/S subjects where BP was not maintained over the duration of the 45-min HUT, ANG II levels tended to be higher compared with HUT(−) subjects. AVP responses were variable in this subgroup. In contrast to the subjects with POTS alone, the NE response was not elevated. This may suggest that those with POTS who progress to syncope may have a disproportionate reliance on the renin-angiotensin-Aldo system compared with other vasoconstrictor systems versus those with POTS alone who may rely on sympathetic nervous system activation. Traditionally, this distinction between POTS and P/S has not been made when analyzing neurohumoral changes in adults, but the unique hemodynamic and autonomic response to HUT may reflect an impairment in the ability to sustain a vasoconstrictive response in those with P/S compared with POTS alone. Thus, our findings support the concept that subjects in this category require additional compensatory mechanisms to maintain sufficient perfusion over the longer HUT timeframe and perhaps in daily activity.
In contrast to POTS subjects, OH and syncope subjects had no change in NE, increased Epi, and exaggerated increases in AVP. There was a failure to significantly increase renin and ANG II in OH subjects with HUT, whereas syncope subjects showed normal renin and ANG II responses. Both groups had a trending increase in Epi and a failure to increase NE, which is consistent with a previous report (18) of sympathoadrenal imbalance in OH and syncope subjects. This study showed drastic increases in Epi before syncopal episodes, suggesting that vasodilation in neurocardiogenic syncope is, at least in part, humorally mediated (10, 18). Furthermore, intraveneous infusion of NE maintained BP in OH subjects during HUT, temporarily eliminating OH (19). Thus, the imbalance of NE and Epi, with higher circulating Epi, most likely contributes to vasodilation or impairments in peripheral vascular resistance by stimulating vasodilating β2-adrenergic receptors in skeletal blood vessels, which results in low BP/cardiac output in OH and syncope subjects (15). However, in the present study, the sympathoadrenal imbalance was accompanied by a trend for exaggerated increases in AVP in syncope and OH subjects and increased Aldo in OH subjects.
Surprisingly, OH subjects exhibited significantly higher serum Aldo compared with HUT(−) subjects, with higher levels in both supine and HUT positions. All other groups had lower Aldo levels while in the supine position, with little to no increase from the supine position to HUT. A trend for higher AVP in the supine position was also observed in the OH group. The higher AVP may be a reflection of a chronically lower BP, as has been suggested when copeptin was used as an indirect measure of chronically elevated AVP (59). The increase in AVP may be due to lower pressures in the OH group compared with the syncope group at the time of the hormonal measurement, given that syncope occurred later in the HUT. A combination of high Aldo and AVP has also recently been associated with hospitalized neonatal foals with clinical hypoperfusion, providing further evidence for these clinical biomarkers to identify subjects with volume depletion and/or poor tissue perfusion (9). Considering that AVP release is most sensitive to reduced serum osmolality followed by sensitivity to decreases in BP, our findings are consistent with the reduction in effective BP and perfusion as the stimulus for release of this hormone. However, the trigger for this response is still unclear. Thus, while accompanied by an imbalance in NE and Epi, higher Aldo and AVP at baseline and the exaggerated increase in AVP in response to HUT in OH subjects appear to be compensating for the lack of response in multiple other vasoconstrictive and fluid retention systems.
The relationship between AVP and reduced BP has been described in both animal and human studies in which increased secretion of plasma AVP is triggered by unloading of arterial baroreceptors in the hypotensive state (26, 54). This is similar to our observations after HUT in both OH and syncope subjects with the reduction in BP. However, stimulation of AVP also occurs independently of the baroreflex (41). Baroreflex sensitivity and HR variability are reduced during HUT in normal subjects, and there is an even greater suppression of these indexes of BP and HR regulation in those with OI and HUT(+) (45). In fact, AVP release is reportedly greater in situations when the baroreflex is impaired (41), which would be consistent with the observed AVP responses during HUT given the negative correlation between AVP and BP during the upright position in HUT(+) subjects. Thus, the trigger for AVP release, low BP, and an exaggerated impairment in baroreflex remains unclear in the present study.
The positive correlation observed between AVP and BP in the supine position was an unexpected finding. In light of the association between dysautonomia and hypertension, this does raise some concern for future elevated BP in HUT(+) subjects (most notably, the OH subgroup with higher supine Aldo) (2, 40). For example, autonomic indexes, including decreased HR variability and baroreflex sensitivity, are accepted as risk factors and indicators for the development and progression of cardiovascular problems such as hypertension and stroke (29, 31, 33). Future studies may include measuring copeptin levels to further elucidate this question. Copeptin, a stable component of AVP, is a marker of treatment effectiveness for OI, with lower levels of copeptin observed in patients effectively treated with vasoconstrictors (59). Whether OI, Aldo, or AVP/copeptin may also serve as biomarkers for the development of hypertension in this patient population warrants further attention, particularly in light of the absence of long-term cardiovascular longitudinal studies in these patients.
The findings in the present study underscore the distinct nature of HUT(+) hemodynamic subgroups, raising the question as to the need for more specific treatment strategies based on individual subgroup characteristics. The exaggerated release of several hormones in the face of minimally maintained or lower pressure in POTS or P/S may suggest that vascular responsiveness to the constrictors is impaired. In contrast, deficits in the release of neurohumoral factors other than AVP appear to have a role in OH. POTS patients do not have high levels of Aldo or AVP either in the supine position or during HUT, suggesting that volume expansion with the use of Aldo-like drugs such as fludrocortisone may be sufficient, particularly when coupled with increased NE, to mitigate their pronounced orthostatic response. In contrast, fludrocortisone may be less effective in OH patients based on the observed increases in Aldo. The exaggerated AVP response in this group supports this possibility. In fact, fludrocortisone is not effective in ∼35% of adolescents treated for OI (12). Therefore, prospective placebo-controlled studies are needed to determine the potential role for other treatment modalities, such as vasoconstrictor therapy (e.g., midodrine), in OH subjects (3, 39, 51).
The limitations of the study include the following. First, while HUT(−) subjects served as controls, all those studied with HUT had reported symptoms of OI before the study. Thus, future study designs require HUT and neurohumoral assessment of asymptomatic, healthy controls. Second, the sample size was relatively small, particularly when considering HUT(+) subgroups. To better define potential differences among patients with POTS, OH, syncope, and P/S as well as the correlative relationship between hemodynamic and neurohumoral measures, a larger cohort of OI patients is required. To better determine the feasibility of conducting such a study, understanding the prevalence of OI in the pediatric population will be necessary. The timing of blood collection also requires further consideration. As discussed above, neurohumoral markers were measured 15 min after HUT to optimally capture orthostatic symptoms. However, by performing a 45-min HUT, several patients with syncope did not manifest orthostatic and cardiovascular changes until later in the study. Therefore, changes in the neurohumoral profile in this subgroup may be more pronounced at different times during the HUT. Finally, while the hormone panel we measured was comprehensive, it did not include cortisol. Cortisol levels may be relevant as anxiety can be a comorbid symptom in pediatric patients with OI (52). Assessing cortisol levels during HUT may help delineate the potential role of physiological stress in these children.
In conclusion, a distinct neurohumoral profile related to OI subgroup diagnoses was demonstrated during HUT in pediatric patients presenting with orthostatic symptoms and gastrointestinal symptoms. These findings support the need for a better understanding of the relationship between these hormones and cardiovascular changes with orthostatic challenge. By studying these potential causes and effects in OI patients, it is anticipated that more focused and possibly more effective treatments can be started in this group of patients, thereby leading to an evidence-based approach.
Perspectives
Since dysautonomia, which can manifest as OI, is a potential risk factor for cardiovascular disease, understanding the mechanisms involved in producing symptoms of OI is warranted, particularly at an early age. The heterogeneity of clinical presentation in OI patients with symptoms ranging from syncope to migraine headaches to gastrointestinal complaints favors a multidisciplinary approach to their care. In the present study, we measured a panel of neurohormones in OI subjects. To determine the impact of orthostatic challenge, we measured these hormones both while in the supine position and during HUT and attempted to define differences among specific HUT(+) subgroups. We demonstrated that elevated AVP was associated with lower BP and that OH subjects have elevated Aldo independent of upright posture. Further evaluation of AVP, the renin-angiotensin-Aldo system, and catecholamines in subjects with orthostatic symptoms may elucidate the mechanisms involved in the cause of these OI subtypes. It may further provide insight into their long-term cardiovascular prognosis, including risk of hypertension and cardiovascular disease.
GRANTS
This study was supported by American Heart Association, National Center Clinical Research Program Grant AHA12CRP9420029.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.L.W., H.A.S., J.E.F., and D.I.D. conception and design of research; A.L.W. and H.A.S. performed experiments; A.L.W., H.A.S., J.E.F., and D.I.D. analyzed data; A.L.W., H.A.S., J.E.F., and D.I.D. interpreted results of experiments; A.L.W. prepared figures; A.L.W., H.A.S., J.E.F., and D.I.D. drafted manuscript; A.L.W., H.A.S., J.E.F., and D.I.D. edited and revised manuscript; A.L.W., H.A.S., J.E.F., and D.I.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Additional support from the Farley Hudson Foundation (Jacksonville, NC) and the Hypertension and Vascular Research Center of Wake Forest University is gratefully acknowledged. The authors thank Anya Brown and the Hypertension Core Assay Laboratory for technical assistance.
REFERENCES
- 1.Andersen LJ, Andersen JL, Schütten HJ, Warberg J, Bie P. Antidiuretic effect of subnormal levels of arginine vasopressin in normal humans. Am J Physiol Regul Integr Comp Physiol 259: R53–R60, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D, Biaggioni I. Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension 61: 701–6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold AC, Shibao C. Current concepts in orthostatic hypotension management. Curr Hypertens Rep 15: 304–12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrod FB, Berlin D. Pregabalin: a new approach to treatment of the dysautonomic crisis. Pediatrics 124: 743–6, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Babic T, Browning KN. The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur J Pharmacol 722: 38–47, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc 87: 1214–25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byku M, Macarthur H, Westfall TC. Inhibitory effects of angiotensin-(1–7) on the nerve stimulation-induced release of norepinephrine and neuropeptide Y from the mesenteric arterial bed. Am J Physiol Heart Circ Physiol 298: H457–H465, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffin ST, Black BK, Biaggioni I, Paranjape SY, Orozco C, Black PW, Dupont WD, Robertson D, Raj SR. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm 9: 1484–90, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.De Hert S. Physiology of hemodynamic homeostasis. Best Pract Res Clin Anaesthesiol 26: 409–419, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Dembek KA, Hurcombe SD, Stewart AJ, Barr BS, MacGillivray KC, Kinee M, Elam J, Toribio RE. Association of aldosterone and arginine vasopressin concentrations and clinical markers of hypoperfusion in neonatal foals. Equine Vet J; doi: 10.1111/evj.12393. [DOI] [PubMed] [Google Scholar]
- 10.Dietz NM, Halliwill JR, Spielmann JM, Lawler LA, Papouchado BG, Eickhoff TJ, Joyner MJ. Sympathetic withdrawal and forearm vasodilation during vasovagal syncope in humans. J Appl Physiol 82: 1785–1793, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Fedorowski A, Burri P, Struck J, Juul-Möller S, Melander O. Novel cardiovascular biomarkers in unexplained syncopal attacks: the SYSTEMA cohort. J Intern Med 273: 359–67, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Fortunato JE, Shaltout HA, Larkin MM, Rowe PC, Diz DI, Koch KL. Fludrocortisone improves nausea in children with orthostatic intolerance (OI). Clin Auton Res 21: 419–23, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Fortunato JE, Wagoner AL, Harbinson RL, D'Agostino RB, Shaltout HA, Diz DI. Effect of fludrocortisone acetate on chronic unexplained nausea and abdominal pain in children with orthostatic intolerance. J Pediatr Gastroenterol Nutr 59: 39–43, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21: 69–72, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Fu Q, Verheyden B, Wieling W, Levine BD. Cardiac output and sympathetic vasoconstrictor responses during upright tilt to presyncope in healthy humans. J Physiol 590: 1839–1848, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 69: 790–798, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Gironacci MM, Longo Carbajosa NA, Goldstein J, Cerrato BD. Neuromodulatory role of angiotensin-(1–7) in the central nervous system. Clin Sci (Lond) 125: 57–65, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DS, Holmes C, Frank SM, Naqibuddin M, Dendi R, Snader S, Calkins H. Sympathoadrenal imbalance before neurocardiogenic syncope. Am J Cardiol 91: 53–58, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein DS, Sewell L, Holmes C, Pechnik S, Diedrich A, Robertson D. Temporary elimination of orthostatic hypotension by norepinephrine infusion. Clin Auton Res 22: 303–306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green EA, Raj V, Shibao CA, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR. Effects of norepinephrine reuptake inhibition on postural tachycardia syndrome. J Am Heart Assoc 2: e000395, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob G, Shannon JR, Costa F, Furlan R, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. Abnormal norepinephrine clearance and adrenergic receptor sensitivity in idiopathic orthostatic intolerance. Circulation 99: 1706–1712, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Jardine DL, Melton IC, Crozier IG, Bennett SI, Donald RA, Ikram H. Neurohormonal response to head-up tilt and its role in vasovagal syncope. Am J Cardiol 79: 1302–1305, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Kanjwal K, Saeed B, Karabin B, Kanjwal Y, Grubb BP. Clinical presentation and management of patients with hyperadrenergic postural orthostatic tachycardia syndrome. A single center experience. Cardiol J 18: 527–531, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann H, Oribe E, Miller M, Knott P, Wiltshire-Clement M, Yahr MD. Hypotension-induced vasopressin release distinguishes between pure autonomic failure and multiple system atrophy with autonomic failure. Neurology 42: 590–393, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Koch KL. A noxious trio: nausea, gastric dysrhythmias and vasopressin. Neurogastroenterol Motil 9: 141–142, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Kohara K, Brosnihan KB, Chappell MC, Khosla MC, Ferrario CM. Angiotensin-(1–7). A member of circulating angiotensin peptides. Hypertension 17: 131–138, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Kohara K, Tabuchi Y, Senanayake P, Brosnihan KB, Ferrario CM. Reassessment of plasma angiotensins measurement: effects of protease inhibitors and sample handling procedures. Peptides 12: 1135–1141, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Lucini D, Mela GS, Malliani A, Pagani M. Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation 106: 2673–2679, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Marc Y, Llorens-Cortes C. The role of the brain renin-angiotensin system in hypertension: implications for new treatment. Prog Neurobiol 95: 89–103, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Maule S, Milan A, Grosso T, Veglio F. Left ventricular hypertrophy in patients with autonomic failure. Am J Hypertens 19: 1049–1054, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Mosqueda-Garcia R, Furlan R, Tank J, Fernandez-Violante R. The elusive pathophysiology of neurally mediated syncope. Circulation 102: 2898–2906, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Mussalo H, Vanninen E, Ikäheimo R, Laitinen T, Laakso M, Länsimies E, Hartikainen J. Baroreflex sensitivity in essential and secondary hypertension. Clin Auton Res 12: 465–471, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Mustafa HI, Garland EM, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm 8: 422–428, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mustafa HI, Raj SR, Diedrich A, Black BK, Paranjape SY, Dupont WD, Williams GH, Biaggioni I, Robertson D. Altered systemic hemodynamic and baroreflex response to angiotensin II in postural tachycardia syndrome. Circ Arrhythm Electrophysiol 5: 173–180, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muth ER, Koch KL, Stern RM. Significance of autonomic nervous system activity in functional dyspepsia. Dig Dis Sci 45: 854–863, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson D, Sutton R, Tas W, Burri P, Melander O, Fedorowski A. Orthostatic changes in hemodynamics and cardiovascular biomarkers in dysautonomic patients. PLos One 10: e0128962, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 111: 1574–1582, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis 55: 425–433, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez CE, Okamoto LE, Arnold AC, Gamboa A, Diedrich A, Choi L, Raj SR, Robertson D, Biaggioni I, Shibao CA. Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension 64: 1235–1240, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro AB, Saad CI, Zanella T, Mulinari RA, Kohlman OG. Vasopressin maintains blood pressure in diabetic orthostatic hypotension. Hypertension 11, Suppl I: I-217–I-219, 1988. [DOI] [PubMed] [Google Scholar]
- 42.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci 317: 75–77, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc 74: 1106–1110, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Schiavone MT, Santos a R, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proc Natl Acad Sci USA 85: 4095–4098, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaltout HA, Wagoner AL, Diz DI, Fortunato JE. Impaired hemodynamic response to head up tilt in adolescents presenting chronic nausea and orthostatic intolerance (Abstract). Hypertension 64: A513, 2014. [Google Scholar]
- 46.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 160: 222–226, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spät A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 84: 489–539, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Stegbauer J, Oberhauser V, Vonend O, Rump LC. Angiotensin-(1–7) modulates vascular resistance and sympathetic neurotransmission in kidneys of spontaneously hypertensive rats. Cardiovasc Res 61: 352–359, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond) 110: 255–263, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1–7) production in postural tachycardia syndrome. Hypertension 53: 767–774, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart JM, Taneja I, Glover J, Medow MS. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 294: H466–H473, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarbell SE, Shaltout HA, Wagoner AL, Diz DI, Fortunato JE. Relationship among nausea, anxiety, and orthostatic symptoms in pediatric patients with chronic unexplained nausea. Exp Brain Res 232: 2645–2650, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, Lennon VA, Shen WK, Low PA. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 82: 308–313, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Thrasher TN. Baroreceptor regulation of vasopressin and renin secretion: low-pressure versus high-pressure receptors. Front Neuroendocrinol 15: 157–196, 1994. [DOI] [PubMed] [Google Scholar]
- 55.Vanderheyden M, Goethals M, Nellens P, Andries E, Brugada P. Different humoral responses during head-up tilt testing among patients with neurocardiogenic syncope. Am Heart J 135: 67–73, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Wang DH, Elijovich F. Modulation and function of extrarenal angiotensin receptors. Cell Biochem 31: 1–17, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Westfall TC, Macarthur H, Byku M, Yang CL, Murray J. Interactions of neuropeptide y, catecholamines, and angiotensin at the vascular neuroeffector junction. Adv Pharmacol 68: 115–139, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Q, Chen X, Li J, Du J. Orthostatic plasma norepinephrine level as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. J Transl Med 12: 249, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao J, Du S, Yang J, Lin J, Tang C, Du J, Jin H. Usefulness of plasma copeptin as a biomarker to predict the therapeutic effectiveness of metoprolol for postural tachycardia syndrome in children. Am J Cardiol 114: 601–605, 2014. [DOI] [PubMed] [Google Scholar]


