While the cardiac benefits of exercise are clear, the underlying mechanism(s) are not completely understood. This is the first study to demonstrate, in intact hearts, isolated myocytes, and isolated mitochondria, that exercise confers an intrinsic protective phenotype by sustaining mitochondrial energetics and reducing reactive oxygen species in early reperfusion.
Keywords: exercise, cardioprotection, mitochondria, arrhythmia, membrane potential
Abstract
Mitochondria influence cardiac electrophysiology through energy- and redox-sensitive ion channels in the sarcolemma, with the collapse of energetics believed to be centrally involved in arrhythmogenesis. This study was conducted to determine if preservation of mitochondrial membrane potential (ΔΨm) contributes to the antiarrhythmic effect of exercise. We utilized perfused hearts, isolated myocytes, and isolated mitochondria exposed to metabolic challenge to determine the effects of exercise on cardiac mitochondria. Hearts from sedentary (Sed) and exercised (Ex; 10 days of treadmill running) Sprague-Dawley rats were perfused on a two-photon microscope stage for simultaneous measurement of ΔΨm and ECG. After ischemia-reperfusion, the collapse of ΔΨm was commensurate with the onset of arrhythmia. Exercise preserved ΔΨm and decreased the incidence of fibrillation/tachycardia (P < 0.05). Our findings in intact hearts were corroborated in isolated myocytes exposed to in vitro hypoxia-reoxygenation, with Ex rats demonstrating enhanced redox control and sustained ΔΨm during reoxygenation. Finally, we induced anoxia-reoxygenation in isolated mitochondria using high-resolution respirometry with simultaneous measurement of respiration and H2O2. Mitochondria from Ex rats sustained respiration with lower rates of H2O2 emission than Sed rats. Exercise helps sustain postischemic mitochondrial bioenergetics and redox homeostasis, which is associated with preserved ΔΨm and protection against reperfusion arrhythmia. The reduction of fatal ventricular arrhythmias through exercise-induced mitochondrial adaptations indicates that mitochondrial therapeutics may be an effective target for the treatment of heart disease.
NEW & NOTEWORTHY
While the cardiac benefits of exercise are clear, the underlying mechanism(s) are not completely understood. This is the first study to demonstrate, in intact hearts, isolated myocytes, and isolated mitochondria, that exercise confers an intrinsic protective phenotype by sustaining mitochondrial energetics and reducing reactive oxygen species in early reperfusion.
cardiovascular disease remains a leading cause of death in the industrialized world (34, 52). One manifestation of cardiovascular disease is sudden cardiac death, which has been estimated to account for ∼1 death per 1,000 in the general population (26). Several factors, including various genetic abnormalities, channelopathies, compromised autonomic function, left ventricular hypertrophy, and acute coronary syndromes, are known to influence the susceptibility to arrhythmia (26, 53, 68). During acute coronary syndromes, the reperfusion of previously ischemic tissue leads to a burst in reactive oxygen species (ROS), a significant contributor to electromechanical dysfunction (3, 10, 48, 75).
Exercise is known to protect against arrhythmia (27, 30, 36, 60), as well as other postischemic damage, such as myocardial stunning (11, 46, 66) and infarction (14, 29, 58, 59). Despite the clear beneficial effect, the underlying cellular mechanisms are not completely understood. The high-oxidative environment during reperfusion collapses mitochondrial energetics and alters cardiac action potential duration, which is known to be arrhythmogenic (3, 6, 7, 13). Among their many functions, mitochondria are centrally involved in ATP production and free radical detoxification through redox reactions, both of which ultimately rely on mitochondrial membrane potential (ΔΨm). Collapses of ΔΨm are known to be associated with the onset of arrhythmia, and pharmacological interventions that preserve ΔΨm have been shown to stabilize sinus rhythm (13, 65). Whether the preservation of ΔΨm is an endogenous adaptation involved in exercise-induced protection has not been determined.
We recently observed that exercise delayed the onset of arrhythmia and decreased the incidence of ventricular fibrillation (VF) through better preservation of redox homeostasis (30). This was attributed to enhanced glutathione reductase (GR) activity, which was essential for cardioprotection (29). While exercise-induced cardioprotection has been repeatedly shown to augment endogenous myocardial antioxidant capacity (29, 30, 44, 60, 70), there is a lack of evidence demonstrating how these adaptations directly protect against reperfusion arrhythmia. Therefore, the objective of the present study was to determine if exercise decreases reperfusion arrhythmia by preserving mitochondrial bioenergetics. Using several different experimental models, we employed a vertically integrated approach to test the hypothesis that exercise protects against reperfusion arrhythmia via better maintenance of ΔΨm, lower mitochondrial ROS production, and preserved redox homeostasis.
METHODS
Animals.
Male Sprague-Dawley rats (250–350 g body wt) were housed on a 12:12-h light-dark cycle, with food and water provided ad libitum. All experiments were conducted in accordance with guidelines established by the National Institutes of Health (Guide for the Care and Use of Laboratory Animals, 8th edition) and the American Veterinary Medical Association (https://www.avma.org/KB/Policies/Documents/euthanasia.pdf) and approved by the East Carolina University Animal Care and Use Committee. For all experiments, rats were anesthetized by intraperitoneal injection of 90 mg/kg ketamine and 10 mg/kg xylazine, and hearts were excised via midline thoracotomy after animals reached a surgical plane of anesthesia. Hearts were placed briefly in 0.9% saline (4°C) and used for isolated heart studies, myocyte isolations, or mitochondrial experiments.
Exercise protocol.
Rats were randomly assigned to exercise (Ex) or sedentary (Sed) groups and exposed to daily exercise or control handling according to established protocols (27, 29). Briefly, rats were acclimated to the treadmill at 15 m/min over a 3-day period, with the time of exercise increased from 5, 10, and 15 min each day. Ex rats underwent 10 days of consecutive treadmill running at 6% grade for 60 min/day: 15 m/min for 15 min, 30 m/min for 30 min, and 15 m/min for 15 min. Sed rats were placed on the nonmoving treadmill for 5 min each day. This exercise protocol mimics a moderate- to high-intensity exercise regimen, characterized by training adaptations with little/no indication of systemic stress (15). All experiments were performed 24 h after the last bout of exercise or handling.
Isolated heart preparation and assessment of arrhythmia.
Excised hearts were rapidly cannulated via the aorta according to our established methods (29, 30) and retrograde-perfused on a modified Langendorff apparatus with gassed (95% O2-5% CO2) Krebs-Henseleit buffer (KHB) containing (mM) 118 NaCl, 24 NaHCO3, 4.8 KCl, 2 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 10 glucose (37°C) at a constant pressure of 75 mmHg. Coronary flow was monitored throughout the protocol with a Transonic flow probe connected in series proximal to the cannula. All measurements were recorded on LabChart 7.0 software (ADInstruments) and stored on a personal computer for subsequent analysis. The definition of ventricular arrhythmia was used in accordance with the methods described by the Lambeth Convention (20).
Two-photon microscopy whole heart imaging during ischemia-reperfusion.
Slight modifications of our previous techniques (13) were used to image instrumented hearts (n = 18) using two-photon microscopy (Olympus FV 1000 multiphoton microscope and Spectra-Physics Mai Tai DeepSee laser) with a ×30 silicone objective lens (UPLSAPO, 1.05 numerical aperture). Hearts were mounted in a 100-mm glass-bottom dish (MatTek) maintained at 37°C for imaging, with ECG obtained via volume-conductance recordings using electrodes placed in the bath. Hearts were enclosed by an on-board incubator maintained at 37°C and imaged at a depth of 800 nm. For the duration of the protocol, 640 × 640-pixel-resolution images were obtained each minute at 2 μs/pixel with low laser power (6.5%). The left ventricle was imaged within 2 mm of the left anterior descending coronary artery on the MatTek dish and stabilized by application of a glass coverslip over the heart to minimize artifacts induced from vibrations.
Isolated hearts were loaded with 100 nM tetramethylrhodamine methyl ester (TMRM; Molecular Probes) for 15 min to measure ΔΨm according to our established methods (13). Our preliminary experiments showed this concentration of TMRM to be optimal to observe collapses of ΔΨm with the mitochondrial uncoupler carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP). TMRM was excited at 800 nm, and emission was collected at 495–540 nm using a two-channel filter cube (model FV10-MRG/R, Olympus). After TMRM loading, hearts were perfused with KHB + blebbistatin (10 μM) to inhibit contraction. Once the image stabilized, a baseline image was captured, and immediately thereafter the hearts were subjected to global, no-flow ischemia (40 min)-reperfusion (10 min). To control for unequal fluorophore loading, TMRM fluorescence was normalized to baseline (F0; prior to ischemia). All images were analyzed using ImageJ, and mean TMRM fluorescence was calculated after thresholding to exclude background for areas not containing sheets of myocardial cells. Four hearts (2 from the Sed group and 2 from the Ex group) were excluded from the imaging analysis because of technical difficulties during image acquisition.
Glutathione levels in cardiac tissue following Langendorff ischemia-reperfusion.
Reduced glutathione (GSH) and glutathione disulfide (GSSG) were measured using high-performance liquid chromatography (HPLC) (25, 33, 40). Left ventricular cardiac tissue was snap-frozen in liquid nitrogen after 20 min of ischemia and 2 h of reperfusion in a subset of rats. Left ventricular tissue was homogenized in a buffer containing 50 mM Trizma base supplemented with 20 mM boric acid, 20 mM l-serine, and 10 mM N-ethylmaleimide (NEM). NEM, an alkylating agent that will both conjugate GSH and inhibit GR, was added to the homogenization buffer to limit autooxidation effects during sample preparation. The tissue homogenate was then split into two derivatization pathways for the detection of GSH and GSSG. For GSH derivatization, 280 μl of the homogenate were deproteinated with 1:10 (vol/vol) 15% trichloroacetic acid and then centrifuged for 5 min at 20,000 g. The supernatant was transferred to an autosampler vial for processing in the HPLC equipment. GSH samples were run on freshly made mobile phase containing 91% of a 0.25% (vol/vol) glacial acetic acid mixed with 9% pure HPLC-grade acetonitrile. Samples were run using a Shimadzu Prominence HPLC system equipped with a Premier C18 column (4.6 × 150 mm, 5 μm; catalog no. 220-91199-12) at a flow rate of 1.0 ml/min. GSH-NEM conjugate was detected by UV chromatography at a wavelength of 265 nm (catalog no. SPD-20A, Shimadzu) (33). Samples were quantified using standards prepared under identical conditions and normalized to the protein content measured in the muscle homogenate by bicinchoninic acid assay.
For GSSG derivatization, 200 μl of the homogenate were deproteinized in 200 μl of 15% perchloric acid and then centrifuged for 5 min at 20,000 g. The resulting supernatant (200 μl) was diluted in 1,000 μl of 0.1 M NaOH twice to ensure that proper pH (∼12) was reached before the reaction with 0.1% o-phthaladehyde (OPA). OPA will react with GSSG at high pH (∼12) to form a fluorescent product detectable at 350-nm excitation and 420-nm emission (catalog no. RF-20A xs, Shimadzu) (40). GSSG samples were processed using a 25 mM sodium phosphate buffer containing 15% HPLC-grade methanol at pH 6. Samples were run through a Shimadzu Prominence HPLC system equipped with a Purospher STAR RP-18 end-capped column (4.6 × 150 mm, 3 μm; EMD Millipore) at a flow rate of 0.5 ml/min. Samples were quantified using standards prepared under identical conditions and normalized to the protein content measured in the muscle homogenate by bicinchoninic acid assay.
Cardiomyocyte cell isolation.
Cardiac ventricular myocytes were isolated using previously published methods with slight modifications (12). Hearts were digested enzymatically on a modified Langendorff apparatus using 1 mg/ml collagenase (type 2; Worthington) and 0.15 mg/ml protease (type XIV; Sigma) dissolved in Tyrode solution. After 8–12 min of digestion, hearts were cut down, minced in Tyrode solution, and passed through a nylon mesh filter. Cells were allowed to gravity-precipitate and then resuspended in Tyrode solution with increasing titrations of Ca2+ to a final concentration of 1.8 mM. Isolated myocytes were incubated (95% O2, 37°C) in DMEM and used for experiments within 8 h of dispersion.
Cardiomyocyte imaging during hypoxia-reoxygenation.
Myocytes were loaded on a perfusion chamber housed on the confocal microscope stage and enclosed in glass to minimize O2 diffusion from room air. The chamber was connected to an in-line solution heater that delivers the superfusate via laboratory tubing with low O2 permeability (Tygon F-4040-A) and is equipped with heating filaments to maintain temperature at 37°C. Pacing electrodes were utilized for field stimulation for the duration of the hypoxia-reoxygenation protocol (4-ms duration, 1-Hz frequency, 10-V amplitude). Myocytes were perfused with Tyrode solution gassed with 100% O2 containing (in mM) 140 NaCl, 10 HEPES, 5 KCl, 1 MgCl2, 1.8 CaCl2, and 10 glucose (pH 7.4, 37°C). For hypoxic Tyrode solution, glucose was excluded, the solution was gassed with 100% argon continuously, and pH was decreased to 6.5 in an attempt to mimic the in vivo cellular environment during ischemia.
Myocytes were incubated for 15 min with 10 nM TMRM and 1 μM CellTracker Blue CMAC (Molecular Probes) for fluorescence imaging of ΔΨm and cellular GSH, respectively. CellTracker Blue CMAC is a GSH-sensitive dye that has been shown to have better cell retention than monochlorobimane in primary cardiomyocytes (42). Myocytes were incubated on a glass coverslip coated with poly-d-lysine, allowed 15 min to adhere, and subjected to 5 min of baseline perfusion. Only rod-shaped myocytes that responded to field stimulation were utilized in the experiments. Preliminary control experiments indicated that a low concentration of TMRM (5 nM) in the Tyrode solutions was required to maintain a stable fluorescent signal for the duration of the protocol. After 5 min of baseline perfusion, the solution was switched to the hypoxic Tyrode solution. After 20 min of hypoxia, the superperfusate was returned to normoxic Tyrode solution for reoxygenation (for 30 min or until cell death). At the end of each experimental protocol, myocytes were perfused with the mitochondrial uncoupler FCCP (1 μM) to verify mitochondrial TMRM specificity. A ×60 water-immersion objective lens was used to image myocytes every minute using 408- and 559-nm argon lasers, and emissions were collected using 430- to 470-nm and 575- to 675-nm band-pass filters, respectively. Images were analyzed with National Institutes of Health ImageJ (http://imagej.nih.gov) in 8-bit grayscale following background subtraction (rolling ball radius 50) with regions of interest drawn around individual cells. NIH “Fire” and “Blue” look-up tables were used for all ΔΨm and GSH images, respectively.
Mitochondria isolation.
Cardiac mitochondria were isolated from hearts from Ex or Sed rats 24 h following the last exercise bout (or control handling) using similar previously published methods (64). Briefly, hearts were excised and minced on ice and trypsin-digested in mitochondria isolation medium (MIM) containing (in mM) 300 sucrose, 10 sodium-HEPES, and 1 EGTA. After 2 min of digestion, 10 ml of MIM with BSA (1 mg/ml) and trypsin inhibitor (100 mg/ml) were added and allowed to gravity-pellet for 8 min. The digested tissue was homogenized and centrifuged at 800 g for 10 min. Supernatant was collected and centrifuged at 12,000 g for 10 min to pellet mitochondria. The pellet was rinsed to remove debris and impurities, suspended in fresh MIM, and centrifuged at 12,000 g for 10 min. The final pellet was resuspended in MIM and kept on ice for experiments.
Mitochondrial O2 consumption and H2O2 emission measurements.
Rates of O2 consumption (JO2) and H2O2 emission (JH2O2) were measured simultaneously using the Oroboros high-resolution respirometry Oxygraph-2k equipped with a custom-made stopper to accommodate a fiber-optic cable for fluorescence measurements (Fluoromax 3, HORIBA Jobin Yvon, Edison, NJ). Mitochondria were energized using complex I and II substrates [glutamate (10 mM), malate (2 mM), pyruvate (2 mM), and succinate (5 mM)] and assayed at 37°C in 2.5 ml of buffer Z assay medium containing (mM) 110 MES potassium salt, 35 KCl, 1 EGTA, 5 K2HPO2, 3 MgCl2·6H2O, 0.5 mg/ml BSA, and 25 creatine monohydrate. JH2O2 was quantified using Amplex UltraRed (25 μM) and horseradish peroxidase (4 U/ml), which was added to the assay buffer. Exogenous superoxide dismutase (SOD, 30 U/ml) was added to convert all generated superoxide to H2O2. The hexokinase (2 U/ml)-2-deoxyglucose (5 mM) “ADP clamp” was used to mimic in vivo conditions. These conditions keep mitochondria in a submaximal phosphorylating state at a fixed ΔΨm by recycling ATP to ADP (75 μM) (72). Anoxia was “self-induced” for 25 min by allowing mitochondria to consume all the O2 in the chamber. After anoxia, mitochondria were reoxygenated by injection of pure O2 into an air bubble above the solution in the chamber. The chamber was then sealed, allowing for measurement of JO2 and JH2O2 during the reoxygenation phase.
The contribution of thioredoxin reductase (TrxR) or GR to mitochondrial ROS production was ascertained in parallel experiments. Mitochondria were energized with 10 mM succinate and treated with 1 μM auranofin (AF) or 100 μM bis-chloroethylnitrosourea (BCNU) to inhibit the thioredoxin and glutathione redox buffering systems, respectively. Endogenous mitochondrial ROS production was monitored as described above.
Statistics.
Data are presented as means ± SE. Arrhythmia analysis was performed using a χ2 test. Mean fluorescence during reperfusion and respiratory control ratios were analyzed using unpaired Student's t-test. Imaging data were analyzed with an ANOVA for reperfusion or reoxygenation using the least significant difference test for matched time comparisons between Ex and Sed groups. All JO2 and JH2O2 data were analyzed using a two-way ANOVA with Tukey's post hoc test. Statistical significance was established when P < 0.05. All data were analyzed and graphed using GraphPad Prism software.
RESULTS
Exercise decreases arrhythmia and preserves ΔΨm during ischemia-reperfusion.
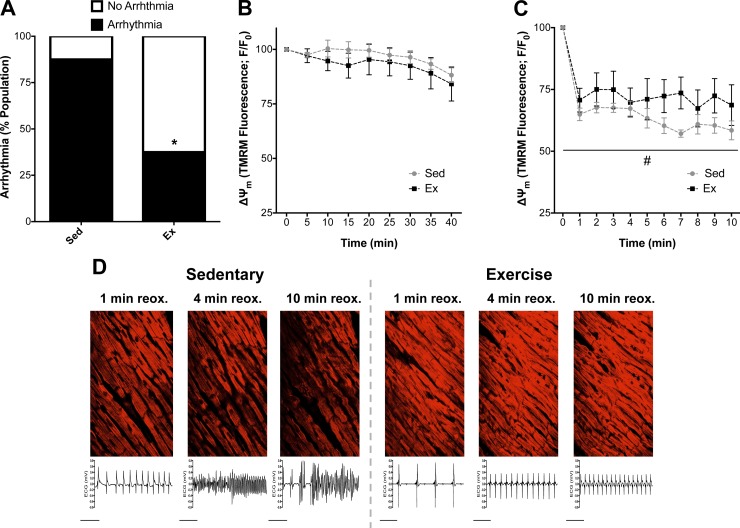
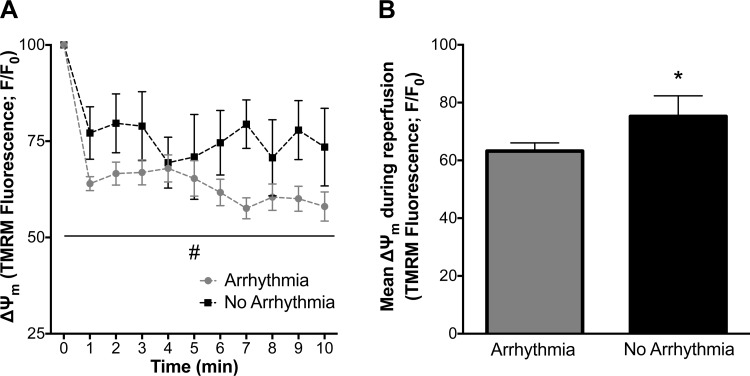
The incidence of ventricular arrhythmia was significantly decreased in hearts from Ex rats: 33% of Ex hearts vs. 88% of Sed hearts transitioned to ventricular tachycardia (VT) and/or VF during early reperfusion (P < 0.05, n = 8 per group; Fig. 1A). In our two-photon studies, nonischemic control hearts showed a stable TMRM fluorescent signal 30 min following TMRM loading, indicating that TMRM washout was not a major contributor to declines in the TMRM signal (data not shown). There was no difference in TMRM signal during ischemia between groups (Fig. 1B). However, Ex hearts better maintained ΔΨm than Sed hearts over the course of reperfusion, which coincided with a decrease in arrhythmia (P < 0.05; Fig. 1). The transition to arrhythmia in Sed hearts was often accompanied by loss of ΔΨm, which was better preserved in Ex hearts that did not transition to arrhythmia during the reperfusion period (Fig. 1D). Underscoring the importance of maintaining ΔΨm during reperfusion, pooled data for all hearts (regardless of Sed vs. Ex group) corroborated the association between ΔΨm loss and electrical dysfunction, with maintenance of ΔΨm associated with protection against arrhythmia (Fig. 2).
Fig. 1.
Arrhythmia and simultaneous 2-photon imaging of mitochondrial membrane potential (ΔΨm) in isolated hearts during ischemia-reperfusion. A: percentage of hearts from exercised (Ex) and sedentary (Sed) rats that transitioned to arrhythmia (ventricular tachycardia/ventricular fibrillation) following 40 min of ischemia. B and C: baseline tetramethylrhodamine methyl ester (TMRM) fluorescence (ΔΨm) values were used to normalize all data (F/F0) during ischemia (B) and reperfusion (C). Values (means ± SE) are expressed as percentage of the population for arrhythmia (n = 7–8 per group). *P < 0.05 vs. Sed; #P < 0.05 vs. Sed main effect. D: representative images of ΔΨm in the ventricular free wall and simultaneous ECG recordings during reperfusion for Sed and Ex. reox, Reoxygenation.
Fig. 2.
Mitochondrial membrane potential (ΔΨm) in isolated hearts that transitioned to arrhythmia vs. no arrhythmia during reperfusion. A: ΔΨm was better maintained in hearts that did not transition to arrhythmia. B: mean ΔΨm fluorescence values during reperfusion. Values are means ± SE. *P < 0.05 vs. arrhythmia; #P < 0.05 vs. arrhythmia main effect.
Glutathione and ΔΨm dynamics.
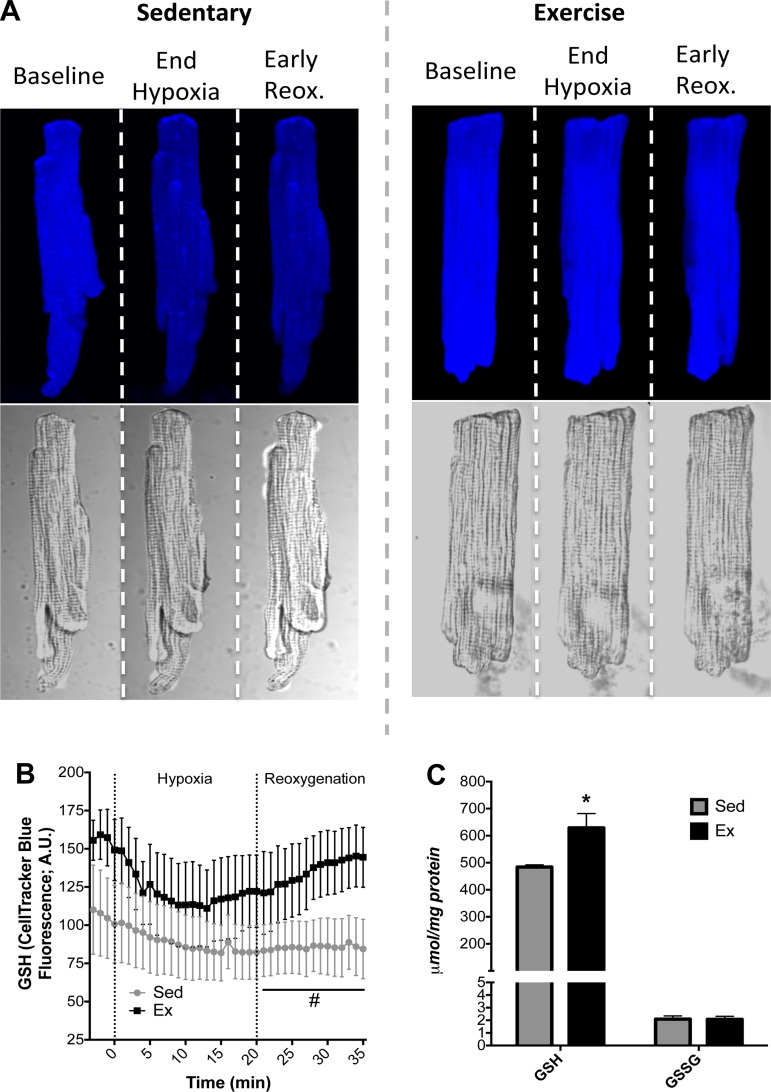
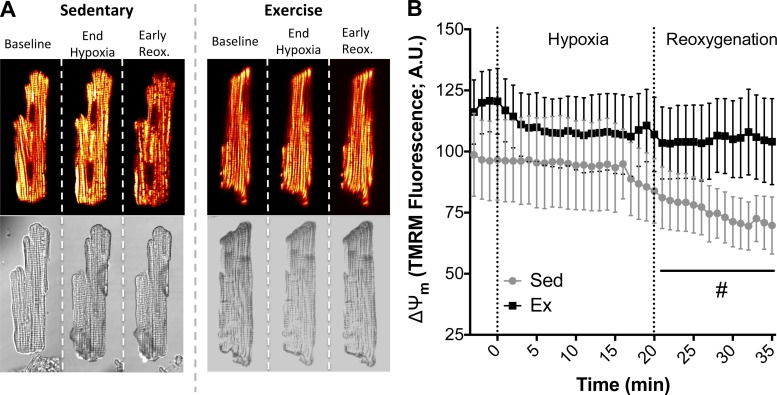
In cardiomyocytes exposed to in vitro hypoxia (20 min)-reoxygenation (30 min), myocytes from Ex hearts maintained higher levels of GSH during reoxygenation and showed an enhanced ability to replenish GSH levels compared with Sed hearts (Fig. 3, A and B). In a more quantitative approach, GSH was measured in whole hearts exposed to ischemia-reperfusion injury (see Fig. 5C). GSH was significantly higher in Ex hearts (Fig. 3C), further demonstrating adaptive maintenance of redox control following a hypoxic or an ischemic insult. The attenuated GSH replenishment in cardiomyocytes from Sed rats coincided with collapse of ΔΨm during reoxygenation, while the enhanced ability of cardiomyocytes from Ex rats to replenish GSH translated into ΔΨm stability during reoxygenation (Figs. 3 and 4). There was a slight decrease in ΔΨm during hypoxia, as shown in Fig. 4B, particularly during late hypoxia, but ΔΨm depolarization was more evident during reoxygenation, which is consistent with our observations in whole heart experiments. The time-lapse images of ΔΨm in paced myocytes exposed to hypoxia-reoxygenation were consistent with the whole heart two-photon data, demonstrating more energetically stable mitochondrial networks in Ex hearts.
Fig. 3.
Cardiac glutathione (GSH) during cellular hypoxia-reoxygenation or cardiac ischemia-reperfusion. A: representative primary cardiomyocyte fluorescence images for Sed and Ex during baseline, at the end of hypoxia, and 6 min into reoxygenation (reox). AU, arbitrary units. B: quantification of GSH levels as measured by CellTracker Blue fluorescence. C: HPLC quantification of GSH and oxidized glutathione (GSSG) in hearts following ischemia-reperfusion. Values are means ± SE. *P < 0.05 vs. Sed. #P < 0.05. vs. Sed main effect.
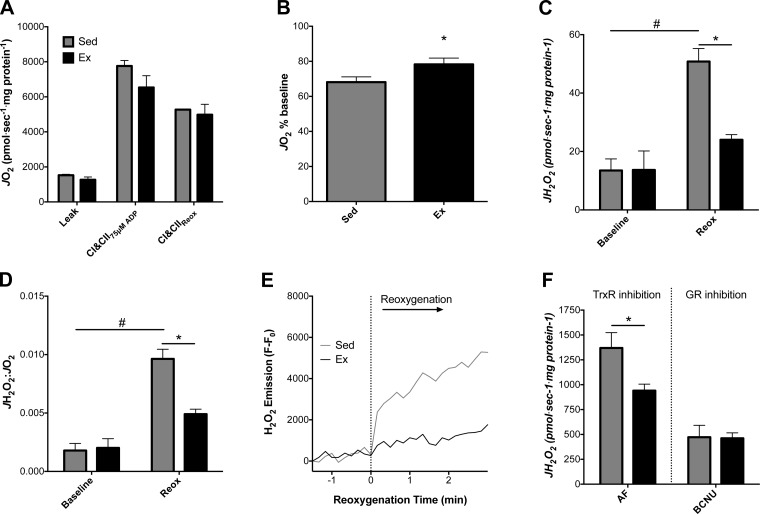
Fig. 5.
Reactive oxygen species (ROS) and isolated mitochondrial energetics during anoxia-reoxygenation. O2 consumption rate (JO2) and H2O2 emission rate (JH2O2) were measured in isolated mitochondria from Sed and Ex hearts. A: JO2 was similar at baseline between Ex and Sed isolated mitochondria respiring on glutamate + malate, pyruvate, and succinate, with ADP clamped at 75 μM (state 3). CI and CII, complexes I and II. B: impairments in state 3 JO2 following anoxia-reoxygenation were determined by comparing relative decreases from baseline for Sed and Ex. C: state 3 JH2O2 before and after anoxia-reoxygenation. D: JH2O2-to-JO2 ratio demonstrates impaired mitochondrial function in Sed mitochondria following anoxia-reoxygenation. E: representative experiment showing a trace of resorufin fluorescence used to calculate JH2O2 during anoxia-reoxygenation. For clarity, data were transformed by subtraction of the anoxic fluorescent value recorded prior to reoxygenation. F: JH2O2 in isolated mitochondria in the presence of the thioredoxin reductase (TrxR) inhibitor auranofin (AF) or the glutathione reductase (GR) inhibitor bis-chloroethylnitrosourea (BCNU). Values are means ± SE. *P < 0.05 vs. Sed main effect; #P < 0.05 vs. Sed baseline.
Fig. 4.
Mitochondrial membrane potential (ΔΨm) during cardiomyocyte hypoxia-reoxygenation. A: representative images of Sed and Ex cardiomyocytes during hypoxia-reoxygenation. Depolarized mitochondrial networks and collapses of ΔΨm are shown during reoxygenation as transition from yellow to red and black. B: quantification of TMRM fluorescence during hypoxia-reoxygenation. Values are means ± SE. #P < 0.05 vs. Sed main effect.
Mitochondrial JO2 and JH2O2 during hypoxia-reoxygenation.
The quality of mitochondria was similar between the groups following isolation as assessed by the respiratory control ratio (5.1 ± 0.4 and 5.2 ± 0.1 in Sed and Ex, respectively). JO2 at a submaximal (75 μM) ADP concentration was not different between Ex and Sed mitochondria prior to anoxia during respiration on complex I and II substrate (Fig. 5A). The decrement in JO2 immediately following anoxia was blunted in mitochondria from Ex animals (P < 0.05; Fig. 5B). Baseline state 3 JH2O2 was not different between Ex and Sed mitochondria before the onset of anoxia (Fig. 5C). JH2O2 was significantly higher after than before anoxia-reoxygenation only for Sed mitochondria (P < 0.05 vs. Sed baseline), while this increase was attenuated in Ex mitochondria and JH2O2 was significantly lower in Ex than Sed mitochondria following anoxia-reoxygenation (P < 0.05 vs. Sed reox; Fig. 5C). The ratio of JH2O2 to JO2 was significantly higher than baseline only for Sed mitochondria following anoxia-reoxygenation (P < 0.05 vs. Sed baseline; Fig. 5D) and significantly higher than for Ex mitochondria following anoxia-reoxygenation (P < 0.05 vs. Sed reox; Fig. 5D). A representative trace for JH2O2 in Fig. 5E demonstrates the lower ROS burst during reoxygenation in mitochondria from Ex hearts.
In parallel experiments, the contributions of the thioredoxin and glutathione redox systems to ROS scavenging were investigated separately. Mitochondria-generated H2O2 was measured under state 4 conditions with succinate, incubated with the TrxR inhibitor AF or the GR inhibitor BCNU. Mitochondrial JH2O2 values were similar in Ex and Sed hearts after inhibition with BCNU, whereas JH2O2 was significantly lower in Ex than Sed hearts only after inhibition with AF (P < 0.05, Fig. 5F).
DISCUSSION
The objective of the present study was to determine the effect of exercise-induced cardioprotection on mitochondrial bioenergetics and redox homeostasis during reperfusion-induced arrhythmia. Our findings indicate that exercise decreases arrhythmia through mitochondria-dependent mechanisms, including better maintenance of ΔΨm and lower ROS production. Several aspects of the present study provide novel insight into mechanisms of exercise cardioprotection. 1) The simultaneous recording of ΔΨm and ECG in the intact heart provides crucial confirmation that mitochondria from exercised hearts have a protective phenotype in situ. We have directly demonstrated that this phenotype correlates with cardiac electrical stability. 2) The continuous, simultaneous recording of mitochondrial O2 consumption and ROS during in vitro hypoxia-reoxygenation allows us to determine the exact time and nature of bioenergetic dysfunction during the metabolic insult itself (as opposed to after the injury has occurred). The present study, along with our previously published data, indicates that the maintenance of redox homeostasis through GR is an exercise-induced adaptation that helps sustain energetic and electrical coupling in the heart (29, 30). 3) Our vertically integrated approach using intact hearts, isolated ventricular myocytes, and isolated mitochondria provides comprehensive insight into the endogenous changes that occur in exercised hearts, indicating that stabilization of mitochondrial energetics is centrally involved in the antiarrhythmic effects of exercise.
Maintenance of ΔΨm and lower reperfusion arrhythmia following exercise-induced cardioprotection.
The maintenance of ΔΨm is an important determinant of ischemia-reperfusion injury, cell death, and arrhythmia (3, 13, 19, 43). Under conditions of metabolic stress, collapses of ΔΨm are known to induce oscillations in cardiac action potential duration due to transient increases in sarcolemmal ATP-sensitive K+ channel currents (3, 7, 55, 71). Lability in K+ current during the repolarization phase of the cardiac cycle can alter the spatiotemporal organization of cardiac electrical activity and increase the susceptibility to abnormal cardiac rhythms (73).
In the present study, exercise-induced cardioprotection led to better preservation of ΔΨm in the intact heart during early reperfusion, with a concomitant decrease in arrhythmia. We also observed a more robust preservation of ΔΨm when hearts were pooled for those that transitioned to arrhythmia vs. no arrhythmia. Heterogeneous collapses of ΔΨm in intact hearts during ischemia-reperfusion have been previously observed using two-photon microscopy (50, 65) or optical mapping (47). In studies of ischemia and reperfusion, more robust collapses of ΔΨm were often observed at the onset of reoxygenation (50, 65), when ROS levels surge and ATP demands resume with the recovery of excitation-contraction coupling. (Ischemic tissue does not contract and, thus, has lower energy demands.) Our present findings are in line with these observations, as we saw the most robust decline in ΔΨm in cells and hearts at the onset of reoxygenation and reperfusion, respectively. While this represents the first direct demonstration of preserved ΔΨm in exercised hearts, our results are consistent with previous studies showing better maintenance of energetics (reviewed in Ref. 27) and delayed opening of ATP-sensitive K+ channels (38) in exercise-conditioned hearts.
The overall reduction of oxidant stress with exercise and maintenance of ΔΨm that we observed are likely interrelated. In beating hearts, ΔΨm helps sustain redox homeostasis through replenishment of endogenous antioxidants via the nicotinamide nucleotide transhydrogenase. The cellular redox environment is then regulated by ROS detoxifying enzymes (e.g., GR and TrxR), the activity and subcellular localization of which control redox-sensitive protein networks. Through second-messenger signaling and posttranslational modifications, redox chemistry has been implicated in cardiac hypertrophy, remodeling, apoptosis, autophagy, and cell death (1, 54, 61). In our study the overall stabilization of energetics contributes to the improved cardiac function after exercise. The redox status of proteins following exercise, such as endothelial nitric oxide synthase (4) and GR (29), appears to play an important role in this protective phenotype. Consistent with our findings are other physiological observations following exercise, whereby redox modification to the ryanodine receptor enhances sarcoplasmic reticulum Ca2+ release (62). Furthermore, enhanced redox control may also prevent aberrant sarcoplasmic reticulum Ca2+-ATPase activity and Ca2+ reuptake by decreasing the oxidation of regulatory thiol-containing residues (57).
Preservation of cellular GSH and ΔΨm during ischemia-reperfusion with exercise.
The GSH pool is an essential part of redox homeostasis and an intricate antioxidant system used in the scavenging of ROS (63). Recent work implicates the cellular redox state in mitochondrial physiology and susceptibility to arrhythmia (6, 13, 30). The collapse of ΔΨm has been observed when GSH levels become oxidized to a critical level, leading to ROS-induced ROS release that can scale to depolarize mitochondrial networks (6, 13, 74). Decreasing the cellular oxidative burden during an oxidative challenge with perfusion of scavengers or a GSH analog prevents the collapse of ΔΨm (32), attenuates shortening of the action potential duration (2), and preserves the function of mitochondria isolated from postischemic hearts (16).
Exercise cardioprotection against arrhythmia has been shown to be dependent on enhanced ROS scavenging through several different endogenous mechanisms acting in parallel (13, 35, 60). Antisense treatment against MnSOD has been shown to abolish the antiarrhythmic effect of exercise (35), corroborating a number of studies that implicate heightened MnSOD in exercise-induced cardioprotection (reviewed in Ref. 27). Since the product of the dismutase reaction, H2O2, must be further processed to keep overall ROS levels low, detoxification by the GSH pool, the largest-capacity thiol buffer in the heart, is also involved. Most studies have found no basal differences in total GSH or the ratio of reduced to oxidized glutathione (GSH/GSSG) after exercise, (30, 37, 39, 45), and our work corroborates these findings. (Although there was a trend for an increase in basal GSH in myocytes from exercised hearts, it did not reach statistical significance.)
Although basal GSH/GSSG changes are rarely observed after exercise, the ability to replenish the GSH pool during an oxidative insult does appear to be involved. In this study the recovery of GSH levels during early reoxygenation correlated with maintained ΔΨm in heart cells and intact hearts. Myocardial GSH levels were also better preserved in hearts as assessed with HPLC (Fig. 3C). The GSSG content was not significantly different in Ex vs. Sed hearts at the end of reperfusion, which we also saw in an earlier study (30). This is likely due to heightened cell permeability of GSSG (67), ostensibly diffusing out of the tissue during the reoxygenation window. We previously showed that the activity of GR, which replenishes GSH, was enhanced after exercise (29, 30). As pharmacological inhibition of GR during ischemia-reperfusion abolished the antiarrhythmic phenotype of exercise (29), the ability to replenish the cellular glutathione pool appears to be centrally involved in exercise cardioprotection. Our observation that isolated cardiomyocytes exposed to hypoxia-reoxygenation displayed enhanced GSH replenishment is also consistent with previous studies in intact hearts/cells where exercise protected against injury after perfusion with the thiol-oxidizing agent diamide (30).
Exercise causes intrinsic mitochondrial adaptations that preserve postischemic function.
We used simultaneous acquisition of mitochondrial O2 consumption and H2O2 emission during anoxia-reoxygenation to determine the extent and time course of endogenous mitochondrial dysfunction. Similar studies using electroparamagnetic spin trapping on isolated mitochondria have shown that anoxia-reoxygenation results in a significant rise in superoxide production during the reoxygenation phase, with concomitant declines in respiratory function (23). Therefore, the anoxia-reoxygenation insult allows removal of cytosolic and compartmentalized cellular defense systems, unmasking mitochondria-specific adaptations.
Several studies have investigated the effect of anoxia-reoxygenation on mitochondrial function (23, 24, 69), but only one following exercise training. Ascensao et al. (9) exercised male Wistar rats for 14 wk at an intensity similar to that used in our protocol and exposed isolated mitochondria to anoxia-reoxygenation 24 h after the last exercise bout. The exercise group maintained higher post-anoxia-reoxygenation state 3 respiratory rates than sedentary controls. However, Ascensao et al. reported no difference in the magnitude of decline in respiration between the two groups (63% and 60% of baseline following anoxia-reoxygenation in exercised and sedentary groups, respectively). Our study demonstrates that exercise maintains mitochondrial energetics following a metabolic insult, assessed by a higher percentage of JO2 recovery following anoxia-reoxygenation (78% and 68% of baseline for Ex and Sed, respectively). Seeking to mimic the in vivo conditions, we used complex I- and II-linked substrates (glutamate, malate, pyruvate, and succinate) and physiologically clamped ADP levels (75 μM), while Ascensao et al. used only complex I substrate and ∼400 μM ADP, which more closely approaches Vmax and may not be as physiologically relevant (18). Recent findings implicate postischemic succinate accumulation as a driver of mitochondrial ROS production through reverse electron transfer (17) in early reperfusion. The inclusion of succinate in our mitochondrial buffers may also explain the differences between this study and previous work (8).
JH2O2 was lower following anoxia-reoxygenation in isolated mitochondria from the Ex than the Sed group, especially in early reoxygenation. These findings are consistent with observations that exercise lowers cardiac ROS accumulation during ischemia-reperfusion (30), protecting against oxidative stress and subsequent collapses of mitochondrial bioenergetics. Furthermore, the JH2O2-to-JO2 ratio was nearly twofold higher in the Sed than the Ex group, implying that exercise induces endogenous mitochondrial adaptations that result in a lower oxidative burden than O2 consumption.
Direct demonstration that mitochondria from exercised animals experience lower levels of oxidative stress corroborates recent findings. Lee et al. (44) reported that exercise significantly decreased H2O2 production in actively respiring mitochondria following ischemia-reperfusion. However, they isolated mitochondria from the myocardium after the ischemia-reperfusion insult, and one cannot ascertain if better mitochondrial function was a cause or a consequence of exercise-induced protection.
Exercise-induced adaptations enhance GSH replenishment through GR.
We determined if lower ROS bursts following anoxia-reoxygenation were due to improved scavenging by GR and/or TrxR. Inhibition of GR abolished the exercise-induced reduction of ROS, but the exercise effect persisted when TrxR was pharmacologically blocked. These data are in line with our previously published data (29, 30) implicating mitochondrial GR in enhanced redox control and stabilization of mitochondrial energetics following exercise-induced cardioprotection. Although few studies have examined TrxR in exercise cardioprotection, the lack of contribution of this scavenging mechanism is consistent with previous studies (21).
Measurement of ROS in living systems often represents the net balance between mitochondrial production and scavenging. Improved endogenous scavenging in the heart following exercise is clear (5, 27). It is plausible that Ex mitochondria also produce less ROS. Mitochondrial ROS production occurs at several different sites along the tricarboxylic acid (Krebs) cycle and electron transport system (56). Mitochondrial complexes I and III and supercomplexes can promote formation of reactive intermediates, especially during pathological conditions (49, 51). Future studies will continue to advance our understanding of how exercise leads to augmented scavenging and, perhaps, lower ROS emission in cardiac mitochondria.
Although exercise studies indicate a role for mitochondrial adaptations in the cardioprotective phenotype, further investigation is required. For example, the energy-sensing mitochondrial ATP-sensitive K+ channel has been implicated in exercise cardioprotection, and channel blockade abolishes the antiarrhythmic effect of exercise (60). Determining if mitochondrial ATP-sensitive K+ channel function directly affects ΔΨm and/or cellular redox status represents an exciting area for future research.
Limitations.
Although there are several limitations in our study, we tried to address these shortcomings with experiments at different levels of tissue organization. First, we used blebbistatin for the whole heart imaging experiments to limit motion artifacts. Blebbistatin inhibits actin-myosin interactions but has no effect on Ca2+ cycling and the cardiac action potential, which allows for recording of electrical activity (22). Still, the clear limitation is that ΔΨm is assessed in a model where the energetic demand of contraction is substantially blunted. For this reason, we used field-stimulated cardiomyocytes as an additional measurement of mitochondrial function during metabolic insult. Two-photon studies are also confounded by the limitation that global, no-flow ischemia provides consistent ventricular arrhythmia but the restoration of coronary flow at reperfusion induces movement artifact as the coronary bed is replenished with fluid. This prevents the continuous monitoring of the same section of ventricular muscle through ischemia and reperfusion, providing a relative signal over time. We also used a mixed population of mitochondria for our experiments and acknowledge that the subsarcolemmal and intermyofibrillar mitochondria may have divergent responses to the ischemic insult (39, 41, 44).
Conclusions.
In summary, our findings demonstrate that exercise helps sustain postischemic mitochondrial bioenergetics and redox homeostasis, which are associated with preserved ΔΨm and protection against reperfusion arrhythmia. This builds on a growing body of literature that indicates a close relationship between the redox environment and stability of ΔΨm. Future work aimed at determining the evolution of specific mitochondrial adaptations may assist in developing therapeutic targets that mimic the adaptive response to exercise-induced cardioprotection.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-123647-01 (to D. A. Brown and S. R. Shaikh), R15 HL-12292201 (to D. A. Brown and S. R. Shaikh), R01 DK-096907 (to P. D. Neufer), and R00 HL-103797 (to J. M. McClung).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.A., A.M.T., T.E.R., J.M.M., E.E.S., S.R.S., P.D.N., and D.A.B. developed the concept and designed the research; R.J.A., A.M.T., and D.J.P. performed the experiments; R.J.A., A.M.T., and D.J.P. analyzed the data; R.J.A., T.E.R., D.J.P., and D.A.B. interpreted the results of the experiments; R.J.A. prepared the figures; R.J.A. drafted the manuscript; R.J.A., J.M.M., E.E.S., and D.A.B. edited and revised the manuscript; R.J.A., A.M.T., T.E.R., J.M.M., E.E.S., S.R.S., P.D.N., and D.A.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Drew Holt for assistance with research animals, Dr. Drew Rockett for technical assistance with the two-photon microscope, and Dr. Christopher J. Wingard for the use of laboratory equipment.
REFERENCES
- 1.Ahsan MK, Lekli I, Ray D, Yodoi J, Das DK. Redox regulation of cell survival by the thioredoxin superfamily: an implication of redox gene therapy in the heart. Antioxid Redox Signal 11: 2741–2758, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiello EA, Jabr RI, Cole WC. Arrhythmia and delayed recovery of cardiac action potential during reperfusion after ischemia. Role of oxygen radical-induced no-reflow phenomenon. Circ Res 77: 153–162, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest 115: 3527–3535, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akita Y, Otani H, Matsuhisa S, Kyoi S, Enoki C, Hattori R, Imamura H, Kamihata H, Kimura Y, Iwasaka T. Exercise-induced activation of cardiac sympathetic nerve triggers cardioprotection via redox-sensitive activation of eNOS and upregulation of iNOS. Am J Physiol Heart Circ Physiol 292: H2051–H2059, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Alleman RJ, Katunga LA, Nelson MA, Brown DA, Anderson EJ. The “Goldilocks Zone” from a redox perspective: adaptive vs. deleterious responses to oxidative stress in striated muscle. Front Physiol 5: 358, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aon MA, Cortassa S, Maack C, O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem 282: 21889–21900, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aon MA, Cortassa S, Marban E, O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278: 44735–44744, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Ascensao A, Ferreira R, Magalhaes J. Exercise-induced cardioprotection: biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. Int J Cardiol 117: 16–30, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Endurance training limits the functional alterations of rat heart mitochondria submitted to in vitro anoxia-reoxygenation. Int J Cardiol 109: 169–178, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Bolli R, Jeroudi MO, Patel BS, DuBose CM, Lai EK, Roberts R, McCay PB. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc Natl Acad Sci USA 86: 4695–4699, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowles DK, Farrar RP, Starnes JW. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am J Physiol Heart Circ Physiol 263: H804–H809, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O'Rourke B. Effects of 4′-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res 79: 141–149, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O'Rourke B. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol 48: 673–679, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol 569: 913–924, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, Fleshner M, Spencer RL, Moore RL. Short-term treadmill running in the rat: what kind of stressor is it? J Appl Physiol 103: 1979–1985, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ceconi C, Curello S, Cargnoni A, Ferrari R, Albertini A, Visioli O. The role of glutathione status in the protection against ischaemic and reperfusion damage: effects of N-acetyl cysteine. J Mol Cell Cardiol 20: 5–13, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515: 431–435, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortassa S, O'Rourke B, Aon MA. Redox-optimized ROS balance and the relationship between mitochondrial respiration and ROS. Biochim Biophys Acta 1837: 287–295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J 341: 233–249, 1999. [PMC free article] [PubMed] [Google Scholar]
- 20.Curtis MJ, Hancox JC, Farkas A, Wainwright CL, Stables CL, Saint DA, Clements-Jewery H, Lambiase PD, Billman GE, Janse MJ, Pugsley MK, Ng GA, Roden DM, Camm AJ, Walker MJ. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol Ther 139: 213–248, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, Naito H. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol 91: 2205–2212, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Dou Y, Arlock P, Arner A. Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. Am J Physiol Cell Physiol 293: C1148–C1153, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Du G, Mouithys-Mickalad A, Sluse FE. Generation of superoxide anion by mitochondria and impairment of their functions during anoxia and reoxygenation in vitro. Free Radic Biol Med 25: 1066–1074, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Du G, Willet K, Mouithys-Mickalad A, Sluse-Goffart CM, Droy-Lefaix MT, Sluse FE. EGb 761 protects liver mitochondria against injury induced by in vitro anoxia/reoxygenation. Free Radic Biol Med 27: 596–604, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, Spigelman AF, Wright RC, Plummer G, Suzuki K, Mackay JP, van de Bunt M, Gloyn AL, Ryan TE, Norquay LD, Brosnan MJ, Trimmer JK, Rolph TP, Kibbey RG, Manning Fox JE, Colmers WF, Shirihai OS, Neufer PD, Yeh ET, Newgard CB, MacDonald PE. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. J Clin Invest 125: 3847–3860, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O'Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng ZJ. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 122: 2335–2348, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frasier CR, Moore RL, Brown DA. Exercise-induced cardiac preconditioning: how exercise protects your achy-breaky heart. J Appl Physiol 111: 905–915, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Frasier CR, Moukdar F, Patel HD, Sloan RC, Stewart LM, Alleman RJ, La Favor JD, Brown DA. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc Res 98: 47–55, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Frasier CR, Sloan RC, Bostian PA, Gonzon MD, Kurowicki J, Lopresto SJ, Anderson EJ, Brown DA. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol 111: 1751–1759, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Ganitkevich V, Reil S, Schwethelm B, Schroeter T, Benndorf K. Dynamic responses of single cardiomyocytes to graded ischemia studied by oxygen clamp in on-chip picochambers. Circ Res 99: 165–171, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Giustarini D, Dalle-Donne I, Milzani A, Fanti P, Rossi R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat Protoc 8: 1660–1669, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129: 399–410, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton KL, Quindry JC, French JP, Staib J, Hughes J, Mehta JL, Powers SK. MnSOD antisense treatment and exercise-induced protection against arrhythmias. Free Radic Biol Med 37: 1360–1368, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Hull SS Jr, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation 89: 548–552, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Husain K, Somani SM. Response of cardiac antioxidant system to alcohol and exercise training in the rat. Alcohol 14: 301–307, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Jew KN, Moore RL. Exercise training alters an anoxia-induced, glibenclamide-sensitive current in rat ventricular cardiocytes. J Appl Physiol 92: 1473–1479, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Judge S, Jang YM, Smith A, Selman C, Phillips T, Speakman JR, Hagen T, Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol 289: R1564–R1572, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Kand'ar R, Zakova P, Lotkova H, Kucera O, Cervinkova Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J Pharm Biomed Anal 43: 1382–1387, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Kavazis AN, McClung JM, Hood DA, Powers SK. Exercise induces a cardiac mitochondrial phenotype that resists apoptotic stimuli. Am J Physiol Heart Circ Physiol 294: H928–H935, 2008. [DOI] [PubMed] [Google Scholar]
- 42.King N, Korolchuk S, McGivan JD, Suleiman MS. A new method of quantifying glutathione levels in freshly isolated single superfused rat cardiomyocytes. J Pharmacol Toxicol Methods 50: 215–222, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Kloner RA, Hale SL, Dai W, Gorman RC, Shuto T, Koomalsingh KJ, Gorman JH 3rd, Sloan RC, Frasier CR, Watson CA, Bostian PA, Kypson AP, Brown DA. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective peptide. J Am Heart Assoc 1: e001644, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y, Min K, Talbert EE, Kavazis AN, Smuder AJ, Willis WT, Powers SK. Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Med Sci Sports Exerc 44: 397–405, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol Regul Integr Comp Physiol 272: R363–R369, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Lennon SL, Quindry JC, Hamilton KL, French JP, Hughes J, Mehta JL, Powers SK. Elevated MnSOD is not required for exercise-induced cardioprotection against myocardial stunning. Am J Physiol Heart Circ Physiol 287: H975–H980, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Lyon AR, Joudrey PJ, Jin D, Nass RD, Aon MA, O'Rourke B, Akar FG. Optical imaging of mitochondrial function uncovers actively propagating waves of mitochondrial membrane potential collapse across intact heart. J Mol Cell Cardiol 49: 565–575, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning A, Bernier M, Crome R, Little S, Hearse D. Reperfusion-induced arrhythmias: a study of the role of xanthine oxidase-derived free radicals in the rat heart. J Mol Cell Cardiol 20: 35–45, 1988. [DOI] [PubMed] [Google Scholar]
- 49.Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 19: 1469–1480, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto-Ida M, Akao M, Takeda T, Kato M, Kita T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation 114: 1497–1503, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 35: 2950–2959, 2014. [DOI] [PubMed] [Google Scholar]
- 53.Nieminen T, Scirica BM, Pegler JR, Tavares C, Pagotto VP, Kanas AF, Sobrado MF, Nearing BD, Umez-Eronini AA, Morrow DA, Belardinelli L, Verrier RL. Relation of T-wave alternans to mortality and nonsustained ventricular tachycardia in patients with non-ST-segment elevation acute coronary syndrome from the MERLIN-TIMI 36 trial of ranolazine vs. placebo. Am J Cardiol 114: 17–23, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Nishida K, Otsu K. Autophagy during cardiac remodeling. J Mol Cell Cardiol. In press. [DOI] [PubMed] [Google Scholar]
- 55.O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science 265: 962–966, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Orr AL, Ashok D, Sarantos MR, Shi T, Hughes RE, Brand MD. Inhibitors of ROS production by the ubiquinone-binding site of mitochondrial complex I identified by chemical screening. Free Radic Biol Med 65: 1047–1059, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin F, Siwik DA, Lancel S, Zhang J, Kuster GM, Luptak I, Wang L, Tong X, Kang YJ, Cohen RA, Colucci WS. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc 2: e000184, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quindry JC, Hamilton KL, French JP, Lee Y, Murlasits Z, Tumer N, Powers SK. Exercise-induced HSP-72 elevation and cardioprotection against infarct and apoptosis. J Appl Physiol 103: 1056–1062, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Quindry JC, Miller L, McGinnis G, Kliszczewicz B, Irwin JM, Landram M, Urbiztondo Z, Nanayakkara G, Amin R. Ischemia reperfusion injury, KATP channels, and exercise-induced cardioprotection against apoptosis. J Appl Physiol 113: 498–506, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quindry JC, Schreiber L, Hosick P, Wrieden J, Irwin JM, Hoyt E. Mitochondrial KATP channel inhibition blunts arrhythmia protection in ischemic exercised hearts. Am J Physiol Heart Circ Physiol 299: H175–H183, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richters L, Lange N, Renner R, Treiber N, Ghanem A, Tiemann K, Scharffetter-Kochanek K, Bloch W, Brixius K. Exercise-induced adaptations of cardiac redox homeostasis and remodeling in heterozygous SOD2-knockout mice. J Appl Physiol 111: 1431–1440, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez G, Escobar M, Pedrozo Z, Macho P, Domenech R, Hartel S, Hidalgo C, Donoso P. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: possible role in cardioprotection. Cardiovasc Res 77: 380–386, 2008. [DOI] [PubMed] [Google Scholar]
- 63.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, Lust RM, Brown DA. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol 52: 1009–1018, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Slodzinski MK, Aon MA, O'Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. J Mol Cell Cardiol 45: 650–660, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor RP, Olsen ME, Starnes JW. Improved postischemic function following acute exercise is not mediated by nitric oxide synthase in the rat heart. Am J Physiol Heart Circ Physiol 292: H601–H607, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Tritto I, Duilio C, Santoro G, Elia PP, Cirillo P, De Simone C, Chiariello M, Ambrosio G. A short burst of oxygen radicals at reflow induces sustained release of oxidized glutathione from postischemic hearts. Free Radic Biol Med 24: 290–297, 1998. [DOI] [PubMed] [Google Scholar]
- 68.Wachtell K, Okin PM, Olsen MH, Dahlof B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thygesen K. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation 116: 700–705, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Willet K, Detry O, Sluse FE. Resistance of isolated pulmonary mitochondria during in vitro anoxia/reoxygenation. Biochim Biophys Acta 1460: 346–352, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med 189: 1699–1706, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan XS, Ma JH, Zhang PH. Modulation of KATP currents in rat ventricular myocytes by hypoxia and a redox reaction. Acta Pharmacol Sin 30: 1399–1414, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu L, Fink BD, Herlein JA, Sivitz WI. Mitochondrial function in diabetes: novel methodology and new insight. Diabetes 62: 1833–1842, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou L, Cortassa S, Wei AC, Aon MA, Winslow RL, O'Rourke B. Modeling cardiac action potential shortening driven by oxidative stress-induced mitochondrial oscillations in guinea pig cardiomyocytes. Biophys J 97: 1843–1852, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci USA 84: 1404–1407, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]