Abstract
Gallstone disease is a widespread disorder costing billions for annual treatment in the United States. The primary mechanisms underlying gallstone formation are biliary cholesterol supersaturation and gallbladder hypomotility. The relative contribution of these two processes has been difficult to dissect, as experimental lithogenic diets cause both bile supersaturation and alterations in gallbladder motility. Importantly, there is no mechanistic explanation for obesity as a major risk factor for cholelithiasis. We discovered that lithogenic diets induce ectopic triacylglycerol (TAG) accumulation, a major feature of obesity and a known muscle contraction impairing condition. We hypothesized that prevention of TAG accumulation in gallbladder walls may prevent gallbladder contractile dysfunction without impacting biliary cholesterol saturation. We utilized adeno-associated virus-mediated knock down of the long-chain fatty acid transporter 2 (FATP2; Slc27A2), which is highly expressed by gallbladder epithelial cells, to downregulate lithogenic diet-associated TAG accumulation. FATP2-knockdown significantly reduced gallbladder TAG, but did not affect key bile composition parameters. Importantly, measurements with force displacement transducers showed that contractile strength in FATP2-knockdown gallbladders was significantly greater than in control gallbladders following lithogenic diet administration, and the magnitude of this effect was sufficient to prevent the formation of gallstones. FATP2-driven fatty acid uptake and the subsequent TAG accumulation in gallbladder tissue plays a pivotal role in cholelithiasis, and prevention of this process can protect from gallstone formation, even in the context of supersaturated bile cholesterol levels, thus pointing to new treatment approaches and targets.
Keywords: contractility, triacylglycerol, gallstones, uptake, FFA
gallstone disease, which is predominated by cholesterol-rich gallstones, has reached a prevalence of ∼15% in the Western world, making it the most common and expensive to treat digestive disease requiring hospitalization (41). A 1999 report (17) estimated, based on the Third National Health and Nutrition Examination Survey, that 6.3 million men and 14.2 million women in the US, aged 20–74 yr, had gallbladder disease, while more recent epidemiological assessments estimate that 20–25 million adults are afflicted with gallstones, requiring annually over 700,000 cholecystectomies at a costs of ∼$6.5 billion (47). Gallstone formation is a multifactorial process that can involve both genetic and environmental risk factors, with genetic factors accounting on average for 25–29% of the risk (25, 35). Common risk factors for gallstone disease include sex, being twice as common in women as in men (16), complex polygenic polymorphisms (56), lack of physical activity (30), obesity (7, 32), hepatic insulin resistance (4), type 2 diabetes (44), and dyslipidemia (41). Mechanistically, the formation of cholesterol gallstones is the result of an incompletely understood interplay of altered bile composition and impaired gallbladder contractility. While cholesterol supersaturation of bile, which can result from an imbalance between the levels of cholesterol, phospholipids, and bile acids, as well as changes in bile acid composition, appears to be required for the formation of cholesterol gallstones, it is clearly not sufficient, as supersaturated bile is also observed in individuals without cholesterol gallstones (22). In addition to bile composition, gallbladder contractility has been suggested to play an important role in cholesterol gallstone formation (53), and gallbladder hypomotility can be detected in both gallstone patients as well as animal models (52). Gallbladder motility is under the control of neuronal as well as endocrine factors. Cholecystokinin (CCK) plays an important role in stimulating postprandial bile release from the gallbladder (37), while FGF19/15 (human/mice) is required for gallbladder filling (10). CCK-1 receptors are found on gallbladder smooth muscle (GBSM) cells, and receptor number has been suggested to correlate with contractile function (62). Indeed, CCK-1 receptor number appears to be decreased in patients with poor gallbladder contractility (51). Another important event linked to impaired gallbladder motility is accumulation of lipids in the gallbladder wall. In fact, several diseases characterized by ectopic lipid accumulation, such as type 2 diabetes mellitus, hypertriglyceridemia (HTG) (49), and insulin resistance, are associated with decreased gallbladder emptying (40). Gallbladder epithelial cells are able to absorb several bile components, including cholesterol (19). In addition to cholesterol, gallbladder wall lipids also include triacylglycerides (TAG), which are greatly increased in animal models of obesity (20). However, which lipids in the gallbladder wall are responsible for the decreased contractile function is unclear, and the origin of the fatty acids that accumulate in the gallbladder is unknown. Bile has been reported to contain between 0.3 and 1.2 g/dl of fatty acids (21); thus it is feasible that fatty acids are absorbed from the bile by the gallbladder epithelium. Previous work by our laboratory (2) and others has demonstrated that cellular uptake of fatty acids, including hepatic (14, 18) and intestinal (50) processes, is predominantly a protein-mediated process. However, which fatty acid transporters are present in the gallbladder and how they relate to lipid accumulation and gallbladder dysfunction have not been investigated thus far. Most importantly, no animal model has been created until now that would allow the determination of the causal role of gallbladder hypermotility by preserving contractile function in the face of a lithogenic diet. Here we show that fatty acid transporter 2 (FATP2; Slc27a2) is expressed in the gallbladder, and that knockdown of FATP2 in combination with a lithogenic diet can be utilized to disentangle the relationships between gallbladder lipid accumulation, contractile dysfunction, and gallstone formation. Loss of FATP2 function decreases gallbladder TAG, but not cholesterol content, while increasing bile fatty acid concentrations. While cholesterol solubility remained unaltered, these animals preserve gallbladder contractile function and are protected from the development of diet-induce cholesterol gallstones, and thus offer a unique model for our understanding of the etiology of cholelithiasis.
MATERIALS AND METHODS
Animals and diets.
Experiments were carried out according to Association for Assessment and Accreditation of Laboratory Animal Care guidelines. Animals were humanly cared for according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (revised 2011). Procedures were approved by the University of California, Berkeley Animal Care and Use Committee. Nine-week-old male C57BL/6J mice were purchased from Jackson Laboratory. Mice were individually housed for a 1-wk acclimation period (temperature: 70 ± 4°F, humidity: 30–70%) so that experiments were conducted on composed 10-wk-old mice. Animals received a tail vein delivered 5 × 1010 units of our validated short hairpin RNA (shRNA) FATP2-adeno-associated virus (AAV) construct no. 6 (50) and were then administered a defined lithogenic diet (TD.90221) (containing 15.8% fat, 1.25% cholesterol, and 0.5% sodium cholate), or a control chow diet (TD.2918) (Harlan Teklad Custom Research) for 8 wk (42, 55, 57). Mice were monitored daily for well-being and weekly for food consumption and weight gain so that handling stress was minimized.
Gallstone measurement.
After 8 wk of lithogenic diet, the mice were fasted overnight and humanely euthanized, and intact gallbladders were harvested. The collected gallbladders were then cut transversely so that their entire contents were collected in preweighed microfuge tubes. The collected gallstone and bile mixture was centrifuged at 10k relative centrifugal force for 10 min. Bile was removed, and the remaining gallstones were dried overnight with an Eppendorf Vacufuge. The gallstones were then weighed with an analytic balance (Mettler Toledo AB54-S) (26).
Immunofluorescence.
Tissues were fixed with 4% paraformaldehyde for 1 h at 4°C. Fixed tissues were incubated overnight at 4°C with 30% sucrose in PBS. Tissues were cryosectioned at a depth of 12 μM. Sections were washed three times with PBS-Tween 20 and then treated with blocking media [HBSS with 5% FBS (Gibco), 0.1% BSA (Fisher), 0.05% saponin (Sigma), and 0.1% Tween 20 (Sigma)] for 1 h. Subsequently, cryosections were incubated with their corresponding primary antibody for 1.5 h at room temperature. Sections were then washed with fresh blocking media for 20 min, and then with PBS-Tween 20 with 0.05% saponin two times for 10 min. Secondary antibodies were diluted into the blocking media and applied to sections for 1 h at room temperature. The sections were washed with the same procedure that followed the primary antibody treatment. Coverslips were mounted with 4,6-diamidino-2-phenylindole (DAPI) mounting medium (Life Technologies). Slides were stored at −20°C and analyzed with a Zeiss LSM710 confocal microscope. Antibodies were as follows: FATP2 polyclonal antibody, which was raised against the COOH-termini of FATP2, as previously described (15, 50), prostaglandin E2 (PGE2) (Sigma), and smooth muscle actin 1a4 (Sigma).
Protein quantification and Western blotting.
Tissues were lysed with RIPA buffer fortified with protease inhibitor P8340 (Sigma). Ten micrograms of protein were loaded into Novex 4–20% Tris glycine precast gels (Life), transferred with the iBlot (Invitrogen) system, and assayed with anti-FATP2 and anti-β-tubulin E7 clone (DSHB). Li-Cor Oddessy was employed to image and quantify bands.
Quantitation of PGE2 staining.
Comparable immunohistochemicals of gallbladders were collected as previously described with a Ziess LSM710. Nuclei were counted with Bitplane's Imaris software. PGE2 fluorescent signal intensity was quantified with BioVision iVision.
Gallbladder contraction assay.
Gallbladders were obtained from 18-wk-old sex-matched mice. After both sides of the gallbladder were gently cut and the lumen was flushed with PBS, gallbladders were attached to the force transducer (Radnoti 8-Chamber Tissue-Organ Bath System) in a warmed, oxygenated organ bath with modified Krebs-Henseleit buffer. After a 30-min equilibration period, concentration curves to methacholine and KCl were obtained. Resting tension of 0.5 g was applied after each treatment (27).
Bile acid species analysis with HPLC-tandem mass spectrometry.
Bile samples were spiked with internal standards and extracted in 5% ammonium hydroxide (NH4OH) in acetonitrile (CH3CN) by mixing with a circular tube rotator for 1 h. Samples were centrifuged for 10 min. The resulting pellets were reextracted with 5% NH4OH in CH3CN and mixed again for 30 min. The combined supernatants were then dried under nitrogen and reconstituted in 1:1 methanol and water. The samples were then injected onto a Javelin C18 guard cartridge fit to a Waters XBridge 2.1 × 50 mm C18 column housed at 40°C on a Shimadzu HPLC system. The HPLC system was programed for a gradient flow of 0.1% formic acid in water and methanol at 0.6 ml/min, with an increase of 5–15% methanol at 0.01 min, 25% methanol at 1 min, 40% methanol at 7 min, and 100% methanol at 10 min. These conditions were held to 12 min when the initial conditions were reestablished and the system equilibrated. A dump valve was used to divert the initial 0.6 min of flow to waste. A Sciex 6500 triple quad mass spectrometer was used in the negative ion mode to detect and quantitate the bile acids. The source conditions were as follows: curtain gas, 20.00; temperature, 550.00; gas 1, 60.00; gas 2, 40.00; collisionally activated decomposition, 8.00; ionspray voltage, −4,500.00; entrance potential, −10.00 (Table 1).
Table 1.
Mass spectrometer settings for the analytes
| Analyte | Q1 | Q3 | Dwell | DP | CE | CXP |
|---|---|---|---|---|---|---|
| Cholic acid | 407.2 | 407.2 | 35 | −180 | −25 | −10 |
| Deoxy cholic acid | 391.2 | 391.2 | 35 | −180 | −25 | −10 |
| Litho cholic acid | 375.2 | 375.2 | 35 | −180 | −25 | −10 |
| Glyco cholic-like | 464.2 | 73.9 | 35 | −180 | −80 | −10 |
| Glyco deoxy cholic-like | 448.2 | 73.9 | 35 | −180 | −80 | −10 |
| Glyco litho cholic-like | 432.2 | 73.9 | 35 | −180 | −80 | −10 |
| Tauro cholic-like | 514.2 | 79.9 | 35 | −250 | −130 | −9 |
| Tauro deoxy cholic-like | 498.2 | 79.9 | 35 | −250 | −130 | −9 |
| Tauro litho cholic-like | 482.2 | 79.9 | 35 | −250 | −130 | −9 |
| d4 Cholic acid | 411.2 | 411.2 | 35 | −180 | −25 | −10 |
| d4 Deoxy cholic acid | 395.2 | 395.2 | 35 | −180 | −25 | −10 |
| d4 Litho cholic acid | 379.2 | 379.2 | 35 | −180 | −25 | −10 |
| d4 Glyco cholic acid | 468.2 | 73.9 | 35 | −180 | −80 | −10 |
| d4 Glyco deoxy cholic acid | 452.2 | 73.9 | 35 | −180 | −80 | −10 |
| d4 Glyco litho cholic acid | 436.2 | 73.9 | 35 | −180 | −80 | −10 |
| d4 Tauro cholic acid | 518.2 | 79.9 | 35 | −250 | −130 | −9 |
| d4 Tauro deoxy cholic acid | 502.2 | 79.9 | 35 | −250 | −130 | −9 |
| d4 Tauro litho cholic acid | 486.2 | 79.9 | 35 | −250 | −130 | −9 |
Q1 and Q3, quadrants 1 and 3, respectively; Dwell, dwell times; DP, declustering potential; CE, collision energy; CXP, collision cell exit potential.
Triacylglycerol content.
Tissues were harvested, lysed, and homogenized with a standard RIPA buffer and a Polytron PT2100 homogenizer. Triglyceride (TG) content was measured with the Infinity Triaglycerides kit (Sigma), as per the manufacturer's instruction. TAG was normalized to protein measured with a BCA kit (Pierce).
Cholesterol content.
The Cholesterol Liquicolor kit (Stanbio 1010-430) was used per the manufacturer's instructions to assess cholesterol content in bile and in RIPA-lysed tissues. Cholesterol content was normalized to protein where applicable, measured with a BCA kit (Pierce).
Phospholipid quantitation.
Biliary phospholipids were measured with the Phospholipid Assay Kit (MAK122; Sigma), as per the manufacturer's instructions.
Nonesterified free fatty acid measurements.
Biliary nonesterified free fatty acid was measured with the Wako Diagnostics NEFA kit (WAKO), as per the manufacturer's instructions.
Total bile acid quantitation (enzymatic).
Total bile acids were measured with the Total Bile Acid Colormetric Assay kit (BQ092A-EALD; Bioquant), as per the manufacturer's instructions.
Statistical analysis.
All data are presented as SE analyzed using Prism (Graphpad). Statistical significance was determined by either one-way ANOVA followed by Tukey posttest or unpaired Student's t-test. Significance is presented at *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
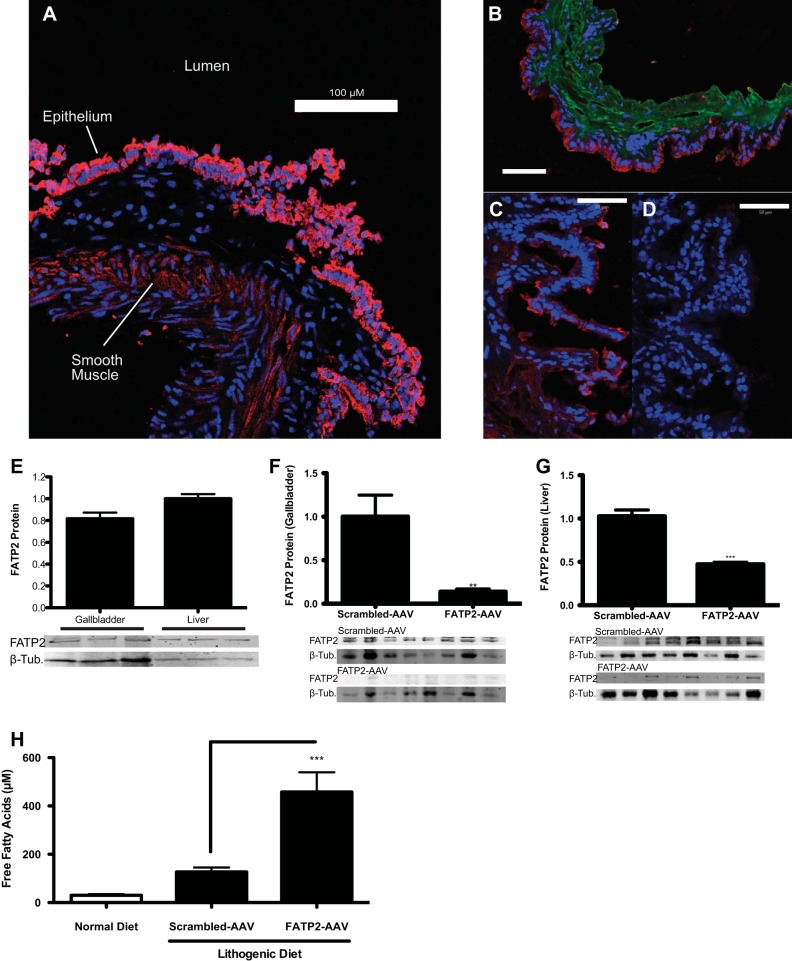
Using RT-PCR and Western blotting, we determined that FATP2 is expressed in murine gallbladder. Immunofluorescence microscopy demonstrated that the protein was found primarily on the apical site of gallbladder epithelial cells and to a lesser extent in the GBSM (Fig. 1, A and B). FATP2 expressed in this location likely facilitates the uptake of long-chain fatty acids from the bile into the gallbladder. To test the contribution of FATP2 expression on gallbladder function and diet-induced cholelithiasis, we utilized our validated and published AAV8-based shRNA knockdown vectors (18). Transduction of animals with 5 × 1010 units of our FATP2 knockdown construct (FATP2-AAV) significantly reduced FATP2 protein expression in gallbladders, rendering FATP2 undetectable by immunofluorescence (Fig. 1, C and D). In homogenized gallbladders, FATP2 is expressed at ∼81% of the level found in liver (Fig. 1E). Following AAV-mediated knockdown, FATP2 protein levels in gallbladders were reduced by 86% (Fig. 1F). Being that AAVs are known to infect hepatic tissues with high specificity, we also looked at FATP2 expression in the liver, which was found to be reduced by ∼53% (Fig. 1G). Interestingly, FATP2 knockdown animals had significantly elevated nonesterified fatty acids in their bile, suggesting that fatty acids translocated by FATP2 are, at least in part, derived from bile rather than circulation (Fig. 1H).
Fig. 1.
Expression and localization of FATP2 in gallbladder. A: immunofluorescence of a cryosectioned gallbladder with anti-FATP2 (red) and DAPI (blue) (scale bar = 100 μm). B: immunofluorescence of a cryosectioned gallbladder with anti-FATP2 (red), anti-smooth muscle actin (green), and DAPI (blue) (scale bar = 100 μm). C and D: immunofluorescence of FATP2 (red) scrambled-AAV-treated or FATP2-AAV-treated gallbladders (scale bar = 50 μm), respectively. E: relative expression of FATP2 in gallbladder and liver, normalized to β-tubulin (n = 3). F: relative expression of FATP2 in gallbladder, normalized to β-tubulin (n = 8). G: relative expression of FATP2 protein in liver, normalized to β-tubulin (n = 6). H: quantitation of free fatty acid content of bile (n = 8). Values are means ± SE. **P < 0.01 and ***P < 0.001.
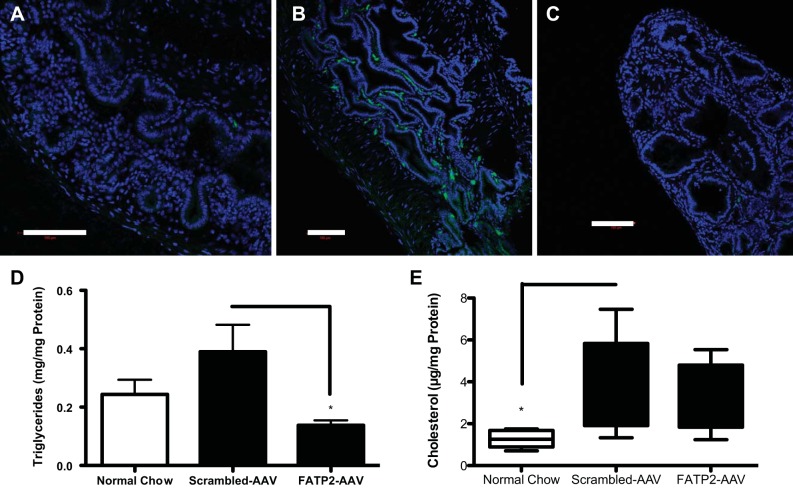
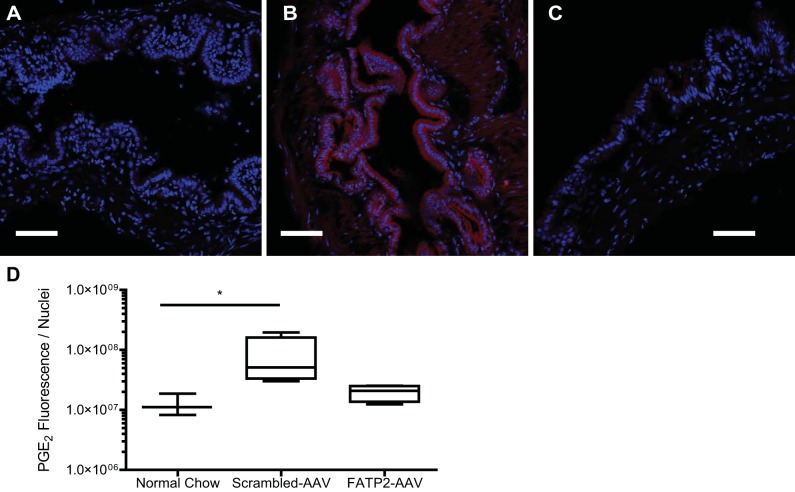
To distill the mechanism of gallstone formation, we first looked at the TG accumulation in the gallbladder on a normal diet (Fig. 2A). Cholelithiasis is strongly correlated with TG deposits found in the gallbladder wall. HTG has also been shown to correlate with gallbladder hypomotility in human gallbladders (49). To determine the effect of FATP2 loss of function on cholelithiasis, we administered an established lithogenic diet (Harlan TD.90221). TG accumulation within the gallbladder was elevated by the lithogenic diet (Fig. 2B) relative to the normal mouse chow diet (Harlan TD.2918). Knockdown of FATP2 reduced the TG content in the gallbladder, which was found to be even lower than in the normal chow-fed animals (Fig. 2, C and D). Total cholesterol content was found to be significantly elevated in gallbladders on the lithogenic diet, unsurprising given the substrate specificity of FATP2 for fatty acids; knockdown of this protein had no effect on cholesterol content (Fig. 2E). TG accumulation in tissues has been associated with the diminished GBSM motility observed with cholelithiasis (20, 32, 49). The mechanism by which TG accumulation affects motility is likely through elevated PGE2 production in the tissue. TG accumulation and lipid droplets have been shown to affect the production of these inflammatory eicosinoids (1). We measured significantly higher PGE2 in gallbladders from animals fed a lithogenic diet relative to normal chow-fed controls (Fig. 3). Knockdown of FATP2 reduced this diet-induced inflammatory effect (FATP2-AAV vs. scrambled-AAV, P = 0.1019).
Fig. 2.
Lipid content of gallbladder tissues. Neutral lipid staining with Bodipy-3922 staining (green) and DAPI (blue) of normal gallbladder on a normal chow diet (A), scrambled-AAV-treated gallbladder on lithogenic diet (B), and FATP2-AAV-treated gallbladder on lithogenic diet (C) (scale bar = 100 μm). D: quantitation of triacylglycerol in gallbladder tissues, normalized to protein concentration (n = 8). E: quantitation of cholesterol in gallbladder tissues, normalized to protein concentration (n = 8). Values are means ± SE. *P < 0.05.
Fig. 3.
Prostaglandin E2 (PGE2; red) and DAPI (blue) immunofluorescence of normal gallbladder on a normal chow diet (A), scrambled-AAV-treated gallbladder on lithogenic diet (B), and FATP2-AAV-treated gallbladder on lithogenic diet (C) (scale bar = 100 μm). D: PGE2 fluorescence normalized to the number of nuclei per field of view (n = 5). Values are means ± SE. *P < 0.05.
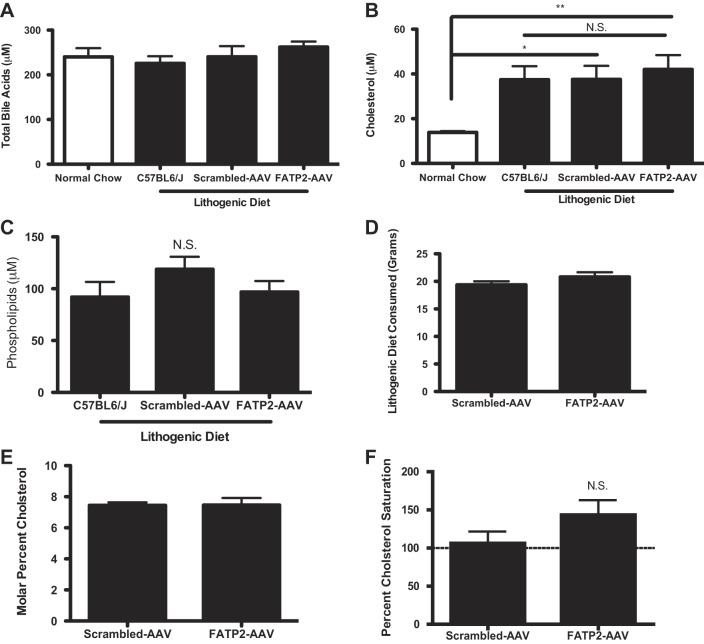
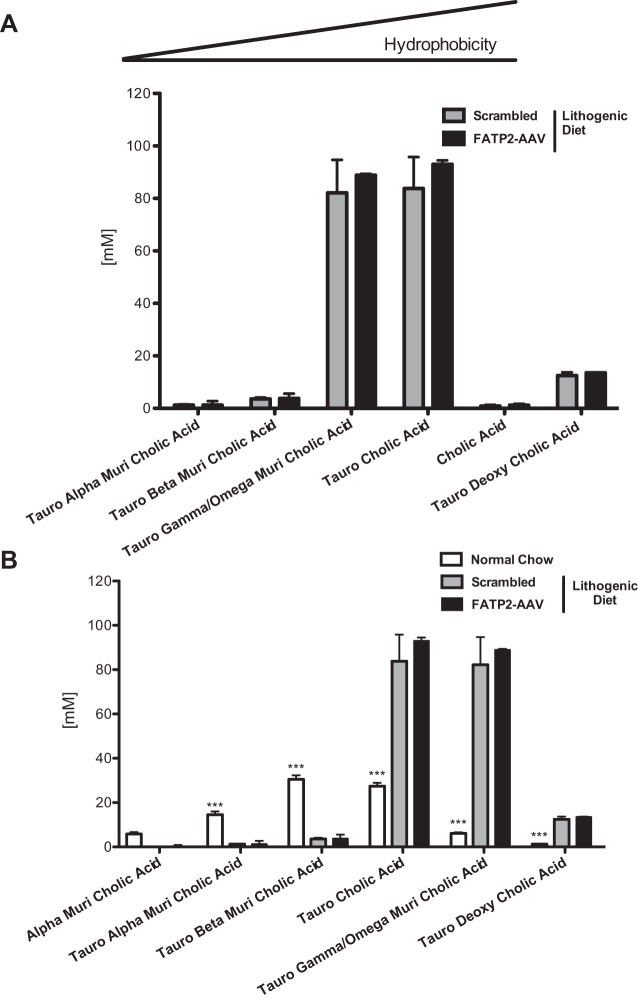
Since AAV-mediated knockdown of FATP2 also targets hepatic FATP2 (31), qualitative and/or quantitative changes in bile acid composition could not be excluded. However, based on enzymatic assays and HPLC-tandem mass spectrometry (MS/MS) measurements, no significant changes in bile acids (Fig. 4A), cholesterol (Fig. 4B), and phospholipids (Fig. 4C) were detected. Importantly, FATP2 knockdown did not alter the feeding behavior of the mice (Fig. 4D). Using the lithogenic index (6) to assess biliary cholesterol saturation, we found that the FATP2 knockdown in lithogenic diet-fed animals resulted in a slightly greater risk of cholesterol saturation than in control mice (Fig. 4, E and F). The lack of any appreciable alteration in total or specific bile acid species contradicts the postulation that FATP2 plays a major role in bile acid metabolism in vivo (33) (Fig. 5A). However, this finding may be a product of knockdown, as opposed to a complete knockout of hepatic FATP2. Unsurprisingly, we found clear differences in the bile composition of animals fed the lithogenic diet relative to the normal chow diet, which appear to bias the bile acid species toward greater hydrophobicity (Fig. 5B) (60). Importantly, knockdown of FATP2 produced no discernible variation in major biochemical influences to gallstone formation. We found that FATP2 knockdown had virtually no impact on bile acid concentration or composition, phospholipid concentration, or cholesterol concentration in the bile of animals fed a lithogenic diet.
Fig. 4.
A: enzymatic measure of total bile acids found in bile (n = 8). B: cholesterol concentration in bile (n = 8). C: phospholipid concentration of mice on lithogenic diet (n = 8). D: lithogenic diet mass consumed (n = 8). E: biliary molar percent cholesterol (n = 8). F: biliary cholesterol saturation percent (n = 8). Values are means ± SE. *P < 0.05 and **P < 0.01. NS, nonsignificant.
Fig. 5.
Bile composition of lithogenic diet-fed animals. A: quantitation of most abundant bile acid species by HPLC-MS/MS (n = 8). B: quantitation of differing bile acid species due to lithogenic diet measured by HPLC-MS/MS (n = 8 for lithogenic diet groups and n = 5 for normal chow group). Values are means ± SE. ***P < 0.001.
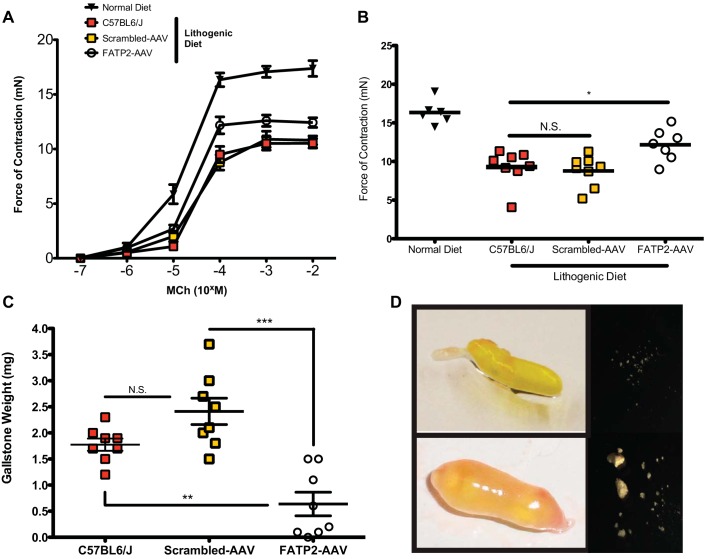
Hypomotility has been shown to be a driving force behind gallstone formation (28). To assess motility of live gallbladders, we directly measured the contractile capabilities with a force displacement transducer (27). The force of contraction of gallbladders from the lithogenic diet-fed animals was significantly reduced relative to normal chow-fed controls (Fig. 6, A and B). FATP2 knockdown rescued the diminished contractile strength that was observed on the lithogenic diet. Despite the cholesterol saturation of the bile, FATP2 knockdown animals were protected from the formation of gallstones (Fig. 6, C and D). These data argue that the principal cause of gallstones with the lithogenic diet model system is neutral lipid-mediated gallbladder hypomotility and suggest targeting FATP2 as a novel treatment option for cholelithiasis.
Fig. 6.
Effect of lithogenic diet on contractile capability of gallbladders and formation of gallstones. A: contractile force generated in response to varied concentrations of methacholine (MCh) (n = 8). B: force of gallbladder contraction stimulated by 100 μM MCh (n = 8). C: gallstone mass (n = 8). Values are means ± SE. *P < 0.05. **P < 0.01. ***P < 0.001. D: representative photographs of gallbladders and recovered gallstones from FATP2-AAV- (top) and scrambled-AAV-treated (bottom) mice.
DISCUSSION
The major driving factors for cholelithiasis are cholesterol supersaturation and gallbladder dysmotility, which often go hand in hand (28, 39, 40, 45, 54, 61). Gallbladder lipid accumulation has been closely linked with the development of hypomotility; however, the underlying molecular mechanisms driving this process have not been well studied. Here we identified the long-chain fatty acid transporter and long-chain acyl synthetase FATP2 (Slc27a2) (14) as being highly expressed in the gallbladder epithelium. Given that other FATP family members have previously been linked to the ectopic accumulation of lipids in skeletal muscle (58) and liver (14), we speculated that FATP2 might contribute both to gallbladder lipid accumulation as well as hypomotility. To dynamically modulate FATP2 function, we utilized an AAV8-based shRNA knockdown strategy that our laboratory previously used for the hepatic knockdown of the transporter (14). Interestingly, the tissue tropism of AAV8, using 5 × 1010 viral particles and tail vein injections, was thought to be liver specific (31); however, we were clearly able to also affect FATP2 expression in the gallbladder. However, since loss of FATP2 function was observed in both liver and gallbladder, we had to take into account a potential contribution of hepatic FATP2, particularly to bile acid formation. Using a cell-based FATP2 overexpression system, Mihalik et al. (33) reported that enhanced FATP2 expression correlated with 3α,7α,12α-trihydroxy-5β-cholestanoic acid CoA ligase activity, an enzymatic step required for bile acid side-chain shortening via β-oxidation. Furthermore, a related transporter, FATP5, had been found to act as a bile acid-CoA ligase required for the reactivation of deconjugated bile acids (13). Since cholesterol supersaturation, which is considered to be essential for cholesterol gallstone formation (7), is a result of the relative proportion of cholesterol, phospholipid, and bile acid concentration, as well as composition, we carefully analyzed the bile acid composition in FATP2 knockdown and control bile using HPLC-MS/MS. To our knowledge, this is the first in vivo analysis of FATP2 and bile acid synthesis. If FATP2 is truly essential for bile acid maturation and side-chain shortening, we would have expected dramatic changes in bile acid composition; however, out of the 27 bile acid species analyzed, none was significantly changed, which makes a role of FATP2 in hepatic bile acid synthesis highly unlikely and, taken together with unaltered biliary cholesterol and phospholipid levels, allowed us to focus on FATP2's function in regard to gallbladder physiology. The gallbladder emptying percentage is strongly correlated with gallstone formation. Impaired emptying enhances retention time of cholesterol-containing vesicles, which promotes nucleation. A number of clinical studies have categorized patients as “strong contractors” or “weak contractors” based on their gallbladder's ability to empty; the “weak” classification is predictive of cholelithiasis (52). Pauletzki et al. (39) found that gallbladder emptying was an independent risk factor for gallstone reoccurrence after lithotripsy. The majority of patients with an ejection fraction of <60% of their biliary content developed gallstones within 3 yr after treatment, which is very impressive due to the fact that it takes considerable time to form symptomatic gallstones in humans.
Gallbladder dysmotility is correlated with cholelithiasis risk states, such as pregnancy, obesity, octreootide (somatostatin analog) treatment, low-calorie dieting, and total parenteral nutrition (54). Gallbladder hypomotility is strongly associated with diseases where excessive deposition of lipid is observed, specifically HTG (49) and obesity (7, 32). CCK receptor expression and integrity (36, 46, 51) have a major role in the prevalence of cholelithiasis and a direct link to motility. Unfortunately, CCK signaling is impaired by cholesterol concentrations, and, therefore, we opted to study GBSM contractility in greater detail via stimulation of muscarinic receptor and a direct force measurement of GBSM contraction. Elevated cholesterol levels have been shown to facilitate sequestration of CCK-1 receptors via a caveolin-3-dependent mechanism (8, 11, 59). Mouse models clearly demonstrate that the lithogenic diet causes cholesterol gallstone formation with impaired CCK secretion and severely impaired gallbladder sensitivity to CCK (48). Being that muscarinic receptors have been shown to play an important role in GBSM contractile capability (5, 9, 29, 38), we chose to use methacholine to test GBSM function. It has already been established that muscarinic receptor agonism produces strong and physiologically relevant contraction of GBSM, independent of receptors sensitive to the high-cholesterol exposure produced by the lithogenic diet (3).
The role of cholesterol in this model may not only be to attenuate CCK receptor expression and alter membrane fluidity. Jennings et al. (23) speculated that gallbladder lipid droplets observed in cholelithiasis models might be primarily cholesterol and cholesterol esters. We now show that they reflect ectopic TAG accumulation, a phenomenon that is frequently observed in other organs in the context of high-fat diets (12). Furthermore, the motility defect in HTG patients can be reversed with TG-lowering therapies, such as fish oil supplementation or fibrate treatment (24). Fibrate treatment is especially interesting because fibrates attenuate bile acid synthesis, thereby increasing the cholesterol saturation margin, which would promote gallstone formation. Fish oil supplementation has been used to address HTG and may affect gallbladder TAG content, but this treatment does not alter the neutral lipid stores independent of alterations in bile composition. Knockdown of FATP2 in the context of a lithogenic diet greatly reduced the accumulation of gallbladder lipid droplets, as well as gallbladder TAG, but not cholesterol content. Concomitantly, we observed an increase in bile nonesterified fatty acids. Thus we propose that the most likely explanation is that neutral lipids in the gallbladder wall are predominantly TAG, and that the source of these lipids is at least in part transport of fatty acids from the bile into the gallbladder tissue. Free fatty acids have been reported to possibly induce cholesterol nucleation in bile (34), which suggests that it is even more impressive that the FATP2 knockdown mice are protected from gallstones. The reduction in gallbladder lipids directly translated into improved gallbladder contractility, further enforcing the mechanistic link between the two, although the molecular mechanisms remain to be elucidated. One possibility would be that FATP2 mediates the uptake and activation of long-chain and very-long-chain fatty acids and thus contributes to the generation and accumulation of C20:4 derived lipid mediators, such as PGE2. Smooth muscle action is strongly regulated by prostacyclins, and contractile impairments have been extensively observed with the production of PGE2 (43). In accordance with this hypothesis, we found that the prostacyclin was significantly elevated by lithogenic diet, but not when FATP2 was knocked down.
Importantly, loss of FATP2 function not only reduced gallbladder TAG and reduced the decline in motility associated with lithogenic diets, but also greatly protected animals from gallstone formation. By knocking out FATP2 in the gallbladder, we have discovered a unique experimental model that allows for the study of TAG-mediated gallbladder hypomotility and its contribution to cholelithiasis, independent of changes in the bile acid pool. To our knowledge, this is the first model to demonstrate that the preservation of gallbladder motility is sufficient to prevent gallstone formation, even in the presence of supersaturated bile cholesterol levels, and should facilitate the development of novel cholelithiasis treatment options.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-066336-07A2-04/14, and P30 DK-026743 (University of California San Francisco Liver Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.M.T., A.F., and A.S. conception and design of research; K.M.T., A.K.-S., H.M.P., D.A.Y., L.W., and R.F. performed experiments; K.M.T., A.K.-S., D.A.Y., K.A., and A.S. analyzed data; K.M.T., A.K.-S., K.A., and A.S. interpreted results of experiments; K.M.T. prepared figures; K.M.T. and A.S. drafted manuscript; K.M.T., A.K.-S., and A.S. edited and revised manuscript; K.M.T. and A.S. approved final version of manuscript.
REFERENCES
- 1.Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res 68: 1732–1740, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CM, Stahl A. SLC27 fatty acid transport proteins. Mol Aspect Med 34: 516–528, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behar J, Lee KY, Thompson WR, Biancani P. Gallbladder contraction in patients with pigment and cholesterol stones. Gastroenterology 97: 1479–1484, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat Med 14: 778–782, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabadak H, Cuadra AE, El-Fakahany EE, Kan B. M2, M3, and M4 muscarinic receptors are expressed in the guinea pig gallbladder. J Recept Signal Transduct Res 29: 63–66, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res 19: 945–955, 1978. [PubMed] [Google Scholar]
- 7.Carey MC, Small DM. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest 61: 998–1026, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Amaral J, Biancani P, Behar J. Excess membrane cholesterol alters human gallbladder muscle contractility and membrane fluidity. Gastroenterology 116: 678–685, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Yu P, de Petris G, Biancani P, Behar J. Distinct muscarinic receptors and signal transduction pathways in gallbladder muscle. J Pharmacol Exp Ther 273: 650–655, 1995. [PubMed] [Google Scholar]
- 10.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA. Identification of a hormonal basis for gallbladder filling. Nat Med 12: 1253–1255, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Cong P, Pricolo V, Biancani P, Behar J. Effects of cholesterol on CCK-1 receptors and caveolin-3 proteins recycling in human gallbladder muscle. Am J Physiol Gastrointest Liver Physiol 299: G742–G750, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 444: 881–887, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Doege H, Baillie RA, Ortegon AM, Tsang B, Wu Q, Punreddy S, Hirsch D, Watson N, Gimeno RE, Stahl A. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology 130: 1245–1258, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Doege H, Grimm D, Falcon A, Tsang B, Storm TA, Xu H, Ortegon AM, Kazantzis M, Kay MA, Stahl A. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem 283: 22186–22192, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doege H, Stahl A. Protein-mediated fatty acid uptake: novel insights from in vivo models. Physiology (Bethesda) 21: 259–268, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Dooley JS. Gallstones and Benign Biliary Diseases. In: Sherlock's Diseases of the Liver and Biliary System. Hoboken, NJ: Wiley-Blackwell, 2011, p. 257–293. [Google Scholar]
- 17.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology 117: 632–639, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Falcon A, Doege H, Fluitt A, Tsang B, Watson N, Kay MA, Stahl A. FATP2 is a hepatic fatty acid transporter and peroxisomal very long-chain acyl-CoA synthetase. Am J Physiol Endocrinol Metab 299: E384–E393, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginanni Corradini S, Ripani C, Della Guardia P, Giovannelli L, Elisei W, Cantafora A, Codacci Pisanelli M, Tebala GD, Nuzzo G, Corsi A, Attili AF, Capocaccia L, Ziparo V. The human gallbladder increases cholesterol solubility in bile by differential lipid absorption: a study using a new in vitro model of isolated intra-arterially perfused gallbladder. Hepatology 28: 314–322, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Goldblatt MI, Swartz-Basile DA, Al-Azzawi HH, Tran KQ, Nakeeb A, Pitt HA. Nonalcoholic fatty gallbladder disease: the influence of diet in lean and obese mice. J Gastrointest Surg 10: 193–201, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Hall JE, Guyton AC. Guyton and Hall Textbook of Medical Physiology. Philadelphia, PA: Saunders/Elsevier, 2011. [Google Scholar]
- 22.Holzbach RT, Marsh M, Olszewski M, Holan K. Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J Clin Invest 52: 1467–1479, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jennings LJ, Xu QW, Firth TA, Nelson MT, Mawe GM. Cholesterol inhibits spontaneous action potentials and calcium currents in guinea pig gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol 277: G1017–G1026, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Jonkers IJ, Smelt AH, Princen HM, Kuipers F, Romijn JA, Boverhof R, Masclee AA, Stellaard F. Fish oil increases bile acid synthesis in male patients with hypertriglyceridemia. J Nutr 136: 987–991, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Katsika D, Grjibovski A, Einarsson C, Lammert F, Lichtenstein P, Marschall HU. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology 41: 1138–1143, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Khanuja B, Cheah YC, Hunt M, Nishina PM, Wang DQ, Chen HW, Billheimer JT, Carey MC, Paigen B. Lith1, a major gene affecting cholesterol gallstone formation among inbred strains of mice. Proc Natl Acad Sci U S A 92: 7729–7733, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudo M, Khalifeh Soltani SM, Sakuma SA, McKleroy W, Lee TH, Woodruff PG, Lee JW, Huang K, Chen C, Arjomandi M, Huang X, Atabai K. Mfge8 suppresses airway hyperresponsiveness in asthma by regulating smooth muscle contraction. Proc Natl Acad Sci U S A 110: 660–665, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavoie B, Nausch B, Zane EA, Leonard MR, Balemba OB, Bartoo AC, Wilcox R, Nelson MT, Carey MC, Mawe GM. Disruption of gallbladder smooth muscle function is an early feature in the development of cholesterol gallstone disease. Neurogastroenterol Motil 24: e313–e324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MC, Yang YC, Chen YC, Huang SC. Muscarinic receptor M3 mediates human gallbladder contraction through voltage-gated Ca2+ channels and Rho kinase. Scand J Gastroenterol 48: 205–212, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Leitzmann MF, Rimm EB, Willett WC, Spiegelman D, Grodstein F, Stampfer MJ, Colditz GA, Giovannucci E. Recreational physical activity and the risk of cholecystectomy in women. N Engl J Med 341: 777–784, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Lisowski L, Dane AP, Chu K, Zhang Y, Cunningham SC, Wilson EM, Nygaard S, Grompe M, Alexander IE, Kay MA. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506: 382–386, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maclure KM, Hayes KC, Colditz GA, Stampfer MJ, Speizer FE, Willett WC. Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N Engl J Med 321: 563–569, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Mihalik SJ, Steinberg SJ, Pei Z, Park J, Kim DG, Heinzer AK, Dacremont G, Wanders RJ, Cuebas DA, Smith KD, Watkins PA. Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J Biol Chem 277: 24771–24779, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Mingrone G, Greco AV, Arcieri Mastromattei E. Free fatty acids stimulate mucin hypersecretion by rabbit gall-bladder epithelium in vitro. Clin Sci (Lond) 78: 175–180, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Nakeeb A, Comuzzie AG, Martin L, Sonnenberg GE, Swartz-Basile D, Kissebah AH, Pitt HA. Gallstones: genetics versus environment. Ann Surg 235: 842–849, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nardone G, Ferber IA, Miller LJ. The integrity of the cholecystokinin receptor gene in gallbladder disease and obesity. Hepatology 22: 1751–1753, 1995. [PubMed] [Google Scholar]
- 37.Otsuki M. Pathophysiological role of cholecystokinin in humans. Journal of gastroenterology and hepatology 15, Suppl: D71–D83, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Parkman HP, Pagano AP, Ryan JP. Subtypes of muscarinic receptors regulating gallbladder cholinergic contractions. Am J Physiol Gastrointest Liver Physiol 276: G1243–G1250, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Pauletzki J, Althaus R, Holl J, Sackmann M, Paumgartner G. Gallbladder emptying and gallstone formation: a prospective study on gallstone recurrence. Gastroenterology 111: 765–771, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Portincasa P, Di Ciaula A, Wang HH, Palasciano G, van Erpecum KJ, Moschetta A, Wang DQ. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 47: 2112–2126, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet 368: 230–239, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Reihner E, Stahlberg D. Lithogenic diet and gallstone formation in mice: integrated response of activities of regulatory enzymes in hepatic cholesterol metabolism. Br J Nutr 76: 765–772, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Ren J, Karpinski E, Benishin CG. Prostaglandin E2 contracts vascular smooth muscle and inhibits potassium currents in vascular smooth muscle cells of rat tail artery. J Pharmacol Exp Ther 275: 710–719, 1995. [PubMed] [Google Scholar]
- 44.Ruhl CE, Everhart JE. Association of diabetes, serum insulin, and C-peptide with gallbladder disease. Hepatology 31: 299–303, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Sato J, Denda M, Chang S, Elias PM, Feingold KR. Abrupt decreases in environmental humidity induce abnormalities in permeability barrier homeostasis. J Invest Dermatol 119: 900–904, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Sato N, Miyasaka K, Suzuki S, Kanai S, Ohta M, Kawanami T, Yoshida Y, Takiguchi S, Noda T, Takata Y, Funakoshi A. Lack of cholecystokinin-A receptor enhanced gallstone formation: a study in CCK-A receptor gene knockout mice. Dig Dis Sci 48: 1944–1947, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Shaffer EA. Gallstone disease: epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol 20: 981–996, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Shahid RA, Wang DQ, Fee BE, McCall SJ, Romac JM, Vigna SR, Liddle RA. Endogenous elevation of plasma cholecystokinin does not prevent gallstones. Eur J Clin Invest 45: 237–246, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smelt AH. Triglycerides and gallstone formation. Clin Chim Acta 411: 1625–1631, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell 4: 299–308, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Upp JR Jr, Nealon WH, Singh P, Fagan CJ, Jonas AS, Greeley GH Jr, Thompson JC. Correlation of cholecystokinin receptors with gallbladder contractility in patients with gallstones. Annal Surg 205: 641–648, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Erpecum KJ, van Berge Henegouwen GP, Stolk MF, Hopman WP, Jansen JB, Lamers CB. Fasting gallbladder volume, postprandial emptying and cholecystokinin release in gallstone patients and normal subjects. J Hepatol 14: 194–202, 1992. [DOI] [PubMed] [Google Scholar]
- 53.van Erpecum KJ, Wang DQ, Moschetta A, Ferri D, Svelto M, Portincasa P, Hendrickx JJ, Schipper M, Calamita G. Gallbladder histopathology during murine gallstone formation: relation to motility and concentrating function. J Lipid Res 47: 32–41, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Venneman NG, van Erpecum KJ. Pathogenesis of gallstones. Gastroenterol Clin North Am 39171–183, vii, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Wang HH, Wang DQ. Reduced susceptibility to cholesterol gallstone formation in mice that do not produce apolipoprotein B48 in the intestine. Hepatology 42: 894–904, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Wittenburg H, Lammert F. Genetic predisposition to gallbladder stones. Semin Liver Dis 27: 109–121, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Wu MK, Hyogo H, Yadav SK, Novikoff PM, Cohen DE. Impaired response of biliary lipid secretion to a lithogenic diet in phosphatidylcholine transfer protein-deficient mice. J Lipid Res 46: 422–431, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Wu Q, Ortegon AM, Tsang B, Doege H, Feingold KR, Stahl A. FATP1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol Cell Biol 26: 3455–3467, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao Z, Schmitz F, Pricolo VE, Biancani P, Behar J. Role of caveolae in the pathogenesis of cholesterol-induced gallbladder muscle hypomotility. Am J Physiol Gastrointest Liver Physiol 292: G1641–G1649, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Xiao ZL, Biancani P, Carey MC, Behar J. Hydrophilic but not hydrophobic bile acids prevent gallbladder muscle dysfunction in acute cholecystitis. Hepatology 37: 1442–1450, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Xiao ZL, Rho AK, Biancani P, Behar J. Effects of bile acids on the muscle functions of guinea pig gallbladder. Am J Physiol Gastrointest Liver Physiol 283: G87–G94, 2002. [DOI] [PubMed] [Google Scholar]
- 62.Zhu J, Han TQ, Chen S, Jiang Y, Zhang SD. Gallbladder motor function, plasma cholecystokinin and cholecystokinin receptor of gallbladder in cholesterol stone patients. World J Gastroenterol 11: 1685–1689, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]