Abstract
Dietary lipids are transported from the intestine through contractile lymphatics. Chronic lipid loads can adversely affect lymphatic function. However, the acute lymphatic pump response in the mesentery to a postprandial lipid meal has gone unexplored. In this study, we used the rat mesenteric collecting vessel as an in vivo model to quantify the effect of lipoproteins on vessel function. Lipid load was continuously monitored by using the intensity of a fluorescent fatty-acid analog, which we infused along with a fat emulsion through a duodenal cannula. The vessel contractility was simultaneously quantified. We demonstrated for the first time that collecting lymphatic vessels respond to an acute lipid load by reducing pump function. High lipid levels decreased contraction frequency and amplitude. We also showed a strong tonic response through a reduction in the end-diastolic and systolic diameters. We further characterized the changes in flow rate and viscosity and showed that both increase postprandially. In addition, shear-mediated Ca2+ signaling in lymphatic endothelial cells differed when cultured with lipoproteins. Together these results show that the in vivo response could be both shear and lipid mediated and provide the first evidence that high postprandial lipid has an immediate negative effect on lymphatic function even in the acute setting.
Keywords: chylomicron, lipid absorption, lymphatic, pump function, viscosity
nearly all dietary lipids enter the venous circulation via the lymphatics (57), which transport chylomicrons from the villi of the small intestine through the lymphatic vessel network to the thoracic duct where lymph is emptied into the blood via the left subclavian vein (24, 40, 58, 73). There is a rapid increase in triglyceride (TG) content in lymph after a lipid-rich meal (74), and how lymphatics respond to functionally handle this increased lipid load remains unclear. Lymph is drained through the intestinal lymphatics in large part through the intrinsic pumping activity of the collecting vessels and one-way valves, which prevent backflow (22, 34). The collecting lymphatic vessels contain lymphatic smooth muscle cells that cause phasic and tonic contractions (38, 79). The collecting vessels' contractility result in a pumping mechanism that provides an active transport system to move lymph from peripheral tissue into venous circulation. It has been shown that this pumping mechanism can be modulated by a variety of substances including nitric oxide (NO) (60, 64), histamine (56), prostaglandins (55, 72), and hormones (66), many of which can be mechanically regulated to alter pump function in response to intraluminal pressure (19) and wall shear stress (28). The pump response of collecting lymphatics in the mesentery after a lipid meal occurs in the context of both a highly dynamic biomechanical and biological microenvironment.
The lymphatic vasculature has recently been implicated in a variety of lipid-related pathologies (24, 36, 83). Several studies have shown strong correlations between obesity, high levels of circulating lipoproteins, and lymphatic dysfunction (4, 10, 16, 32, 33, 35, 37, 39, 52, 54, 61, 63, 65, 77, 82, 84). There has been a growing interest lately in the role that lymphatics play in the development and progression of lipid-related diseases and in quantitatively describing the evidently strong interplay between lipids and lymphatic vessel structural and functional behavior (24, 83). Although recent studies provide significant evidence that lymphatic function and the local lipid environment are highly influenced by one another, it is less clear how the main collecting lymphatics that drain the lipid-rich lymph of the intestine functionally respond to the rapid increase in lipid load that occurs in response to a lipid-rich meal. Given the regional heterogeneity of lymphatic pump performance (28, 30), knowledge of the contractile response to lipid exposure in the lymphatic tissue bed that encounters the largest potential lipid load will provide a framework for understanding the global lymphatic response to pathological lipid levels. Although it is well established that circulating lipoproteins interact with the vascular endothelium to elicit endothelium-mediated vascular responses that have been attributed to vascular disease (69, 75), it is not known whether such interactions also occur within the lymphatic endothelium and whether they cause measurable changes in the pump function of the vessel that could result in pathologically low lymph transport capacity. Previously, we reported the development of an intravital imaging system capable of simultaneously tracking lymphatic pump function and lipid load in vivo in the rat mesentery (45). In this study we utilized this system to quantify the phasic and tonic contractile response of mesenteric prenodal lymphatics as they transitioned from a state of fasting to a postprandial lipid load. We hypothesized that as lipid concentration in the mesenteric vessel increases postprandially, the vessel will functionally respond to acute lipid exposure in a manner that is similar to pathological nonmesenteric vessels and exhibit a decrease in intrinsic lymphatic pump function. To determine whether the responses seen postprandially were shear mediated, we further quantified mesenteric duct lymph flow rates and lymph viscosity and reported the first measurements of transient postprandial lymph viscosity values. To further investigate the potential of an endothelial mediated response we quantified intracellular Ca2+ in response to shear stress following incubation of lymphatic endothelial cells with lipoproteins.
MATERIALS AND METHODS
Animal model and ethical approval.
Male Sprague-Dawley rats weighing 180–280 g (Charles River, Wilmington, MA) were chosen to facilitate comparative studies of lymphatic contractility to previous studies performed on the same strain. Both lipid (n = 8) and saline (n = 6) groups were provided with the same standard chow diet. Rats were fasted for 48 h before each experiment while water was available ad libitum. One sugar cube was provided per rat the day after fasting began. All experiments were carried out under general isoflurane anesthesia and animals were continuously monitored for signs of distress. Internal body temperature was maintained at 37–38°C with a feedback-controlled setup. Following the experimental procedure rats were euthanized with a cut in the diaphragm while still under anesthesia. All animal procedures were approved by the Georgia Institute of Technology Internal Animal Care and Use Committee (IACUC) and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preimaging surgical preparation.
After a surgical area around the abdominal cavity was shaved, a 2-cm incision was made at the midline starting 1 cm below the xiphoid process. The stomach was located and gently moved to the outside of the abdomen to expose the duodenum. A small incision was then made in the duodenum with a surgical scalpel and a small silicone tube was inserted into the incision and fixed to the outside of the duodenum with topical tissue adhesive (GLUture, Abbott, Worcester, MA). The stomach and duodenum were then placed back into the abdominal cavity. A single surgical suture was used to reduce the opening of the abdominal incision to ∼1 cm. A segment of the small intestine distal to the duodenum was exteriorized and stabilized in a groove between two acrylic plates, thus exposing the mesentery over an imaging window covered with a glass slide. An albumin physiological salt solution (APSS; in mM: 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 sodium pyruvate, 0.02 EDTA, 3.0 MOPS, and 1 g/l BSA) (all reagents from Sigma, St. Louis, MO) with pH adjusted to 7.4 ± 0.1 at 38°C was temperature controlled to 36–39°C and flowed at a rate of 21 ml/min to bathe the mesentery. A total of 1 liter of APSS was recirculated for each rat throughout the experiment. The APSS bath recapitulated the oncotic extracellular environment found around the mesentery. The temperature of the rat was monitored and recorded with a rectal thermometer (Kent Scientific, Torrington, CT). Internal body temperature was maintained at 37–38°C via a feedback control mechanism by continuously monitoring the rat with a rectal probe and automatically adjusting a circulating water bath that flowed warmed water through tubing integrated within the custom-designed surgical board. A lymphatic vessel was then located and placed over the imaging window. The vessel was given 10 min to equilibrate under the given conditions and then imaging began.
A lipid solution containing Intralipid-20% fat emulsion (30% of total volume, Sigma), oleic acid (0.89 mg/ml, Sigma), saline (0.9% NaCl, 70% of total volume), and BODIPY C16 (40 μg/ml) (Life Technologies, Grand Island, NY) was infused through the duodenal cannula at a flow rate of 5 ml/h. The infusion was stopped when the solution reached the part of the small intestine that was being imaged (evident by the intestinal segment turning white and a noticeable distension). For the saline control group, saline was infused at the same flow rate as the lipid solution and infusion stopped when the intestine appeared relatively distended. The color of the intestine remained the same owing to the fact that saline, unlike the lipid emulsion, was clear and not white. Image acquisition was carried out for an average of 90 min for both groups.
In vivo imaging and processing of mesenteric lymphatic vessel function.
A dual-channel optical imaging system and customized image-processing algorithms were used to acquire both high-speed video and fluorescence intensity to simultaneously track lymphatic contraction and lymph lipid levels as described previously (45). The high-speed video was captured at a frame rate of 250 fps and the fluorescence images at 0.2 fps (i.e., 1 frame every 5 s). Continuous 1-min video sequences were captured at 1-min intervals (i.e., capturing a minute of video every other minute). Following acquisition, videos were digitally stabilized and vessel diameter tracings were obtained. Mean fluorescence intensity was also quantified following image stabilization. (Supplemental Material for this article is available online at the Journal website.)
Mesenteric duct lymph collection.
Male Sprague-Dawley rats (weighing ∼300 g) (Harlan Laboratories, Indianapolis, IN) were housed individually and maintained in a temperature and humidity controlled facility, on a 12-h light/dark cycle. Animals had free access to water and standard chow (Harlan Teklad 7012 Mouse/Rat Sterilizable Diet, Harlan Laboratories, Indianapolis, IN) prior to all procedures. All animal procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Prior to placement of lymph cannulas, the animals were fasted overnight with free access to water. Under isoflurane anesthesia, a midline incision was made and a cannula (polyvinyl chloride tubing, 0.5-mm inner diameter, 0.8-mm outer diameter, Tyco Electronics, Castle Hill, Australia) was placed in the major mesenteric lymphatic duct as described by Bollman et al. (5). The lymphatic cannula was secured with cyanoacrylate glue (Krazy Glue, Columbus, OH). A silicone feeding tube (1.02-mm inner diameter, 2.16-mm outer diameter, VWR International, West Chester, PA) was introduced into the stomach and advanced slightly beyond the pylorus into the duodenum. The feeding tube was secured with a purse-string ligature in the stomach. Both the lymph cannula and the duodenal feeding tube were exteriorized through the right flank; the abdomen was then closed in two layers. After surgery, the animals were placed in Bollman restraint cages and allowed to recover overnight (18 h). The animals were kept in a temperature-regulated chamber (24°C) to prevent hypothermia. To compensate for fluid and electrolyte loss due to lymphatic drainage, a 5% glucose-saline solution was infused into the duodenum at 3 ml/h for 6–7 h, followed by an overnight infusion of saline only at 3 ml/h.
Following overnight recovery, fasting lymph was collected for 1 h prior to a 3-ml duodenal bolus of lipid (2.2 ml Liposyn II and 0.8 ml saline). At 30 min following the bolus, a 0.9% saline infusion was provided at 3 ml/h for the remainder of the study period. Lymph samples were continuously collected on ice every 15 min for 4 h. The anticoagulant-containing samples were treated in the same manner except upon collection a cocktail of 10% by volume of an antiproteolytic cocktail (0.25 M EDTA, 0.80 mg/ml aprotinin and 80 U/ml heparin) was added to each collection tube.
Triglyceride and viscosity measurements of mesenteric lymph.
Lymphatic TG concentrations were determined by use of a commercially available kit (Randox TG, Randox Laboratories, Crumlin, Northern Ireland, UK). Lymph samples were shipped on ice overnight from Cincinnati, OH to Atlanta, GA for viscosity measurements. Fluorescent carboxylate-modified polystyrene (PS) FluoSpheres (Molecular Probes) with 1.0-μm diameters and 3% polydispersity were used; Pluronics F-127 (EO100PO70EO100, molecular weight ∼12,600) from Sigma Chemicals was used without further purification for surface modification of the PS particles to prevent nonspecific interactions between the lymph and the tracer particles (46). Samples were prepared by combining 1.5 μl of tracer particle suspension with 50 μl of lymph and gently mixing the samples by repeated loading and dispensing of the pipette. The samples were then loaded into ∼100-μm-thick sample chambers, which were created by placing parafilm spacers between a microscope slide and coverslip and sealed with vacuum grease to prevent evaporation. The samples were placed on the Peltier-controlled thermal microscope stage (PE100-LI2, Linkam Scientific Instruments) that was used for effective temperature control of the sample during measurements; the sample temperature was carefully monitored via a thermocouple (HH11B, Omega) that was mounted inside the sample chamber.
Viscosity information of the samples was obtained by performing statistical analysis of the mobility of colloidal tracer particles via particle tracking video microscopy (PTVM) (17). The Brownian motion of fluorescent tracer particles in the samples was monitored at 38°C (rat internal body temperature) via an optical microscope (Leica DM-IRB) with a ×63 objective, and movies were captured by using a CCD camera (Cohu 4920, Poway, CA; 30 fps and 640 × 480 pixel resolution). Subsequently the recorded movies were analyzed with software developed with Interactive Data Language (ITT Visual Information Solutions, Boulder, CO). Because Brownian motion leads to small particle displacements on these time scales and is highly sensitive to external vibrational noise, all experiments were performed on a vibration-isolated optical table. After obtaining video images, we utilized a standard brightness-weighted centroid method to identify the particle trajectories. The method uses four major steps: restoring the image, locating possible particle centers, refining particle positions/eliminating unwanted particles, and linking particle positions in subsequent frames into trajectories (8). A control sample with well-documented viscosity (deionized water at 38°C) was used to confirm the accuracy of our protocol.
Through the Einstein-Stokes relation it is possible to relate the mean squared displacement (MSD) of the particles during the time interval τ and the viscosity of the fluid by using the following expression:
| (1) |
where d is the dimensionality of the trajectories (typically d = 2 in PTVM), kB is Boltzman's constant, T is the absolute temperature, η is the viscosity, and a is the radius of the particle. Therefore, a linear relationship is expected between MSD and τ with the slope, which can be obtained from data fitting of MSD measurements as a function of τ, being:
| (2) |
Solving for the viscosity, η, yields the following relation:
| (3) |
Lymphatic endothelial cell culture.
Human dermal microvascular endothelial neonatal lymphatic cells (HMVEC-dlyNeo, Lonza, New York, NY) were cultured in T25 polystyrene flasks at a seeding density of 5,000 cells/cm2. Flasks were coated for 1 h at room temperature with a collagen solution containing type I rat tail collagen (BD Biosciences, San Jose, CA) at a concentration of 50 μg/ml in 0.1% acetic acid (Sigma). The cells were grown in EBM-2 supplemented with the EGM-2 BulletKit (both from Lonza). Cells in the flasks were grown to passage 6 and trypsinized at 60–90% confluency preceding seeding in the flow chambers. Cells used for all experiments were at passage 7 within the flow chambers.
Flow chamber setup and Fluo-4 dye loading.
To impose flow, cells were seeded in a polystyrene-based flow chamber measuring 3.8 mm in width and 0.4 mm in height (μ-Slide VI 0.4 ibiTreat, IBIDI, Munich, Germany). The flow chambers were coated with the same collagen solution as above for 1 h. Cells were then seeded at a density of 20,000 cells/cm2 and given 48 h to reach full confluency. Experiments were carried out 48 h postseeding. EBM-2 culture media was replenished at 24 h. For the case of the VLDL-treated group, cells were incubated for 24 h with 10 mg/ml TG content (in EBM-2) from human plasma (Lee Biosolutions, St. Louis, MO). Immediately before the start of an experiment, cells within the flow chambers were rinsed 2× with prewarmed serum-free DMEM-F12 (Life Technologies). DMEM-F12 with HEPES was used for pH stability at room temperature and CO2 levels. In addition, because of autofluorescence of phenol red in the green channel, the medium chosen was phenol red free. All uses of DMEM-F12 in this study were serum free. To image intracellular calcium dynamics, Fluo-4 AM (Life Technologies) with a final concentration of 10 μM in DMEM-F12 as a buffer was incubated with the cells for 30 min at 37°C. Cells were then washed 2× with DMEM-F12 at room temperature and incubated in the same medium for 20 min at room temperature to allow complete deesterification of the AM esters.
Shear-induced intracellular Ca2+ measurements.
A custom-built LabVIEW virtual instrument was created to control a 12-roller Ismatec REGLO Digital MS-4/12 peristaltic pump (IDEX Health and Science, Glattbrugg, Switzerland) by using RS-232 commands sent at a sampling period of 200 ms to the pump in an approach similar to that previously published (48). The program has the capability of imposing any arbitrary flow waveform. For this study an upward ramp going from 0 to 4 dyn/cm2 was used. The stimulus lasted for 1 min. Imaging was carried out on a Zeiss Axioobserver inverted microscope (Carl Zeiss Microimaging, Jena, Germany) with a ×20 objective. A back-illuminated CCD camera (PIXIS 1024B, Princeton Instruments, Trenton, NJ) was used to acquire fluorescent images with 500-ms integration time at 1-s intervals. A mercury lamp source set at 20% intensity (X-Cite, Lumen Dynamics, Mississauga, ON, Canada) was used to continuously excite the Fluo-4 dye. Fluorescence intensity was quantified using ImageJ by drawing a small region of interest (ROI) inside the nucleus of each cell. For each field of view, 40–45 cells were randomly chosen for quantification. An ROI over an area clear of cells was used to correct for background fluorescence for each image individually. The output metric used (F/F0) represents the fluorescence signal, F, divided by the average fluorescence for all images preceding the stimulus, F0. Images were saved and analyzed as 16-bit TIFFs. All image acquisition was carried out in Micro-Manager (26). All Ca2+ experiments were carried out within a temperature-controlled incubator at 27°C because Fluo-4 showed very high leakage rates out of lymphatic endothelial cells at 37°C, making it extremely difficult to run these studies at physiological temperatures. We captured 30 s of baseline (F0) before stimulating the cells.
Statistics.
Eight rats were used for the lipid-infused group and six for the saline controls. For each rat a single vessel was imaged. The Pearson correlation coefficient was used as a correlation index. Data for the in vivo lipid treatment study were not normally distributed as determined by a D'Agostino and Pearson omnibus normality test; thus a nonparametric one-way ANOVA with Kruskal-Wallis test of comparison was used. ANOVA was followed by correction for multiple comparisons by using a Dunn's test. In the case of the high lipid load segments only four rats/vessels reached this threshold. When comparing the percent changes between the saline and lipid groups multiple unpaired t-tests were run and the Holm-Sidak method was used to correct for multiple comparisons. Six independent Ca2+ imaging experiments were carried out for each group. For each experiment, 40–45 randomly chosen cells within the field of view were quantified to give an average for that experiment. The fluorescence intensity signal representing intracellular Ca2+ was divided into upstroke and downstroke regions. The upstroke was fitted to an exponential growth function while the downstroke was fitted to an exponential decay function. All results are reported as means ± standard deviation unless otherwise noted. For all tests, statistical significance was defined as P ≤ 0.05. All statistical analyses were performed with GraphPad Prism v6 (GraphPad Software, La Jolla, CA).
RESULTS
Imaging vessel contractile behavior in response to lipid load.
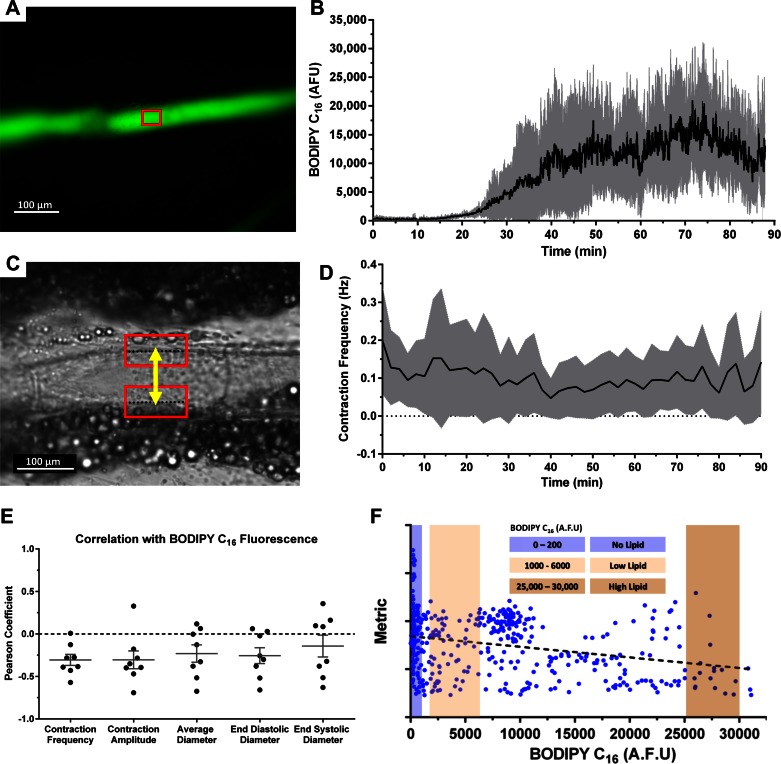
By imaging the fluorescence emission of BODIPY C16 in a mesenteric vessel (Fig. 1A), we were able to track the lipid uptake dynamics of a vessel after lipid arrival in the corresponding intestinal segment drained by that vessel (Fig. 1B). Previously, we demonstrated that the kinetics of BODIPY C16 into lymph closely follows that of TG, making it a suitable reporter for tracking lymph TG levels over time (45). Lipid is first detected in the collecting vessel immediately draining the intestine within 15 min of appearing in the corresponding intestinal segment. This detection was followed by a rapid rise in lymph lipid level that peaked between 60 and 80 min postarrival of the lipid in the intestinal loop section being imaged. The high-speed bright-field video provided a clear view of the mesenteric vessel walls and was used to quantify diameter changes over time for every 1-min interval (Fig. 1C), allowing us to extract and quantify various parameters of vessel pump function including contraction frequency (Fig. 1D), contraction amplitude, average diameter, and end-diastolic/systolic diameters. Calculating the Pearson correlation coefficient between the relative lipid concentration over a 1-min interval and the respective pump function parameter over that same time interval suggested that increases in lipid load resulted in a decrease in the contractile activity of the lymphatic vessel [correlations ranged between −0.15 and −0.30 (Fig. 1E)]. To analyze this in more detail, we utilized the BODIPY fluorescence intensity signal and segmented the experimental data into three discrete groups [1) no lipid, 2) low lipid, and 3) high lipid (Fig. 1F)] and calculated vessel pump functional parameters for each of these loads. The fluorescence interval that we chose to represent each of these groups was chosen as to have extreme lipid values, noting that only four vessels reached the extreme high lipid loads. For the purpose of statistical analysis, the data points from all rats were pooled and each 1-min data segment with its corresponding lipid content was treated as a data point.
Fig. 1.
Simultaneous high-speed video and fluorescence acquisition provides the ability to assess the effect of a lipid load on lymphatic pump function. A: fluorescent image of a rat mesenteric prenodal collecting lymphatic vessel. BODIPY C16 is used as a fluorescent indicator for TG concentration within the vessel. The red window indicates a typical region in which fluorescence intensity was quantified following image stabilization to remove motion artifacts. B: BODIPY C16 fluorescence intensity in the vessel over time following duodenal infusion of a fat emulsion, Intralipid, along with BODIPY C16. C: a single bright-field frame from a video sequence taken at 250 fps. The lymphatic vessel wall can be clearly seen and is typically surrounded by adipocytes. The red boxes represent a region of interest (ROI) around each wall that was tracked by cross-correlation. The distance measurement, yellow line, provided diameter tracings that were used to quantify various functional parameters such as contraction frequency (D). E: the Pearson correlation coefficient calculated for each metric as a function of BODIPY C16 fluorescence. Negative correlations were observed for all 5 metrics in a majority of the rats. F: a representative distribution for a certain metric (average diameter shown in this case) as a function of BODIPY fluorescence. Three discrete segments were chosen to represent cases where there was no lipid in the vessel, low lipid, and high lipid; n = 8, gray error bands and bars represent SD. AFU, arbitrary fluorescence units.
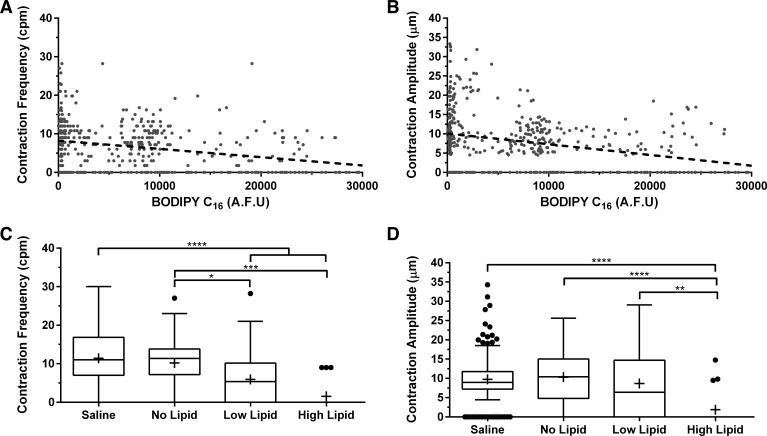
Contraction frequency and amplitude (phasic response) decrease in response to lipid.
Utilizing the obtained diameter tracings, we quantified two key parameters describing the phasic response of the lymphatic vessel, contraction frequency and contraction amplitude, and showed that they both decrease with an increase in lipid load. Contraction frequency showed a slightly negative correlation with BODIPY concentration, R2 = 0.1 (Fig. 2A). The points where contraction frequency is zero represented video segments in which the vessel did not physically contract within the 1-min video segment being quantified. The contraction amplitude also exhibited a similar negative correlation, R2 = 0.1 (Fig. 2B). As described earlier we further divided the lipid load into 1) no load, 2) low lipid, and 3) high lipid. Contraction frequency was inversely related to lipid load and decreased from an average of 10.2 ± 6.9 cpm when no lipid was present in the vessel to 1.8 ± 3.4 cpm with a high lipid load (P = 0.0001), with several vessels exhibiting no contraction at that load. Even low lipid loads caused a significant decrease in contraction frequency compared with no lipid (P = 0.0174). There was no difference between the contraction frequency of vessels prior to lipid uptake compared with vessels draining from the intestines that were infused with saline (P > 0.9999) (Fig. 2C). Contraction amplitude averaged ∼10.0 ± 4.9 μm in saline-infused animals and 10.3 ± 7.4 μm in lipid-infused animals when there was no lipid in the vessel, whereas in vessels with high lipid loads the contraction amplitude significantly decreased fivefold to 1.9 ± 4.5 μm (P < 0.0001). Taken together these results suggest a reduction in the phasic contractility of rat mesenteric lymphatics after an intraduodenal infusion of lipid.
Fig. 2.
Mesenteric lymphatic vessels exhibit a decrease in their phasic response as evident by a decrease in both contraction frequency and amplitude. A: contraction frequency decreased as a function of BODIPY C16 fluorescence (R2 = 0.1). B: contraction amplitude also decreased (R2 = 0.1). C: contraction frequency exhibited a lipid load-dependent effect where it decreased from 10.2 ± 6.9 cpm when no lipid was present to 1.8 ± 3.4 cpm with the highest lipid load (P = 0.0001). D: contraction amplitude also showed a similar dependency on lipid load where it decreased from 10 ± 4.9 μm under no lipid load to 1.9 ± 4.5 μm (P < 0.0001) under the high load; n = 8, error bars represent SD. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
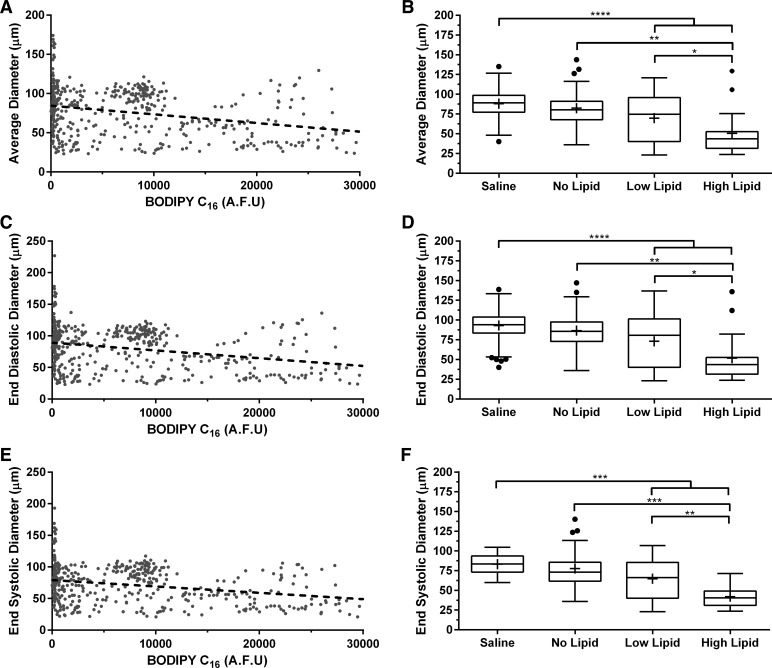
Average and end-diastolic/systolic diameters (tonic response) also decrease in response to lipid.
In addition to a phasic contractile response, lymphatics are also known to exhibit a tonic contractile response with different underlying molecular mechanisms regulating the phasic and tonic responses (78). To track changes in vessel tone over time, we quantified three parameters that are most reflective of the tonic response that have been reported in isolated, perfused lymphatic vessels: average diameter, end-diastolic diameter, and end-systolic diameter. All three parameters decrease with an increase in lipid load, suggesting a measurable tonic response of the vessel to an infusion of lipid (Fig. 3, A, C, and E). The average diameter decreased from 82 ± 25 to ∼50 ± 27 μm under high lipid vs. no lipid (P = 0.0088). There was no statistical difference between no lipid and the saline control group (P = 0.2940) (Fig. 3B). Following a similar trend to the average diameter, the end-diastolic diameter decreased from 86 ± 26 to 52 ± 30 μm under high lipid (P = 0.0096). There was no difference in average end-diastolic diameter between the no lipid and saline controls (P = 0.2843) (Fig. 3D). The end-systolic diameter decreased from 78 ± 24 to 42 ± 12 μm under high lipid (P = 0.0001). Similar to the end-diastolic diameter, there was no difference between the no lipid and saline controls (P = 0.5439) (Fig. 3F).
Fig. 3.
Mesenteric lymphatic vessels exhibit a decrease in their tonic response as evident by a decrease in both average and end-diastolic/systolic diameters. A: average diameter decreased as a function of BODIPY C16 fluorescence (R2 = 0.1). C and E: end-diastolic and end-systolic diameters also decreased (R2 = 0.1). B: average diameter exhibited a lipid load-dependent effect where it decreased from 82 ± 25 μm when no lipid was present to 50 ± 27 μm with the highest lipid load (P = 0.0096). D: end-diastolic diameter also showed a similar dependency on lipid load where it decreased from 86 ± 26 μm under no load to 52 ± 30 μm (P = 0.0096) under the high load. F: end-systolic diameter decreased from 78 ± 24 to 42 ± 12 μm under high lipid (P = 0.0001); n = 8, error bars represent SD. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
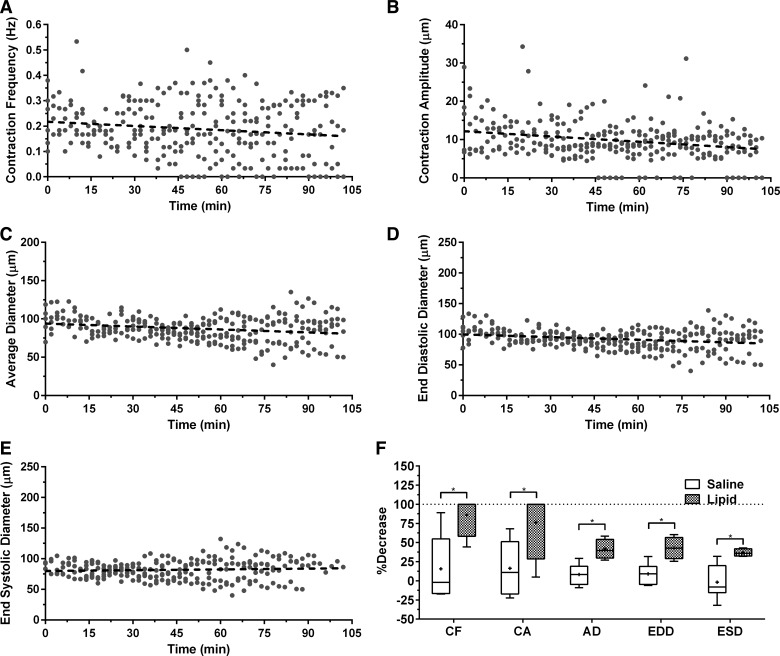
Lymphatics maintain constant pump function throughout imaging procedure.
Since the rat is maintained under anesthesia and the mesenteric vessels are exposed to imaging for over 2 h, there was some concern that loss of contractile activity could occur over time and thus result in the reduction of pump function that was observed during periods of high lipid vessel content. Thus we tested various anesthesia protocols including ketamine/xylazine, fentanyl/droperidol, and isoflurane. For all of our experiments we used isoflurane. We chose isoflurane because other anesthesia significantly reduced intestinal peristalsis and caused the lipid solution to not reach the area of the intestine we were imaging within any reasonable amount of time (data not shown). Intestinal peristalsis is also important for the digestive and absorptive process of lipid and hence inhibiting that will greatly affect the process. The contraction frequency we obtained for the control rats (under isoflurane) is similar to those reported in previous studies using a different anesthesia consisting of fentanyl/droperidol cocktail (84). All animals were kept under a constant plane of anesthesia throughout all procedures. In addition to anesthesia concerns, the mesenteric bed becomes stretched because of the significant distention of the intestinal wall as the bolus of lipid arrives, which could also potentially alter contractile function since lymphatic vessels are likely highly sensitive to axial stretch. To account for these effects, we designated a group of control rats that underwent the same procedure except they were given an equal volume infusion of saline instead of lipid. The contraction frequency and amplitude showed a very modest decrease over time in the saline control rats (Fig. 4, A and B), but comparing the phasic response metrics at the same interval in time when we typically see the high lipid load in the lipid-infused rats showed that the percentage decrease for both metrics was significantly higher in the lipid group. Specifically, there was an 86% decrease in contraction frequency compared with 16% in controls (P = 0.019) and 76% decrease in contraction amplitude compared with 16% in controls (P = 0.049) (Fig. 4F). The average, end-diastolic, and end-systolic diameters also showed a slight decrease in the saline control rats (Fig. 4, C–E), but comparing the tonic response metrics at the same interval in time when we typically see the high lipid load in the lipid-infused rats showed that the percentage decrease for all three metrics was significantly higher in the lipid group. Specifically, there was a 41% decrease in average diameter compared with 8% in controls (P = 0.005), a 43% decrease in end-diastolic diameter compared with 9% in controls (P = 0.006), and a 37% decrease in end-systolic diameter compared with an actual increase of 3% in controls (P = 0.01) (Fig. 4F). All this suggests that the phasic and tonic responses observed in the presence of high lipid loads are not an indirect result of the infusion procedure itself.
Fig. 4.
Rats infused with saline show minimal decrease in phasic and tonic response. Control rats were infused with saline only instead of the lipid emulsion. Contraction frequency (R2 = 0.02) (A), contraction amplitude (R2 = 0.07) (B), average diameter (R2 = 0.05) (C), end-diastolic diameter (R2 = 0.06) (D), and end-systolic diameter (R2 = 0.005) (E) showed minimal decrease over time. F: contraction frequency showed a much higher percentage decrease in the lipid-infused rats than in the controls (86 vs. 16%, P = 0.019), as did contraction amplitude (76 vs. 16%, P = 0.049), average diameter (41 vs. 8%, P = 0.005), end-diastolic diameter (43 vs. 9%, P = 0.006) and end-systolic diameter (37 vs. an increase of 3%, P = 0.01); n = 6, error bars represent SE. *P ≤ 0.05. CF, contraction frequency; CA, contraction amplitude; AD, average diameter; EDD, end-diastolic diameter; ESD, end-systolic diameter.
Lymph flow and viscosity increase with lipid uptake.
Although we sought to measure flow rates in the vessel utilizing our previously published approach (21, 45), we were only able to accomplish this in the control rats where the average flow rate for the 120-min period was 19.43 ± 13.45 μl/h (Fig. 5A) with high variability among individual rats (Fig. 5B) but no evident changing trend. These measurements were not possible in the lipid-infused rats because of the low contrast between lymphocytes and the surrounding lymph. This low contrast was most likely due to the dramatic increase in chylomicron concentration, which acted as light scattering agents (note, lymph turns milky white after a high-fat meal) (71). Therefore, we utilized a lymph collection technique to determine flow rate changes from the intestine in response to our lipid meal by cannulating the mesenteric duct on a set of different lipid-infused rats (see materials and methods).
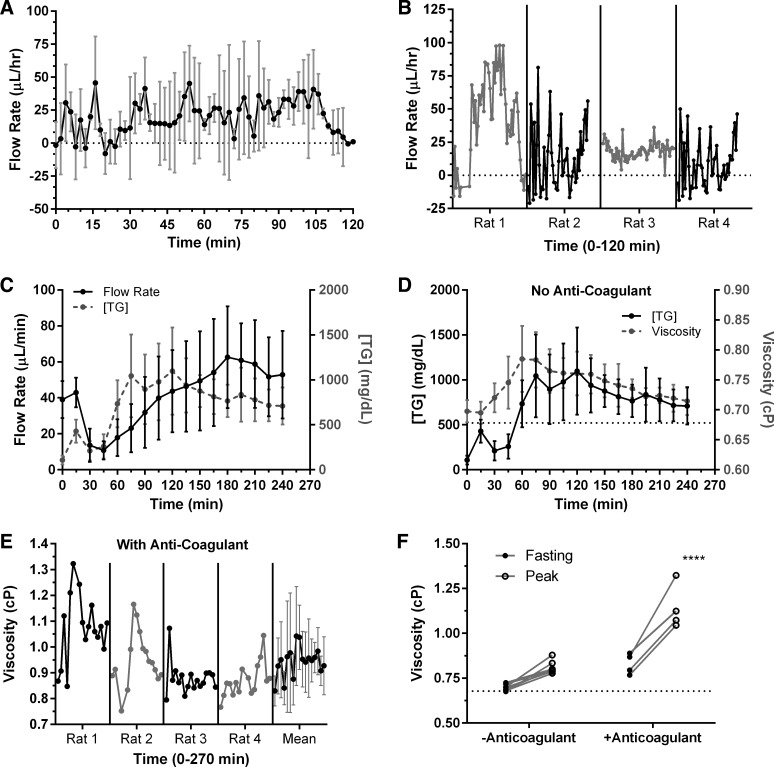
Fig. 5.
Measuring changes in flow rate, viscosity, and TG content postprandially. A: flow rate for the control rats can be measured thanks to the fact that there is large inherent contrast between lymphocytes and the surrounding lymph. Similar measurements cannot be made, however, on the lipid-infused rats. Flow rate in control rats (infused with saline) does not significantly change upon infusion; n = 5, error bars represent SD. B: a more detailed view of A where each rat is plotted individually to show the large intra-animal variability. C: mesenteric duct flow rate and lymph TG both increase upon duodenal lipid infusion. D: viscosity increases with an increase in lipid content. E: average lymph viscosity when an anticoagulant is added to the samples is ∼1.04 ± 0.2 cP with an average peak of ∼1.14 ± 0.13 cP. F: peak viscosity increases after lipid infusion compared with fasting lymph.
The mesenteric duct lymph flow rate varied with different rats. The typical trend showed an initial decrease in flow rate from 39 ± 10 to 13.6 ± 9 μl/min after 30 min from the start of lymph collection and then began a gradual increase to a peak of 63 ± 28 μl/min at ∼180 min. Flow rate did not return to baseline value within the 4-h data collection period (Fig. 5C). To determine the transient changes in viscosity following a high-fat meal, TG concentration and lymph flow rate samples were collected at 15-min intervals for up to 4 h following the start of duodenal lipid infusion. The average lymph TG concentration ranged from 107 ± 48 mg/dl at the start of lymph collection to 709 ± 205 mg/dl at 4 h with a peak mean of 1,098 ± 487 mg/dl occurring at 2 h. TG concentration increased relatively steeply up to 2 h and then started a gradual decline (Fig. 5C). The average lymph viscosity ranged from 0.70 ± 0.02 cP at the start of lymph collection to 0.71 ± 0.02 cP at 4 h with a peak mean of 0.79 ± 0.05 cP occurring at 1 h. Viscosity values followed a similar trend to TG concentration where they increased steeply up to 1 h and then began a gradual decline (Fig. 5D). TG concentration did not return to baseline fasting values within the 4-h data collection interval.
Lymph has been shown to contain small amounts of coagulating factors that tend to promote coagulation upon collection and thus affect viscosity (12, 27). So we sought to quantify the changes in viscosity that might result from a modified procedure through the addition of an anticoagulant. We found that in one of the rats the viscosity peak was as high as 1.32 cP (Fig. 5E) whereas the average lymph viscosity for all rats combined peaked at ∼1.04 cP ± 0.2 compared with 0.79 ± 0.05 cP when no anticoagulant was present in the samples. The mean fasting and maximum viscosities were 0.83 ± 0.06 and 1.14 ± 0.13 cP, respectively (Fig. 5F).
Shear sensitivity of lipid-conditioned lymphatic endothelial cells.
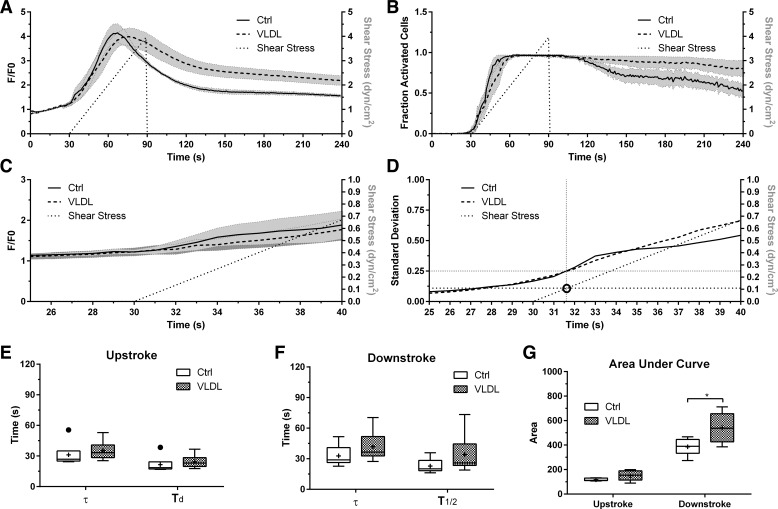
Calcium has been shown to be a secondary messenger in lymphatic endothelial cells (LECs) upon activation with wall shear stress (WSS) (41). To test whether exposure to lipoproteins affected the cells' calcium response to WSS, we incubated lymphatic endothelial cells with VLDL at 10 mg/ml TG concentration (typical elevated levels occurring in rat mesenteric lymph after a high-fat meal, Fig. 5C) for 24 h. To determine what shear stress level activated the calcium response we used a ramp profile going from 0 to 4 dyn/cm2 over a 1-min period and quantified Fluo-4 fluorescence for both control and VLDL-treated conditions. The shear stimulus was then stopped and imaging continued for an additional 2.5 min to capture the kinetics of intracellular calcium decay. Lipid exposure resulted in a slower decay of the intracellular calcium response mostly due to higher average intensity per cell before the 120-s mark and a higher number of cells remaining in their “activated” state after that (Fig. 6, A and B). Looking more closely at the time frame when we start seeing an increase in fluorescence there was no difference between both conditions (Fig. 6C). We calculated the moving standard deviation over time, where the standard deviation is that of the cell population within one experiment (i.e., FOV). We defined a standard deviation of 0.25 as indicating a response by the cells (the higher the SD the more cells are activated within the FOV). With this we were able to determine that for both conditions a shear stress value of 0.11 dyn/cm2 elicited a response, which is well within the physiological range of WSS reported in mesenteric lymphatics (22, 45) (Fig. 6D). We further wanted to quantify the fluorescence intensity signal and divided the typical intensity profile, such as in Fig. 6A, into a upstroke and downstroke region in the similar approach to a previous study (41). We fit the upstroke region to an exponential growth function and quantified the time constant, τ, and doubling time Td. There was no significant difference between the VLDL-treated group and the control for either parameter (P = 0.46 and 0.60, respectively) (Fig. 6E). Similarly, we fit the downstroke region to an exponential decay function and quantified τ and the half-life T1/2 (P = 0.28 and 0.18, respectively) (Fig. 6F). Although these parameters were not significantly different, the area under the curve for the downstroke region was significantly larger for the VLDL-treated group (P = 0.0017), indicating a more prolonged Ca2+ response in LECs treated with VLDL (Fig. 6G).
Fig. 6.
Effects of lipid exposure on the intracellular calcium response of LECs exposed to fluid shear in vitro. Intracellular calcium response by LECs to cells incubated for 24 h with VLDL (TG concentration of 10 mg/ml) exhibit sustained intracellular calcium concentration compared with controls (Ctrl) when exposed to a ramp shear stress profile going from 0–4 dyn/cm2 for 1 min. B: fraction of “activated” cells exhibiting a detectable intracellular calcium concentration. Although there was no difference in the rate of activation of the 2 LEC populations upon exposure to wall shear, a significantly higher percentage of cells exposed to VLDL continued to have detectable levels of intracellular calcium compared with controls (C). To further clarify this the standard deviation between cells in a given experiment was calculated over time and a value of 0.25 was defined the threshold for detecting a shear response. This threshold was calculated to be 0.11 dyn/cm2 for both groups (D). E: the time constant, τ, and doubling time, Td, showed no difference between the control and VLDL groups during the upstroke portion of the signal (P = 0.46 and 0.60, respectively). F: similarly, τ and the half-life T1/2 also showed no difference during the downstroke (P = 0.28 and 0.18, respectively). G: there was a significant difference in the area under the curve for the VLDL-treated group vs. control during the downstroke region (P = 0.0017) indicating a prolonged Ca2+ response; n = 6, error bands represent SE in A and B, and SD in C. E, F, and G are Tukey box-and-whisker plots. *P ≤ 0.05.
DISCUSSION
Although lipid uptake kinetics by lymphatics has been extensively studied and reported in literature, most of these studies were carried out by collecting lymph via the mesenteric lymphatic duct (47) or though in vitro cultures (23, 59). Recent work has provided great insight into the mechanism of lipid uptake into the intestinal lacteal and showed for the first time that the lacteals, and not just the collecting vessels, exhibit contractility (15). Previous studies looking at lymph lipid content used in vitro assays on collected lymph based on the hydrolysis of TG and a fluorescence colorimetric readout (11). Despite the fact that TG assays are more sensitive and provide a more direct measurement of TG, they cannot be used for real-time intraluminal TG monitoring. We have previously shown that lymph fluorescence of BODIPY C16 correlates quite strongly with total lymph TG (45), thus giving us a real-time measurement of the lipid content in the vessel and allowing us to quantify the instantaneous vessel response upon lipid exposure. In addition, and since our technique allows us to visualize when lipid first appears in the intestinal region drained by a given lymphatic, we were able to determine the total time for lipid absorption, packaging of chylomicrons, release into the villi and uptake into the lacteal. Lipid can first be detected in the mesenteric collecting vessel within 10–20 min and reaches a maximum concentration around 60–80 min. The time frame previously reported for chylomicrons to first appear in the mesenteric lymphatic duct was reported to be at around 14–22 min and to peak around 2–3 h (74). The data in our study, taken in conjunction with in vitro studies reporting the kinetics of chylomicron synthesis and release by enterocytes, suggest that uptake of chylomicrons into the lacteal is quite rapid and that enterocyte absorption and chylomicron synthesis are the primary rate-limiting steps of lipid uptake. In fact, a very recent study has shown that lipid can be seen in both the enterocytes and lacteal within 1 min and rises quickly within 15 min (15).
After the infusion of a lipid emulsion the vessel responded by reducing the phasic contraction frequency and amplitude and through reducing the overall average vessel diameter, the end-diastolic, and the end-systolic diameters. The reduction in phasic contractility appears to agree with similar results reported in mesenteric collecting lymphatic vessels isolated from a rat metabolic syndrome model (84) and in vivo in obese mice (4) but is in disagreement with one in vivo study in which rats infused with olive oil demonstrated a significant increase in collecting lymphatic contraction frequency (54). Their study was limited in its quantitative description of vessel luminal lipid content and thus it is difficult to compare their results with those presented here but when they binned their data as to have three levels of lipid concentration there was a statistically significant decrease in vessel contraction frequency with the increase in lipid concentration. It is worth noting that the fatty acid content for both Intralipid and olive oil is similar except that Intralipid has a higher level of the polyunsaturated omega-6 linoleic acid than olive oil whereas olive oil is richer in the monounsaturated omega-9 oleic acid (53). The decrease in phasic contractility presented in our study could be attributable to a variety of factors including oxidative stress due to lipoprotein oxidation, through induced nitric oxide (iNOS)-mediated NO (51) or histamine (56), both of which are released by activated lymph resident immune cells that double in density after a lipid meal (54). However, these factors would not explain the strong tonic response we observed in the lymphatics in response to high lipid, as both NO and histamine are known to have strong vasorelaxation effects on lymphatics. Lymphatic vessel tone is usually defined as being the percent difference between the passive diameter of the vessel under Ca2+-free conditions at a given pressure and the end-diastolic diameter with the presences of Ca2+ at the same pressure (84). Although we are unable to directly quantify vessel tone in this manner in vivo, because we have no control over the pressure or the Ca2+ concentration, we interpret a reduction in the end-diastolic diameter as indicative of a tonic response. A variety of factors have been previously shown to cause vessel constriction in lymphatics; these include elevated downstream pressures (66), increased local vessel pressure (18), inhibition of VEGFR-3 (9), certain concentrations of histamine (25), and arachidonic acid metabolites (44). Interestingly, both arachidonic acid and prostaglandin H2 (PGH2) produced very similar responses when added to isolated sheep lymphatics as seen in our study, namely a reduction in phasic contractility while increasing vessel tone (44). Thromboxane (a metabolite of PGH2) has been shown to be increased in response to a fatty meal in humans (20), and thromboxane has been previously shown to act as vasoconstrictor of blood vessels in the gut to limit food-induced increases in capillary exchange capacity (2). The extent that the observed lipid induced changes in lymphatic contractile function could be mediated through arachidonic acid metabolites warrants future attention.
Perhaps most surprising is that lymph flow is significantly elevated in the downstream mesenteric lymphatic duct during lipid absorption even though pump function in the prenodal collectors is reduced. Although apparently contradictory at first, there are two possible explanations for how this could occur. One possibility is that in the case of lipid absorption the intrinsic pump is no longer the primary driver of lymphatic flow, since lymph could be driven by elevated extrinsic factors such as enhanced lymph formation from microvascular filtration and enhanced intestinal peristalsis. Thus changes in lymphatics in classical definitions of lymphatic pump performance (such as fractional pump flow) are not representing the actual flow in the vessel. Another possibility could be that flow in the prenodal collectors is actually reduced and the elevated flow in the mesenteric duct is due to fluid exchange at the lymph node going from the blood vasculature to the lymphatic. It has been demonstrated both experimentally and computationally that under certain conditions Starling's forces favor fluid leaving the circulation and entering the lymphatics in the lymph node (1, 42).
Lymph flow within collecting vessels continuously exposes the lymphatic endothelium to wall shear stress (22), which has been shown to play a primary role in vessel development and function. Low shear stress levels are required for both the formation of structurally sound lymphatic vessels (14, 43, 50, 62) and maintenance of lymphatic endothelial cell identity (14). Shear stress also actively modulates the pumping response of the vessel, and hence transport, through the differential release of vasoactive substances, such as NO and histamine (31, 68, 80). This response seems to vary with the physiological region of the vessels whereby the thoracic duct has been shown to be the most sensitive to shear stress, drastically reducing its pumping when exposed to flow and thus lowering its resistance to flow and behaving as a passive conduit. Mesenteric vessels, on the other hand, are less sensitive to shear stress, maintaining some of their function as a pump even in the presence of fluid flow (28, 29). Regardless, fluid shear stress within the lymphatic is crucial in understanding lymphatic behavior particularly in the context of intestinal absorption. For example, any changes in microvascular filtration that occur postprandially would produce substantial changes on the fluid load and thus wall shear stress experienced by the lymphatics. Thus it is difficult to definitively say whether the observed changes in lymphatic function under high lipid load are a direct result of lipid altering LEC function or an indirect result due to changes in capillary filtration. It is well established that lipid absorption enhances capillary filtration and thus increases lymph flow (74) and was confirmed in our lymph cannulation studies here as well (Fig. 5C). Given that flow rate is elevated in the lymphatics after lipid absorption, it is surprising that the vessel exhibited a tonic response rather than dilating, which would suggest that the observed effects are not merely a wall shear stress response due to capillary filtration changes. Although, unfortunately, we did not monitor central venous pressure through the entire procedure, we did control for tissue hydration and intestinal distension through the infusion of saline controls.
Lymph viscosity, along with flow rate and vessel diameter, is an important contributing factor to shear stress. One of the most dramatic changes in lymph content occurs in the mesenteric lymphatic vessels that transport absorbed lipids from the small intestine to the mesenteric lymphatic duct. These changes are especially dramatic after a high-fat meal when lipid concentration dramatically increases compared with the fasting state. Although there are currently methods to measure the changes in lymph flow rates and vessel diameter (22, 45) and to impose arbitrary lymphatic flow waveforms in vitro (48), it has been very difficult to estimate the contribution of viscosity changes to shear stress resulting from the increase in lymphatic lipid concentration. Most studies estimating shear stress in lymphatics refer to a 1917 study to provide an estimate for lymph viscosity, where Burton-Opitz and Nemser (12) measured the viscosity of dog lymph after feeding a high-fat meal. They accomplished this by collecting lymph from the thoracic duct and measuring the time required for the passage of lymph through a capillary tube at a given pressure. Unfortunately, this method cannot be used with small lymph volumes, preventing measurements in smaller animal models, regional measurements from various lymph formation sources, and temporal measurements over short drainage intervals. More recently, Bouta et al. (6, 7), reported in vivo lymph viscosity in the collecting lymphatic draining the hindlimb by calculating the diffusion coefficient of fluorescently labeled albumin following in vivo multiphoton fluorescence recovery after photobleaching (MP-FRAP) and then utilizing the Stokes-Einstein equation to estimate the viscosity. However, this technique is problematic for producing absolute measurements of viscosity due to the numerous assumptions that are violated when this technique is utilized in vivo. The main hindrance in measuring lymph viscosity has been the relatively small sample volumes collected through the various lymph collection techniques (47). These small volumes make it difficult to obtain complex fluid properties through conventional rheology measurements, which typically require sample volumes larger than a milliliter (13). As a result of miniaturization, improvements in imaging technology and computing power, a new category of rheology, microrheology, has emerged. Microrheology probes the material response on micrometer length scales and typically requires less than 10 μl of sample (8), with the ability to even probe intracellular rheological properties (81). Here we utilized a passive microrheology technique that uses the Brownian motion of embedded florescence particles to accurately measure the viscosities of rat lymph during a fasting state as well as the transient changes occurring over a 4-h period following a high-fat meal. We showed that the viscosity of lymph increases by as much as 50%, which translates to an equal increase in shear stress at a constant held flow rate and vessel diameter.
Taking into consideration that both flow rate and viscosity are increased as a function of lipid uptake, one would expect that shear would increase and classical shear-sensitive pathways to increase vessel diameter and decrease contractility would be activated. The observation that the vessel diameter significantly decreases with high lipid suggests that the pump response observed in vivo is not shear mediated. Our in vitro studies suggest that levels of lipid exposure similar to those observed in vivo can alter the calcium response of lymphatics by increasing cytoplasmic calcium retention of calcium (as a result of either increased extracellular influx or mobilization from intracellular stores) and the percentage of activated cells. Our combined observations of elevated LEC cytoplasmic calcium after VLDL exposure and a significant loss of diastolic relaxation in vivo in the presence of high lipid suggest that, similar to the blood vasculature, lipid can directly alter shear-mediated events in the lymphatics (69, 75). The consequence of high lipid on downstream signaling events that modulate the vessel diameter is an important area of research that warrants further exploration as the work here is the first to report the interactions between shear and lipid in the lymphatic endothelium.
Quantifying the acute functional response of mesenteric lymphatic vessels during lipid absorption of a high-fat meal is crucial to understanding numerous clinical pathologies where lipids have been implicated in malformations and dysfunctional lymphatic vessels (67). For example, in some protein-losing enteropathies such as primary intestinal lymphangiectasia (PIL), the blind-ended initial lymphatics in the intestinal villi are significantly dilated and cause lymph leakage into the intestinal lumen. A standard procedure in managing the disease is to prescribe a low-fat diet supplemented with medium- (MCT) and short-chain TGs (SCT), which minimizes the lipid load of the lymphatics since they primarily absorb long-chain TGs (LCT) (76). The absence of high lipid load prevents the rupture of lacteals and hence reduced lymph leakage, yet the extent that the effectiveness of this therapy is a result of beneficial effects on the collecting mesenteric lymphatic pump remains unknown. Using a long-chain fluorescent fatty acid analog such as BODIPY C16 can help to better understand how downstream impairment of collecting lymphatic vessel lipid clearance might contribute to the observed overloading of the lacteals. Even before PIL symptoms develop, patients have shown delayed transport of lipid from the intestine, suggesting that lymphatic lipid transport function is compromised at an early stage of the disease (67). In addition, lymphatic phasic contraction was shown to be hindered in an isolated vessel model of gut inflammation, suggesting that lymphatic function might be compromised in inflammatory bowel diseases such as Crohn's disease (3). Although alleviating the lipid burden on lymphatics is clinically beneficial in many of these intestinal disorders, the exact mechanisms of lymphatic failure and the interplay between the lipid absorption process and lymphatic function in these disease states is still unclear. The results presented here suggest that high concentrations of lipid can directly reduce lymphatic pump function, which would likely exacerbate the condition in a disease physiology where there is already substantial inflammation.
Obesity is one of the few established factors that predisposes patients to developing secondary lymphedema (33, 63). Additionally, several recent studies have demonstrated impaired function of the lymphatics in obesity (4, 33, 63, 82). LCTs constitute ∼90% of the fat content in a typical Western diet so if lipid content is adversely affecting lymphatic pump function, it is likely to increase the chances of lymphedema since patients who have a reduced lymphatic functional capacity at baseline are at higher risk for developing lymphedema (82). It has also been shown that reducing the amount of LCT in diets of lymphedema patients and replacing them with MCT reduced peripheral edema (70). This edema reduction could be due to reduced formation of lymph, and hence less leakage in the affected limb, or to the fact that lower lipid content in lymph possibly restores lymphatic vessels to their normal functional capacity.
While our in vivo animal model along with the developed imaging tools provide a good platform to study the effect of lipids on lymphatic pump response, there were several limitations to our understanding of the physiological postprandial response. The procedure is highly invasive, requires anesthetic, and alters the vessel's mechanical environment. Care was taken to minimize these effects by including control rats that underwent all of the same surgical and mechanical perturbations but received a bolus of saline in the intestine rather than lipid. Since we cannot measure lymphatic flow in the local vessel, it makes it difficult to ascertain whether alterations in fluid shear stress during lipid absorption might be partially responsible for the pump function response. However, this seems unlikely, as there are no reports of shear stress in lymphatics causing a decrease in the contractile frequency and a reduction in the end-diastolic diameter. Typically, elevated fluid shear reduces contraction frequency and dilates the vessel (29, 49, 56). Additionally, in vitro experiments utilized VLDL as the source of lipid exposure since it is readily commercially available and human chylomicrons from intestinal lymph would be difficult to obtain. Although we matched the total TG content as closely as possible it is important to recognize that the apolipoprotein content on the surface of VLDL (ApoB-100) and chylomicrons (ApoB-48) is different, as well as their physiological origin (VLDL is synthesized primarily in the liver).
In conclusion, we report here for the first time the kinetics of lipid uptake from the intestine to the mesenteric collecting lymphatic vessel immediately draining the region in the small intestine where the lipid is being absorbed. We go on to show, using a novel multimodal intravital microscopy approach that, in the context of a lipid meal, high lipid loads within a lymphatic vessel alter lymphatic function by reducing phasic contractions and causing an overall tonic constriction in the vessel. This is accompanied by an increase in lymph viscosity and flow rate, which would also alter the shear stress environment on the lymphatic.
GRANTS
Funding support for this work was provided by the National Institutes of Health (R00HL091133, R01HL113061) and an American Heart Association Predoctoral Fellowship (T. Kassis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.K. and J.B.D. conception and design of research; T.K., S.C.Y., and A.B.K. performed experiments; T.K., S.C.Y., A.B.K., V.B., and J.B.D. analyzed data; T.K. and J.B.D. interpreted results of experiments; T.K. prepared figures; T.K. drafted manuscript; T.K., S.C.Y., A.B.K., P.T., V.B., and J.B.D. edited and revised manuscript; T.K., S.C.Y., A.B.K., P.T., V.B., and J.B.D. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Rachel Cornelius for help with establishing and troubleshooting the in vivo lipid uptake experimental protocol. The authors also acknowledge the University of Cincinnati Mouse Metabolic Phenotype Center (DK059630) for providing the lymph samples. Current affiliations of the authors are as follow:
Timothy Kassis, Research Laboratory of Electronics, and Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA.
Sri Charan Yarlagadda, Sealed Air Corporation, Greenville, SC.
Alison B. Kohan, Department of Nutritional Sciences, University of Connecticut, Storrs, CT.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1.Adair TH, Moffatt DS, Paulsen WA, Guyton A. Quantitation concentration of changes in lymph protein during lymph node transit. Am J Physiol Heart Circ Physiol 243: H351–H359, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Alemayehu A, Chou CC. Thromboxane plays a role in postprandial jejunal oxygen uptake and capillary exchange. Am J Physiol Gastrointest Liver Physiol 259: G430–G435, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Alexander JS, Chaitanya GV, Grisham MBB, Boktor M. Emerging roles of lymphatics in inflammatory bowel disease. Ann NY Acad Sci 1207: E75–E85, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, Detmar M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One 9: e94713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollman J, Cain J, Grindlay J. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 33: 1349, 1948. [PubMed] [Google Scholar]
- 6.Bouta EM, Wood RW, Brown EB, Rahimi H, Ritchlin CT, Schwarz EM. In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice. J Physiol 592: 1213–1223, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouta EM, Wood RW, Perry SW, Brown EB, Ritchlin CT, Xing L, Schwarz EM. Measuring intranodal pressure and lymph viscosity to elucidate mechanisms of arthritic flare and therapeutic outcomes. Ann NY Acad Sci 1240: 47–52, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breedveld V, Pine D. Microrheology as a tool for high-throughput screening. J Mater Sci 8: 4461–4470, 2003. [Google Scholar]
- 9.Breslin JW, Gaudreault N, Watson KD, Reynoso R, Yuan SY, Wu MH. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am J Physiol Heart Circ Physiol 293: H709–H718, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Brorson H, Ohlin K, Olsson G, Karlsson MK. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol 7: 3–10, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 19: 476–482, 1973. [PubMed] [Google Scholar]
- 12.Burton-Opitz R, Nemser R. The viscosity of lymph. Am J Physiol 45: 25–29, 1917. [Google Scholar]
- 13.Carpen IC, Brady JF. Microrheology of colloidal dispersions by Brownian dynamics simulations. J Rheol (N Y N Y) 49: 1483, 2005. [Google Scholar]
- 14.Chen C, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vincente A, Mericko P, Levy RJ, Makinen T, Oliver G, Kahn ML. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest 122: 2006–2017, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe K, Jang JY, Park I, Kim Y, Ahn S, Park D, Hong Y, Alitalo K, Koh GY, Kim P. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest 125: 4042–4052, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke CJ, Nanjee MN, Stepanova IP, Olszewski WL, Miller NE. Variations in lipid and apolipoprotein concentrations in human leg lymph: effects of posture and physical exercise. Atherosclerosis 173: 39–45, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Crocker J, Grier D. Methods of digital video microscopy for colloidal studies. J Colloid Interface Sci 310: 298–310, 1996. [Google Scholar]
- 18.Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296: H293–H302, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol 303: H795–H808, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgado-Lista J, Lopez-Miranda J, Cortés B, Perez-Martinez P, Lozano A, Gomez-Luna R, Gomez P, Gomez MJ, Criado J, Fuentes F, Perez-Jimenez F. Chronic dietary fat intake modifies the postprandial response of hemostatic markers to a single fatty test meal. Am J Clin Nutr 87: 317–322, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Dixon JB, Gashev AA, Zawieja DC, Moore JE Jr, Cote G, Moore JE, Coté GL. Image correlation algorithm for measuring lymphocyte velocity and diameter changes in contracting microlymphatics. Ann Biomed Eng 35: 387–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon JB, Moore JE Jr, Cote GL, Gashev AA, Zawieja DC, Greiner ST, Moore JE. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13: 597–610, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Dixon JB, Raghunathan S, Swartz MA. A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol Bioeng 103: 1224–1235, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon JB. Lymphatic lipid transport: sewer or subway? Trends Endocrinol Metab 1: 480–487, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobbins DE, Buehn MJ, Dabney JM. Constriction of perfused lymphatics by acetylcholine, bradykinin and histamine. Microcirc Endothelium Lymphatics 6: 409–425, 1990. [PubMed] [Google Scholar]
- 26.Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N. Advanced methods of microscope control using μManager software. J Biol Methods 1: 1–10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fantl P, Nelson J. Coagulation of lymph. J Physiol 122: 33–37, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11: 477–492, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gashev AA, Zhang RZ, Muthuchamy M, Zawieja DC, Davis MJ. Regional heterogeneity of length-tension relationships in rat lymph vessels. Lymphat Res Biol 10: 14–19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol 575: 821–832, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannattasio C, Zoppo A, Gentile G, Failla M, Capra A, Maggi FM, Catapano A, Mancia G. Acute effect of high-fat meal on endothelial function in moderately dyslipidemic subjects. Arterioscler Thromb Vasc Biol 25: 406–410, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Greene AK, Grant FD, Slavin SA, Maclellan RA. Obesity-induced lymphedema: clinical and lymphoscintigraphic features. Plast Reconstr Surg 135: 1715–1719, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol Heart Circ Physiol 233: H57–H65, 1977. [DOI] [PubMed] [Google Scholar]
- 35.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 37: 1072–1081, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Harvey NL. The link between lymphatic function and adipose biology. Ann NY Acad Sci 1131: 82–88, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi H, Sato Y, Kanai S, Ichikawa M, Funakoshi A, Miyasaka K. Increased lymphatic lipid transport in genetically diabetic obese rats. Am J Physiol Gastrointest Liver Physiol 282: G69–G76, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Helden D, Zhao J. Lymphatic vasomotion. Clin Exp Pharmacol Physiol 27: 1014–1018, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J 16: 48–54, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab 296: E1183–E1194, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jafarnejad M, Cromer WE, Kaunas RR, Zhang SL, Zawieja DC, Moore JE. Measurement of shear stress-mediated intracellular calcium dynamics in human dermal lymphatic endothelial cells. Am J Physiol Heart Circ Physiol 308: H697–H706, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jafarnejad M, Woodruff MC, Zawieja DC, Carroll MC, Moore JE. Modeling lymph flow and fluid exchange with blood vessels in lymph nodes. Lymphat Res Biol 13: 234–247, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22: 3282–3291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston MG, Gordon JL. Regulation of lymphatic contractility by arachidonate metabolites. Nature 293: 294–297, 1981. [DOI] [PubMed] [Google Scholar]
- 45.Kassis T, Kohan AB, Weiler MJ, Nipper ME, Cornelius R, Tso P, Dixon JB. Dual-channel in-situ optical imaging system for quantifying lipid uptake and lymphatic pump function. J Biomed Opt 17: 086005, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim AJ, Manoharan VN, Crocker JC. Swelling-based method for preparing stable, functionalized polymer colloids. J Am Chem Soc 127: 1592–1593, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Kohan A, Howles P, Tso P. Methods for studying rodent intestinal lipoprotein production and metabolism. Curr Protoc Mouse Biol 2: 219–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kornuta JA, Nipper ME, Dixon JB. Low-cost microcontroller platform for studying lymphatic biomechanics in vitro. J Biomech 46: 183–186, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kornuta JA, Dixon JB. Ex vivo lymphatic perfusion system for independently controlling pressure gradient and transmural pressure in isolated vessels. Ann Biomed Eng 42: 1691–1704, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lahdenranta J, Hagendoorn J, Padera TP, Hoshida T, Nelson G, Kashiwagi S, Jain RK, Fukumura D. Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res 69: 2801–8, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, Ruddle NH. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA 108: 18784–18789, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ, Angeli V. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol 175: 1328–1337, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lovegrove JA, Griffin BA. The acute and long-term effects of dietary fatty acids on vascular function in health and disease. Curr Opin Clin Nutr Metab Care 16: 162–167, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Miura S, Sekizuka E, Nagata H, Oshio C, Minamitani H, Suematsu M, Suzuki M, Hamada Y, Kobayashi K, Asakura H. Increased lymphocyte transport by lipid absorption in rat mesenteric lymphatics. Am J Physiol Gastrointest Liver Physiol 253: G596–G600, 1987. [DOI] [PubMed] [Google Scholar]
- 55.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol Regul Integr Comp Physiol 274: R790–R796, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, Chatterjee V, Meininger CJ, Gashev AA. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 21: 640–648, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phan CT, Tso P. Intestinal lipid absorption and transport. Front Biosci 6: D299–D319, 2001. [DOI] [PubMed] [Google Scholar]
- 58.Randolph G, Miller N. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest 124: 929–935, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reed AL, Rowson SA, Dixon JB. Demonstration of ATP-dependent, transcellular transport of lipid across the lymphatic endothelium using an in vitro model of the lacteal. Pharm Res 30: 3271–3280, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribera J, Pauta M, Melgar-Lesmes P, Tugues S, Fernández-Varo G, Held KF, Soria G, Tudela R, Planas AM, Fernández-Hernando C, Arroyo V, Jiménez W, Morales-Ruiz M. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut 62: 138–145, 2013. [DOI] [PubMed] [Google Scholar]
- 61.Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol 176: 1122–1129, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hägerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes J-M, Adams RH, Mäkinen T, Kiefer F, Kwak BR, Petrova TV. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell 22: 430–445, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, Joseph WJ, Mehrara BJ. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol 307: H165–H172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol 591: 2139–2156, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scallan JP, Hill MA, Davis MJ. Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signaling. Cardiovasc Res 107: 89–89, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scallan JP, Wolpers JH, Davis MJ. Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J Physiol 591: 443–459, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Servelle M. Congenital malformation of the lymphatics of the small intestine. J Cardiovasc Surg (Torino) 32: 159–165, 1991. [PubMed] [Google Scholar]
- 68.Shirasawa Y, Ikomi F, Ohhashi T. Physiological roles of endogenous nitric oxide in lymphatic pump activity of rat mesentery in vivo. Am J Physiol Gastrointest Liver Physiol 278: G551–G556, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest 93: 50–55, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soria P, Cuesta A, Romero H, Martinez F. Dietary treatment of lymphedema by restriction of long-chain triglycerides. Angiology 45: 703–707, 1994. [DOI] [PubMed] [Google Scholar]
- 71.Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev 50: 3–20, 2001. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi N, Kawai Y. Effects of vasoconstrictive and vasodilative agents on lymphatic smooth muscles in isolated canine thoracic ducts. J Pharmacol Exp Ther 254: 165–170, 1990. [PubMed] [Google Scholar]
- 73.Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol Gastrointest Liver Physiol 250: G715–G726, 1986. [DOI] [PubMed] [Google Scholar]
- 74.Tso P, Pitts V, Granger DN. Role of lymph flow in intestinal chylomicron transport. Am J Physiol Gastrointest Liver Physiol 249: G21–G28, 1985. [DOI] [PubMed] [Google Scholar]
- 75.Vedernikov YuP, Lankin VZ, Tikhaze AC, Vikhert AM. Lipoproteins as factors in vessel tone and reactivity modulation. Basic Res Cardiol 83: 590–596, 1988. [DOI] [PubMed] [Google Scholar]
- 76.Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis 3: 5, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, Heinonen SE, Samaranayake H, Heikura T, Alitalo K, Ylä-Herttuala S. Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol 34: 1162–1170, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol 297: H726–H734, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.von der Weid P-Y, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol 36: 1147–1153, 2004. [DOI] [PubMed] [Google Scholar]
- 80.von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol 280: H2707–H2716, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Weihs D, Mason TG, Teitell MA. Bio-microrheology: a frontier in microrheology. Biophys J 91: 4296–4305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D, Mehrara BJ. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One 8: e70703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ying LH, Pin YK, Veronique A. Lipid biology and lymphatic function: a dynamic interplay with important physiological and pathological consequences. J Clin Cell Immunol 5: 5, 2014. [Google Scholar]
- 84.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 302: H643–H653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.