Abstract
The diagnosis and treatment of liver disease remain a major health concern worldwide because of the diverse etiologies of this disease. For this reason, new therapeutic targets are greatly needed to halt the progression of this damaging disease. Upon initiation of liver injury by viral infection, autoimmune disease or toxin, and/or hepatitis, chronic disease may develop, which can progress to cirrhosis, hepatocellular carcinoma (HCC), cholangiocarcinoma, liver failure, or death. The Lin28/lethal-7 (let-7) molecular switch has emerged as a central regulator of multiorgan injuries and cancer development. Lin28 is a stem cell marker vital to initiation or maintenance of a stem cell phenotype. Lin28 has not been extensively studied in the liver, despite its ability to induce tissue regeneration via reprogramming of oxidative enzymes in other tissues and its involvement with numerous upstream regulators and downstream targets in liver disease. Theoretically, overexpression of Lin28 in certain forms of liver disease could be a potential treatment that aids in liver regeneration. Alternatively, Lin28 has been implicated numerous times in the progression of diverse cancer types and is associated with increased severity of disease. In this case, Lin28 could be a potential inhibitory target to prevent malignant transformation in the liver. This review seeks to characterize the role of Lin28 in liver disease.
Keywords: lethal-7, liver disease, hepatic disorders, hepatocellular carcinoma, primary biliary cholangitis, primary sclerosing cholangitis, liver repair
liver diseases remain a major health concern in the United States and worldwide. The term “liver disease” includes >100 etiologies of hepatic and biliary diseases, all of which can ultimately lead to liver failure. Liver and biliary cancer combined represent the fifth leading cause of death in men and the ninth leading cause of death in women in the United States (69). Unfortunately, mortality from liver and intra- and extrahepatic bile duct cancer continues to rise and is projected to be the third leading cause of cancer-related deaths in the United States by 2030 (59). Current treatment strategies merely aim to prevent disease progression; therefore, more targets are needed to develop curative therapies. Many studies are underway to examine the mechanisms of liver and biliary tumorigenesis in an effort to identify novel treatment modalities.
MicroRNAs (miRNAs) are small noncoding RNAs that influence gene expression independent of any other regulation, typically by direct binding. These small regulatory RNAs have recently garnered a great deal of attention from the scientific community as potential targets for treatment of human disease. Several miRNAs have been shown to be altered during the progression of liver diseases (5). For example, lethal-7 (let-7), one of the first miRNAs discovered, is altered in several forms of liver disease (27). Additionally, let-7 is a major regulator of cellular differentiation in the developing organism.
A main regulator of let-7 is an RNA-binding protein and stem cell marker, Lin28 (48). Lin28 and let-7 have been shown to control cellular differentiation in a see-saw-type regulatory mechanism (72). An abundance of let-7 correlates with a differentiated cellular phenotype, whereas increased expression of Lin28 indicates an undifferentiated phenotype (61). Elevation of Lin28 and decrease of let-7 are linked with highly aggressive disease in other organs, and their importance in development points to a high likelihood of their importance in liver diseases. Alterations in let-7 family members and Lin28 have been shown in various cancers, including prostate and breast cancers. Additionally, Lin28 upregulation has been characterized in highly aggressive and metastatic tumors (86). This review aims to evaluate the relationship between liver disease and Lin28 and its associated miRNAs.
Lin28
Lin28 is a small RNA-binding protein that contains three RNA-binding domains (41). In humans and other mammals, Lin28 has two forms: Lin28A and Lin28B. In humans, these proteins are coded by two loci: Lin28A, located on chromosome 1, and Lin28B, located on chromosome 6 (51). Lin28A is a 209-amino acid monomeric protein with a mass of 22,743 Da; Lin28B is a 250-amino acid with a mass of 27,084 Da (41).
Lin28A is a translational enhancer, capable of promoting protein synthesis by chaperoning its target mRNAs to polysomes (29). Lin28A is predominantly a cytoplasmic protein, but it is found in the nucleus 10–15% of the time because of its ability to shuttle between the cytoplasm and the nucleus. Typically, Lin28A tends to localize to cytoplasmic processing bodies and stress granules (55). Although Lin28A is primarily a cytoplasmic regulator, methylation of Lin28 increases its nuclear retention and protein stability. A methylated nuclear form of Lin28A is able to sequester pri-let-7 and block its processing. This nuclear form of Lin28A is able to regulate transcriptional changes in the V-myc avian myelocytomatosis viral oncogene homolog pathway, which leads to maintenance of the stem cell phenotype and inhibition of differentiation (32). Also, Lin28 is able to directly interact with eukaryotic translation initiation factor 3 subunit 2, a component of the eukaryotic translation initiation factor 3 complex, which is required for several steps to initiate protein synthesis (42). Additionally, Lin28A directly interacts with nucleolin, which is the main nuclear protein of growing cells (53). In humans, Lin28 is typically found in embryonic stem cells, placenta, and testes. During development, Lin28 is highly expressed in fetal liver; however, this high level of expression typically decreases as cells become further differentiated.
Lin28B is primarily an inhibitor of miRNA biogenesis in the nucleus. It inhibits the precursors of let-7 and, possibly, miR-107, miR-143, and miR-200c by binding to the pri-miRNA in the nucleus and sequestering the pri-miRNA from the microprocessing complex. In this way, the miRNA is prevented from maturing and performing its function (55). Lin28B is highly expressed in the placenta and is expressed to a lesser extent in the testes and fetal liver (21). Two isoforms of Lin28B are formed by alternative splicing: the 250-amino acid isoform 1 and the 180-amino acid isoform 2. Lin28B isoform 1 is highly upregulated in the placenta and in poorly differentiated forms of hepatocellular carcinoma (HCC). Lin28B isoform 2 is expressed in the fetal liver and benign liver tissues, as well as in well-differentiated tumor tissues. Lin28B isoform 2 is upregulated in triple-negative breast cancers and in liver, ovarian, and thyroid carcinomas (55). In vitro, Lin28B exhibits an oncofetal expression pattern, where overexpression upregulates stemness markers [octamer-binding transcription factor 4, Nanog, and SRY (sex-determining region Y)-box 2] and enhances tumor sphere formation. Inhibition of Lin28B does the opposite (11).
Mechanisms and Actions of Lin28
Lin28 was discovered in Caenorhabditis elegans as a developmental regulator. In this model, Lin28 expression decreased as cells differentiated (4). Its downstream target is typically the miRNA let-7, but it has been shown to bypass let-7 and affect cell cycle regulators and a myriad of other mRNAs independently. Lin28 is regulated by miR-125 (lin-4 in lower animals) and let-7 (46). The mechanisms of Lin28, its targets, and its regulators are discussed below.
miR-125-Lin28-let-7 pathway.
Lin28 typically acts through a universal pathway that involves direct regulation of let-7 and regulation by miR-125. Lin28 is able to directly bind to insulin-like growth factor 2 (IGF2) mRNA, myogenic differentiation 1 mRNA, acidic ribosomal phosphoprotein P0 [acidic ribosomal phosphoprotein P0 (ARBP/36B4)] mRNA, and its own mRNA (67).
The main miRNA target of Lin28, the developmental timing regulator miRNA let-7, was one of the first miRNAs discovered and was characterized as an important developmental regulator in C. elegans. In this organism, deletion of let-7 inhibited maturation, whereas overexpression of let-7 caused early maturation (62). This ability to regulate cellular differentiation points to the possibility that let-7 has the ability to promote cellular transformation in a repairing liver. let-7 has been expanded into a family of miRNAs known as the let-7 family, which regulates cell development and differentiation in vertebrates.
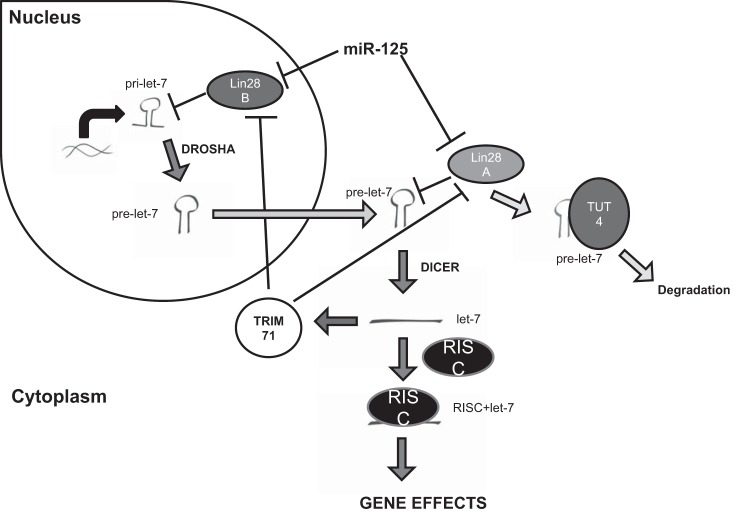
miRNAs, including let-7, typically regulate genes by binding to the 3′-untranslated region of the target mRNA, which destabilizes the mRNA and prevents translation (7). RNA polymerase transcribes primary miRNAs from DNA in the nucleus. These primary miRNAs are cleaved by the Drosha-DGCR8 complex into pre-miRNAs that are exported from the nucleus. In the cytoplasm, pre-miRNAs are cleaved once again by the Dicer-TRBP complex into its mature duplex length. The miRNA then binds to the RNA-induced silencing complex, which guides the mature miRNA to its target (77) (Fig. 1).
Fig. 1.
Lin28 is able to affect let-7 maturation by utilization of several methods. Lin28B is able to bind to pri-let-7 in the nucleus and prevent cleavage by Drosha. Lin28A binds to pre-let-7, preventing cleavage by Dicer. In addition, Lin28A is able to recruit RNA uridylyltransferase 4 (TUT4) to bind to pre-let-7, which leads to degradation of let-7. Lin28 is regulated by miR-125 in the nucleus and cytoplasm. Additionally, upregulation of let-7 leads to activation of tripartite motif containing 71 (TRIM71), which inhibits both forms of Lin28. RISC, RNA-induced silencing complex.
Lin28 regulates let-7 expression by three separate methods: 1) inhibition of pri-let-7 processing by binding to Drosha (73, 74), 2) inhibition of pre-let-7 cleavage by Dicer via direct binding to pre-let-7 and subsequent unwinding at the Dicer binding region (38, 64), and 3) recruitment of RNA uridylyltransferase 4 to the Lin28-pre-let-7 complex, which leads to uridylation and degradation of pre-let-7 (22, 23, 36).
miR-125 is a regulatory miRNA that controls the expression of several downstream signals involved in cellular proliferation, metastasis, immunity, apoptosis, and differentiation, including Lin28. miR-125 directly targets and inhibits Lin28 mRNA, which stops translation of the Lin28 protein, effectively blocking its expression (78). Interestingly, let-7 has also been shown to be an inhibitor of Lin28 in a feedback-loop mechanism (64). Higher levels of let-7 expression are associated with increased organism differentiation and decreased Lin28 expression. let-7 is able to accomplish this inhibition of Lin28 by activation of tripartite motif containing 71, which targets Lin28 for ubiquitin-mediated proteasomal degradation (35) (Fig. 1).
Alternate Lin28 signaling pathways.
Outside the miR-125-Lin28-let-7 pathway, there are alternative downstream targets of Lin28. For example, IGF2, a protein secreted by the liver, is a known target of Lin28, and this interaction is primarily independent of let-7 (6, 56). IGF2 is mostly active during development and displays a strong antiapoptotic effect on cells (54). Although Lin28 can bind IGF2 directly, it has also been shown that transcription factor RelB, a member of the nuclear factor-κ light chain enhancer of activated B cells (NF-κB) family, is able to form a multiprotein complex with Lin28 and IGF2 mRNA-binding protein (IMP3) to enhance the translation efficiency of IGF2 (50, 70).
Several miRNAs share the same tetranucleotide sequence motif (GGAG) as let-7 in the terminal loop and are regulated by Lin28 in the same way as let-7 (23): miR-107, miR-143, and miR-200c. miR-107 has been shown to promote tumor growth and cancer progression. Interestingly, miR-107 achieves this ability to promote tumor progression by negative regulation of let-7 via direct binding (9). miR-143 is typically portrayed as a tumor suppressor gene and is important in the progression of cancer in numerous studies (28, 31, 85).
Lin28 in cellular transformation.
Cellular transformation, transition from an epithelial phenotype to a mesenchymal or stem cell-like phenotype, is vital to liver repair and maintenance. Lin28 has been shown to be integral to cellular transformation, when it was shown to be a key factor in the creation of induced pluripotent stem cells (83). Thus, cellular transformation is a main function of Lin28 and is most likely important in cellular transformation required for liver repair. Recent studies have shown that miR-200c, another miRNA that is regulated by Lin28, may be important in the epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET). Overexpression of miR-200c promotes MET, which is important in termination of cancer progression (24, 25). It has been theorized that the EMT-MET balance dictates the ability to either recover from injury or progress to a cirrhotic condition. More EMT promotes liver fibrosis, whereas more MET promotes liver healing and regeneration (12). In addition, this morphological transition is vital to induce the final repair of the liver and prevent subsequent tumor formation (80). These earlier studies have suggested that cellular transformation and the EMT-MET balance, which is regulated by miR-200c via Lin28, may be important in liver pathology and therapy, although further studies are required to clarify the link between cellular transformation and liver conditions.
The liver is a plastic organ that is able to respond to external or internal damage and maintain its own homeostasis. A controversial theory is that, in order for the liver to regenerate itself, mature hepatocytes and/or cholangiocytes must transform into a more stem-like state to produce replacements for injured areas. Unfortunately, the same transformation responsible for repair can also be dysregulated, leaving the cells in a mesenchymal phenotype. These cells undergo malignant transformation and proliferate, forming tumors and replacing healthy tissue. For transformation from an epithelial to a mesenchymal phenotype, cells dedifferentiate to move to new locations. Typically, when the cell relocates, it undergoes a redifferentiation to reintegrate into the tissue. Because Lin28 expression promotes a stem cell-like phenotype, it is vital to cellular transformation.
An alternate theory of liver repair is that hepatocytes or cholangiocytes simply undergo mitosis to replace the damaged area of the liver, which would not involve Lin28. In all likelihood, liver repair is a combination of several different repair mechanisms, which may involve the contribution of mitosis of uninjured cells, cellular transformation, and/or liver stem cell differentiation.
Regulation of cellular transformation during development.
Lin28 was initially shown in C. elegans to regulate the fate of cells both in early development and terminal differentiation (3, 4, 46). In more recent years, Lin28 has been seen as a regulator of stem cell pluripotency in all organisms and is downregulated as stem cells differentiate into their terminal cell types (17, 47, 63, 81).
In the development of the liver, the expression levels of the transcription factors hepatocyte nuclear factor (HNF) 4α and HNF6 are inversely correlated with those of let-7. Inhibition of let-7b was shown to increase the levels of HNF4α, HNF6, and miR-122 and to cause an accumulation of mesenchymal stem cells, which triggered initiation of hepatic commitment (2).
Upregulation of Lin28 and downregulation of let-7 are essential to the maintenance of a stem cell phenotype. In an immature organism, the Lin28-to-let-7 ratio will lean toward Lin28; however, as the organism matures, the ratio changes to favor let-7. In other words, cell differentiation occurs when Lin28 decreases and let-7 increases, whereas cells become less differentiated when Lin28 expression increases and levels of let-7 decrease. This see-saw-type mechanism is essential not only to the development of an organism, but also to cellular transformation, as these are the mechanisms critical for liver repair.
Cell cycle regulation.
Cell cycle regulation is important in the maintenance of normal growth, as well as stability of the organism, regulation of repair, and cellular proliferation. The Lin28-let-7 pathway has been shown to be vital for maintenance of the cell cycle in several instances. For example, overexpression of let-7 has been shown to lead to the loss of cell growth and subsequent induction of apoptosis, which is vital to the developing organism's ability to form organs and limbs.
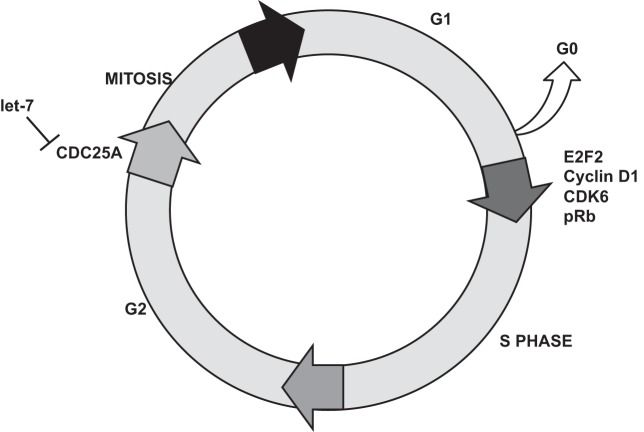
The mechanisms by which Lin28 and let-7 regulate the cell cycle are varied. For example, let-7c has been shown to directly target the 3′-untranslated region of the M-phase inducer cell division cycle 25A (CDC25A), leading to its inhibition and causing the cells to remain in the G1 phase and making them unable to divide. Inhibition of CDC25A, in turn, causes decreases in other cell cycle regulators such as cyclin D1, cyclin-dependent kinase 6, retinoblastoma protein, and E2F transcription factor 2 (87) (Fig. 2). Overexpression of let-7g or let-7i has been shown to suppress DNA replication, which leads to inhibition of proliferation and promotion of apoptosis by inhibition of the antiapoptotic protein Bcl-xL (79). The ability of Lin28 to regulate the cell cycle points to an important role for the Lin28-let-7 pathway not only during development, but also during liver repair and progression to liver cancer.
Fig. 2.
Upregulation of let-7 is able to regulate the cell cycle by inhibiting the mitotic checkpoint regulator cell division cycle 25A (CDC25A). Inhibition of CDC25A causes downregulation of the G1 regulators cyclin D1, cyclin-dependent kinase 6 (CDK6), retinoblastoma protein (pRb), and E2F transcription factor 2 (E2F2). This combined effect leads to cells being halted in the G0 phase and unable to divide.
Lin28 in Liver Disease
Hepatitis.
Inflammation of the liver, hepatitis, is induced by viral infection, autoimmune disorders, toxin exposure, or other etiologies and is characterized by heat, edema, and an influx of inflammatory cells. Theoretically, Lin28 should be actively involved in repair mechanisms following acute liver injury; however, most studies performed in hepatitis characterize transformation to HCC via Lin28 expression. For example, when the subatomic genome of the hepatitis C virus was expressed in cultured cells, expression of cancer stem cell markers, including Lin28, was increased (1). Similarly, it has been shown that the hepatitis B virus decreases let-7 expression by modification of Lin28B expression (79).
Other targets of Lin28 have also been studied in hepatitis. For example, reduced expression of miR-107 was found in chronically infected hepatitis C patients (65). In addition, miR-200c is upregulated in hepatitis C patients with fibrotic lesions. miR-200c reduces Fas-associated phosphatase 1 expression, which subsequently increases expression of the protooncogene tyrosine-protein kinase Src (Src) kinase and liver fibrosis (60).
Polymorphisms in IGF2, a downstream target of Lin28, are associated with decreased clearance of the hepatitis B virus and increased risk of HCC (33). Additionally, IGF2 has been found to be hypomethylated in patients with cirrhotic hepatitis C that progressed to HCC. Therefore, this hypomethylation could be predictive of HCC progression (14).
Although not in the liver, Lin28 has been shown to interact with other inflammatory molecules, which are often found in hepatitis. For example, in an acute spinal contusion model in rats, Lin28 was able to act via NF-κB to participate in lipopolysaccharide (LPS)-induced inflammatory responses in astrocytes (84). Lin28 and interleukin (IL)-6 interact in a unique way. It has been shown that Src, a proto-oncogene, is able to activate NF-κB, which directly activates Lin28. Activation of Lin28 leads to repression of let-7. Since let-7 typically represses IL-6, activation of Src causes an increase in IL-6, which promotes inflammation and activates signal transducer and activator of transcription 3. This pathway upregulates NF-κB, promoting a positive-feedback loop (26). IL-1β is another inflammatory marker in hepatitis. In non-small cell lung cancer cells, IL-1β acted through cyclooxygenase 2-hypoxia-inducible factor 1α to repress miR-101, a miRNA involved in tumor suppression, which subsequently increased Lin28B and allowed for cellular transformation to a cancer cell phenotype (75) (Fig. 3).
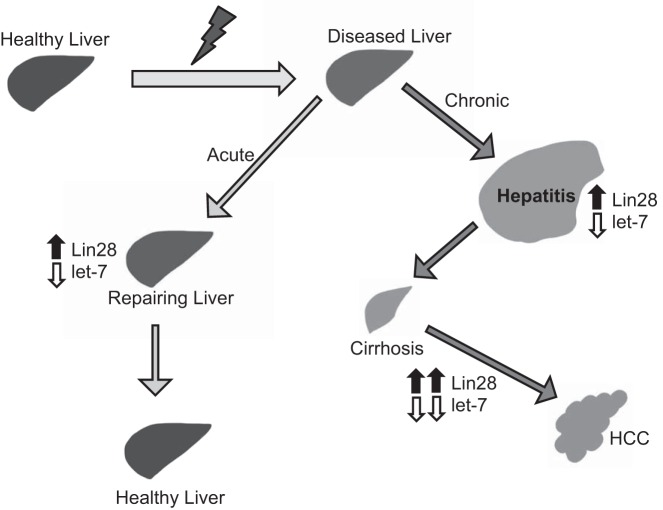
Fig. 3.
When a healthy liver is injured by disease or other injury, it can follow an acute disease route where repair is successful via upregulation of Lin28 and downregulation of let-7. If, instead, the disease becomes chronic, it leads to hepatitis, where the liver again tries to repair the injury via activation of Lin28 and deactivation of let-7. If repair is unsuccessful, the liver progresses to cirrhosis and then, upon a large increase in Lin28 and decrease in let-7, the liver can progress to HCC.
Alcoholic and nonalcoholic fatty liver disease.
Alcoholic liver disease (ALD) occurs as a result of excess alcohol consumption over time and is characterized by steatosis (fatty liver) and the sequelae of injury and inflammation leading to fibrosis (scarring) and, eventually, cirrhosis. Patients with alcoholic liver injury are also more prone to other hepatic insults, such as nonalcoholic fatty liver disease (NAFLD) and chronic viral hepatitis (19). Although the role of Lin28 has not been studied in ALD directly, it can be speculated that Lin28 is active during repair mechanisms induced by alcoholic liver injury. In addition, Lin28 should be upregulated as ALD progresses to cirrhosis and cells take on a malignant phenotype.
This can be observed indirectly through Lin28 targets. In the serum of ethanol (EtOH)-fed mice, let-7a, let-7b, and let-7g are decreased, indicating an upregulation of Lin28 (44). In addition, miR-107 is elevated in rats that were fed EtOH over 5 wk (15). Because Lin28 typically blocks the action of miR-107, this indicates that EtOH has the ability to suppress Lin28 expression, which leads to upregulation of miR-107. This result could be indicative of EtOH repressing the liver's ability to repair.
NAFLD is characterized by steatosis of the liver typically correlated with obesity but can be a result of certain medications. The incidence is increasing in the United States because of excessive consumption of fat- and sugar-laden foods. In addition to NAFLD, sugary foods can lead to type 2 diabetes. The combination of NAFLD and diabetes often leads to nonalcoholic steatohepatitis (NASH), which is characterized by progressive hepatitis. Left untreated, NASH can progress to cirrhosis and, ultimately, liver failure.
NAFLD causes excessive inflammation as a result of increased steatosis. NF-κB-mediated inflammation has been linked to a strong increase in Lin28, which quickly decreases let-7 and subsequently induces cellular transformation (26). Therefore, it is quite likely that Lin28 increases as NAFLD progresses to the more severe NASH. In addition, comparison of steatosis with steatohepatitis has shown a contrast in let-7 levels: let-7b expression is increased in steatohepatitis, but let-7d is decreased in steatosis (30). In addition, the downstream Lin28 target miR-200c was upregulated in a rat model of steatohepatitis (18). The reduced levels of let-7 in steatosis indicate an increased level of Lin28 in this acute disease, indicating an active role of liver repair. In contrast, the increased levels of the downstream targets of Lin28, miR-200c and let-7, in steatohepatitis indicate that liver repair is reduced or nonexistent as the liver progresses to cirrhosis.
HCC.
HCC is an aggressive malignancy that arises from hepatocytes, typically in the setting of chronic hepatitis or cirrhosis. Typically, HCC is a result of the liver's efforts to repair cirrhosis by unrestricted cellular proliferation. HCC is frequently locally advanced or metastatic at the time of diagnosis, and most patients succumb to the disease within 3–6 mo as a result of late diagnosis and/or inability to resect the tumor (69). Stem-like cells play a role in HCC development, as Lin28 and let-7 have been implicated multiple times in this disease both in basic science and clinical studies.
Multiple cell lines have been derived from HCC and are easily testable for Lin28 and let-7 levels, as well as manipulation of these markers. For example, members of the let-7 family have been shown to be decreased in HCC cell lines: HepG2, Hep3B, and Huh7 (34). Additionally, the Hep3B cell line has been shown to express high levels of Lin28 (71). The ability of let-7 to inhibit the HCC phenotype was examined by let-7 overexpression was shown to inhibit the viability and mobility of HCC cells. In this model, let-7 appeared to remain in the cytoplasm to induce changes in organelles, including autophagy (39). Additionally, HCC proliferation was inhibited after transfection of let-7g mimics, while let-7g inhibitors showed the opposite effect (34).
Targeting of Lin28 in HCC cell lines also shows promise. For instance, RNAi knockdown of Lin28B in the HCC cell line HCC36 decreased cellular proliferation in vitro and reduced tumor growth in the xenograft model. On the other hand, overexpression of Lin28B in the HCC cell line HA22T resulted in heightened tumorigenicity and induced EMT, leading to tumor invasion (76). This is expected, as increased Lin28 should induce more of a stem-like phenotype. Additionally, in this study, knockdown of Lin28B by RNAi in the HCC cell line HCC36 suppressed proliferation in vitro and reduced in vivo tumor growth in nonobese diabetic/severe combined immunodeficiency mice. In contrast, overexpression of Lin28B in the HCC cell line HA22T enhanced tumorigenicity (76). This indicates that Lin28 is a promising potential therapeutic target in HCC.
In vivo, Lin28B has been implicated as the prime generator of hepatoblastoma and HCC in mouse models. Overexpression of Lin28B alone was sufficient to initiate tumor formation, whereas liver-specific knockout of both Lin28A and Lin28B reduced tumor burden and extended survival. In the same study, systemic inhibition of Lin28B with siRNA reduced tumor burden and extended survival as well (49). In a separate study in a mouse xenograft model using HCC cells, cholesterol-conjugated let-7 overexpression inhibited tumor growth and invasion, as well as metastasis (39). In the same model but in a separate study, tumor growth was suppressed in the mouse xenograft model using let-7c-overexpressed HepG2 cells (87).
In human patients, higher levels of Lin28B have been found in the circulating peripheral blood nucleated cells of HCC patients than non-HCC patients. In addition, increased Lin28B expression was also associated with a larger tumor size, higher tumor grade, high American Joint Committee on Cancer stage, and high Barcelona Clinic Liver Cancer stage (11). Upon tumor resection, Lin28 was shown to be higher in HCC than non-HCC tumor tissues, and high levels of Lin28 were also correlated with larger HCC tumors (58). When tested for let-7, these same HCC tissues had significantly lower levels of let-7c than adjacent normal tissue, and a correlation was seen between low let-7c levels and poor tissue differentiation (88). Along the same lines, high-grade HCC tumors with high α-fetoprotein levels have also been shown to express Lin28B more abundantly than normal tissue (76). These patient studies underline the importance of Lin28 in the development and maintenance of the cancer phenotype and point out that Lin28 could be a strong potential therapeutic target in the fight against HCC.
Biliary diseases.
In primary biliary cholangitis, also known as primary biliary cirrhosis (PBC), the intrahepatic small- to medium-sized bile ducts are destroyed by an autoimmune mechanism, which leads to cholestasis (40). PBC is typically found in a 10:1 ratio of women to men. Patients are typically 35–70 yr of age (8). Primary sclerosing cholangitis (PSC) is a biliary disease that leads to cholestasis via inflammation and obstructive biliary fibrosis. PSC is commonly associated with inflammatory bowel disease and occurs in men 70% of the time; patients are, on average, 41 yr of age (16). PSC will progress to cirrhosis and liver failure without hepatic transplantation and can be a mitigating factor in the development of HCC or cholangiocarcinoma.
Although Lin28 expression has not been studied extensively in cholangiopathies, numerous studies examining members of the let-7 family have been published, allowing an extrapolation of the results to speculate on the activities of Lin28 in biliary diseases. As in most liver diseases, there are two points where cellular transformation and, thus, Lin28 would be active: 1) repair after injury and 2) progression to cholangiocarcinoma.
Because inflammation is a critical element in the etiology of diverse cholangiopathies, it is important to examine its effects on cholangiocytes. LPS release by intestinal bacteria upon liver injury continues to be an important mediator of bile duct inflammation due to the link between the gut and the liver. In vitro it has been shown that let-7i has the ability to regulate expression of the LPS receptor Toll-like receptor in cholangiocytes, indicating the ability of let-7 to regulate immune responses due to specific bacterial infections (10). The ability of let-7i to regulate this inflammation indicates that Lin28 may be overexpressed during inflammatory processes in the bile ducts. During cancer transformation, let-7 would typically be downregulated and Lin28 would be upregulated in a cholangiocarcinoma tumor. Interestingly, using cholangiocarcinoma cells, we have shown that overexpression of let-7a increases cell survival following chemotherapy (45).
In vivo, we showed that inhibition of let-7a or miR-125b in bile duct-ligated mice increased intrahepatic bile duct mass and increased expression of nerve growth factor (20). This is expected, because without let-7 or miR-125 to inhibit Lin28, cellular transformation, especially EMT, should be upregulated and there should be a proliferation of cholangiocytes. Diethylnitrosamine-treated mice that were subjected to bile duct ligation surgery and subsequently formed cholangiocarcinoma showed repression of let-7 and upregulation of Lin28B (82). This is expected and also is consistent with the HCC data, where let-7 is decreased and Lin28 is upregulated in tumors.
In humans, most of the work on let-7 has been done in PBC patients. For instance, levels of let-7b were lower in the peripheral blood cells of patients with PBC than healthy controls. Additionally, let-7b levels decreased in parallel to the increases in disease severity (57). In a separate study of end-state PBC patients, let-7d was downregulated in PBC liver tissue compared with normal tissue (52). This is expected in these diseased samples, because Lin28 should be upregulated due to the liver's attempts to repair itself in the early stages of disease or in end-stage disease, the progression to HCC or cholangiocarcinoma. Although miRNAs have been examined in human PSC samples, let-7 family members were either not selected as miRNAs of interest or the results were unclear (37, 66).
Polycystic liver disease.
Polycystic liver diseases (PCLD) encompass a spectrum of autosomal-dominant and -recessive disorders that may occur in association with polycystic kidney diseases. Multiple gene mutations have been associated with disease progression that often leads to multiple hepatic cysts and, ultimately, hepatic fibrosis. The mutated genes encode proteins primarily expressed in cilia, which leads to ciliary malformation and malfunction. Isolated liver disease includes autosomal-dominant PCLD, which occurs in ∼1 in 100,000 people (13, 43). Recent evidence demonstrates that cholangiocytes involved in PCLD have the ability to undergo EMT, which indicates a probable role of Lin28 in this disease (43). Recent microarray analysis confirmed that 68 miRNAs are differentially expressed in cystic cholangiocytes of polycystic kidney rats. The majority of these miRNAs had decreased expression, which included many members of the let-7 family (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, and let-7i) (43). Lin28 has not been studied in PCLD; however, the let-7 data suggest that Lin28 would be upregulated, promoting cellular transformation and cholangiocyte proliferation. More research is needed to confirm the roles of Lin28 and the let-7 family in hepatic ciliopathies; however, these current findings suggest that targeting Lin28 may prove to be beneficial in halting cyst growth and subsequent hepatic fibrosis.
Therapeutic Potentials
Theoretically, since Lin28 is a progenitor cell regulator, it should be involved in two distinct stages of liver disease: 1) liver repair by cellular transformation and 2) progression to cancer. In the mouse, Lin28A was shown to promote tissue repair of hair follicles, bone, and cartilage by reprograming oxidative enzymes. In these models, let-7 repression was necessary, but not sufficient, to repair tissue injuries independently of Lin28A (68). Similar mechanisms could be utilized in liver repair as a wound healing-like response to injury.
Since overexpression of Lin28 is able to regulate the regeneration of other mesenchymal tissues, it is conceivable that targeted overexpression of Lin28 in the progression of liver disease will aid in the organ's regenerative capabilities. Overexpression of Lin28 could progress to cancer if unchecked; therefore, it may be necessary to follow the Lin28 overexpression with an expression vector directed to let-7 and/or miR-125 to silence the Lin28 followed by excess cellular transformation.
In vivo studies have shown that repression of Lin28 in xenograft and other tumorigenic models has the ability to inhibit and/or slow tumor formation. This demonstrates that Lin28 is a good and viable target for potential therapeutics to prevent the progression of chronic liver diseases to cancer. Although it would be best for a targeted liver therapy to repress Lin28 overexpression, because Lin28 is rarely expressed in adult tissues, it would be feasible to repress Lin28 systemically in liver cancer patients.
Conclusions/Future Perspectives
Lin28 regulation shows great promise as a potential therapeutic for treatment of liver disease. Recent studies have suggested that Lin28 is integral to the transformation of cancer cells and Lin28 is most likely involved during the regulation of repair in the liver following injury. Lin28 has been shown in this review to be critical in the liver's ability to undergo cellular transformation and maintain homeostasis.
Unfortunately, Lin28 appears to be a rather potent signal and may potentially have the ability to dedifferentiate cells to an almost stem-like state, as shown by its requirement in induction of pluripotent stem cells. This strong signal to dedifferentiate and undergo cellular transformation has been shown to induce HCC and other types of cancers. In addition, higher expression of Lin28 has been shown to correlate with more malignant tumors, which also correlate with a less differentiated cell.
It is possible that, in many cases, liver injury is not severe enough to activate the Lin28-let-7 pathway. However, when liver injury is severe, the liver may decrease let-7 expression, allowing an increase in Lin28 in an effort to repair the injury by induction of cellular transformation and more active proliferation. As Lin28 levels increase, there may be a checkpoint that does not occur and that could lead to cancer as less differentiated cells overtake the liver. Overall, there seems to be a fine balance between Lin28 and let-7 in liver homeostasis and repair. More Lin28 and let-7 studies need to be performed in the liver to elucidate the influence of these important factors on liver disease and repair.
GRANTS
This work was supported in part by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a Department of Veterans Affairs Research Career Scientist Award, Department of Veterans Affairs Merit Award 5I01BX000574 (to G. Alpini), Department of Veterans Affairs Biomedical Laboratory Research Grants 1I01BX001724 (to F. Meng) and 5I01BX002192 (to S. Glaser), and National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-058411, DK-076898, DK-107310, and DK-062975 (to G. Alpini, F. Meng, and S. Glaser). This material is the result of work supported by resources at the Central Texas Veterans Health Care System.
DISCLAIMERS
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M., C.H., and K.S. prepared the figures; K.M. and K.S. drafted the manuscript; C.H., K.S., T.L., M.M., S.G., F.M., and G.A. edited and revised the manuscript; T.L., M.M., S.G., F.M., and G.A. approved the final version of the manuscript; G.A. developed the concept and designed the research.
REFERENCES
- 1.Ali N, Allam H, May R, Sureban SM, Bronze MS, Bader T, Umar S, Anant S, Houchen CW. Hepatitis C virus-induced cancer stem cell-like signatures in cell culture and murine tumor xenografts. J Virol 85: 12292–12303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alizadeh E, Akbarzadeh A, Eslaminejad MB, Barzegar A, Hashemzadeh S, Nejati-Koshki K, Zarghami N. Up regulation of liver-enriched transcription factors HNF4a and HNF6 and liver-specific microRNA (miR-122) by inhibition of let-7b in mesenchymal stem cells. Chem Biol Drug Des 85: 268–279, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. A hierarchy of regulatory genes controls a larva-to-adult developmental switch in C. elegans. Cell 57: 49–57, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226: 409–416, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Arrese M, Eguchi A, Feldstein AE. Circulating microRNAs: emerging biomarkers of liver disease. Semin Liver Dis 35: 43–54, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Balzer E, Heine C, Jiang Q, Lee VM, Moss EG. LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development 137: 891–900, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? Biochem Soc Trans 36: 1224–1231, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 386: 1565–1575, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Chen PS, Su JL, Cha ST, Tarn WY, Wang MY, Hsu HC, Lin MT, Chu CY, Hua KT, Chen CN, Kuo TC, Chang KJ, Hsiao M, Chang YW, Chen JS, Yang PC, Kuo ML. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest 121: 3442–3455, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen XM, Splinter PL, O'Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem 282: 28929–28938, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SW, Tsai HW, Lin YJ, Cheng PN, Chang YC, Yen CJ, Huang HP, Chuang YP, Chang TT, Lee CT, Chao A, Chou CY, Chan SH, Chow NH, Ho CL. Lin28B is an oncofetal circulating cancer stem cell-like marker associated with recurrence of hepatocellular carcinoma. PLos One 8: e80053, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology 50: 2007–2013, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cnossen WR, Drenth JP. Polycystic liver disease: an overview of pathogenesis, clinical manifestations and management. Orphanet J Rare Dis 9: 69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couvert P, Carrie A, Paries J, Vaysse J, Miroglio A, Kerjean A, Nahon P, Chelly J, Trinchet JC, Beaugrand M, Ganne-Carrie N. Liver insulin-like growth factor 2 methylation in hepatitis C virus cirrhosis and further occurrence of hepatocellular carcinoma. World J Gastroenterol 14: 5419–5427, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dippold RP, Vadigepalli R, Gonye GE, Patra B, Hoek JB. Chronic ethanol feeding alters miRNA expression dynamics during liver regeneration. Alcohol Clin Exp Res 37 Suppl 1: E59–E69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 145: 521–536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faas L, Warrander FC, Maguire R, Ramsbottom SA, Quinn D, Genever P, Isaacs HV. Lin28 proteins are required for germ layer specification in Xenopus. Development 140: 976–986, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Feng YY, Xu XQ, Ji CB, Shi CM, Guo XR, Fu JF. Aberrant hepatic microRNA expression in nonalcoholic fatty liver disease. Cell Physiol Biochem 34: 1983–1997, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser S, Meng F, Han Y, Onori P, Chow BK, Francis H, Venter J, McDaniel K, Marzioni M, Invernizzi P, Ueno Y, Lai JM, Huang L, Standeford H, Alvaro D, Gaudio E, Franchitto A, Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology 146: 1795–1808, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, Chen Y, Ito H, Watanabe A, Ge X, Kodama T, Aburatani H. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene 384: 51–61, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol 16: 1021–1025, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, Yeom KH, Han J, Kim VN. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138: 696–708, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Hurteau GJ, Carlson JA, Roos E, Brock GJ. Stable expression of miR-200c alone is sufficient to regulate TCF8 (ZEB1) and restore E-cadherin expression. Cell Cycle 8: 2064–2069, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Hurteau GJ, Carlson JA, Spivack SD, Brock GJ. Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res 67: 7972–7976, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-κB, Lin28, Let-7 microRNA, and IL6 links inflammation to cell transformation. Cell 139: 693–706, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of microRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res 14: 419–427, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang S, Zhang LF, Zhang HW, Hu S, Lu MH, Liang S, Li B, Li Y, Li D, Wang ED, Liu MF. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J 31: 1985–1998, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin J, Jing W, Lei XX, Feng C, Peng S, Boris-Lawrie K, Huang Y. Evidence that Lin28 stimulates translation by recruiting RNA helicase A to polysomes. Nucleic Acids Res 39: 3724–3734, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin X, Chen YP, Kong M, Zheng L, Yang YD, Li YM. Transition from hepatic steatosis to steatohepatitis: unique microRNA patterns and potential downstream functions and pathways. J Gastroenterol Hepatol 27: 331–340, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Kent OA, Chivukula RR, Mullendore M, Wentzel EA, Feldmann G, Lee KH, Liu S, Leach SD, Maitra A, Mendell JT. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev 24: 2754–2759, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SK, Lee H, Han K, Kim SC, Choi Y, Park SW, Bak G, Lee Y, Choi JK, Kim TK, Han YM, Lee D. SET7/9 methylation of the pluripotency factor LIN28A is a nucleolar localization mechanism that blocks let-7 biogenesis in human ESCs. Cell Stem Cell 15: 735–749, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YJ, Yoon JH, Kim CY, Kim LH, Park BL, Shin HD, Lee HS. IGF2 polymorphisms are associated with hepatitis B virus clearance and hepatocellular carcinoma. Biochem Biophys Res Commun 346: 38–44, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Lan FF, Wang H, Chen YC, Chan CY, Ng SS, Li K, Xie D, He ML, Lin MC, Kung HF. Hsa-let-7g inhibits proliferation of hepatocellular carcinoma cells by downregulation of c-Myc and upregulation of p16(INK4A). Int J Cancer 128: 319–331, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Cho S, Kim MS, Choi K, Cho JY, Gwak HS, Kim YJ, Yoo H, Lee SH, Park JB, Kim JH. The ubiquitin ligase human TRIM71 regulates let-7 microRNA biogenesis via modulation of Lin28B protein. Biochim Biophys Acta 1839: 374–386, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Lehrbach NJ, Armisen J, Lightfoot HL, Murfitt KJ, Bugaut A, Balasubramanian S, Miska EA. LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat Struct Mol Biol 16: 1016–1020, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, Georgiades C, Singh VK, Khashab M, Amateau S, Li Z, Okolo P, Lennon AM, Saxena P, Geschwind JF, Schlachter T, Hong K, Pawlik TM, Canto M, Law J, Sharaiha R, Weiss CR, Thuluvath P, Goggins M, Shin EJ, Peng H, Kumbhari V, Hutfless S, Zhou L, Mezey E, Meltzer SJ, Karchin R, Selaru FM. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 60: 896–907, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lightfoot HL, Bugaut A, Armisen J, Lehrbach NJ, Miska EA, Balasubramanian S. A LIN28-dependent structural change in pre-let-7g directly inhibits dicer processing. Biochemistry 50: 7514–7521, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YM, Xia Y, Dai W, Han HY, Dong YX, Cai J, Zeng X, Luo FY, Yang T, Li YZ, Chen J, Guan J. Cholesterol-conjugated let-7a mimics: antitumor efficacy on hepatocellular carcinoma in vitro and in a preclinical orthotopic xenograft model of systemic therapy. BMC Cancer 14: 889, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lleo A, Maroni L, Glaser S, Alpini G, Marzioni M. Role of cholangiocytes in primary biliary cirrhosis. Semin Liver Dis 34: 273–284, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loughlin FE, Gebert LF, Towbin H, Brunschweiger A, Hall J, Allain FH. Structural basis of pre-let-7 miRNA recognition by the zinc knuckles of pluripotency factor Lin28. Nat Struct Mol Biol 19: 84–89, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Masutani M, Sonenberg N, Yokoyama S, Imataka H. Reconstitution reveals the functional core of mammalian eIF3. EMBO J 26: 3373–3383, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol 25: 265–271, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng F, Glaser SS, Francis H, Yang F, Han Y, Stokes A, Staloch D, McCarra J, Liu J, Venter J, Zhao H, Liu X, Francis T, Swendsen S, Liu CG, Tsukamoto H, Alpini G. Epigenetic regulation of miR-34a expression in alcoholic liver injury. Am J Pathol 181: 804–817, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng F, Henson R, Wehbe-Janek H, Smith H, Ueno Y, Patel T. The microRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J Biol Chem 282: 8256–8264, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell 88: 637–646, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol 258: 432–442, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Nam Y, Chen C, Gregory RI, Chou JJ, Sliz P. Molecular basis for interaction of let-7 microRNAs with Lin28. Cell 147: 1080–1091, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen LH, Robinton DA, Seligson MT, Wu L, Li L, Rakheja D, Comerford SA, Ramezani S, Sun X, Parikh MS, Yang EH, Powers JT, Shinoda G, Shah SP, Hammer RE, Daley GQ, Zhu H. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models. Cancer Cell 26: 248–261, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1: a000034, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, Kimura K, Makita H, Sekine M, Obayashi M, Nishi T, Shibahara T, Tanaka T, Ishii S, Yamamoto J, Saito K, Kawai Y, Isono Y, Nakamura Y, Nagahari K, Murakami K, Yasuda T, Iwayanagi T, Wagatsuma M, Shiratori A, Sudo H, Hosoiri T, Kaku Y, Kodaira H, Kondo H, Sugawara M, Takahashi M, Kanda K, Yokoi T, Furuya T, Kikkawa E, Omura Y, Abe K, Kamihara K, Katsuta N, Sato K, Tanikawa M, Yamazaki M, Ninomiya K, Ishibashi T, Yamashita H, Murakawa K, Fujimori K, Tanai H, Kimata M, Watanabe M, Hiraoka S, Chiba Y, Ishida S, Ono Y, Takiguchi S, Watanabe S, Yosida M, Hotuta T, Kusano J, Kanehori K, Takahashi-Fujii A, Hara H, Tanase TO, Nomura Y, Togiya S, Komai F, Hara R, Takeuchi K, Arita M, Imose N, Musashino K, Yuuki H, Oshima A, Sasaki N, Aotsuka S, Yoshikawa Y, Matsunawa H, Ichihara T, Shiohata N, Sano S, Moriya S, Momiyama H, Satoh N, Takami S, Terashima Y, Suzuki O, Nakagawa S, Senoh A, Mizoguchi H, Goto Y, Shimizu F, Wakebe H, Hishigaki H, Watanabe T, Sugiyama A, Takemoto M, Kawakami B, Yamazaki M, Watanabe K, Kumagai A, Itakura S, Fukuzumi Y, Fujimori Y, Komiyama M, Tashiro H, Tanigami A, Fujiwara T, Ono T, Yamada K, Fujii Y, Ozaki K, Hirao M, Ohmori Y, Kawabata A, Hikiji T, Kobatake N, Inagaki H, Ikema Y, Okamoto S, Okitani R, Kawakami T, Noguchi S, Itoh T, Shigeta K, Senba T, Matsumura K, Nakajima Y, Mizuno T, Morinaga M, Sasaki M, Togashi T, Oyama M, Hata H, Watanabe M, Komatsu T, Mizushima-Sugano J, Satoh T, Shirai Y, Takahashi Y, Nakagawa K, Okumura K, Nagase T, Nomura N, Kikuchi H, Masuho Y, Yamashita R, Nakai K, Yada T, Nakamura Y, Ohara O, Isogai T, Sugano S. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet 36: 40–45, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Padgett KA, Lan RY, Leung PC, Lleo A, Dawson K, Pfeiff J, Mao TK, Coppel RL, Ansari AA, Gershwin ME. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J Autoimmun 32: 246–253, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parada CA, Roeder RG. A novel RNA polymerase II-containing complex potentiates Tat-enhanced HIV-1 transcription. EMBO J 18: 3688–3701, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrik J, Reusens B, Arany E, Remacle C, Coelho C, Hoet JJ, Hill DJ. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 140: 4861–4873, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Piskounova E, Polytarchou C, Thornton JE, LaPierre RJ, Pothoulakis C, Hagan JP, Iliopoulos D, Gregory RI. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 147: 1066–1079, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polesskaya A, Cuvellier S, Naguibneva I, Duquet A, Moss EG, Harel-Bellan A. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev 21: 1125–1138, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian C, Chen SX, Ren CL, Zhong RQ, Deng AM, Qin Q. [Abnormal expression of miR-let-7b in primary biliary cirrhosis and its clinical significance]. Zhonghua Gan Zang Bing Za Zhi 21: 533–536, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Qiu JL, Huang PZ, You JH, Zou RH, Wang L, Hong J, Li BK, Zhou K, Yuan YF. LIN28 expression and prognostic value in hepatocellular carcinoma patients who meet the Milan criteria and undergo hepatectomy. Chin J Cancer 31: 223–232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74: 2913–2921, 2014. [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran S, Ilias Basha H, Sarma NJ, Lin Y, Crippin JS, Chapman WC, Mohanakumar T. Hepatitis C virus induced miR200c down modulates FAP-1, a negative regulator of Src signaling, and promotes hepatic fibrosis. PLos One 8: e70744, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rehfeld F, Rohde AM, Nguyen DT, Wulczyn FG. Lin28 and let-7: ancient milestones on the road from pluripotency to neurogenesis. Cell Tissue Res 359: 145–160, 2015. [DOI] [PubMed] [Google Scholar]
- 62.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403: 901–906, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Richards M, Tan SP, Tan JH, Chan WK, Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells 22: 51–64, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol 10: 987–993, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Sarma NJ, Tiriveedhi V, Crippin JS, Chapman WC, Mohanakumar T. Hepatitis C virus-induced changes in microRNA 107 (miRNA-107) and miRNA-449a modulate CCL2 by targeting the interleukin-6 receptor complex in hepatitis. J Virol 88: 3733–3743, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shigehara K, Yokomuro S, Ishibashi O, Mizuguchi Y, Arima Y, Kawahigashi Y, Kanda T, Akagi I, Tajiri T, Yoshida H, Takizawa T, Uchida E. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLos One 6: e23584, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12: 395–406, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shyh-Chang N, Zhu H, Yvanka de Soysa T, Shinoda G, Seligson MT, Tsanov KM, Nguyen L, Asara JM, Cantley LC, Daley GQ. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 155: 778–792, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 65: 5–29, 2015. [DOI] [PubMed] [Google Scholar]
- 70.Thakar NY, Ovchinnikov DA, Hastie ML, Gorman J, Wolvetang EJ. RELB alters proliferation of human pluripotent stem cells via IMP3- and LIN28-mediated modulation of the expression of IGF2 and other cell-cycle regulators. Stem Cells Dev 24: 1888–1900, 2015. [DOI] [PubMed] [Google Scholar]
- 71.Tian N, Han Z, Li Z, Zhou M, Fan C. Lin28/let-7/Bcl-xL pathway: the underlying mechanism of drug resistance in Hep3B cells. Oncol Rep 32: 1050–1056, 2014. [DOI] [PubMed] [Google Scholar]
- 72.Tsialikas J, Romer-Seibert J. LIN28: roles and regulation in development and beyond. Development 142: 2397–2404, 2015. [DOI] [PubMed] [Google Scholar]
- 73.Van Wynsberghe PM, Kai ZS, Massirer KB, Burton VH, Yeo GW, Pasquinelli AE. LIN-28 co-transcriptionally binds primary let-7 to regulate miRNA maturation in Caenorhabditis elegans. Nat Struct Mol Biol 18: 302–308, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science 320: 97–100, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Zhang LF, Wu J, Xu SJ, Xu YY, Li D, Lou JT, Liu MF. IL-1β-mediated repression of microRNA-101 is crucial for inflammation-promoted lung tumorigenesis. Cancer Res 74: 4720–4730, 2014. [DOI] [PubMed] [Google Scholar]
- 76.Wang YC, Chen YL, Yuan RH, Pan HW, Yang WC, Hsu HC, Jeng YM. Lin-28B expression promotes transformation and invasion in human hepatocellular carcinoma. Carcinogenesis 31: 1516–1522, 2010. [DOI] [PubMed] [Google Scholar]
- 77.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228–234, 2009. [DOI] [PubMed] [Google Scholar]
- 78.Wu L, Belasco JG. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Mol Cell Biol 25: 9198–9208, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu L, Wang Q, Yao J, Jiang H, Xiao C, Wu F. MicroRNA let-7g and let-7i inhibit hepatoma cell growth concurrently via downregulation of the anti-apoptotic protein B-cell lymphoma-extra large. Oncol Lett 9: 213–218, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie G, Diehl AM. Evidence for and against epithelial-to-mesenchymal transition in the liver. Am J Physiol Gastrointest Liver Physiol 305: G881–G890, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang DH, Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns 3: 719–726, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Yang H, Li TW, Peng J, Tang X, Ko KS, Xia M, Aller MA. A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involved in progression. Gastroenterology 141: 378–388, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920, 2007. [DOI] [PubMed] [Google Scholar]
- 84.Yue Y, Zhang D, Jiang S, Li A, Guo A, Wu X, Xia X, Cheng H, Tao T, Gu X. LIN28 expression in rat spinal cord after injury. Neurochem Res 39: 862–874, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Liu S, Hu T, Liu S, He Y, Sun S. Up-regulated microRNA-143 transcribed by nuclear factor κB enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology 50: 490–499, 2009. [DOI] [PubMed] [Google Scholar]
- 86.Zheng YW, Nie YZ, Taniguchi H. Cellular reprogramming and hepatocellular carcinoma development. World J Gastroenterol 19: 8850–8860, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu X, Wu L, Yao J, Jiang H, Wang Q, Yang Z, Wu F. MicroRNA let-7c inhibits cell proliferation and induces cell cycle arrest by targeting CDC25A in human hepatocellular carcinoma. PLos One 10: e0124266, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Zhu XM, Wu LJ, Xu J, Yang R, Wu FS. Let-7c microRNA expression and clinical significance in hepatocellular carcinoma. J Int Med Res 39: 2323–2329, 2011. [DOI] [PubMed] [Google Scholar]