Abstract
Nicotine and its derivatives, by binding to nicotinic acetylcholine receptors (nAChRs) on bronchial epithelial cells, can regulate cellular signaling and inflammatory processes. Delineation of nAChR subtypes and their responses to nicotine stimulation in bronchial epithelium may provide information for therapeutic targeting in smoking-related inflammation in the airway. Expression of nAChR subunit genes in 60 bronchial epithelial biopsies and immunohistochemical staining for the subcellular locations of nAChR subunit expression were evaluated. Seven human bronchial epithelial cell lines (HBECs) were exposed to nicotine in vitro for their response in nAChR subunit gene expression to nicotine exposure and removal. The relative normalized amount of expression of nAChR α4, α5, and α7 and immunohistochemical staining intensity of nAChR α4, α5, and β3 expression showed significant correlation with lung function parameters. Nicotine stimulation in HBECs resulted in transient increase in the levels of nAChR α5 and α6 but more sustained increase in nAChR α7 expression. nAChR expression in bronchial epithelium was found to correlate with lung function. Nicotine exposure in HBECs resulted in both short and longer term responses in nAChR subunit gene expression. These results gave insight into the potential of targeting nAChRs for therapy in smoking-related inflammation in the airway.
Keywords: nicotine, nicotinic acetylcholine receptor, quantitative polymerase chain reaction, lung function, bronchial epithelium
tobacco smoking is an important cause of lung function impairment, more commonly manifest as airflow obstruction on lung function tests. Nicotine in tobacco smoke is also one major component to cause damage to bronchial epithelium as well as being a potential carcinogen for the lung (12, 16). There were previous reports of nicotine stimulation of normal bronchial epithelium giving rise to aberrant Akt or NF-κB signaling that may contribute to the development of airway inflammation. Recent discoveries of functional acetylcholine receptors on lung epithelial cells and lung tumors raise the question of whether exposure to nicotine could participate in pathogenesis of airway disorders, one example would be chronic obstructive pulmonary disease (COPD). COPD is defined as an airway disorder resulting from chronic exposure to noxious gaseous substances like tobacco smoke and it usually manifests with respiratory symptoms together with the detection of nonreversible airflow obstruction on lung function tests (5). The ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC), i.e., FEV1/FVC ratio, of less than 70% is an indicator of airflow obstruction (5). Studying the pattern of nicotinic acetylcholine receptor (nAChR) expression in bronchial epithelium might provide information that nicotine is playing a role via specific subtypes of nAChRs in development of airflow obstruction.
nAChRs are found in nonneuronal tissues, such as α3, α5, and α7 in cultured bronchial epithelial cells (10, 22), but the pattern of nAChR expression is not completely delineated in human bronchial epithelial tissue. The mechanisms and contributions toward development of lung disease by these various nAChR subunits are not well defined.
Recent meta-analysis has implicated the CHRNA3-CHRNB4-CHRNA5 region on chromosome 15q25 to be associated with nicotine dependence, and this region is known to encode the different nAChR α subunits (21) (CHRN being the gene symbol for nAChR). In one study, genetic variants in this 15q24/25 region are associated with emphysema (8, 13), and a large-scale genomewide association study suggested that variants in that region could be genetic risk factor for the development of airflow obstruction independent of smoking (20). There is also evidence from Genome-Wide Association Study (GWAS) that these nAChR clusters may act on COPD development via susceptibility to smoking but not directly on development of COPD (1).
In this study, we aimed to delineate the expression pattern of nAChR subunits in bronchial epithelium, from normal-looking areas at bronchoscopy with autofluorescence imaging (AFI), in correlation with clinical characteristics and lung function. Immortalized normal bronchial epithelial cell lines were established from bronchial epithelial biopsy and were tested for nAChR expression in response to nicotine exposure for 9 days. The results from these experiments would provide insight into the following scientific questions: What are the cellular locations for different nAChR subunit expression and what could be the anatomical and functional implications? Can these immortalized bronchial epithelial cell lines be models for further study of the functional role of nAChR in bronchial epithelium? Understanding the pattern and regulatory mechanisms of nAChR subunit expression in human airway may give insight into their roles in inflammatory airway disorders and relevant lung function changes.
MATERIALS AND METHODS
This was a prospective cohort study. Consecutive subjects, having sputum atypia undergoing bronchoscopy with AFI (Fig. 1), were recruited. The inclusion criteria were sputum cytology examination demonstrating atypical cells but recent chest imaging did not reveal localizing lesion accountable for the sputum detection of atypical cells and ability to give informed, written consent. The exclusion criteria were patients having active hemoptysis or patients with bleeding tendency or on oral anticoagulation, which may increase the risk of bleeding from bronchoscopic biopsies even if oral anticoagulation was stopped before bronchoscopy. Chest radiographs and computed tomography (CT) thorax scans were done in the 6 wk before they were referred for AFI. Spirometry was performed 3 days before bronchoscopy. The study was approved by HKU/HKHA HKWC EC/IRB local Institutional Review Board (IRB) of the University of Hong Kong (HKU)/Hong Kong Hospital Authority Hong Kong West Cluster (HK HA HKWC 09-120), and the research has been carried out in accordance with the Declaration of Helsinki (2008). Nonsmokers were patients who had never smoked for their lifetime. Smokers included current smokers, who were current active chronic smokers, and ex-smokers, who had smoked daily for more than 12 mo in the past but had quit smoking at least 12 mo before bronchoscopy. Chronic smokers were asked to stop smoking, in particular on the day before lung function tests and bronchoscopy.
Fig. 1.
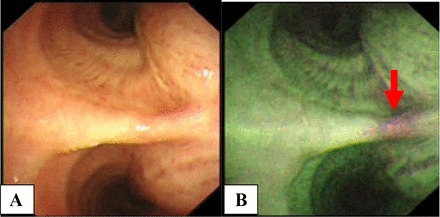
Side-by-side comparison of endoscopic view, with A and B showing ordinary white-light bronchoscopy (WLB) and the corresponding view under autofluorescence imaging (AFI), with the green fluorescence in AFI representing normal bronchial epithelium while the magenta areas (arrow) indicate the potential sites of abnormalities not visualized on the corresponding WLB views. Bronchial biopsies for this study were taken adjacent to AFI magenta areas where the areas appeared green in fluorescence under AFI.
At bronchoscopy with AFI, suspicious areas will show up as areas of magenta fluorescence color, in contrast to the green fluorescence color for normal bronchial epithelium (Fig. 1). After diagnostic specimens were biopsied from magenta areas, four additional bronchial biopsies were taken from the adjacent green fluorescent areas for this study. The first piece of these four additional biopsies was cultured, the next two pieces were paraffinized for histological sectioning, and the final piece was saved in RNAlater solution (Qiagen, Hilden, Germany). Total RNA was extracted from the piece of bronchial biopsy kept in RNAlater solution. Lung function tests were performed with the Vmax Encore lung function test system (CareFusion, San Diego, CA), and test results were interpreted according to the American Thoracic Society-European Respiratory Society recommendations (11).
Immortalized bronchial epithelial cell lines.
Four immortalized bronchial epithelial cell lines, HBEC-KT 2–5, were used (from John Minna MD, University of Texas Southwestern Medical Center at Dallas) (15). Three cell lines were established from recruited subjects with immortalization using the same laboratory protocol (15) and they were all beyond 100 passages at the time of these experiments. These new cell lines were named HKBS62N-KT, HKBS65.2N-KT, and HKBS150N-KT.
Complementary DNA synthesis.
Total RNA was extracted from tissue specimens and cell lines. RNA samples (1 μg) were reverse transcribed in 20 μl of reaction mix [5× First-Strand Buffer (ThermoFisher Scientific, Waltham, MA), DTT (100 mM, Promega, Madison, WI), dNTP (1 mM, Amersham Biosciences, Bath, UK), oligo-dT12-18 primers (ThermoFisher Scientific) and random hexamer (Promega, Madison, WI), RNaseOUT Recombinant Ribonuclease Inhibitor (ThermoFisher Scientific), and Superscript II Reverse Transcriptase (ThermoFisher Scientific)] with 1-h reaction at 42°C.
Quantitative PCR reactions.
The primers spanning across intron-exon junctions were designed for quantitative PCR targeting nAChR subunit genes α3, α4, α5, α6, α7, β2, β3, and β4 with 18S as the reference gene. The methodology used was the same as described before (7). Quantitative PCR reactions were carried out in triplicate. SYBR Green I [SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich, St. Louis, MO)] was used as the detection dye and ribosomal 18S was used as the reference gene. Final reaction volume was 10 μl with 1.25 μl of 1/10 Tris-EDTA-diluted cDNA from RT reaction, 0.1 μM of each specific primer, 5 μl SYBR Green Jumpstart Taq ReadyMix. Quantitative PCR cycles were set at 10 min denaturation, followed by 40 cycles of 95°C for 15 s, 60°C for 5 s, and 72°C for 20 s; and 72°C for 10 min as the final extension step. Dissociation curves were inspected for each pair of primers and only one dissociation peak must be present for each run of reaction before the results were considered to be valid. A fivefold serial dilution of a reference sample was used for construction of standard curves with respect to each pair of primers. Quantitative PCR was run for each tumor or cell line cDNA sample and the cycle time (Ct) for a particular sample was obtained from the standard curves for a specific pair of primers. The Ct for unknown samples was compared with Ct of reference samples to obtain the normalized relative amount for unknown samples. The normalized relative amount was then log2 transformed and approximation to normal distribution estimated. For qPCR data, the normalized relative amount for all samples was multiplied by a common factor of 1,024 and then log2 transformed, and the transformed qPCR data was used for direct visual inspection and comparison (7). The relative normalized amount of each nAChR subunit gene expression was correlated with lung function parameters.
Immunohistochemistry.
nAChR antibodies (Santa Cruz, Dallas, TX) targeting different subunits α3, α4, α5, α6, α7, β2, and β3 were used for tissue sections of bronchial epithelium. The immunohistochemical staining intensity (Grade 0–3) of nAChR at different cellular locations (bronchial cell cytosol, bronchial gland serous membrane, bronchial gland mucus component) was scored by an independent pathologist (K-H Fu) who was blinded to the clinical information of all recruited subjects (Fig. 2). The scored grading of each bronchial biopsy was correlated with lung function parameters.
Fig. 2.
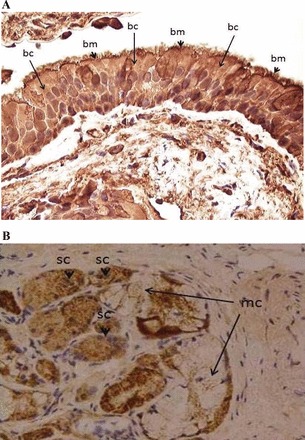
A: immunostaining pattern of bronchial epithelial cells. Arrowheads, apical cell membrane (bm); long arrows, cytosol (bc). There is accentuation of the staining at the apical cell membrane and terminal web (arrows) of the ciliated bronchial cells compared with the cytosol. Immunostain for nAChRs α4, Nikon Eclipse Ni-U with Plan Achromat ×40 objective, c-mount 0.7×, and 2/3″ charge-coupled device (CCD) camera with 5 megapixels. B: immunostaining pattern of bronchial gland acini. Arrowheads, serous cells (sc); long arrows mucous cells (mc). The staining reaction is seen mainly within the cytosol. Immunostain for nAChRs α3, Nikon Eclipse Ni-U with Plan Achromat ×20 objective, c-mount 0.7×, and 2/3″ CCD camera with 5 megapixels.
Response to nicotine exposure in normal HBEC lines.
Seven NHBE cell lines, i.e., HBEC-KT 2–5 (15), HKBS62N-KT, HKBS65N-KT, and HKBS150N-KT, were cultured and equal number of passages for each cell lines were randomized into nicotine group and control group. The HBECs were seeded and incubated for 24 h before addition of nicotine. On day 0, nicotine (100 nM) was added to the nicotine group and the same volume of culture medium without nicotine was added to the control group. Nicotine at 100 nM was found to be an easily achievable serum level in smokers (9). Both groups were further incubated for 9 days, after which the nicotine was removed, with one interim change of medium with or without nicotine, respectively, for each group. On day 9, both groups will switch to plain medium and were allowed to grow until day 12. Cells were harvested on days 0, 3, 6, 9, and 12. Nine days was chosen because the usual population doubling time was ∼10 days for these cultured bronchial epithelial cells. Total RNA was extracted for reverse transcription and quantitative PCR as described above.
Statistical analysis.
Student's t-tests, with assumptions of two tails and unequal variance, were used for comparison of expression level of different subunit genes between groups of samples with different sex distribution and smoking history. All expression levels were log2 transformed to achieve data normality, and χ2 tests were used for comparing the distribution of smokers and nonsmokers with high or low mean expression of individual or combinations of nAChR subunit genes. Bonferroni adjustments were applied when multiple comparisons of the expression levels or immunohistochemical staining resulted across different nAChR subunits. Statistical tests were carried out with IBM PASW for Windows version 21 (IBM, Armonk, NY).
RESULTS
Demographics of recruited subjects.
Seventy consecutive patients were referred for bronchoscopy with AFI for sputum atypia (Fig. 1). Six declined participation in the study. Four subjects were not recruited because of recent treatment with oral anticoagulation. Sixty subjects were recruited with informed, written consent obtained. There were 52 men and 8 women. The mean age was 61.8 ± 10.8 yr; 32 of them were chronic smokers (53.3%), 19 were ex-smokers (31.7%), and 9 were nonsmokers (15%). The mean amount of smoking was 24.9 ± 20.3 pack-years. The bronchial biopsies were reviewed by an independent pathologist (K. H. Fu) who was not involved in subject recruitment and was blinded to their clinical characteristics. Of the 60 bronchial biopsy specimens, 56 (93.3%) showed normal bronchial epithelium while two biopsies from smokers showed squamous metaplasia and two other biopsies from smokers showed moderate squamous dysplasia. The demographic characteristics of these patients are summarized in Table 1. These recruited subjects were not previously diagnosed to have COPD and they had no significant respiratory symptoms. Airflow obstruction (defined as FEV1/FVC < 70% on lung function tests) (5) was found in 37 (61.7%) of subjects with FEV1/FVC of 65.8 ± 12.9%, most of which were considered mild degree of airflow obstruction with FEV1 (% of predicted) at 87 ± 17.9%. CT scan of thorax for all the subjects showed no abnormalities.
Table 1.
Demographics
| All Subjects | IHC Evaluable | |
|---|---|---|
| Sex (F:M) | 8:52 | 4:35 |
| F | 8 (13.3%) | 4 (10.3%) |
| M | 52 (86.7%) | 35 (89.7%) |
| Mean age, yr (±SD) | 61.8 ± 10.8 | 63.1 ± 9.9 |
| Range | 30–83 | 33–79 |
| Smoking status (NS:CS:EX) | ||
| NS | 9 (15.0%) | 6 (15.3%) |
| CS | 32 (53.3%) | 19 (48.7%) |
| EX | 19 (31.7%) | 14 (35.9%) |
| Amount of smoking, pack-year | 24.9 ± 20.3 | 22.1 ± 18.6 |
| FEV1/FVC, % | 65.8 ± 12.9 | 68.7 ± 10.1 |
| Without AFO | 23 (38.3%) | 14 (35.9%) |
| With AFO | 37 (61.7%) | 25 (64.1%) |
| FEV1, liters | 2.01 ± 0.56 | 2.63 ± 0.49 |
| FEV1, % pred) | 87.0 ± 17.9 | 88.3 ± 15.4 |
| Mild (>80%) | 41 (68.3%) | 28 (71.8%) |
| Moderate (50–80%) | 19 (31.7%) | 11 (28.2%) |
| FVC, liters | 3.21 ± 0.73 | 3.44 ± 0.66 |
| FVC, % pred | 102.5 ± 15.2 | 99.8 ± 13.3 |
| Histology | ||
| No abnormality detected | 56 (93.3%) | 35 (89.8%) |
| Moderate squamous | 2 (3.4%) | 2 (5.1%) |
| Squamous metaplasia | 2 (3.4%) | 2 (5.1%) |
Basic demographics of all recruited subjects (n = 60); corresponding demographics of subjects with biopsies evaluable by immunohistochemistry (IHC evaluable; n = 39) are also listed in parallel.
F, female; M, male; NS, nonsmoker; CS, chronic smoker; EX, ex-smoker; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; AFO, airflow obstruction; % pred, percentage of predicted values.
Quantitative PCR analysis of nAChR subunit gene expression.
The relative normalized amount of nAChR mRNA expression of nAChR α4, α5, and α7 showed significant correlation with lung function, adjusted for sex and smoking status (Table 2). The mRNA expression level of nAChR α4 was found to correlate with most spirometry parameters of FEV1 (r = 0.325, P = 0.023) (Fig. 3A), FEV1 (% pred) (r = 0.375, P = 0.010), FVC (r = 0.485, P = 0.041), FVC (% pred) (r = 0.593, P = 0.022). The same correlation patterns were found for nAChR α5 mRNA expression level, correlating with FEV1 (r = 0.338, P = 0.049) (Fig. 3B), FEV1 (% pred) (r = 0.403, P = 0.026), FVC (r = 0.469, P = 0.024), FVC (% pred) (r = 0.587, P = 0.011), and for nAChR α7 mRNA correlating with FEV1 (r = 0.370, P = 0.034) (Fig. 3C), FEV1 (% pred) (r = 0.425, P = 0.011), FVC (% pred) (r = 0.576, P = 0.048). The nAChR β2 mRNA was found to correlate with FEV1/FVC ratio (r = −0.219, P = 0.011).
Table 2.
Partial correlations of relative normalized amount of each CHRN subunit gene mRNA expression levels with lung function parameters
| CHRNA3 | CHRNA4 | CHRNA5 | CHRNA6 | CHRNA7 | CHRNB2 | CHRNB3 | CHRNB4 | |
|---|---|---|---|---|---|---|---|---|
| FEV1 | 0.019 (P = 0.587) | 0.325* (P = 0.014*) | 0.338* (P = 0.010*) | −0.002 (P = 0.059) | 0.370* (P = 0.004*) | −0.061 (P = 0.302) | −0.108 (P = 0.833) | −0.053 (P = 0.263) |
| FEV1, % pred | 0.040 (P = 0.355) | 0.375* (P = 0.043*) | 0.403* (P = 0.011*) | −0.071 (P = 0.071) | 0.425* (P = 0.023*) | −0.080 (P = 0.456) | −0.181 (P = 0.111) | −0.126 (P = 0.112) |
| FVC | 0.038 (P = 0.217) | 0.485* (P = 0.041*) | 0.469* (P = 0.032*) | −0.144 (P = 0.231) | 0.471 (P = 0.054) | −0.179 (P = 0.125) | −0.050 (P = 0.542) | −0.061 (P = 0.255) |
| FVC, % pred | 0.086 (P = 0.116) | 0.593* (P = 0.021*) | 0.587* (P = 0.011*) | −0.033 (P = 0.067) | 0.576* (P = 0.013*) | −0.211 (P = 0.433) | −0.119 (P = 0.113) | −0.234 (P = 0.132) |
| FEV1/FVC | 0.154 (P = 0.213) | 0.037 (P = 0.313) | 0.056 (P = 0.543) | −0.120 (P = 0.121) | 0.076 (P = 0.073) | −0.419* (P = 0.043*) | −0.079 (P = 0.217) | −0.072 (P = 0.562) |
r values are shown correlating lung function parameters with quantitative expression of various nicotinic acetylcholine receptors (nAChRs). Significant P values (
P < 0.05) are underlined in bold.
Fig. 3.
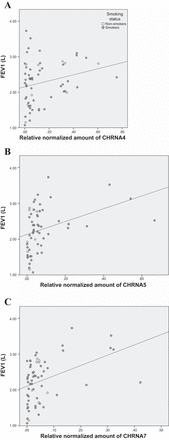
Scatterplots showing significant correlations of forced expiratory volume in 1 s (FEV1) with relative normalized amount of CHRNA4 (A), CHRNA5 (B), and CHRNA7 (C) mRNA expression level.
Immunohistochemical staining.
Of 60 subjects recruited, 39 endobronchial biopsies were found to be of sufficient evaluable quality after histological examination to proceed on to immunohistochemistry. The age and sex distribution, smoking status, and lung function parameters of these 39 subjects were found to be similar and not significantly different from those characteristics of the whole group of 60 recruited subjects (Table 1). The staining intensity of nAChR α4 bc (surface epithelial cytosol staining), α5 sc (bronchial gland serous cell cytosol staining), and β3 mc (bronchial gland mucous cell staining) intensity (Fig. 2) was found to correlate with lung function parameters. The nAChR α4 bc staining intensity was found to correlate with FVC (% pred) (r = 0.552, P = 0.025), while nAChR α5 sc staining intensity was found to correlate with FEV1 (% pred) (r = 0.369, P = 0.026) and nAChR β3 mc staining intensity correlated with FEV1 (r = 0.367, P = 0.035), adjusted for the effects of age, sex, and smoking status of included subjects (Table 3).
Table 3.
Correlations of expression level of different nAChR by immunohistochemical intensity with lung function parameters
| bm | bc | dm | dc | sm | sc | mm | mc | |
|---|---|---|---|---|---|---|---|---|
| nAChR α4 | ||||||||
| FVC, % pred | −0.439 | 0.552* | 0.019 | −0.025 | 0.363 | 0.180 | 0.363 | 0.123 |
| nAChR α5 | ||||||||
| FEV1, % pred | 0.121 | 0.066 | 0.073 | −0.295 | 0.077 | 0.369* | 0.014 | 0.244 |
| nAChR β3 | ||||||||
| FEV1 | 0.177 | 0.210 | −0.088 | −0.270 | −0.148 | 0.317 | −0.081 | 0.367* |
Staining by cellular location: bm, surface epithelial cell membrane; bc, surface epithelial cytosol; dm, bronchial gland duct membrane; dc, bronchial gland duct cytosol; sm, bronchial gland serous cell membrane; sc, bronchial gland serous cell cytosol; mm, bronchial gland mucous cell membrane; mc, bronchial gland mucous cell cytosol; r values are shown with significant values (*P < 0.05) indicated.
Correlations of nAChR subunit gene mRNA expression levels with immunohistochemical staining intensity.
The mRNA expression levels of nAChR subunit gene α4, α5, α7, and β2 showed significant and highest correlation coefficient with the corresponding immunohistochemical staining intensity (Table 4). There were also significant but weaker correlations between the mRNA expression levels with immunohistochemical staining intensity for nAChR α4, α5, α6, and α7 (Table 4), and the significance remains after adjustment for multiple comparisons.
Table 4.
Immunohistochemical expression
| mRNA expression | CHRNA3 | CHRNA4 | CHRNA5 | CHRNA6 | CHRNA7 | CHRNB2 | CHRNB3 |
|---|---|---|---|---|---|---|---|
| α3 | 0.067 | 0.161 | 0.183 | 0.086 | 0.132 | 0.150 | 0.292 |
| α4 | 0.254 | 0.579* | 0.359* | 0.141 | 0.401* | 0.175 | 0.126 |
| α5 | 0.063 | 0.373* | 0.595* | 0.079 | 0.433* | 0.149 | 0.234 |
| α6 | 0.139 | 0.500* | 0.360* | 0.090 | 0.252 | 0.197 | 0.293 |
| α7 | 0.083 | 0.366* | 0.194 | 0.082 | 0.552* | 0.152 | 0.227 |
| β2 | 0.067 | 0.139 | 0.138 | 0.029 | 0.031 | 0.531* | 0.253 |
| β3 | 0.093 | 0.128 | 0.051 | 0.003 | 0.118 | 0.277 | 0.076 |
Correlation matrix of nAChR mRNA expression levels with immunohistochemical intensity. The header row lists the mRNA expression of different nAChR subunits correlating with the corresponding nAChR subunit detection by immunohistochemistry in each column. Significant correlations are in bold
(P < 0.05).
Exposure of normal HBEC lines to nicotine.
When HBEC lines were exposed to 100 nM nicotine and then RNA harvested at days 0, 3, 6, 9, and 12, a significant rise in the expression levels of CHRN A5 and A7 was found with rapid return to baseline levels of expression upon nicotine removal (Fig. 4, A and C). There was a sharp rise in nAChR α5 subunit gene expression upon exposure to nicotine, and a similar sharp but slightly delayed rise in nAChR α7 subunit gene expression. Both these responses dropped with withdrawal of nicotine on day 9 (Fig. 4, A and C). There was also a sharp but not sustained rise in nAChR α6 subunit upon nicotine exposure and it dropped after day 6 before nicotine was removed on day 9 (Fig. 4B). No significant rise or fall was found in the levels of expression of other nAChR subunit genes analyzed, including CHRN A3, A4, B2, B3, and B4.
Fig. 4.
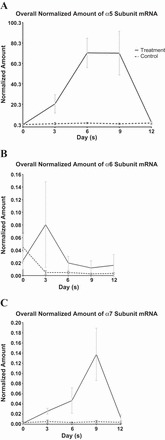
Graphic representations of the results of nicotine exposure for 9 days in immortalized bronchial epithelial cells [with the solid lines summarizing mean ± SE of cell lines exposed to nicotine (treatment group) and the dashed lines representing the respective cell lines without exposure to nicotine (control group)] showing sharp rise in nAChR α5 subunit gene expression upon exposure to nicotine (A), with a similar sharp but a slightly delayed rise in nAChR α7 subunit gene expression (C). Both these responses dropped with withdrawal of nicotine on day 9. B: there was also a sharp but not sustained rise in nAChR α6 subunit upon nicotine exposure but it dropped after day 6 before nicotine was removed on day 9.
DISCUSSION
The distribution, function, and ligand-binding affinity of nAChR depends on the composition of nAChR subunits, although the exact function and physiological roles of individual nAChR subtypes are not completely understood. To our knowledge, the correlation of expression of these nAChR subunits with lung function has not been reported before. The quantitative PCR analysis of nAChR subunit gene expression levels allowed for quantitative comparison between groups of samples with respect to sex distribution and smoking habits.
In this study the most significant correlations between lung function parameters and nAChR α4, α5, and α7 expression levels or the corresponding immunohistochemical staining intensity of each nAChR subunit expression. CHRNA5 is known to be associated with nicotine dependence (21) and the presence of emphysema in smokers (8, 13). The result of this study provided direct support to previous GWAS study that CHRNA5 expression was linked to the development of airflow obstruction (20) independent of effects of age, sex, and smoking status. The subjects included in this subjects were either having no airflow obstruction or just mild degree of it [FEV1 (% of predicted value) between 50–80%]. The presence of nAChR α5 subunit in nAChR has been shown to alter the calcium permeability and nicotine sensitivity in vitro (4). nAChR α7 subunit was found to be important in the control of nicotine-induced calcium-influx in SCLC (17) and was thus thought to be important in growth signal transduction induced by nicotine binding to nAChR. The expression of nAChR α5 and α7 in normal bronchial epithelium suggested that they may play similar roles in nAChR signaling and such correlation with airflow parameters on lung function tests would suggest that there could be activated nAChR signaling in response to nicotine in smokers correlating with airflow obstruction.
Although we have found significant reduction in nAChR α4 expression in lung cancer (7), there is so far no further evaluation of its role in normal bronchial epithelium. There is recent evidence that nAChR α7 and α4β2 activation (16) could be associated with transactivation via cyclic adenosine monophosphate (cAMP) stimulation of the epidermal growth factor receptor (EGFR) signaling pathways inducing cell growth and proliferation, thus may contribute to carcinogenesis (16). This cAMP activation could also be a potential target for phosphodiesterase inhibition, which is one therapeutic strategy in targeting inflammation in COPD (5). The transient but not sustainable nAChR α6 that dropped before nicotine withdrawal was also intriguing and deserves further investigation into the roles of nAChR α6 in normal bronchial epithelial cells.
CHRNA5 and A7 showed significant reversible induction of expression on in vitro exposure to nicotine, providing direct evidence that these subunit genes respond to acute nicotine exposure and could mediate the immediate or short-term effects of nicotine. Little is known about the functional significance of the expression these specific CHRNA5 and A7 in the central nervous system or in lung cancer, although CHRNA7 had been reported to be expressed in SCLC cell lines (18) and was thought to be related to smoking in schizophrenic patients (3). Upregulation of functional CHRNA7 has also been demonstrated in normal HBECs on exposure to nicotine (19). The return of the expression levels of those three subunits, of nAChR α5, α6, and α7, to baseline upon cessation of nicotine exposure may reflect that continued or chronic exposure to nicotine (usually taken to be more than 10 days) of continuous exposure to nicotine (14) was required for nicotine addiction or other cellular effects of nicotine mediated by specific nAChR subunits. The effects of chronic exposure to nicotine on nAChR subunit gene expression in these normal HBECs and whether the same response is maintained, or other nAChR subunit genes would be involved, in chronic nicotine exposure warrant further evaluation.
In neural tissues, the upregulation of nAChRs in response to chronic nicotine is consistently reported as posttranslational events without changes in mRNA (2, 6). In this study, we have demonstrated nicotine induced increases in the expression levels of both nAChR mRNA and protein in culture bronchial epithelial cells, nicotine-induced upregulation of nicotinic receptors in bronchial epithelial cells may include both transcriptional and posttranslational mechanisms.
Although this is a small sample size, it did show correlation between different nAChR subunits in terms of mRNA expression level and the protein expression level by immunohistochemical staining. nAChR expression was correlated with lung function parameters that are suggestive of airflow obstruction. The other parts of lung function tests including lung capacity assessment and diffusion capacity measurements were not performed in this study. This supported our initial hypothesis of correlations between nAChR expression and lung functions, contributing to the development of airflow obstruction, which could be one characteristic of smokers with COPD. Validation in a larger cohort of smoker and nonsmoker subjects would be warranted but this initial sample of 60 apparently healthy subjects was already difficult to recruit. The chance of validation in COPD subjects would be even more difficult because there is seldom indication for bronchoscopic biopsy or surgical biopsy in COPD subjects, adding to the difficulties in performing similar clinical studies. The functional roles of different nAChR subunits, working on individual basis or in combination, in bronchial epithelium and how their expression levels translate into lung function abnormalities and ultimately clinical airflow obstruction requires further work.
In summary, there are correlations between nAChR subunit expression in the bronchial epithelium with lung function parameters. These nAChR subunit genes could be contributing to the development of airflow obstruction in smokers. Further evaluation of the functions and roles played by these nAChR subunit genes in the development of tobacco-related airway disorders are warranted.
GRANTS
The work described in this manuscript was supported by the University of Hong Kong Seed Funding 2010 and NCI SPORE in Lung Cancer (P50CA70907).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.C.-L.L., I.I.W., M.S.-M.I., and J.D.M. conception and design of research; D.C.-L.L., K.-H.F., M.M.-S.L., K.-H.C., I.I.W., B.G., S.-W.T., M.S.-M.I., and J.D.M. analyzed data; D.C.-L.L., S.Y.L., K.-H.F., M.M.-S.L., K.-H.C., I.I.W., B.G., S.-W.T., M.S.-M.I., and J.D.M. interpreted results of experiments; D.C.-L.L. and K.-H.F. prepared figures; D.C.-L.L., S.Y.L., M.M.-S.L., K.-H.C., I.I.W., B.G., S.-W.T., M.S.-M.I., and J.D.M. drafted manuscript; D.C.-L.L., S.Y.L., K.-H.F., M.M.-S.L., K.-H.C., I.I.W., B.G., S.-W.T., M.S.-M.I., and J.D.M. edited and revised manuscript; D.C.-L.L., S.Y.L., K.-H.F., M.M.-S.L., K.-H.C., I.I.W., B.G., S.-W.T., M.S.-M.I., and J.D.M. approved final version of manuscript; S.Y.L. and K.-H.F. performed experiments.
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance by Kenneth M. K. Lau and Arthur C. F. Leung and the statistical support provided by Crystal Kwan.
REFERENCES
- 1.Budulac SE, Vonk JM, Postma DS, Siedlinski M, Timens W, Boezen MH. Nicotinic acetylcholine receptor variants are related to smoking habits, but not directly to COPD. PloS One 7: e33386, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo SF, Mazzo F, Pistillo F, Gotti C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem Pharmacol 86: 1063–1073, 2013. [DOI] [PubMed] [Google Scholar]
- 3.De Luca V, Likhodi O, Van Tol HH, Kennedy JL, Wong AH. Regulation of alpha7-nicotinic receptor subunit and alpha7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatr Scand 114: 211–215, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther 286: 311–320, 1998. [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Available from http://www.goldcopd.org, 2015.
- 6.Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 78: 756–765, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam DC, Girard L, Ramirez R, Chau WS, Suen WS, Sheridan S, Tin VP, Chung LP, Wong MP, Shay JW, Gazdar AF, Lam WK, Minna JD. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res 67: 4638–4647, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Lambrechts D, Buysschaert I, Zanen P, Coolen J, Lays N, Cuppens H, Groen HJ, Dewever W, van Klaveren RJ, Verschakelen J, Wijmenga C, Postma DS, Decramer M, Janssens W. The 15q24/25 susceptibility variant for lung cancer and chronic obstructive pulmonary disease is associated with emphysema. Am J Respir Crit Care Med 181: 486–493, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth Differ 5: 1033–1040, 1994. [PubMed] [Google Scholar]
- 10.Maus AD, Pereira EF, Karachunski PI, Horton RM, Navaneetham D, Macklin K, Cortes WS, Albuquerque EX, Conti-Fine BM. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol 54: 779–788, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Minna JD. Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer. J Clin Invest 111: 31–33, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai SG, Kong X, Edwards LD, Cho MH, Anderson WH, Coxson HO, Lomas DA, Silverman EK; ECLIPSE and ICGN Investigators . Loci identified by genome-wide association studies influence different disease-related phenotypes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 1498–1505, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prendergast MA, Harris BR, Mayer S, Littleton JM. Chronic, but not acute, nicotine exposure attenuates ethanol withdrawal-induced hippocampal damage in vitro. Alcohol Clin Exp Res 24: 1583–1592, 2000. [PubMed] [Google Scholar]
- 15.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res 64: 9027–9034, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer 9: 195–205, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Sheppard BJ, Williams M, Plummer HK, Schuller HM. Activation of voltage-operated Ca2+-channels in human small cell lung carcinoma by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Int J Oncol 16: 513–518, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Song P, Sekhon HS, Jia Y, Keller JA, Blusztajn JK, Mark GP, Spindel ER. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res 63: 214–221, 2003. [PubMed] [Google Scholar]
- 19.Wang Y, Pereira EF, Maus AD, Ostlie NS, Navaneetham D, Lei S, Albuquerque EX, Conti-Fine B. Human bronchial epithelial and endothelial cells express α7 nicotinic acetylcholine receptors. Mol Pharmacol 60: 1201–1209, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Wilk JB, Shrine NR, Loehr LR, Zhao JH, Manichaikul A, Lopez LM, Smith AV, Heckbert SR, Smolonska J, Tang W, Loth DW, Curjuric I, Hui J, Cho MH, Latourelle JC, Henry AP, Aldrich M, Bakke P, Beaty TH, Bentley AR, Borecki IB, Brusselle GG, Burkart KM, Chen TH, Couper D, Crapo JD, Davies G, Dupuis J, Franceschini N, Gulsvik A, Hancock DB, Harris TB, Hofman A, Imboden M, James AL, Khaw KT, Lahousse L, Launer LJ, Litonjua A, Liu Y, Lohman KK, Lomas DA, Lumley T, Marciante KD, McArdle WL, Meibohm B, Morrison AC, Musk AW, Myers RH, North KE, Postma DS, Psaty BM, Rich SS, Rivadeneira F, Rochat T, Rotter JI, Artigas MS, Starr JM, Uitterlinden AG, Wareham NJ, Wijmenga C, Zanen P, Province MA, Silverman EK, Deary IJ, Palmer LJ, Cassano PA, Gudnason V, Barr RG, Loos RJ, Strachan DP, London SJ, Boezen HM, Probst-Hensch N, Gharib SA, Hall IP, O'Connor GT, Tobin MD, Stricker BH. Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. Am J Respir Crit Care Med 186: 622–632, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Summah H, Zhu YG, Qu JM. Nicotinic acetylcholine receptor variants associated with susceptibility to chronic obstructive pulmonary disease: a meta-analysis. Respir Res 12: 158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zia S, Ndoye A, Nguyen VT, Grando SA. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res Commun Mol Pathol Pharmacol 97: 243–262, 1997. [PubMed] [Google Scholar]


