Abstract
Renin is synthesized in the principal cells of the collecting duct (CD), and its production is increased via cAMP in angiotensin (ANG) II-dependent hypertension, despite suppression of juxtaglomerular (JG) renin. Vasopressin, one of the effector hormones of the renin-angiotensin system (RAS) via the type 2-receptor (V2R), activates the cAMP/PKA/cAMP response element-binding protein (CREB) pathway and aquaporin-2 expression in principal cells of the CD. Accordingly, we hypothesized that activation of V2R increases renin synthesis via PKA/CREB, independently of ANG II type 1 (AT1) receptor activation in CD cells. Desmopressin (DDAVP; 10−6 M), a selective V2R agonist, increased renin mRNA (∼3-fold), prorenin (∼1.5-fold), and renin (∼2-fold) in cell lysates and cell culture media in the M-1 CD cell line. Cotreatment with DDAVP+H89 (PKA inhibitor) or CREB short hairpin (sh) RNA prevented this response. H89 also blunted DDAVP-induced CREB phosphorylation and nuclear localization. In 48-h water-deprived (WD) mice, prorenin-renin protein levels were increased in the renal inner medulla (∼1.4- and 1.8-fold). In WD mice treated with an ACE inhibitor plus AT1 receptor blockade, renin mRNA and prorenin protein levels were still higher than controls, while renin protein content was not changed. In M-1 cells, ANG II or DDAVP increased prorenin-renin protein levels; however, there were no further increases by combined treatment. These results indicate that in the CD the activation of the V2R stimulates renin synthesis via the PKA/CREB pathway independently of RAS, suggesting a critical role for vasopressin in the regulation of renin in the CD.
Keywords: intrarenal renin-angiotensin system, collecting duct, water deprivation, distal tubular renin, prorenin, PKA/CREB
the renin-angiotensin system (RAS) plays a pivotal role in the regulation of blood pressure (BP) and in the maintenance of body sodium and fluid balance. There is growing evidence demonstrating the presence of the RAS in the tissues of renal and cardiovascular systems (5–7, 35), supporting the importance of the tissue RAS in the accomplishment of its functions. The kidneys generate angiotensin II (ANG II) de novo (46, 47). The luminal presence of angiotensinogen (AGT) in the proximal tubules (PT) (24), angiotensin-converting enzyme (ACE) in the collecting duct (CD) (3, 15, 25, 39), and prorenin and renin in the distal nephron has been established (40, 41). These findings further support the formation of intratubular ANG II from sequential coordinated actions of tubular renin and ACE that act on AGT substrate delivered from the proximal segments. As evidence, ANG II, AGT, and renin contents are increased in the kidneys from ANG II-infused hypertensive rats (12), which contribute to sodium reabsorption (48). However, the mechanisms regulating renin in the CD and its physiological implications are not well understood.
There is consensus evidence showing that the cAMP-PKA and cAMP response element-binding protein (CREB) pathway constitute the central pathway for renin regulation in JG cells (17, 26). Klar et al. (23) showed that cAMP formation mediated by forskolin, an activator of adenylate cyclase (AC), increased the activity of renin promoter thus stimulating renin gene transcription in juxtaglomerular (JG) cells. In the distal nephron, renin synthesis and transcription are upregulated by ANG II through the activation of the AT1 receptor and PKC (8).
Vasopressin (AVP) is a potential effector of the RAS (16) in CD cells. The activation of the AVP type 2 receptor (V2R) stimulates the cAMP/PKA/CREB pathway and aquaporin-2 (AQP2) expression in the apical plasma membrane of principal cells in the CD (28). AT1 receptor blockade in rats treated with the specific V2R agonist desmopressin (DDAVP) and subjected to dietary NaCl restriction decreased urine osmolality and blunted the DDAVP-induced upregulation and phosphorylation of AQP2 via PKA (27). This further supports a role for ANG II in the regulation of AQP2. Nonetheless, it has not been determined whether the V2R regulates CD renin abundance.
In the present study, we tested the hypothesis that V2R activation in CD cells stimulates renin synthesis via the PKA/CREB pathway independently of the AT1 receptor. We performed in vitro studies using M-1 cells to determine the effects of DDAVP treatment on renin and the signaling pathways involved. To establish in vivo relevance, we evaluated the renin and prorenin responses in renal inner medullary tissues, devoid of renin from JG cells, from mice subjected to 48-h water deprivation and RAS blockade.
MATERIALS AND METHODS
M-1 cell cultures.
The M-1 cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were harvested after 6 h of treatments. DDAVP (catalog no. 16679-58-6, Sigma-Aldrich, St. Louis, MO) was tested at doses of 10−10 to 10−6 M. Forskolin (catalog no. 66575-29-9, Sigma-Aldrich) was used at 10−6 M. H89 (catalog no. B1427, Sigma-Aldrich) was used at 10−6 M. ANG II (catalog no. 4474-91-3, Sigma-Aldrich), was used at 10−7 M, and tolvaptan (catalog no. 150683-30-0, Sigma-Aldrich) was used at 10−6 M. Calphostin C (catalog no. 121263-19-2, Santa Cruz Biotechnology, Santa Cruz, CA) was used at 10−7 and 10−6 M. Losartan (catalog no. 124750-99-8, Sigma) was used at 10−6 M.
PKA assay and cAMP measurements.
M-1 cells were seeded and treated with DDAVP, and the reaction was stopped by removal of the medium and addition of a cocktail of protease inhibitors (catalog no. P9599, Sigma-Aldrich). PKA activity was measured using a PKA kinase activity ELISA kit from Enzo-Life Sciences (catalog no. ADI-EKS-390A, Glasgow, UK) after 20-min treatment. The cAMP levels of M-1 cells were determined with cAMP ELISA (Cayman, Ann Arbor, MI) according to the manufacturer's protocol. Briefly, cells were stimulated with DDAVP or DDAVP plus inhibitors or vehicle (PBS) for 20 min and harvested in 100 μl of 0.1 M HCl containing 10−9 M IBMX to prevent the degradation of cAMP. The ANG II+IBMX group (see Fig. 5B) was also harvested in the presence of IBMX. After centrifugation at 1,000 g for 10 min, the supernatants were decanted into clean new tubes to process immediately or store at −80°C. For cAMP assay, the samples were diluted two- to threefold using a kit provided for ELISA buffer. Fifty microliters of the diluted samples and the cAMP standards were added to each well of the plate precoated with mouse anti-rabbit IgG cAMP ELISA (Cayman) using equal amounts of protein following the manufacturer's instructions.
Fig. 5.

A: DDAVP- and ANG II-independent upregulation of prorenin and renin in vitro. M-1 cells were treated with DDAVP (10−6 M), ANG II (10−7 M), or DDAVP+ANG II for 4 h. Combined treatment did not enhance prorenin and renin protein levels. B: cAMP levels in M-1 cells subjected to various treatments. DDAVP increased cAMP levels, as well as was observed by ANG II treatment alone. ANG II+DDAVP increased cAMP levels to the same extent in M-1 cells without an additive effect. AT1 receptor blockade with losartan (Los) did not affect the increases in cAMP. Calphostin C (PKC inhibitor, 10−7 M) in the presence of ANG II and DDAVP partially reduced cAMP formation. Higher dose of calphostin C blunted this effect, indicating that the stimulation of cAMP formation by DDAVP and ANG II is dependent of PKC. *P < 0.05. **P < 0.01 vs. vehicle-treated group. #P < 0.05 vs. ANG II+DDAVP and DDAVP groups.
RNA isolation and real-time quantitative PCR for renin mRNA.
Total RNA was extracted by using the RNeasy Mini Kit (Qiagen, Valencia, CA). Primers and probes used to amplify renin mRNA using the TaqMan System were forward: 5′-AGTACTATGGTGAGATCGGCATT-3′, reverse: 5′-AGATTCACAACCTCTATGACTCCTC-3′, and a fluorogenic probe: 5′6′-FAM-TTCAAAGTCATCTTTGACACGGGTTCAG-BHQ1-3. A mouse β-actin gene was used as an internal standard: forward: 5′-ATCATGAAGTGTGACGTTGA-3′, reverse: 5′-GATCTTCATGGTGCTAGGAGC-3′, and a fluorogenic probe: 5′-6-HEX-TCTATGCCAACACAGTGCTGTCTGGT-BHQ2-3′. The qRT-PCR was performed in the Mx3000P system (Stratagene, Santa Clara, CA).
Renin, prorenin, and CREB protein expression by western blot analysis.
Twenty micrograms of total protein were separated and transferred to a nitrocellulose membrane (Invitrogen, Carlsbad, CA). Anti-phospho-CREB and total CREB were obtained from Cell Signaling (catalog no. mAb 9198, Danvers, MA). For prorenin and renin detection, polyclonal IgG B-12 antibody was used (Santa Cruz Biotechnology). Results were expressed as the ratio between the abundance of the protein of interest and β-actin. Recombinant mouse prorenin (AnaSpec, Fremont, CA) and renin (Lee Biosolutions St. Louis, MO) were used as standards. Cell culture media (1 ml) was centrifuged at 3,000 rpm for 5 min, and the supernatant was concentrated (10×) using Amicon ultrafilter units (Millipore, Temecula, CA) after 20–30 min of centrifugation. Final recovery volume was 40 μl, and 40 μg of total protein were used for Western blot analysis.
CREB knockdown in M-1 cells using short hairpin RNA.
Plasmids containing four different sequences [SureSilencing short hairpin (sh) RNA Plasmids, catalog no. 336311, Qiagen] were purified (see Fig. 4A) and transfected (1 μg of DNA) using Lipofectamine LTX (Invitrogen, Carlsbad, CA) for 36 h. Efficiency of transfection was confirmed by green fluorescent protein (GFP) detection (see Fig. 4B). Reduction of CREB protein expression reached 67 ± 9% quantified by the CREB vs. β- actin ratio (see Fig. 4C). A scrambled shRNA sequence containing GFP was used as a negative control.
Fig. 4.
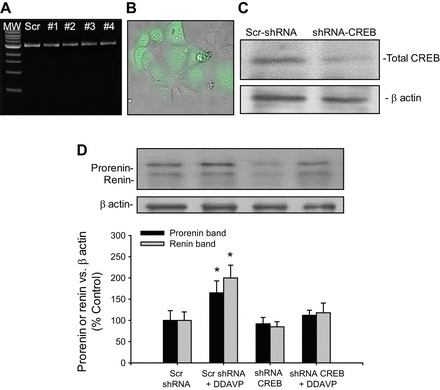
A: integrity of the CREB-short hairpin (sh)RNA plasmids (1–4) and scrambled control (Scr) after purification assessed by agarose gels. MW, molecular weight standard. B: green fluorescent protein (GFP)-containing shRNA plasmid (green) showing transfection efficiency. C: representative Western blot showing the knockdown of CREB protein (67 ± 9%). D: CREB-shRNA prevented the DDAVP-mediated increase in prorenin and renin upregulation in M-1 cells. *P < 0.05 vs. control group (Scr shRNA).
Renin and CREB immunofluorescence.
M-1 cells were fixed in cold methanol, blocked, stained with either rabbit anti-renin (B-12, Santa Cruz Biotechnology) or total CREB (Cell Signaling, New England Biolabs, Beverly, MA), and detected with Alexa Fluor 594-conjugated to anti-rabbit IgG (Invitrogen). Samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). Negative controls were obtained by omission of the specific primary antibody. NIS Elements analysis software (Nikon) was used to quantify renin-prorenin immunostaining (object count) and nuclei CREB localization based on their DAPI (nuclei) colocalization by measuring the merging area of CREB-DAPI in n = 6 fields (×100). Results are expressed as the percentage of nuclei-CREB colocalization (see Fig. 3B).
Fig. 3.
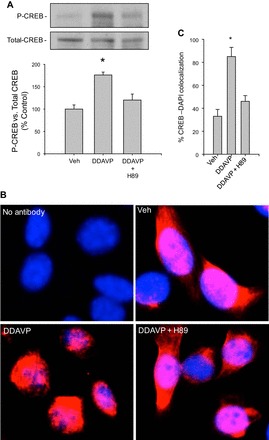
Inhibition of PKA activity diminished the DDAVP-induced CREB phosphorylation and nuclear localization in M-1 cells. A: levels of the phosphorylated form of CREB normalized by total CREB after immunoblotting. B: CREB-4′,6-diamidino-2-phenylindole (DAPI) colocalization by immunofluorescence showing that CREB is undergoing nuclear destination after DDAVP treatment; however, H89 pretreatment blunted this effect as judged by immunofluorescence colocalization (red) with DAPI (blue) staining. C: percentage of CREB-DAPI colocalization in each group *P < 0.05 vs. vehicle-treated group; n = 6.
In vivo treatments, water restriction protocol, and animal tissue sampling.
The Institutional Animal Care and Use Committees approved all animal protocols. Male CF-1 mice (18–20 g, n = 5, Charles River, Wilmington, MA) were subjected to 48-h water deprivation or ad libitum tap water supplementation. Another set of animals (n = 5) was subjected to a combined treatment with losartan (30 mg·kg−1·day−1, Cozaar) and captopril (40 mg·kg−1·day−1, Fisher Scientific, Houston, TX) supplemented in food, with free access to water or water restriction for 48 h. To increase plasma osmolality, we performed intraperitoneal injection of 20% mannitol (34) equivalent to ∼1.6 g/kg in saline solution in an additional set of mice (n = 5). Mice were euthanized after 1 h, and prorenin and renin protein abundance was compared with control animals receiving saline injection. Overall, all mice were euthanized using CO2 following treatments. Renal tissues were stereo microdissected, washed in PBS three times, and 15 mg of inner medullary tissues devoid of renin from JG cells were used to measure renin mRNA or protein. As previously demonstrated (37), no differences between the amount of prorenin or renin protein levels were found in perfused kidneys vs. freshly isolated dissected medullary tissues (data not shown).
Immunostaining.
Renin immunostaining was performed in paraformaldehyde-fixed kidney consecutive sections with the peroxidase technique using renin antibody provided by Dr. Tadashi Inagami (Vanderbilt University) as previously described (37). AQP2 immunostaining was performed with the peroxidase technique using an anti-AQP2 antibody (sc-28829, Santa Cruz Biotechnology). Periodic acid-Schiff (PAS) staining was performed for better characterization of the structures.
Plasma osmolality measurements.
Plasma osmolality was measured using a vapor pressure osmometer (model 5520; Wescor, Logan, UT). Blood samples were centrifuged at 14,000 rpm, and 10 μl were used. Measurements were made from filter disks absorbed with plasma fluid. Appropriate osmometer calibration was verified by measurement of standards before the sampling.
Statistical analyses.
The mRNA measurements were done in triplicate. For Western blotting, an average number of five to six independent observations were performed for each treatment. Data were evaluated by the Grubb test followed by paired and unpaired Student t-tests or by one-way ANOVA with Tukey's posttest. For mRNA and protein data, control levels were defined as 100%. Significance was defined as P < 0.05. Results are expressed as means ± SE.
RESULTS
DDAVP increases renin synthesis in M-1 cells.
The immunoblotting data revealed two bands, one at ∼45 and the other at 38 kDa (Fig. 1A), consistent with previous reports (12, 19, 32). DDAVP elicited dose-related increases in renin mRNA (306 ± 30 vs. 100 ± 12%, P < 0.001), prorenin (165 ± 23 vs. 100 ± 10%, P < 0.05), and renin (200 ± 13 vs. 100 ± 15%, P < 0.05) in cell lysates (Fig. 1, B and C). At 10−8 M, we observed slight increases in prorenin protein levels but significant increases in renin protein (142 ± 15%, P < 0.05) and renin mRNA (135 ± 19%, P < 0.05) levels. PKA activity was increased at a dose of 10−8 M of DDAVP (Abs-450: 0.67 ± 0.11 vs. vehicle: 0.45 ± 0.12, n = 4, P < 0.05) and 10−6 M (Abs 450: 0.95 ± 0.14, n = 4, P < 0.05 vs. vehicle). These results agree with the increased phosphorylation of CREB at the same doses (p-CREB/T-CREB ratio: 135 ± 13 and 150 ± 15%, P < 0.05, Fig. 1D). Immunofluorescence in M-1 cells (Fig. 1E) showed a marked increase in prorenin-renin immunostaining after DDAVP treatment (object count: 435 ± 13 vs. control: 27 ± 8). DDAVP significantly increased the prorenin band, starting at 10−10 M [arbitrary units (AU)/mg protein: 28 ± 4 vs. 12 ± 3, P < 0.05] in the cell culture media. We did not detect a renin band at basal conditions; however, the band was present at 10−10 M DDAVP (Fig. 1F). Tolvaptan, a V2R antagonist, blunted DDAVP-mediated inductions on prorenin and renin protein abundances (Fig. 1, E and G) and prorenin-renin immunofluorescence (object count: 15 ± 4 vs. control: 27 ± 8), confirming the receptor specificity of DDAVP.
Fig. 1.
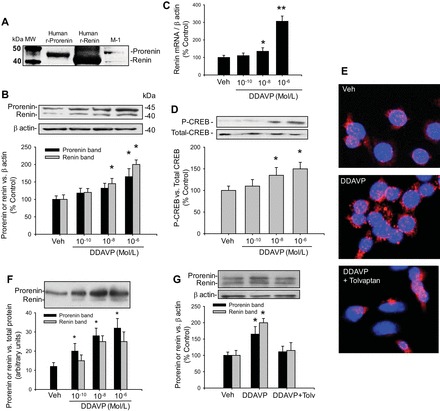
Renin expression in M-1 collecting duct cells is increased by desmopressin (DDAVP) treatment. A: renin and prorenin protein bands were visualized by Western blotting using 40 μg of protein extracts. The identity of renin (∼38 kDa) and prorenin (∼45 kDa) was based on the comparison with positive controls using human recombinant renin and prorenin proteins. B: dose-response curve for DDAVP (10−10, 10−8, and 10−6 M) showed prorenin and renin protein expression levels in cell lysates. C: renin mRNA expression at 3 different doses of DDAVP. D: cAMP response element-binding protein (CREB) phosphorylation at 3 different doses of DDAVP showed a significant increase in phospho-CREB at 10−8 and 10−6 M DDAVP. E: immunofluorescence (×100) using a prorenin/renin antibody showing the increased punctuated red staining (anti-mouse IgG secondary antibody Alexa Fluor 594) in response to DDAVP (10−7 M). F: dose-response curve for DDAVP showed prorenin and renin expression levels in cell culture media (×10) concentrated. G: tolvaptan, a V2 receptor antagonist, blunted the DDAVP-mediated increase in prorenin and renin protein abundances. *P < 0.05 vs. vehicle-treated group. **P < 0.01 vs. vehicle-treated group; n = 6.
DDAVP increases renin in M-1 cells through the PKA/CREB pathway.
To investigate the intracellular pathways involved in the V2R-dependent upregulation of renin in the CD, we examined the effects of H89, a PKA inhibitor, in the presence of DDAVP treatment. As shown in Fig. 2, pretreatment (20 min) with H89 completely prevented the DDAVP-induced expression of prorenin [DDAVP+H89: 95 ± 20% vs. control: 100 ± 10%, P = not significant (NS)] and renin (DDAVP+H89: 98 ± 18 vs. control: 100 ± 13%, P = NS). No effects were observed with H89 alone (prorenin: 89 ± 20%, renin 82 ± 15%, P = NS). Forskolin, an enhancer of intracellular cAMP formation, greatly increased prorenin (230 ± 23%, P < 0.05) and renin (255 ± 17%, P < 0.05) protein abundance. Because cAMP-dependent activation of PKA leads to CREB phosphorylation and CREB localization in the nucleus, we further investigated the effects of H89 on the CREB pathway. DDAVP increased the phosphorylation levels of CREB after 20 min of treatment, while H89 pretreatment prevented this effect (Fig. 3A). Nuclear localization of CREB protein was analyzed by immunofluorescence after 20 min of DDAVP treatment. As shown in Fig. 3, B and C, the increased nuclear localization of CREB immunostaining was blunted by H89 treatment. To further confirm the role of CREB in this pathway, we used a CREB shRNA to knock down CREB expression. As shown in Fig. 4D, CREB knockdown (67 ± 9%) prevented the DDAVP-mediated increase in prorenin (119 ± 9%, P = NS vs. control) and renin protein bands (121 ± 16%, P = NS vs. control).
Fig. 2.
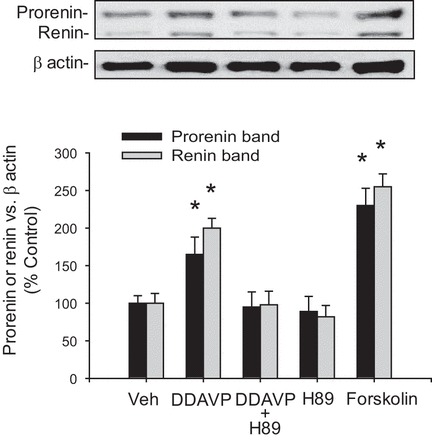
Activation of V2 receptor (V2R) increases prorenin and renin protein levels via PKA pathway in M-1 cells. Augmentation of prorenin and renin protein was blunted by H89 a PKA inhibitor, while forskolin treatment increased prorenin and renin expression. *P < 0.05 vs. vehicle-treated group; n = 6.
ANG II and DDAVP independently increase prorenin and renin in M-1 cells.
We next examined the effects of ANG II treatment plus DDAVP in M-1 cells. ANG II mediated renin upregulation in CD cells at submicromolar concentrations in vitro (19). M-1 cells were incubated with DDAVP (10−6 M), ANG II (10−7 M), or both for 4 h. As shown in Fig. 5A, DDAVP treatment increased prorenin and renin protein levels to the same extent observed with ANG II incubations compared with controls (prorenin: 195 ± 23%, P < 0.05 and renin: 200 ± 20% P < 0.05). Treatment of cells with ANG II plus DDAVP caused a similar induction of (prorenin: 172 ± 13% P < 0.05 and renin: 189 ± 15%, P < 0.05 vs. control). Figure 5B shows the cAMP levels in M-1 cells subjected to various treatments. DDAVP increased cAMP levels to a similar extent as observed by ANG II treatment alone. ANG II plus DDAVP increased cAMP levels to the same extent, and AT1 receptor blockade does not affect the DDAVP/ANG II-mediated increases in cAMP levels, demonstrating that there is not an additive effect. Calphostin C (PKC inhibitor) partially prevented the cAMP formation in the ANG II+DDAVP group at 10−7 M. This effect was even greater at a higher calphostin C concentration (10−6 M), indicating that the stimulation of cAMP formation by DDAVP and ANG II is dependent on PKC. As a positive control, we tested whether phosphodiesterase inhibition may show further increases in measurable cAMP. As shown in Fig. 5B, cAMP levels were even greater in M-1 cells treated with ANG II plus IBMX.
Water deprivation stimulates renin expression in medullary CDs.
To examine the in vivo effects of V2R activation on renin in the CD from the renal inner medulla, we used 48-h water-deprived mice. To avoid contamination of the JG renin component, we exclusively used microdissected renal inner medullary tissues to measure renin mRNA and prorenin and renin protein abundance. As shown in Fig. 6A, renin mRNA levels were augmented in the tissues of water-deprived mice compared with controls (300 ± 56 vs. 100 ± 15%, P < 0.05). Similarly, the prorenin (165 ± 23 vs. 100 ± 13%, P < 0.05) and renin (254 ± 18 vs. 100 ± 11%, P < 0.05) protein levels (Fig. 6B) were augmented, and there was increased specific positive immunostaining in the renal cortical and medullary CD cells (Fig. 6C). To confirm the physiological effect of water deprivation, we performed AQP2 immunostaining in consecutive kidney sections. As expected, water deprivation increased apical AQP2 abundance. Figure 6 also shows PAS staining to better appreciate the structures.
Fig. 6.
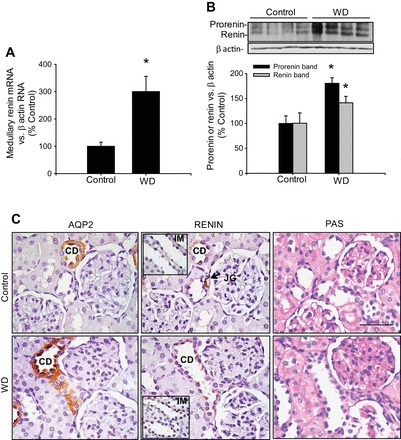
Renin expression is increased in the medullary collecting ducts following water deprivation. A: mice subjected to water deprivation (WD) showed increased renin mRNA levels in inner medullary tissues, which are not of juxtaglomerular origin. *P < 0.05 vs. control group; n = 4. B: prorenin and renin protein levels in inner medullary tissues were augmented by WD. *P < 0.05 vs. control group; n = 4. C: consecutive kidney sections (3 μm) from a control mouse (top) and a WD mouse (bottom) showing aquaporin-2 (AQP2), renin, and periodic acid-Schiff (PAS) staining. Kidney sections are from cortical regions to show juxtaglomerular (JG) renin as positive control (renin, top middle, arrow). Middle: renin staining in collecting ducts (CD) from cortex and inner medulla (IM; insets). Pictures were captured using a digital camera and an Eclipse Nikon microscope. Bar = 400 μm.
Increased renin mRNA and prorenin protein expression in renal medullary tissues following water deprivation is independent of AT1 receptor activation.
Figure 7A shows the expression of medullary prorenin and renin in mice subjected to 48 h of water deprivation with and without losartan plus captopril in the food. Despite RAS blockade, renin mRNA was significantly higher than in control mice (285 ± 56%, P < 0.05). Prorenin protein levels were significantly higher than controls (171 ± 10 vs. 100 ± 23%, P < 0.05) but not those from renin (113 ± 13%). As previously demonstrated, ACE inhibition or AT1 receptor blockade does not affect prorenin renin expression at basal conditions (14, 37). These data indicate that renin mRNA and prorenin protein levels are augmented by water deprivation independently of AT1 receptor activation. The posttranslated unchanged abundance of renin also suggests that the AT1 receptor might mediate processing and cleavage of prorenin in CD cells since losartan plus captopril treatment prevented the increase in renin protein.
Fig. 7.
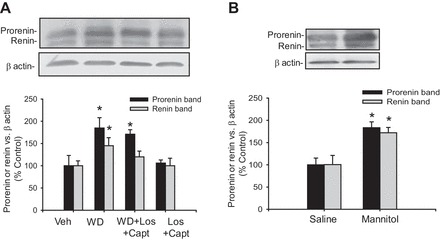
WD increases prorenin and renin expression independently of renin-angiotensin system (RAS) activation. A: in an additional experiment, mice were subjected to WD or WD plus losartan (Los) and captopril (Capt) treatment in food for 48 h. Prorenin and renin were augmented in medullary tissues of WD mice. WD plus RAS blockade caused increased expression of prorenin, but renin was not different from control, suggesting posttranscriptional processing mediated by AT1 receptor activation. No effects were observed in mice treated with losartan plus captopril alone. B: mice subjected to intraperitoneal injection of mannitol (20%, 1 h) showed increased expression of prorenin and renin in the renal medulla. *P < 0.05.
Intraperitoneal injection of mannitol induces upregulation of renin in the renal medulla.
To assess the acute AVP effect on CD renin, we used mice subjected to a single intraperitoneal injection of hypertonic mannitol to increase osmotic-mediated vasopressin release. Mice were euthanized after 1 h, and medullary tissues and plasma osmolality were analyzed. Plasma osmolality was slightly but significantly increased in the mannitol-treated group (mannitol: 315 ± 4 vs. control: 298 ± 6 mosmol/l, P < 0.05, n = 5). As shown in Fig. 7B, prorenin and renin protein levels were significantly higher than in controls (prorenin: 182 ± 13 vs. 100 ± 15%, P < 0.05; renin: 172 ± 12 vs. 100 ± 9%, P < 0.05).
DISCUSSION
The present study demonstrates that 1) V2R activation stimulates renin synthesis and secretion in the CD via the PKA/CREB pathway; 2) renin and prorenin are secreted by M-1 cells in response to DDAVP stimulation; 3) renin is augmented in the renal inner medullary CD of mice after 48 h of water deprivation; 4) renin is stimulated in the renal inner medullary CD by water deprivation even in the presence of AT1 receptor blockade; and 5) rats with mannitol-induced increases in plasma osmolality exhibit rapid increases in prorenin and renin synthesis in the renal medulla.
Although the mechanisms of renin regulation in JG cells are well established, the regulation of distal tubular renin remains unclear. It is known that the cAMP/PKA/CREB pathway constitutes the central pathway for the stimulation of renin expression in JG cells (17, 26). However, ANG II suppresses renin synthesis through PKC and Ca2+ in JG cells, as part of the physiological negative feedback regulation of renin release (36). In the CD, increases in intracellular Ca2+ and in cAMP levels are required to target AQP2 to increase osmotic water permeability (4). However, the lack of a potentiation effect of thapsigargin on AVP-dependent cAMP accumulation indicates that the increases in intracellular Ca2+ may not play a major role on the responses elicited by AVP (20). Previous studies demonstrated that CD renin is upregulated by PKC activation and that the ANG II-mediated upregulation of renin is suppressed by PKC inhibitors (12). Studies in rat inner medullary CD cells showed that PKC inhibitors decrease cAMP accumulation (29). These findings suggest that the activation of PKC plays a role in stimulation of AC, cAMP accumulation, and subsequent renin upregulation in the CD. In the present study, we demonstrated that cAMP accumulation in M-1 cells also depends on intact PKC activity, since PKC inhibition partially suppressed cAMP accumulation in response to ANG II plus DDAVP treatment.
Kang et al. (15) showed that M-1 cells treated with ANG II showed an increase in renin mRNA levels. They also demonstrated that the CD is the main source of prorenin in diabetic rats (15). Our data agree with these results; however, we detected the presence of two protein bands (renin and prorenin) in cell lysates and cell culture media of M-1 cells, both augmented by DDAVP and ANG II treatments (Fig. 4). We previously showed that freshly isolated rat inner medullary CD cells express mainly prorenin and that its protein levels are stimulated by ANG II (12). In the present study using M-1 cells treated with either DDAVP or ANG II, we showed increases in prorenin and renin to the same extent, but not in response to a combined treatment, which only caused further increases in prorenin. This suggests that lower concentrations of both hormones may interact in vivo to enhance renin production in the CD. Interestingly, increases in PKA activity were only observed in response to a micromolar dose of DDAVP, consistent with previous reports using cultured rat inner medullary CD cells (9) (28). Lee et al. (28) demonstrated that ANG II plays a role in the regulation of AQP2 targeting to the plasma membrane in CD cells. This effect is mediated by the activation of the AT1 receptor (28). The effect of activation of the AT1 receptor is probably mediated by the activation of PKC (12). Rozengurt et al. (43) showed that PKC activation enhanced the cAMP accumulation induced by forskolin and that this effect can be prevented by downregulation of PKC. This modulatory effect of ANG II may be mediated by direct activation of AC (1, 2).
Previous studies have ruled out the involvement of AC3 and AC4 in the vasopressin-dependent stimulation of cAMP (21, 22) and suggested that the most likely AC involved is AC6 (42), which is the most abundant AC in the CD. However, the present study did not allow defining which of the ACs is involved in the V2R-dependent stimulation of renin in the CD. Future studies are needed to address this question.
Interestingly, we observed granule-like patches with positive immunofluorescence for prorenin/renin in M-1 cells treated with DDAVP (Fig. 1). This observation along with the presence of prorenin and, to a lesser extent of renin in the cell culture media, strongly suggests that prorenin and renin are secreted in response to V2R stimulation. Secretion of renin from the CD of rats has been previously reported (31). The apical secretion of renin by the CD may favor the luminal interaction with AGT delivered from proximal segments to contribute to intratubular ANG II de novo generation during water deprivation.
ANG II increases AVP release from the supraoptic nucleus via the pituitary gland into the blood (38). It is possible that during the water deprivation period of 48 h, intrarenal RAS activation may also exert stimulatory effects on CD renin. In the microdissected inner medullary tissues, renin transcript was markedly augmented by water deprivation. RAS activation due to volume depletion may also contribute to renin upregulation in the renal medulla (12). In this regard, the role of the AT1 receptor was clearly demonstrated in the AT1aR knockout mice, which exhibit increased 24-h urine excretion (30). This effect is not suppressed by exogenous AVP or water restriction, and it was associated with reductions in AC protein expression (44), suggesting the importance of cross talk between AVP and ANG II in the mechanisms of urinary concentration. Our current findings demonstrate that both mechanisms also regulate renin in the CD. Indeed, renin and prorenin in the CD of the inner medulla were stimulated by water deprivation in mice; however, pharmacological blockade of RAS during water deprivation did not change renin protein. These results suggest that intrarenal RAS stimulation by water deprivation may further enhance prorenin cleavage, thus leading to increases in renin formation and release from the CD cells.
The physiological significance of these studies is highlighted by the fact that volume depletion and augmented plasma osmolality can act synergistically through the stimulation of intrarenal RAS, contributing to sodium and water retention. Indeed, the demonstrations that ANG II and AVP can directly stimulate epithelial sodium channels in the CD (10, 33) and suggest a parallel pathway for the stimulation of synthesis and secretion of prorenin-renin, as well as of AQP-2 expression, in the principal cells. Furthermore, the binding of renin and prorenin to the (pro)renin receptor on the apical side of the CD may help prevent the losses of these proteins in the urine (11) and may also contribute to increase their catalytic activity to further increase intratubular ANG II generation (11, 13). Importantly, it has been shown that plasma AVP concentrations are increased in some patients with heart failure, despite hyponatremia and low plasma osmolality (45). Thus the increases in plasma AVP levels may result from the activation of pressure mechanoreceptors due to low cardiac output (18). However, it is likely that the increases in CD renin may help to restore water and salt equilibrium in volume-depletion conditions or during low cardiac output through the enhancement of intratubular ANG II formation.
In summary, the present study provides clear evidence for the V2R-mediated regulation of CD renin. V2R activation in the M-1 cells increases renin synthesis and secretion. Furthermore, the demonstration of increased medullary renin/prorenin formation following 48 h of water deprivation suggests that V2R activation also regulates CD renin in vivo; thus AVP may contribute to intratubular ANG II formation, leading to further stimulation of water and sodium reabsorption in the distal nephron segments.
GRANTS
Funds were received from FONDECYT-Chile 11121217 and 79112017 grants to A. A. Gonzalez; from a National Institute of General Medical Sciences National Institutes of Health (NIH: CoBRE P30GM-103337) grant to L. G. Navar; and from NIH Grants DK104375-01 and LA-CaTS (U54-GM104940) to M. C. Prieto.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.A.G., L.G.N., and M.C.P. provided conception and design of research; A.A.G., F.C.-A., C.I.-G., A.G.-V., L.Z., R.H., and C.B.R. performed experiments; A.A.G. and M.C.P. analyzed data; A.A.G., L.G.N., and M.C.P. interpreted results of experiments; A.A.G. prepared figures; A.A.G. and M.C.P. drafted manuscript; A.A.G., L.G.N., and M.C.P. edited and revised manuscript; A.A.G., L.G.N., and M.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Molecular Core of the Tulane Renal Hypertension Center of Excellence for providing the physical infrastructure to perform some of the experiments of this study.
REFERENCES
- 1.Beazely MA, Alan JK, Watts VJ. Protein kinase C and epidermal growth factor stimulation of Raf1 potentiates adenylyl cyclase type 6 activation in intact cells. Mol Pharmacol 67: 250–259, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Beazely MA, Watts VJ. Galphaq-coupled receptor signaling enhances adenylate cyclase type 6 activation. Biochem Pharmacol 70: 113–120, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol 272: F405–F409, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct—roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Danser AHJ, Saris JJ. Prorenin uptake in the heart: a prerequisite for local angiotensin generation? J Mol Cell Cardiol 34: 1463–1472, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Danser AH, van Kats JP, Admiraal PJ, Derkx FH, Lamers JM, Verdouw PD, Saxena PR, Schalekamp MA. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension 24: 37–48, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Dellabruna R, Pinet F, Corvol P, Kurtz A. Opposite regulation of renin gene-expression by cyclic-amp and calcium in isolated mouse juxtaglomerular cells. Kidney Int 47: 1266–1273, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Ecelbarger CA, Chou CL, Lolait SJ, Knepper MA, DiGiovanni SR. Evidence for dual signaling pathways for V-2 vasopressin receptor in rat inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 270: F623–F633, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro) renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 57: 594–599, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez AA, Prieto MC. Renin and the (pro)renin receptor in the renal collecting duct: role in the pathogenesis of hypertension. Clin Exp Pharmacol Physiol 42: 14–21, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension 53: 351–355, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in angiotensin II-induced hypertensive rats. Am J Physiol Renal Physiol 281: F19–F25, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogarty DC, Tran DN, Phillips MI. Involvement of angiotensin receptor subtypes in osmotically induced release of vasopressin. Brain Res 637: 126–132, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Jensen BL, Schmid C, Kurtz A. Prostaglandins stimulate renin secretion and renin mRNA in mouse renal juxtaglomerular cells. Am J Physiol Renal Fluid Electrolyte Physiol 271: F659–F669, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Kalra PR, Anker SD, Coats AJS. Water and sodium regulation in chronic heart failure: the role of natriuretic peptides and vasopressin. Cardiovasc Res 51: 495–509, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension 51: 1597–1604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsura T, Verbavatz JM, Farinas J, Ma TH, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin-1 and aquaporin-2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA 92: 7212–7216, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kittikulsuth W, Stuart D, Kohan DE. Adenylyl cyclase 4 does not regulate collecting duct water and sodium handling. Physiol Rep 2: e00277, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kittikulsuth W, Stuart D, Van Hoek AN, Stockand JD, Bugaj V, Mironova E, Blount MA, Kohan DE. Lack of an effect of collecting duct-specific deletion of adenylyl cyclase 3 on renal Na+ and water excretion or arterial pressure. Am J Physiol Renal Physiol 306: F597–F607, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klar J, Sandner P, Muller MW, Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflügers Arch 444: 335–344, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol 12: 431–439, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension 42: 195–199, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kurtz A, Wagner C. Cellular control of renin secretion. J Exp Biol 202: 219–225, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Kwon TH, Nielsen J, Knepper MA, Frøkiær J, Nielsen S. Angiotensin II AT1 receptor blockade decreases vasopressin-induced water reabsorption and AQP2 levels in NaCl-restricted rats. Am J Physiol Renal Physiol 288: F673–F684, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lee YJ, Song IK, Jang KJ, Nielsen J, Frøkiær J, Nielsen S, Kwon TH. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol 292: F340–F350, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Lee YJ, Shin SJ, Tan MS, Hsieh TJ, Tsai JH. Increassed renal atrial natriuretic peptide synthesis in rats with deoxycorticosterone acetate-salt treatment. Am J Physiol Renal Fluid Electrolyte Physiol 271: F779–F789, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Li XC, Shao Y, Zhuo JL. AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int 76: 169–177, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol Renal Physiol 301: F1195–F1201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Lara LS, Gonzalez AA, Bourgeois CR, Seth DM, Prieto MC. Angiotensin II stimulates renin synthesis and secretion via protein kinase C activation and cAMP accumulation in collecting duct M-1 cells. J Invest Med 60: 456–457, 2012. [Google Scholar]
- 33.Mamenko M, Zaika O, Pochynyuk O. Direct regulation of ENaC by bradykinin in the distal nephron. Implications for renal sodium handling. Curr Opin Nephrol Hypertens 23: 122–129, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manninen PH, Lam AM, Gelb AW, Brown SC. The effect of high-dose mannitol on serum and urine electrolytes and osmolality in neurosurgical patients. Can J Anaesth 34: 442–446, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol 21: 1093–1096, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qadri F, Culman J, Veltmar A, Maas K, Rascher W, Unger T. Angiotensin-II-induced vasopressin release is mediated through alpha-1-adrenoceptors and angiotensin-II AT1 receptors in the supraoptic nucleus. J Pharmacol Exp Ther 267: 567–574, 1993. [PubMed] [Google Scholar]
- 39.Redublo Quinto BM, Camargo de Andrade MC, Ronchi FA, Santos EL, ves Correa SA, Shimuta SI, Pesquero JB, Mortara RA, Casarini DE. Expression of angiotensin I-converting enzymes and bradykinin B-2 receptors in mouse inner medullary-collecting duct cells. Int Immunopharmacol 8: 254–260, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, Hillas E, Zhang S, Ward K, Bloch-Faure M, Meneton P, Lalouel JM. Renin and kallikrein in connecting tubule of mouse. Kidney Int 64: 2155–2162, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Roos KP, Bugaj V, Mironova E, Stockand JD, Ramkumar N, Rees S, Kohan DE. Adenylyl cyclase VI mediates vasopressin-stimulated ENaC activity. J Am Soc Nephrol 24: 218–227, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozengurt E, Murray M, Zachary I, Collins M. Protein kinase C activation enhances cAMP accumulation in Swiss 3T3 cells: inhibition by pertussis toxin. Proc Natl Acad Sci USA 84: 2282–2286, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrier RW. Interactions between angiotensin II and arginine vasopressin in water homeostasis. Kidney Int 76: 137–139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrier RW. Use of diuretics in heart failure and cirrhosis. Semin Nephrol 31: 503–512, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol 296: F1067–F1071, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao W, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension 56: 378–383, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension 54: 120–126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


