Abstract
Pediatric TB meningitis (TBM) is a highly-morbid, oft-fatal disease. Standard treatment includes isoniazid, rifampin, pyrazinamide, and ethambutol. Current rifampin dosing achieves low cerebrospinal fluid (CSF) concentrations, and CSF penetration of ethambutol is poor. In adult trials, higher-dose rifampin and/or a fluoroquinolone reduced mortality and disability. To estimate optimal dosing of rifampin and levofloxacin for children, we compiled plasma and CSF pharmacokinetic and outcomes data from adult TBM trials plus plasma pharmacokinetic data from children. A population pharmacokinetic/pharmacodynamic model using adult data defined rifampin target exposures (plasma AUC0–24=92 mg*h/L). Levofloxacin targets and rifampin pediatric drug disposition information were literature-derived. To attain target rifampin exposures, children require daily doses of at least 30 mg/kg orally or 15 mg/kg intravenously. From our pediatric population PK model, oral levofloxacin doses needed to attain exposure targets were 19–33 mg/kg. Our results provide data-driven guidance to maximize pediatric TBM treatment while we await definitive trial results.
Keywords: tuberculous meningitis, pediatric dosing, developmental pharmacology, rifampin, levofloxacin
INTRODUCTION
Tuberculous meningitis (TBM) is a devastating illness, with a particularly high morbidity and mortality in young children (1). In Cape Town, South Africa, among 554 children with TBM, 13% died and 71% suffered neurologic sequelae (2). Other studies confirm that only about 20% of children with TBM fully recover (3). Moreover, the risk of developmental complications related to TBM is unique to children and is common (4). Given the high risk of death or neurologic impairment following pediatric TBM, treatment optimization has the potential to significantly impact survival and quality of life of affected children.
The World Health Organization (WHO) recommends treating pediatric TBM with two months of isoniazid, rifampin, pyrazinamide, and ethambutol, followed by ten months of isoniazid and rifampin (5). The recommended dose of rifampin (15 mg/kg, given orally) is the same as that suggested for intrathoracic TB, despite the fact that cerebrospinal fluid (CSF) to serum ratios of rifampin total drug concentration range from 0.04 to 0.11, and that, at recommended current doses, CSF concentrations of rifampin barely exceed the minimum inhibitory concentration (MIC) against Mycobacterium tuberculosis (6). Since rifamycins drive TB treatment response (7–9), higher doses of rifampin are likely required for optimal TBM therapy. In addition, ethambutol has negligible CSF penetration and contributes marginally, if at all, to combination treatment for TBM (10–12). A more potent fourth drug, with bactericidal activity against M. tuberculosis and good CSF penetration, may be needed to optimize therapy. For example, some groups replace ethambutol with ethionamide and report clinical success (13). Pediatric dosing recommendations for TB are largely based on adult trial results and pediatric TBM regimens vary widely by country; there has only been one clinical trial evaluating regimens for TBM in children (14).
In a recent Phase 2 factorial design clinical trial among 60 adults with TBM in Indonesia evaluating standard-dose oral rifampin (450 mg daily, corresponding to 10 mg/kg in Indonesian patients) versus higher-dose rifampin (600 mg) given intravenously, with or without moxifloxacin, use of high-dose intravenous rifampin for two weeks was associated with a remarkable reduction in 6-month mortality, from 65% to 35% (9). The change in rifampin dose and mode of delivery was associated with a 3-fold increase in plasma area under the time-concentration curve (AUC0–6h) and maximum concentration (Cmax), and a 3-fold increase in CSF concentrations, from 0.21 to 0.60 μg/mL. Mortality differences could be seen as little as two weeks into treatment, demonstrating that optimizing antimicrobial therapy has the potential to save lives, particularly when highly-effective regimens are provided early. In another Phase 2 trial, 61 adults with TBM in Vietnam were randomized to receive WHO-recommended TBM treatment with the addition of oral ciprofloxacin, levofloxacin, gatifloxacin, or no additional drug. Pharmacokinetic/pharmacodynamic (PK/PD) analyses showed a U-shaped exposure-response relationship across a broad range of outcome variables (i.e. if CSF drug exposures were too low or too high, higher rates of disability and mortality resulted) (15). Levofloxacin has excellent CSF penetration (74%) and is available in child-friendly formulations. The plasma and CSF AUC/MIC associated with improved survival in the adult trial in Vietnam were 112–220 and 14–252 h, respectively. Confirmatory trials of higher-dose rifampin and/or fluoroquinolones for adult TBM are underway. How and whether these recent adult findings will translate into improved treatment of pediatric TBM is unknown.
Here, we collected rifampin plasma PK, rifampin CSF PK, and outcomes data from the adult TBM treatment trial in Indonesia (9); information about drug disposition among children receiving rifampin for treatment of drug-sensitive TB (16,17); and plasma PK data from children receiving levofloxacin for treatment of drug-resistant TB (18). We used a modeling approach and clinical trials simulations to estimate the following: (1) pediatric rifampin doses needed to reach target exposures associated with reduction in mortality in adults, and (2) pediatric levofloxacin doses needed to match target exposures in adults. Suggested doses by age and weight for rifampin and levofloxacin for children with TBM are provided.
RESULTS
Data
Raw data from 53 individuals in the adult TBM trial and 23 children in the pediatric levofloxacin PK study and literature-derived information about levofloxacin PK targets, rifampin disposition in children, and kidney maturation in young children were included in the models (9,15,19). Demographic information about study participants in the adult TBM trial and the pediatric PK study has been described previously (9,18). In total, 234 adult rifampin plasma, 69 adult rifampin CSF and 128 pediatric levofloxacin plasma samples were used in the analyses.
Population PK/PD analyses—target rifampin and levofloxacin exposures
Population PK of rifampin in adults; defining rifampin PK targets to maximize efficacy
The final rifampin adult PK model was a two compartment disposition model parameterized using CL and V, Q and Vp allometrically scaled and linked to the first order absorption model to describe rifampin absorption following oral dosing. Rifampin bioavailability (based on oral and IV PK data) was estimated to be 60%. The model was linked to the CSF compartment (See Methods and Figure 1). CSF distribution appeared to be constant over time, that is, there was no evidence of time-dependent change in rate or extent of CSF uptake. The fraction of rifampin in CSF, or CSF:plasma ratio, was 0.082. Final model parameters are provided in Table 1. Goodness of fit plots are provided in Supplemental Figure 1.
Figure 1.
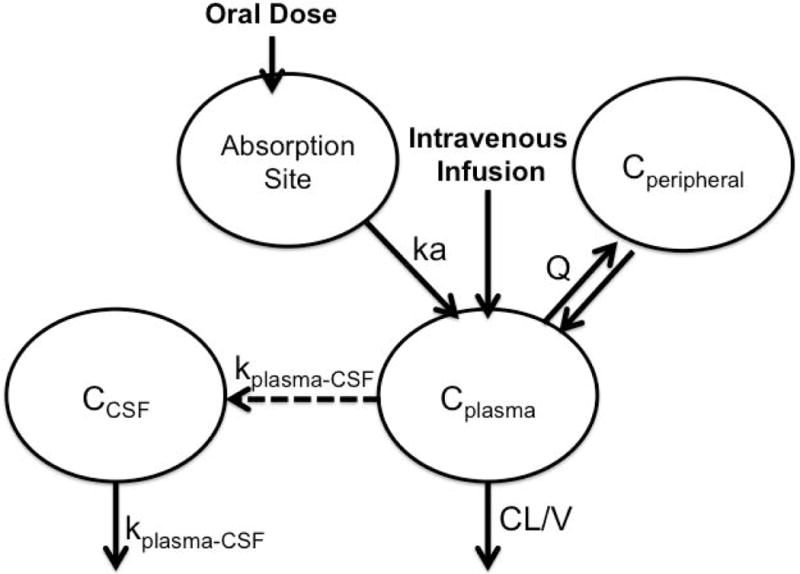
Population PK model to describe plasma and CSF data from adults taking rifampicin as part of multidrug treatment for tuberculous meningitis.
Table 1.
Final model parameter estimates for adult population pharmacokinetics of rifampin.
| Population PK Parameters | Typical Value (R.S.E) for a 70 kg Adult | Median (95% CI) |
|---|---|---|
| CL* (L/h) | 5.71 (7%) | 5.67 (4.88, 6.41) |
| V* (L) | 24.9 (16%) | 24.1 (15.6, 33.1) |
| ka (h−1) | 0.644 (13%) | 0.638 (0.474, 0.832) |
| F (%) | 60.0 (10%) | 59.7 (47.8, 72.3) |
| Q* (L/h) | 9.46 (38%) | 10.0 (3.08, 29.0) |
| V2* (L) | 12.4 (22%) | 13.4 (8.92, 18.5) |
| kplasma-CSF (h−1) | 0.120 (79%) | 0.137 (0.060, 0.688) |
| Ratioplasma-CSF | 0.0807 (30%) | 0.073 (0.048, 0.107) |
|
| ||
| Between-Subject Variability (BSV)
| ||
| BSV - CL (%) | 34% (16%) | 35 (24, 47) |
| BSV – V (%) | 60% (29%) | 67 (36, 145) |
| Ratioplasma-CSF (%) | 28% (32%) | 31 (10, 51) |
|
| ||
| Residual Error
| ||
| Additive Plasma mg/L | 2.18 (12%) | 2.12 (1.71, 2.55) |
| Proportional CSF | 0.280 (24%) | 0.255 (0.116, 0.377) |
|
| ||
| Correlation
| ||
| Correlation CL-V | 0.53 (25%) | |
Concentrations of rifampin refer to total concentration (protein-bound plus unbound) in plasma and in cerebrospinal fluid.
Clearance and Volume were scaled allometrically using 0.75 and 1, respectively
PK/PD analysis demonstrated that a plasma AUC0–24 of 92 mg*h/L maximized survival (Figure 2). Survival data was best described using time-varying Weibull distribution (P<0.001). The exposure-response models (i.e., using AUC and Cmax) performed significantly better than the dose-response model (P<0.05). In addition, plasma AUC0–24 was the PK parameter that best described treatment response in the PK/PD model; that is, there was a slight improvement in the PK/PD model OFV using AUC0–24 in plasma compared to other PK predictors (Supplemental Table 1). Importantly, this result suggests that plasma AUC0–24 may be used as a surrogate predictor of survival for TBM outcomes instead of CSF AUC0–24 or Cmax. From the final PK/PD model, the predicted percentage of patients alive at six months was simulated for plasma AUC values ranging from 0 to 200 mg*h/L (Figure 2). Final exposure-response model parameters are provided in Table 2. Goodness of fit plots are provided in Supplemental Figure 2. The target exposure of AUC0–24 of 92 mg*h/L is in good agreement with the AUC0–24 attained in the superior arm of the adult TBM trial (20).
Figure 2.
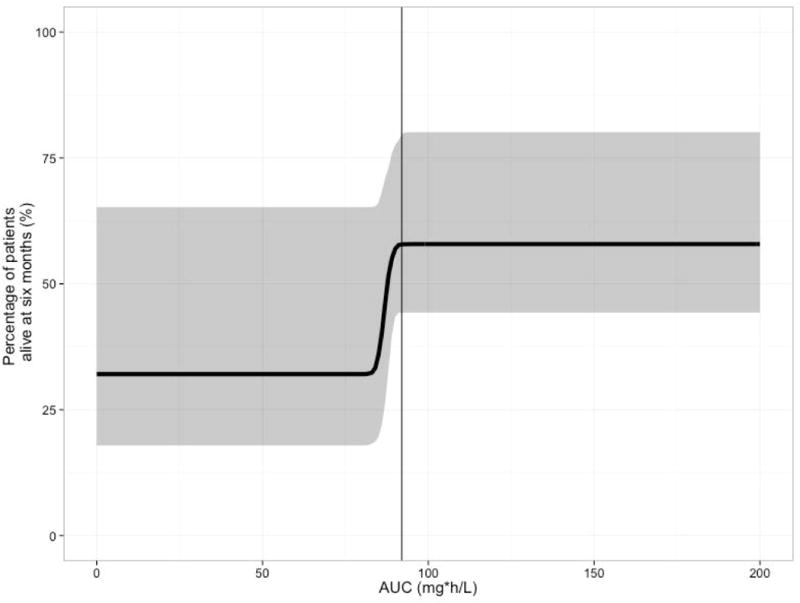
Exposure-response relationship between rifampin plasma AUC0–24 and survival in tuberculosis meningitis patients. The continuous line represents the median for the simulated data and shaded region represents the 90% confidence interval for the median of the simulated data. The AUC that predicts 99% of maximal response is 92 mg*h/L, which is indicated in the figure by the x-intercept line.
Table 2.
Final model parameter estimates for adult population pharmacokinetic/pharmacodynamics of rifampin.
| Hazard Model Parameters | Typical Value (%R.S.E) | Median (95% CI) |
|---|---|---|
| Hazard (λ) day−1 | 0.0070 (60%) | 0.0078 (0.0017, 0.049) |
| Shape (α) | 0.499 (11%) | 0.502 (0.400, 0.654) |
| Emax | 0.729 (17%) | 0.788 (0.429, 0.953) |
| AUC50 mg*h/L | 86.4 (1%) | 84.1 (50.0, 120) |
| Gamma (γ) | 118 (Fixed) | Fixed |
Population pharmacokinetics of levofloxacin in children
A one-compartment disposition model fit the data best (see Methods). Final model parameters are provided in Table 3. Goodness of fit plots are found in Supplemental Figure 3. Following a 15 mg/kg dose of levofloxacin, drug exposures were substantially lower in all children (AUC0–24 of approximately 30 μg*h/mL) compared to exposures in adults following a standard 750 mg oral dose (AUC of 101 μg*h/mL, as defined in the Levofloxacin package insert). We observed no clear effect of age, although the sample was small and only included children <8 years of age and very few very young children. Modeling confirmed that levofloxacin CL in children requires allometric scaling to account for weight-dependent changes.
Table 3.
Final model parameter estimates for pediatric population pharmacokinetics of levofloxacin.
| Population PK Parameters | Typical Value (R.S.E) for a 14 kg Child |
|---|---|
| CL/F (L/h) | 5.43 (6%) |
| V (L) | 25.4 (8%) |
| ka (h−1) | 1.79 (22%) |
|
| |
| Between-Subject Variability (BSV)
| |
| BSV - CL (%) | 29% (21%) |
| BSV - V (%) | 35% (24%) |
| BSV - ka (%) | 61% (20%) |
|
| |
| Residual Error
| |
| Additive mg/L | 0.2813 (0.1818) |
| Proportional | 6.2% (0.2985) |
|
| |
| Correlation
| |
| CL-V | 0.84 (20%) |
Estimated pediatric doses required to reach target rifampin and levofloxacin exposures
Clearance values from pediatric patients receiving intravenous rifampin therapy vary by weight but do not vary significantly by age (independent of weight) over the age range of 3 months to 12 years in the beginning of treatment prior to full autoinduction (16), and the extent of oral rifampin absorption (F) is about 50% in children (17). To reach a plasma AUC target of 92 mg*h/L at treatment initiation, we estimate that children will require an oral dose of at least 30 mg/kg or an IV dose of 15 mg/kg (Figure 3). Higher doses would be needed to ensure every child reaches the target exposure, i.e, AUC > 92 mg*h/L (Supplemental Table 2); of note, the safety of these higher doses must be considered. Predicted oral doses of levofloxacin required to reach a plasma AUC goal of 101 μg*h/L in children are provided in Table 4.
Figure 3.
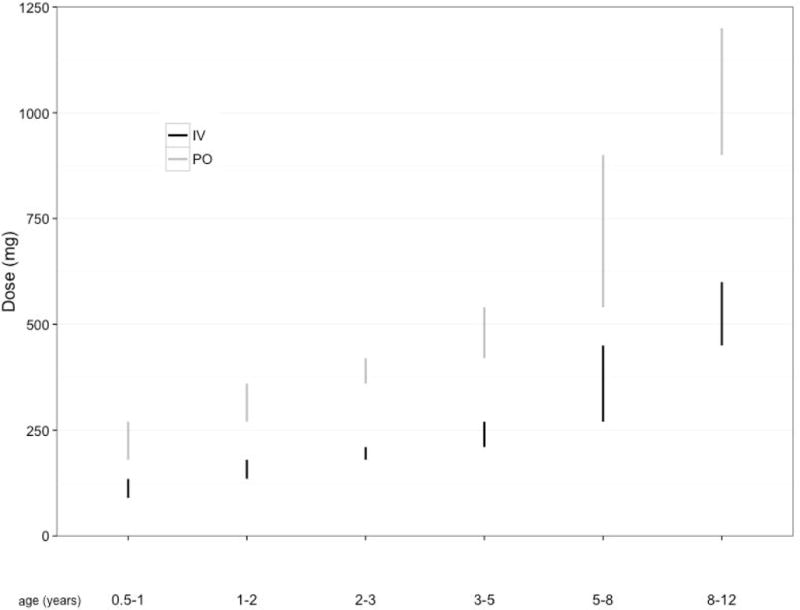
Range of rifampin doses in a simulated population of children with TB meningitis needed to achieve the target area under the concentration-time curve (AUC) of 92 mg*h/L, by weight and age. Black bars represent the range for intravenous dosing and grey bars represent the range for oral dosing.
Table 4.
Predicted oral doses of levofloxacin required to reach a plasma AUC goal of 101 mcg*h/L for children.
| Weight in kg | Dose needed to reach adult target (in mg/kg) | Dose needed to reach adult target (in mg) |
|---|---|---|
|
| ||
| 2–3 | 19.2 | 48.0 |
| 3–5 | 23.7 | 95.0 |
| 5–10 | 26.3 | 197.3 |
| 10–15 | 27.2 | 326.4 |
| 15–20 | 33.3 | 582.2 |
| 20–30 | 30.4 | 760.8 |
| 30–40 | 28.0 | 979.2 |
DISCUSSION
Our study has several key results. First, using innovative modeling approaches and adult and pediatric PK/PD data, we show that children require higher rifampin mg/kg doses than adults, at least 30mg/kg oral or 15mg/kg IV, and that penetration of rifampin into CSF is low. We estimate that higher doses of oral levofloxacin will be needed to reach adult target exposures that are associated with significant reduction in morbidity and mortality.
Recently, WHO launched a campaign aimed at reducing deaths from childhood TB from 75,000 per year to zero (21). If the global community is committed to eliminating TB-related deaths in children, strategies aimed at optimizing treatment for the most severe form of pediatric TB, TBM, are needed. Recent adult studies showing significant reductions in mortality from TBM from changing the antimicrobial regimen are encouraging because they provide information about drug combinations and exposure targets to test in children and because they demonstrate that even patients who present with late-stage TBM may benefit from optimized treatment. Currently, there is wide variability in national treatment guidelines because there are few empiric data to drive regimen and dosing choices (14). Only one randomized clinical trial of TBM treatment has ever been conducted in children, in 1986 (22). The optimal treatment of adult and pediatric TBM is poorly-established because of sizeable knowledge gaps: drug doses for first-line drugs (especially rifampin) to achieve adequate exposures at the site of infection; the best fourth agent to use with isoniazid, rifampin, and pyrazinamide; and the duration of treatment required for cure. Determining the optimal treatment regimen for TBM using PK/PD data is an important initial step towards improving treatment and outcomes.
Estimates of the oral doses of rifampin needed to optimize TBM treatment in children are high given that bioavailability of rifampin, as estimated in adults in the current study, is only about 60% and is 50% in children (17). Whether or not target exposures can be achieved with orally-administered rifampin given to sick children, who are often receiving their medications via nasogastric tube or crushed, is unknown and must be tested prospectively. The potential benefits of IV treatment (higher maximum concentrations, circumventing issues of bioavailability and drug delivery in sick children) must be weighed against the logistical challenges of its use including cost, accessibility, and the risk of infections from intravenous lines. The safety of higher doses of rifampin also requires careful investigation. In 2011, WHO recommended that the oral pediatric dose of rifampin be increased to 10–20 mg/kg for all forms of childhood TB based on data from intrathoracic TB (5,21). Doses at least that high are routinely used in some settings (for example at least 20 mg/kg daily for 6 months for TBM in Cape Town, South Africa)(13,23), and IV doses of 12–20 mg/kg have been used safely in children (16,24). In adults, oral doses of 35 mg/kg appear to be safe in a recently-completed twelve-week Phase 2B clinical trial (25).
There is mounting evidence that rifamycin drug exposure drives treatment response, both in pulmonary TB and TBM (7,8,9,25,26) ; it is therefore crucial to optimize the dose of rifampin. Up to now, no other agents have been identified that have equivalent sterilizing activity, so rifampin cannot simply be replaced by other drugs that penetrate into the CSF more readily. Rather, rifampin dosing must be adjusted to deliver the required exposures to the compartment of interest, provided that safety is preserved. With WHO standard treatment doses in adults, CSF concentrations of rifampin barely exceed the MIC against M. tuberculosis, whether or not the meninges are inflamed (6). Our modeling suggests that the doses we propose for children will deliver CSF levels higher than the MIC for rifampin against M. tuberculosis from very early in treatment. The blood:brain barrier and blood:CSF barrier fully mature shortly after birth and CSF penetration of medications in adults and children older than 4 months of age are similar (27–31). Detailed information about the effects of age on CSF penetration of rifampin in children should be studied prospectively (32). Whether or not higher doses will be required later in treatment (to account for autoinduction) requires further investigation.
Fluoroquinolones, including levofloxacin or moxifloxacin, are part of standard treatment for MDR-TB for adults and children (33, 34). Fluoroquinolone-related musculoskeletal side effects are rare in children and are of mild to moderate severity and are reversible (34–37). Current American Academy of Pediatrics guidelines recommend fluoroquinolone use in children with severe illnesses or infections with limited treatment options (35). Fluoroquinolones penetrate well into CSF and have potent activity against M. tuberculosis. If levofloxacin is given to children at higher than the currently-used doses, additional safety data will be needed to confirm that higher doses pose no additional risk among growing children. Oral doses of 15–20 mg/kg daily for MDR-TB treatment or prophylaxis have been well-tolerated (34).
For pediatric TB, it is generally reasonable to prove efficacy of a new treatment regimen in adults and then conduct PK and safety studies in children in order to choose doses that achieve adult-equivalent exposures. However, in some cases, efficacy studies in children may be needed. For pediatric TBM, the spectrum of disease appears to be different (less mortality and more neurocognitive sequelae), so in that case, clinical trials evaluating not only PK and safety but also longitudinal efficacy may be particularly important. Mathematical modeling using efficacy data from adults and PK data from children can help clinical trialists make decisions about treatments and doses to test. This is particularly important in settings where standard of care is clearly suboptimal, unmet clinical need is high, and there are public health implications.
Our study has several limitations. Levofloxacin PK data in the youngest children, in whom renal function is rapidly developing, were limited; however, there is a wealth of literature describing levofloxacin developmental pharmacology in very young children that we used in our models to enable estimation of optimal doses in this population (19), and, our model parameters and dosing recommendations aligned nicely with those described for children receiving single-dose levofloxacin in a different setting, giving reassurance about the generalizability of results (19). With regards to rifampin, there may be regional differences in PK. PK parameters early in treatment in our adult study population, though, were similar to values among patients taking single dose rifampin in past trials (38). Rifampin PK may also differ depending on dosing scheme, as autoinduction magnitude is influenced by frequency of dosing (data not shown). For that reason, we did not include PK data from children in India where treatment is given thrice-weekly. In addition, PK data from children early in treatment are limited. Because we were trying to match adult PK targets from early in treatment, we could not use available raw data from children in South Africa (where our PK model was originally developed) in our models because those data were collected at steady state when autoinduction was complete; rather we used information about rifampin disposition in children from early in treatment from the literature. However, having robust steady state PK data in hand gave us better understanding of developmental pharmacology of rifampin and more confidence in dosing recommendations. It should also be emphasized that our suggested doses require prospective testing to assess safety and confirm that rifampin PK targets can be met, particularly with oral dosing in sick children in different geographic settings.
Pediatric TBM has high morbidity and mortality with currently-recommended treatment. Improved treatment strategies are sorely needed. Given this data, it is imperative to now prospectively evaluate increased doses of rifampin plus levofloxacin for pediatric TBM.
METHODS
Data sources for rifampin
Data from clinical studies in adults with TBM (deidentified prior to use) and model information from children with TB were obtained (9,18,38). The studies were conducted in accordance with Good Clinical Practice standards and local ethical legislation.
Rifampin plasma and CSF PK and outcome data from adults with TBM
In an open-label, factorial design, Phase 2 clinical trial, adults with TBM in Indonesia were randomized to receive rifampin 450 mg orally or rifampin 600 mg IV (for two weeks) and then further randomized to receive moxifloxacin 400 mg, moxifloxacin 800 mg, or ethambutol 750 mg orally (also for two weeks). Study treatments were given once daily in combination with other first-line TB drugs; adjunctive treatment with dexamethasone was provided. PK sampling for rifampin was performed in the first three days of drug treatment with plasma samples collected pre-dose and at 1, 2, 4, 6 and 24 hours after dosing and CSF samples collected at 3–6 and 6–9 hours post-dose. Patients were followed for 6 months for safety and mortality (9).
Population PK of rifampin in plasma and CSF of adults with TBM
Using dosing, plasma PK, CSF PK, and outcomes data from the clinical trial in adults in Indonesia, target plasma and CSF exposures associated with beneficial treatment outcome were determined. A nonlinear mixed-effects analysis was performed by simultaneously modeling all available plasma and CSF drug concentration data. With this population approach, the central tendency in the population, i.e., the typical value, as well as the variability, e.g., interindividual variability (IIV) and residual error, could be described. The model-building process was performed in a stepwise fashion, developing first the structural plasma PK model following IV dosing, including variability. In a second step, data following oral rifampin were included to describe rifampin absorption rate and extent (bioavailability). Then, a full model also describing CSF penetration was developed, keeping the parameters of the plasma PK model fixed. As a last step, all parameters were re-estimated simultaneously using all data. The likelihood ratio test (LRT) was used to evaluate statistical significance for inclusion of additional parameters in nested models, assuming that the objective function value (OFV) was chi-squared distributed; thus, a decrease in OFV of 3.84 points between hierarchical models with one parameter differing is considered a statistical difference at a 5% significance level. Goodness-of-fit plots computed in the Xpose (version 4.0) program were also used to guide model selection.
Plasma PK model. One- and two-compartment models with a first-order elimination, parameterized in terms of clearance (CL), volume of distribution (V), intercompartmental clearance (Q), and peripheral volume of distribution (V2) and bioavailability (F), were fitted to the data. Interindividual variability was allowed on all plasma PK parameters and assumed to be log-normally distributed. A full covariance-variance structure was initially estimated with reductions allowed on the basis of the magnitude of estimates and the LRT.
Residual error model. Several models describing the residual variability were investigated: additive and proportional error models and a slope-intercept model. Different residual errors were estimated for plasma and CSF.
- Tissue penetration model. CSF drug penetration was described using effect compartment models similar to those of Sheiner et al. (43) (Figure 1), with the following equation:
where C is concentration, kplasma-CSF is the time rate constant for the transfer of drug from the plasma to CSF, PCCSF is the penetration coefficient between plasma and CSF and Aplasma/Vplasma is the concentration of drug predicted in the plasma compartment at time t, with Aplasma being the amount of drug in plasma and Vplasma the apparent volume of the plasma compartment. Inter-individual variability was investigated on the parameters belonging to the CSF penetration model, but as only single observation sample was available from each individual at a given occasion, separation of between-subject variability and residual error was not possible. Model evaluation. Percentile confidence intervals for all estimated parameters in the final model were computed from the estimation of 500 resampled nonparametric bootstrap data sets. For each individual, the predicted area under the concentration-time curve (AUC) and maximum concentration (Cmax) in plasma, and CSF were computed. Visual predictive checks (VPCs) were performed to evaluate the simulation properties of the final model.
Software. Data were analyzed using the first-order conditional estimation method as implemented in the software NONMEM, version 7.3 (ICON Development Solutions, Ellicott City, MD). Graphical, statistical, and exploratory analyses were conducted using the R package (version 2.11.1), while Xpose (version 4.0) was used for data set checkout and graphical evaluations of the modeling output (40). Nonparametric bootstrap and visual predictive check (VPC) were performed using PsN software (version 3.2.12) (41, 42), and scripts were created using R.
Pharmacokinetic/pharmacodynamics model for rifampin in adults with TBM
A pharmacokinetic-pharmacodynamic (PK/PD) model was developed using PK and survival (all-cause mortality over 6 months) data from the adult TBM trial. Individual estimates of plasma and CSF pharmacokinetics (AUC and Cmax) were computed using integration techniques in NONMEM (39). A time-to-event base model was developed from survival data using a cumulative hazard distribution function in NONMEM. Both constant and time-varying hazard functions were explored, including exponential and Weibull distributions as defined below.
Constant hazard (exponential distribution):
Time-varying hazard (Weibull distribution):
Where h0 (t) is the base hazard function, with λ and α representing the scale and shape factors, respectively.
Additionally, step models, as well as linear, Emax and sigmoidal Emax relationships to the baseline hazard, were analyzed to explore the impact of explanatory covariates, e.g., age, weight, sex, HIV, dose and exposure (AUC and Cmax) in plasma and CSF on time to death in TBM patients. Continuous covariates were modeled as change in hazard according to the equation, exemplified for AUC, below.
h1(t) is the base hazard model with inclusion of covariates, where Emax is the maximal effect, AUC50 is the AUC required to produce 50% of Emax and gamma (γ) represents the steepness factor that accommodates the steepness of the curve about AUC50.
Model evaluation was based on likelihood ratio tests as described above and graphical assessment of time-to-event VPCs.
Data sources for levofloxacin
HIV-infected and uninfected children aged 3 months to 15 years routinely started on second-line treatment for multidrug-resistant (MDR) TB treatment (mainly intrathoracic) or preventive therapy which included levofloxacin were included (18). A levofloxacin dose of 15 mg/kg body weight once daily was given. PK sampling was performed 2–8 weeks after treatment initiation. Blood samples were collected pre-dose, then 1, 2, 4, 6 and 8 hours post-dose.
Population PK of levofloxacin in children
Levofloxacin PK for children was described using a one compartment disposition model parameterized using CL and V and linked to the first order absorption model as shown in the following equations:
Disposition parameters were allometrically scaled using the following equations and centered at the median weight in this cohort of 14kg:
A function describing maturation of the kidney function was included as described in Li et al. for simulation purposes, since we had data from very few young children to support estimation of parameters (19). Parameters were assumed to be log normally distributed. Correlations between CL and V was estimated. Residual error model was assumed to be combined of proportional and additive error. Model evaluation and software are described above.
Simulation of doses of rifampin and levofloxacin required to achieve target exposures in plasma and CSF in children
Modeling and simulation was performed to determine the doses of rifampin that would most likely achieve exposure targets in children, by age and weight. A PK/PD model was developed to determine the best predictor of survival (e.g. dose, AUC, Cmax). The PK predictor that corresponded to 99% of maximal survival in 50% of children was targeted. Furthermore, when more aggressive treatment may be acceptable, dosages at which up to 99% of children are expected to achieve the target were calculated. For extrapolation to pediatric patients, clearance and bioavailability information from the first three-days of rifampin treatment (i.e., before auto-induction) were derived from intravenous and oral data in the literature (16,17).
For levofloxacin, we developed a model using pediatric data from Thee et al. study as well as information from analyses conducted by the U.S. Food and Drug Administration in the general pediatric population (levofloxacin package insert, pediatric data) to determine the doses required for children to achieve AUC values associated with TB treatment success in adults (15,18). Specifically, a dose of 750 mg in adults achieves an AUC 101 μg*h/mL, within the desired AUC/MIC range of 112–220, assuming an MIC for levofloxacin against M. tuberculosis of 0.5–1 μg/mL.
Supplementary Material
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Pediatric TB meningitis (TBM) has high morbidity and mortality. Optimal treatment is not established for children, but recent clinical trials in adults suggest higher doses of rifamycins and use of fluoroquinolones may improve outcomes.
What question did this study address?
In this study, we used plasma and cerebrospinal pharmacokinetic data and outcomes data from a successful adult TBM clinical trial to establish target exposures associated with reduced mortality and to describe CSF penetration of rifampin in adults with TBM. We then gathered rifampin and levofloxacin plasma PK data from children with tuberculosis to determine pediatric doses required to achieve target concentrations.
What this study adds to our knowledge?
The target plasma AUC0–24 for rifampin for treating adult TBM is 92 mg*h/L. The fraction of rifampin in CSF, or CSF:plasma ratio, was 0.082. To reach adult exposure targets, we estimate that children need daily rifampin doses of at least 15 mg/kg IV or 30 mg/kg orally. Levofloxacin doses required to achieve targets s are provided.
How this might change clinical pharmacology and therapeutics?
Rifampin given at currently-recommended doses for pediatric TBM fails to achieve target concentrations, and CSF exposures are unlikely to surpass the mean inhibitory concentration of rifampin against M. tuberculosis. Higher doses of rifampin and/or use of levofloxacin may improve survival. Our study provides dosing recommendations for upcoming trials aimed at optimizing pediatric TBM treatment, in which the PK, safety, and efficacy of these doses can be confirmed.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, under award number R01HD074944 (KED, SS, GR, AG, RMS). Other sources of support were: Bristol-Myers Squibb ‘Secure the Future’ Foundation (HM) and the South African National Research Foundation (HM), NIH/NIAID UM1AI069465 (AG), KNAW and ANDALAN UNPAD research grant for conducting adult trials (RR), National Institutes of Health R01: 069169-01 (AH), the German Leprosy and Tuberculosis Relief Association (AH), and a VIDI grant from the Netherlands Foundation for Scientific Research (RvC).
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
The authors report no Conflicts of Interest.
AUTHOR CONTRIBUTIONS
K.E.D., R.S., R.R., J.E.H., A.H., G.R., A.R.G., S.S., H.M., A.G., K.T., R.V.C., and R.A. wrote the manuscript; K.E.D., R.S., R.R., A.R.G., H.M., and R.A. designed the research; R.R., A.H., A.R.G., R.V.C., and R.A. performed the research; R.S. and J.E.H. analyzed the data.
References
- 1.Thwaites G, Chau TT, Mai NT, Drobniewski F, McAdam K, Farrar J. Tuberculous meningitis. J Neurol Neurosurg Psychiatry. 2000 Mar;68(3):289–299. doi: 10.1136/jnnp.68.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Well GT, Paes BF, Terwee CB, Springer P, Roord JJ, Donald PR, et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics. 2009;123(1):e1–8. doi: 10.1542/peds.2008-1353. [DOI] [PubMed] [Google Scholar]
- 3.Karande S, Gupta V, Kulkarni M, Joshi A. Prognostic clinical variables in childhood tuberculous meningitis: an experience from Mumbai, India. Neurol India. 2005 Jun;53(2):191–5. doi: 10.4103/0028-3886.16407. discussion 195–6. [DOI] [PubMed] [Google Scholar]
- 4.Schoeman J, Wait J, Burger M, van Zyl F, Fertig G, van Rensburg AJ, et al. Long-term follow up of childhood tuberculous meningitis. Dev Med Child Neurol. 2002 Aug;44(8):522–526. doi: 10.1017/s0012162201002493. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Rapid advice: Treatment of tuberculosis in children. 2010;13 /WHO/HTM/TB/2010. [PubMed] [Google Scholar]
- 6.Ellard GA, Humphries MJ, Allen BW. Cerebrospinal fluid drug concentrations and the treatment of tuberculous meningitis. Am Rev Respir Dis. 1993 Sep;148(3):650–655. doi: 10.1164/ajrccm/148.3.650. [DOI] [PubMed] [Google Scholar]
- 7.Savic RM, Weiner M, Mac Kenzie W, Heilig C, Dooley K, Engle M, et al. PK/PD analysis of rifapentine in patients during intensive phase treatment for tuberculosis from Tuberculosis Trials Consortium studies 29 and 29X; Denver, CO. September 9; 6th International Workshop on Clinical Pharmacology of Tuberculosis Drugs; 2013. [Google Scholar]
- 8.Boeree MJ, Plemper van Balen G, Aarnoutse RA. High-dose rifampicin: how do we proceed? Int J Tuberc Lung Dis. 2011 Aug;15(8):1133. doi: 10.5588/ijtld.11.0198. [DOI] [PubMed] [Google Scholar]
- 9.Ruslami R, Ganiem AR, Dian S, Apriani L, Achmad TH, van der Ven AJ, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013 Jan;13(1):27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 10.Pilheu JA, Maglio F, Cetrangolo R, Pleus AD. Concentrations of ethambutol in the cerebrospinal fluid after oral administration. Tubercle. 1971 Jun;52(2):117–122. doi: 10.1016/0041-3879(71)90017-1. [DOI] [PubMed] [Google Scholar]
- 11.Gundert-Remy U, Klett M, Weber E. Concentration of ethambutol in cerebrospinal fluid in man as a function of the non-protein-bound drug fraction in serum. Eur J Clin Pharmacol. 1973 Aug;6(2):133–136. doi: 10.1007/BF00562440. [DOI] [PubMed] [Google Scholar]
- 12.Radenbach KL. Minimum daily efficient dose of ethambutol: general review. Bull Int Union Tuberc. 1973 Jun;48(0):106–111. [PubMed] [Google Scholar]
- 13.Donald PR, Schoeman JF, Van Zyl LE, De Villiers JN, Pretorius M, Springer P. Intensive short course chemotherapy in the management of tuberculous meningitis. Int J Tuberc Lung Dis. 1998 Sep;2(9):704–711. [PubMed] [Google Scholar]
- 14.Chiang SS, Khan FA, Milstein MB, Tolman AW, Benedetti A, Starke JR, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2014 Oct;14(10):947–957. doi: 10.1016/S1473-3099(14)70852-7. [DOI] [PubMed] [Google Scholar]
- 15.Thwaites GE, Bhavnani SM, Chau TT, Hammel JP, Torok ME, Van Wart SA, et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother. 2011 Jul;55(7):3244–3253. doi: 10.1128/AAC.00064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koup JR, Williams-Warren J, Weber A, Smith AL. Pharmacokinetics of rifampin in children. I. Multiple dose intravenous infusion. Ther Drug Monit. 1986;8(1):11–16. doi: 10.1097/00007691-198603000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Koup JR, Williams-Warren J, Viswanathan CT, Weber A, Smith AL. Pharmacokinetics of rifampin in children. II. Oral bioavailability. Ther Drug Monit. 1986;8(1):17–22. doi: 10.1097/00007691-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Thee S, Garcia-Prats AJ, McIlleron HM, Wiesner L, Castel S, Norman J, et al. Pharmacokinetics of ofloxacin and levofloxacin for prevention and treatment of multidrug-resistant tuberculosis in children. Antimicrob Agents Chemother. 2014 May;58(5):2948–2951. doi: 10.1128/AAC.02755-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Nandy P, Chien S, Noel GJ, Tornoe CW. Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob Agents Chemother. 2010 Jan;54(1):375–379. doi: 10.1128/AAC.00667-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Te Brake L, Dian S, Ganiem AR, Ruesen C, Burger D, Donders R, et al. Pharmacokinetic/pharmacodynamic analysis of an intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis. Int J Antimicrob Agents. 2015 Feb 7; doi: 10.1016/j.ijantimicag.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Roadmap for childhood tuberculosis: toward zero deaths. 2013 URL: http://apps.who.int/iris/bitstream/10665/89506/1/9789241506137_eng.pdf.
- 22.Ramachandran P, Duraipandian M, Nagarajan M, Prabhakar R, Ramakrishnan CV, Tripathy SP. Three chemotherapy studies of tuberculous meningitis in children. Tubercle. 1986 Mar;67(1):17–29. doi: 10.1016/0041-3879(86)90028-0. [DOI] [PubMed] [Google Scholar]
- 23.Schoeman J, Wait J, Burger M, van Zyl F, Fertig G, van Rensburg AJ, et al. Long-term follow up of childhood tuberculous meningitis. Dev Med Child Neurol. 2002 Aug;44(8):522–526. doi: 10.1017/s0012162201002493. [DOI] [PubMed] [Google Scholar]
- 24.Nahata MC, Fan-Havard P, Barson WJ, Bartkowski HM, Kosnik EJ. Pharmacokinetics, cerebrospinal fluid concentration, and safety of intravenous rifampin in pediatric patients undergoing shunt placements. Eur J Clin Pharmacol. 1990;38(5):515–517. doi: 10.1007/BF02336694. [DOI] [PubMed] [Google Scholar]
- 25.High-dose rifampin, SQ109 and moxifloxacin for treating TB: the PanACEA MAMS-TB trial; Conference on Retroviruses and Opportunistic Infections; Seattle, WA. February 25; 2015. [Google Scholar]
- 26.Diacon AH, Patientia RF, Venter A, van Helden PD, Smith PJ, McIlleron H, et al. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob Agents Chemother. 2007 Aug;51(8):2994–2996. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013 Dec;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heideman RL, Gillespie A, Ford H, Reaman GH, Balis FM, Tan C, et al. Phase I trial and pharmacokinetic evaluation of fazarabine in children. Cancer Res. 1989 Sep 15;49(18):5213–5216. [PubMed] [Google Scholar]
- 29.Kellie SJ, Barbaric D, Koopmans P, Earl J, Carr DJ, de Graaf SS. Cerebrospinal fluid concentrations of vincristine after bolus intravenous dosing: a surrogate marker of brain penetration. Cancer. 2002 Mar 15;94(6):1815–1820. doi: 10.1002/cncr.10397. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs RF, Kearns GL, Brown AL, Longee DC. Cerebrospinal fluid penetration of imipenem and cilastatin (primaxin) in children with central nervous system infections. Antimicrob Agents Chemother. 1986 Apr;29(4):670–674. doi: 10.1128/aac.29.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowe ES, Kitchen BJ, Erdmann G, Stork LC, Bostrom BC, Hutchinson R, et al. Plasma pharmacokinetics and cerebrospinal fluid penetration of thioguanine in children with acute lymphoblastic leukemia: a collaborative Pediatric Oncology Branch, NCI, and Children’s Cancer Group study. Cancer Chemother Pharmacol. 2001 Mar;47(3):199–205. doi: 10.1007/s002800000229. [DOI] [PubMed] [Google Scholar]
- 32.Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb) 2010 Sep;90(5):279–292. doi: 10.1016/j.tube.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Bamrah S, Brostrom R, Dorina F, Setik L, Song R, Kawamura LM, et al. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis. 2014 Aug;18(8):912–918. doi: 10.5588/ijtld.13.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thee S, Garcia-Prats AJ, Draper HR, McIlleron HM, Wiesner L, Castel S, et al. Pharmacokinetics and Safety of Moxifloxacin in Children With Multidrug-Resistant Tuberculosis. Clin Infect Dis. 2014 Oct 30; doi: 10.1093/cid/ciu868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley JS, Jackson MA, Committee on Infectious Diseases, American Academy of Pediatrics The use of systemic and topical fluoroquinolones. Pediatrics. 2011 Oct;128(4):e1034–45. doi: 10.1542/peds.2011-1496. [DOI] [PubMed] [Google Scholar]
- 36.Bamrah S, Brostrom R, Dorina F, Setik L, Song R, Kawamura LM, et al. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis. 2014 Aug;18(8):912–918. doi: 10.5588/ijtld.13.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loos U, Musch E, Jensen JC, Mikus G, Schwabe HK, Eichelbaum M. Pharmacokinetics of oral and intravenous rifampicin during chronic administration. Klin Wochenschr. 1985 Dec 2;63(23):1205–1211. doi: 10.1007/BF01733779. [DOI] [PubMed] [Google Scholar]
- 38.Zvada SP, Denti P, Donald PR, Schaaf HS, Thee S, Seddon JA, et al. Population pharmacokinetics of rifampicin, pyrazinamide and isoniazid in children with tuberculosis: in silico evaluation of currently recommended doses. J Antimicrob Chemother. 2014 May;69(5):1339–1349. doi: 10.1093/jac/dkt524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979 Mar;25(3):358–371. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- 40.Jonsson EN, Karlsson MO. Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Computer methods and programs in biomedicine. 1999;58(1):51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 41.Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed. 2005 Sep;79(3):241–257. doi: 10.1016/j.cmpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007 Jul;82(1):17–20. doi: 10.1038/sj.clpt.6100241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


