Abstract
Background:
Hippocampal volume data from India have recently been reported in younger adults. Data in older adults are unknown. The present paper describes hippocampal volume from India among older adults and compares the same with patients having Alzheimer's disease (AD) and mild cognitive impairment (MCI).
Materials and Methods:
A total of 32 cognitively normal subjects, 20 patients with AD, and 13 patients with MCI were enrolled. Patients were evaluated for the diagnosis of AD/MCI using the National Institute of Neurological and Communicative Disorders and Stroke and the Related Disorders Association criteria and the Clinical Dementia Rating (CDR) Scale (score = 0.5), respectively. Hippocampal volume was measured using magnetic resonance imaging (MRI) machine by manual segmentation (Megnatom Symphony 1.5T scanner) three-dimensional (3D) sequences.
Results:
Age and duration of illness in the MCI group were 70.6 ± 8.6 years and 1.9 ± 0.9 years, respectively. In the AD group, age and duration of illness were 72 ± 8.1 years and 3.1 ± 2.2 years, respectively. In cognitively normal subjects, the age range was 45-88 years (66.9 ± 10.32) years. Mean mini–mental status examination (MMSE) score of healthy subjects was 28.28 ± 1.33. In the MCI group, MMSE was 27.05 ± 1.79. In the AD group, MMSE was 13.32 ± 5.6. In the healthy group, the hippocampal volume was 2.73 ± 0.53 cm3 on the left side and 2.77 ± 0.6 cm3 on the right side. Likewise, in MCI, the volume on the left side was 2.35 ± 0.42 cm3 and the volume on the right side was 2.36 ± 0.38 cm3. Similarly, in the AD group, the volume on the right side was 1.64 ± 0.55 cm3 and on the left side it was 1.59 ± 0.55 cm3. Post hoc analysis using Tukey's honestly significant difference (HSD) showed, using analysis of variance (ANOVA) that there was a statistically significant difference between healthy and AD (P ≤ 0.01), and between healthy and MCI (P ≤ 0.01) subjects. There was a correlation between MMSE score and hippocampal volume in the AD group.
Conclusion:
The volume of the hippocampus in older Indian adults was 2.77 ± 0. 6 cm3 on the right side and 2.73 ± 0.52 cm3 on the left side. There was a significant hippocampal volume loss in MCI/AD compared to cognitively normal subjects.
Keywords: Alzheimer's disease (AD), depression, hippocampal volume, mild cognitive impairment (MCI), normative data, older Indian adults
Introduction
The hippocampus is a plastic and vulnerable structure.[1] It can get damaged in a variety of conditions, such as Alzheimer's disease (AD), mild cognitive impairment (MCI), epilepsy, depression, posttraumatic stress disorders, Cushing's disease, and hypertension. Assessment (both qualitative and quantitative) of hippocampus can be done via several methods. The role of quantitative volumetry is growing.[1,2] For example, in diagnosis of mesial temporal lobe sclerosis, volumetry can help in lateralization and prognostication of seizure control.[3] Likewise, it can differentiate between various types of dementias and differentiate true dementia from pseudodementia, which is a common diagnostic confusion. Bilateral shrinkage of hippocampii is a hallmark of AD. Patients with AD present with cognitive decline and inability to form new memories. The value of this structure in learning and memory is therefore indisputable. Interest in radiological measurement of the hippocampus has grown exponentially in recent times. Presently, the hippocampus is an early radiological marker of cognitive decline, is a predictor of conversion of MCI to AD, and can also help in early diagnosis of AD. Of late, there have been suggestions that radiological measurements of hippocampal volumes will become a matter of routine for the diagnostic evaluation of dementia of AD type.
Normative data for hippocampal volume have recently been given in younger adults from India.[3] While the data in young adults help in cases of epilepsy, no such data exist for older adults that have direct relevance to AD/MCI and several other related diseases. The purpose of this study was to provide hippocampal volume measurements in cognitively normal healthy older Indian adults and compare them with AD/MCI.
Materials and Methods
Patient selection and diagnostic evaluation
Patients with memory complaints presenting to the Department of Neurology were selected randomly (simple random sampling) and asked to attend the Memory Clinic for detailed neuropsychological and neurological examination for diagnosis of MCI/AD. A detailed general physical examination was done in all cases to rule out systemic disease. Patients were evaluated for the diagnosis of AD using the National Institute of Neurological and Communicative Disorders and Stroke and the Related Disorders Association criteria.[4] Diagnosis of MCI was done as per Clinical Dementia Rating (CDR) scale (score = 0.5).[5] Dementias other than AD and memory complaints not meeting CDR criteria for diagnosis of MCI were excluded for the present study.
Healthy controls
Healthy older adults (45-85 years) were recruited from among the staff workers of a tertiary care institute. Written, informed consent was obtained from all participants. Demographic details such as age, sex, and educational background was noted. Codes for education (0 = High school, 1 = Undergraduates, and 2 = Postgraduates) were assigned. This was done to convert nominal data into ordinal data for studying correlation between education and hippocampal volume. Healthy controls were subjected to magnetic resonance imaging (MRI) of the brain using the protocol detailed below. Thorough history taking, clinical examination, and inspection of written and records was done to diagnose diabetes, hypertension, seizures, and depression in healthy subjects. The Cornell Scale for Depression in Dementia (CSDD) was used to screen participants for depression. The study was approved by the Institutional Ethics Committee.
Scales
The mini–mental status examination (MMSE) was used to divide patients into “mild moderate” and “severe” categories. The CSDD was used to screen participants for depression.
Radiology protocol
Volumes have been calculated using a region of interest (ROI) approach, using magnetization prepared rapid acquisition gradient echo (MPRAGE) sequences, coronal oblique, perpendicular to the long axis of the hippocampus [Figures 1 and 2]. Areas of the hippocampus on subsequent sections were added and multiplied by the section thickness and interslice gap for an estimate of the volume.
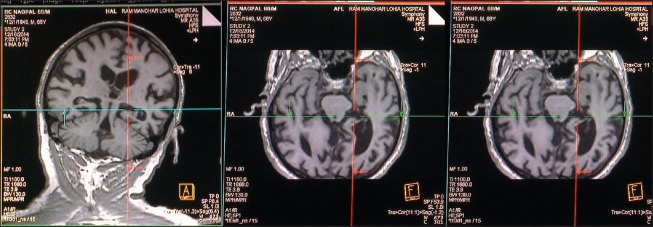
Figure 1.
MRI of brain showing oblique coronal images. ROI approach showing the area of hippocampus highlighted in T1-weighted images. Area in the consecutive slides has been summed up by manually outlining hippocampal area and multiplying by interslice gap and slice thickness to obtain the volume in cubic centimeter (cm3)
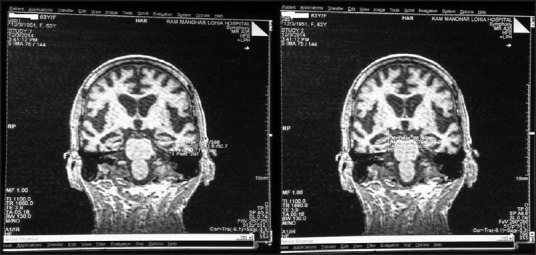
Figure 2.
Localization of hippocampus using T2 sagittal section for planning coronal oblique sections perpendicular to long axis of hippocampus
In the current study, hippocampal volume has been measured using a 1.5 Tesla Magnetic Resonance machine (Megnatom Symphony 1.5T scanner, Germany). Images were acquired in T1- and T2-weighted, fluid attenuation inversion recovery (FLAIR) sequences in the axial, coronal, and sagittal planes. A T2 sagittal section was used to plan the three-dimensional (3-D) sequences by 3-D MPRAGE for estimation of hippocampal volumes.
Images perpendicular to the long axis of the hippocampus, oblique coronal section were taken for delineation of the selected area (ROI). T1-weighted coronal images were used in all slides wherever the hippocampus was visible. Image parameters were as follows: A 3-D image reconstruction was done using fast low angle shot (FLASH) MRI. Slice thickness was 1 mm with a repetition time of 14 s (total scan time = 5.22 min). The hippocampus was defined as cornu ammonis, dentate gyrus, and subiculum. The hippocampus was delineated using anatomical landmarks as described below.
In the first slide of coronal section-T1 weighted images, the hippocampus was first visualized, and the area bordering the amygdala was considered to be the most anterior part of the hippocampus. The alveus was used as a landmark to separate the amygdala from the hippocampus. Precaution was taken not to include part/s of the amygdala. Then, 3-D viewing images were used to clearly define hippocampal boundaries [Figure 3].
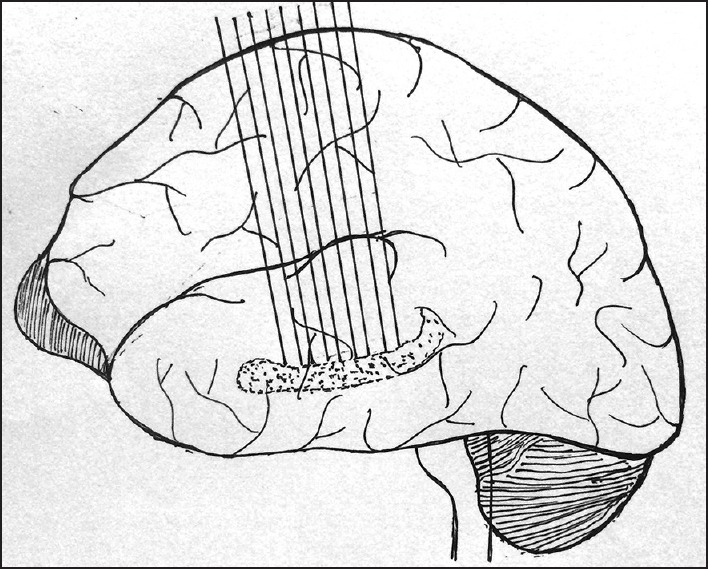
Figure 3.
3-D planes used for outlining the hippocampus in the current study
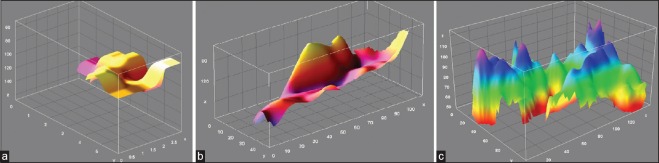
Figure 4.
Three dimensional (3-D) outlines of three hippocampal images (Pseudo images generated by Image-J, National Institute of Health, USA, downloaded free at http://imagej.nih.gov/ij. Figures-A, B & C have been drawn from present patients to show comparison of normal hippocampus (A) in healthy individuals with those having MCI (B), and AD. Those with AD have high crests and troughs and uneven volume loss (C)
The alveus was visualized as a band of white matter and taken as the border between the hippocampus and the amygdala. Crux of the fornix was taken as the posterior boundary of the hippocampus. Hippocampal volume was measured by summing up the area that had been delineated using the manual cursor. The area thus obtained was multiplied by 0.15 (1 mm slice thickness + 0.5 mm interslice gap).
This yielded values in cubic centimeter. Intrarater and interrater reliability were calculated using Cohen's kappa between two raters. Intrarater reliability was calculated by Vikas Dhikav (n = 10) and interrater reliability was calculated between Sharmila Duraswamy and Vikas Dhikav (n = 10). Ten cases within and in between raters were selected randomly between raters and the same were rated twice. They consisted of healthy, MCI, and AD group patients.
Statistical analysis
Statistical Package for Social Sciences (SPSS®, SPSS Inc., Chicago, IL, USA) ver. 17 was used for statistical data analysis. Normality of data was checked using q-q plot. Correlation and regression were performed with dependent variable (hippocampal volumes) and Pearson correlation coefficient with P value was calculated. Analysis of variance (ANOVA) with post hoc analysis was used to analyze the differences between the three groups. Differences between left and right hippocampal volumes were compared using the paired t- test. A P value of 0.05 was considered to be significant. > Linear regressionwas used to know the correlation between hippocampal volumes, age, and MMSE, and logistic regression was used for correlation of education and hippocampal volume. Cohen's kappa was used to estimate interrater reliability. Two-sided P value <0.05 was used to test the level of significance.
Results
Demographic and clinical details of the study participants are summarized in Table 1. Age and duration of illness for the MCI group were 70.6 ± 8.6 years and 1.9 ± 0.9 years, respectively. In the AD group, age and duration of illness were 72 ± 8.1 years and 3.1 ± 2.2 years, respectively. In the healthy group, the age range was 45-88 (66.93 ± 10.32) years.
Table 1.
Demographic and clinical data of the study participants
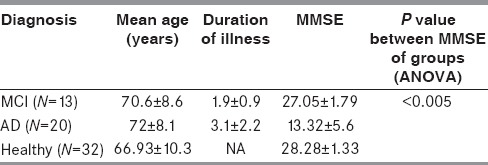
Mean MMSE scores of healthy subjects and of the AD group were statistically different (P value ≤ 0.001). Tukey's honestly significant difference (HSD) showed the difference to be significant at (P value ≤ 0.01) between MCI and AD and healthy group AD. Correlations between age, education, and MMSE with hippocampal volume are given in Table 2.
Table 2.
Correlation coefficients between planned variables
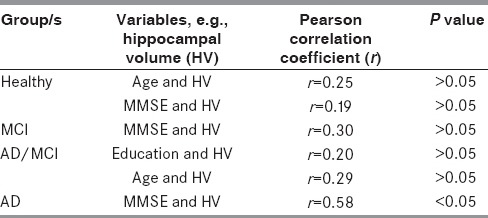
There was no significant difference between the MMSE scores of those in the MCI and healthy groups. In the MCI and AD groups, decreasing hippocampal volumes were correlated with decreasing MMSE scores, but the same was not seen in the healthy group cases. A total of 10 patients out of 20 with the diagnosis of AD (50%) had hippocampal atrophy in the present study [Table 3].
Table 3.
Summary characteristics of hippocampal volumes* in the present study

Hippocampal atrophy[3,5] was defined using the mean hippocampal volume ± 2 standard deviation deviations. Only 1 case out of 13 had hippocampal atrophy in the MCI group [odds ratio (OR) = 6.5, 95% confidence interval (CI) = 0.7414-56.9872]. None in the healthy group had hippocampal atrophy. In the current study, the percentage of hippocampal volume loss in AD was 47% compared to the healthy individuals. Likewise, in the MCI group, there was a 15% volume loss compared to the healthy group. That means that from MCI to AD there is a volume loss of almost 35%. A high intra- and interrater agreement (VD and SD) was obtained between two raters who independently rated images (Cohen's kappa = 0.69). Cohen's kappa was calculated as the agreement between the raters when the calculated means of hippocampal volumes values did not differ by more than 5% between raters and was taken as the disagreement when the difference was more than 5%. A high correlation was obtained between low education (<10th standard or 10 years of formal school education) and low hippocampal volume (Pearson correlation coefficient = 0.6, P value <0.05).
Post hoc analysis using Tukey's HSD showed, using ANOVA, that there was a statistically significant difference between healthy and AD (P ≤ 0.01), and healthy and MCI (P ≤ 0.01) subjects.
Discussion
The hippocampus[1,2,3] and loss of its volume[3] has received attention in the diagnostic and prognostic evaluation of neurocognitive disorders.[1] Hippocampal volume has also been used in the research setting,[4] where it has been shown to differentiate cognitively normal elderly from those with AD and in clinical drug trials related to cognition enhancers.[5] Longitudinal results confirm that initial hippocampal volume is predictive of conversion to AD[6] and can also differentiate AD from MCI.[7] It can also differentiate dementia from pseudodementia;[8] different types of dementias can also be differentiated, in combination with clinical and other supportive laboratory data.[9] A variety of manual and automatic techniques have been used for measurements.[10,11] Though the automatic method is faster and less likely to be affected by rater bias, manual measurements are considered the gold standard.[12] Recently, normative data of hippocampal volumes in many countries have been published[11] and in some countries they have been known for some time, but such data in older Indian adults are currently unknown.
There are several risk factors that are known to damage the hippocampus, such as, stress, depression, seizures, hypertension, depression, and diabetes.[1] Likewise, evidence is emerging regarding the association of biochemical factors such as vitamin D3, serum cortisol, and homocysteine with hippocampal volume loss. For the current study, individuals have been selected who have normal values of biochemical factors that have been known to cause damage to the hippocampus. These factors include serum cortisol, homocysteine, vitamin D, and vitamin B12. Likewise, these subjects/patients have also been screened for clinical risk factors potentially or actually damaging hippocampus, e.g., diabetes, hypertension, seizures, and depression. Therefore, the present study reports the hippocampal volumes of healthy older adults and compares those with MCI/AD cases to observe the extent of volume loss in the absence of putative or actual factors known to damage the hippocampus. Hippocampal volume data from a small number of children[2] and young adults[3] in India exist. Clinical challenges exist when defining hippocampal atrophy in Indian patients using Western data, as the hippocampal volume values differ significantly.[4] Volumes obtained in the present study are not significantly different from the volumes reported earlier from India.[3] One study had reported on healthy elderly subjects from India, but it had a small sample size and calculations were not done using power analysis.[9] Another study was done in Indian children, the volumes reported from which are similar to those from our own. In the current study, the percentage of hippocampal volume loss in AD patients was almost 50% compared to the healthy individuals. Likewise, in the MCI group, there was a 15% volume loss compared to the healthy group. That means that from MCI to AD, there is a volume loss of almost 35%.
Our values differ from the ones reported from Western countries.[4] This is consistent with a small study of Malay children, where it was shown that volumes in the Asian region could be naturally smaller. Likewise, a study of young adults from India has shown a volume of 2.4 cm3. Our results differ by 12% from the volumes reported by this earlier study. A relatively different hippocampal volume measurement protocol[13] may have been responsible for the small variation. In a large study, no significant correlation between age and hippocampal volume has been found.[14] However, in other initial studies, correlation between age and volume was found.[15] We have included the entire length of hippocampus, as suggested by Bhatia et al.[15] Right and left asymmetry is consistent with the findings earlier by Jack et al.[16] Our data in older adults are not significantly different from older Chinese adults.[17] Differences in normative data and volume in AD have been reported elsewhere; in India, such data are not known.[18] Though the current sample size is not very large, for a first-time study its sample size is almost the same as that in many studies reported previously.[17,18,19]
This study reports that a decreasing MMSE score correlates with decreasing hippocampal volumes in AD. The same has been demonstrated earlier.[20] This supports the notion that decrease in MMSE indicates that the severity of dementia is higher, and it has also been correlated with the Visual Rating Scale of Scheltens.[21]
The major strengths of the study are as follows. Hippocampal volumes have been given for the first time in older adults from the Indian subcontinent with good inter- and intrarater reliability. Subjects from wide age ranges have been selected. A comparison has been obtained using cases of MCI/AD to show that hippocampal volume loss occurs in both of the subgroups. Moreover, reported values are not significantly different from an earlier reported study.[3] Power analysis has been employed for calculating the requisite sample size in this study, and the same can be used for normative data from older adults in the Indian subcontinent.
The present study is the first study from India describing the volumes of the hippocampus in older adults. Though it included carefully selected patients, use of a standardized hippocampal measurement protocol, and standardized diagnostic criteria in Indian settings, it nevertheless has some limitations. Patients who have been patients visiting hospitals have been studied but not the older adults living in the community. Controls working in the institute setting as staff workers have been included. Hospital staff are prone to a number of stresses: factors that are known to affect hippocampal volumes. Notwithstanding the limitations, the present study provides the normative and comparative data of hippocampal volumes in Indian patients that can be used as reference for defining atrophy in this age group.
There are several uses of volumetry.[6,7] It can help to differentiate dementia from pseudodementia. The extent of volume loss in the latter is less than in the former. Likewise, different subtypes of dementias can be differentiated on the basis of volumetry.[8] The extent of volume loss in AD has been seen to be higher compared to other dementias such as frontotemporal, normal pressure hydrocephalus and vascular dementias. Notably, this is one of the common diagnostic confusions in identifying subtypes of demented patients, as it may have prognostic implications. Volumetric analysis of the hippocampus can also be used for clinical trial purposes when a new drug is under evaluation.[5] There is a reason to believe that those with smaller baseline hippocampal volumes are more likely to convert compared to those with larger volumes.[6,7] Therefore, overall, volumetry can enhance the accuracy of MRI as a diagnostic modality in diagnosing dementia subtypes in a significant way. The only impediment is that manually outlining the hippocampus in both sides could be a tedious and labor-intensive process. However, it should be noted that in patients with AD, because there is a significant shrinkage of hippocampal volume, it takes just 5-15 min for an experienced observer to calculate hippocampal volume on a 1.5 Tesla machine. In those with a large hippocampal volume, as it will be visible in several cuts, it would take a longer time. Though automatic segmentation methods are available, manual delineation of the hippocampus is still considered to be the “gold standard” method.[8] The definition of the hippocampus has to be done properly and one should be cautious not to leave out important areas and not to include something that is not important, e.g., parahippocampal area, amygdala, and choroid plexus. The current study provides baseline and comparative data of hippocampal volumes in older adults from the Indian subcontinent.
A total of 10 patients out of 20 with the diagnosis of AD had hippocampal atrophy in the present study (50%), while only MCI patients had hippocampal volume loss in the range of atrophy. As the data from India have been reported only from young adults[3] and there were some issues with the paper,[22] the authors of the paper agreed that data from an older age group were needed.[23] The importance of the data in older adults is that the hippocampal volumetry values have been included as research criteria for the diagnosis of preclinical AD.[24]
The role of hippocampal volumetry is growing. The volume of the hippocampus reported in the present study and that in a study done from India earlier[4] are smaller compared to a similar series[25] in the Western population (3.32 cm3 vs 3.20 cm3). The percentage of difference is approximately 15%. Such differences have also been noted in Malay[2] and Chinese studies. Different data acquisition techniques, analysis of software, and different types of anatomical boundaries may have been responsible for this. It has been realized that comparing results of MRI studies from different centers could be difficult because of this,[25] but the use of standardized hippocampal outlining protocols[26] has helped in minimizing errors.[27]
Conclusion
Hippocampal volumes in healthy controls over the age of 45 years have been provided in the Indian subcontinent. For comparison, volumes of hippocampus have been given for AD and MCI. The percentage of hippocampal volume loss in AD was almost 50% compared to the healthy individuals. In the AD group, there was a correlation between decrease in MMSE score and decrease in hippocampal volume. There was good agreement between raters on hippocampal volume values.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors wish to acknowledge the help of Ms. Pinki Mishra for making the hand-drawn diagram of MRI sections taken perpendicular to the long axis of the hippocampus. In addition, the help of Love Gandhi and Mr. Virender Arya of AITBS Publishers, who helped in making 3-D curves using Image-J, is appreciated. Ms. Dolly Prajapati helped in the finalization of the correlation coefficient tables. The help of Ms. Rashmi Nain, Nanchang University, People's Republic of China in statistical analysis and approving the manuscript for final publication is appreciated.
References
- 1.Dhikav V, Anand K. Potential predictors of hippocampal atrophy in Alzheimer's disease. Drugs Aging. 2011;28:1–11. doi: 10.2165/11586390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Mulani SJ, Kothare SV, Patkar DP. Magnetic resonance volumetric analysis of hippocampi in children in the age group of 6-to-12 years: A pilot study. Neuroradiology. 2005;47:552–7. doi: 10.1007/s00234-005-1379-3. [DOI] [PubMed] [Google Scholar]
- 3.Mohandas AN, Bharath RD, Prathyusha PV, Gupta AK. Hippocampal volumetry: Normative data in the Indian population. Ann Indian Acad Neurol. 2014;17:267–71. doi: 10.4103/0972-2327.138482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak HK, Qian W, Ng KS, Chan Q, Song YQ, Chu LW, et al. Combination of MRI hippocampal volumetry and arterial spin labeling MR perfusion at 3-Tesla improves the efficacy in discriminating Alzheimer's disease from cognitively normal elderly adults. J Alzheimers Dis. 2014;41:749–58. doi: 10.3233/JAD-131868. [DOI] [PubMed] [Google Scholar]
- 5.Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou YY, Toga AW, et al. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer disease. Alzheimer Dis Assoc Disord. 2012;26:17–27. doi: 10.1097/WAD.0b013e3182163b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anstey KJ, Maller JJ. The role of volumetric MRI in understanding mild cognitive impairment and similar classifications. Aging Ment Health. 2003;7:238–50. doi: 10.1080/1360786031000120732. [DOI] [PubMed] [Google Scholar]
- 7.Cavedo E, Redolfi A, Angeloni F, Babiloni C, Lizio R, Chiapparini L, et al. The Italian Alzheimer's Disease Neuroimaging Initiative (I-ADNI): Validation of structural MR imaging. J Alzheimers Dis. 2014;40:941–52. doi: 10.3233/JAD-132666. [DOI] [PubMed] [Google Scholar]
- 8.Dolek N, Saylisoy S, Ozbabalik D, Adapinar B. Comparison of hippocampal volume measured using magnetic resonance imaging in Alzheimer's disease, vascular dementia, mild cognitive impairment and pseudodementia. J Int Med Res. 2012;40:717–25. doi: 10.1177/147323001204000236. [DOI] [PubMed] [Google Scholar]
- 9.Vijayakumar A, Vijayakumar A. Comparison of hippocampal volume in dementia subtypes. ISRN Radiol. 2012;2013:174524. doi: 10.5402/2013/174524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–10. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Theodore WH, Cook M, McCarthy G. MRI-based hippocampal volumetrics: data acquisition, normal ranges, and optimal protocol. Magn Reson Imaging. 1995;13:1057–64. doi: 10.1016/0730-725x(95)02013-j. Review. [DOI] [PubMed] [Google Scholar]
- 12.Apostolova LG, Dinov ID, Dutton RA, Hayashi KM, Toga AW, Cummings JL, et al. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain. 2006;129:2867–73. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- 13.de Flores R, La Joie R, Landeau B, Perrotin A, Mézenge F, de La Sayette V, et al. Effects of age and Alzheimer's disease on hippocampal subfields: Comparison between manual and free surfer volumetry. Hum Brain Mapp. 2015;36:463–74. doi: 10.1002/hbm.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouiha A, Duchesne S. Multi-decade hippocampal and amygdala volume analysis: Equal variability and limited age effect. Neurosci Lett. 2011;499:93–8. doi: 10.1016/j.neulet.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 15.Bhatia S, Bookheimer SY, Gaillard WD, Theodore WH. Measurement of whole temporal lobe and hippocampus for MR volumetry: Normative data. Neurology. 1993;43:2006–10. doi: 10.1212/wnl.43.10.2006. [DOI] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: Normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–54. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- 17.Zou L, Xiao J, Zhou X, Sun C, Xiong Y. Hippocampal formations, amygdala and anterior temporal lobes: Normative volumetric measurements from MR imaging in normal adults of China. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:719–22. [PubMed] [Google Scholar]
- 18.Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR Am J Neuroradiol. 1999;20:207–11. [PMC free article] [PubMed] [Google Scholar]
- 19.Gonçalves-Pereira PM, Oliveira E, Insausti R. Quantitative volumetric analysis of the hippocampus, amygdala and entorhinal cortex: Normative database for the adult Portuguese population. Rev Neurol. 2006;42:713–22. [PubMed] [Google Scholar]
- 20.Adachi M, Kawakatsu S, Sato T, Ohshima F. Correlation between volume and morphological changes in the hippocampal formation in Alzheimer's disease: Rounding of the outline of the hippocampal body on coronal MR images. Neuroradiology. 2012;54:1079–87. doi: 10.1007/s00234-012-1019-7. [DOI] [PubMed] [Google Scholar]
- 21.Dhikav V, Sethi M, Anand KS. Medial temporal lobe atrophy in Alzheimer's disease/mild cognitive impairment with depression. Br J Radiol. 2014;87:20140150. doi: 10.1259/bjr.20140150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand KS, Dhikav V, Doraswamy S, Garga UC. Issues with normative data of hippocampal volumetry in Indian population. Ann Indian Acad Neurol. 2015;18:259. doi: 10.4103/0972-2327.150607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohandas AN, Bharath RD, Prathyusha PV, Gupta AK. Author's Reply: Regarding issues with article on hippocampal volumetry. Ann Indian Acad Neurol. 2015;18:260–1. doi: 10.4103/0972-2327.152088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besson FL, La Joie R, Doeuvre L, Gaubert M, Mézenge F, Egret S, et al. Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer's disease. J Neurosci. 2015;35:10402–11. doi: 10.1523/JNEUROSCI.0150-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: Minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–42. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka Y, Mori E, Inagaki M, Kozaki Y, Nakano T, Wenner M, et al. Manual tracing guideline for volumetry of hippocampus and amygdala with high-resolution MR. No To Shinkei. 2003;55:690–7. [PubMed] [Google Scholar]
- 27.Wang D, Guo ZH, Liu XH, Li YH, Wang H. Examination of hippocampal differences between Alzheimer disease, amnestic mild cognitive impairment and normal aging: Diffusion kurtosis. Curr Alzheimer Res. 2015;12:80–7. doi: 10.2174/1567205012666141218142422. [DOI] [PubMed] [Google Scholar]