Abstract
Cerebellar agenesis (CA) is an extremely rare entity. We present two adult patients with CA. The 61-year-old man had ataxia, dysarthria, abnormalities in cerebellar tests, severe cognitive impairment, and moderate mental retardation. The 26-year-old woman had dysmetria, dysdiadochokinesia, and dysarthria as well as mild cognitive impairment and mild mental retardation. Magnetic resonance imaging (MRI) showed complete absence of the cerebellum with small residual vermis. Brainstem was hypoplastic and structures above tentorium were normal. Supratentorial white matter bundles were unaffected in diffusion tensor tractography. Only few adult patients with CA have so far been published. These cases show that patients with CA present with a variety of developmental, clinical, and mental abnormalities; and emphasize the role of the cerebellum in normal motor, language, and mental development.
Keywords: Cerebellar agenesis, diffusion tensor tractography, magnetic resonance imaging
Introduction
Cerebellar agenesis (CA) is an extremely rare entity. There is a debate in the literature as to whether a normal or near normal life is possible in case of complete CA. While supporters of one side of the debate say that such patients always have severe motor deficits,[1] others believe the myth that they may not have any observable symptoms. Most cases with CA are diagnosed in the prenatal or early postnatal period and are associated with other central nervous system abnormalities.[2,3,4] Only few children or adults with this disorder have so far been published. While most of these cases were diagnosed in autopsy,[1,5,6] others were living patients diagnosed by magnetic resonance imaging (MRI).[7,8,9,10,11,12,13,14] Detailed clinical evaluation was available only in three cases.[11,13,14] We report clinical, neuropsychiatric, and advanced neuroimaging findings of two new cases of CA in adult living patients to shed more light on this subject.
Case Report
Case 1
We diagnosed complete agenesis of the cerebellum in a 61-year-old male patient presenting with difficulty in walking and disturbed speech. He had a history of developmental delay, had not learned to read or write, and had not worked. He was severely dysarthric andataxic. He had bilateral dysmetria, dysdiadochokinesia, and reduced arm and leg coordination. His muscle strength and tone was normal without any motor deficits with normal deep tendon reflexes and sensory system. He had congenital esotropia and strabismic amblyopia. His family history was unremarkable. His full scale intelligent quotient (IQ) level, as assessed using Kent E-G-Y Test and the Porteus Maze Test, was 35 showing that he had moderate mental retardation. He also had severe cognitive impairment (15/59) as tested by using Cognitive Status Schedule. Review of his available medical reports, dating back to 5 years earlier, disclosed that he had been issued a disability certificate for his moderate mental retardation, and that his neurologic and mental status had been assessed several times in the meantime showing only mild ataxia and an IQ score of 45. We noted that deterioration of his neurological and mental status had started 1 year prior to his latest presentation to our hospital. Cranial MRI (1.5 T GE HDxt) showed almost complete absence of the cerebellum with only small residual cerebellar tissue corresponding to vermis. Brainstem and middle cerebellar peduncles were hypoplastic; posterior fossa was normal in size. Absent cerebellum resulted in a large cerebrospinal fluid (CSF) space representing a huge cisterna magna. Supratentorial structures were normal [Figure 1a and b]. Diffusion tensor imaging (DTI) was performed with the following parameters: time repetition (TR) = 6,500 ms, time echo (TE) = 90 ms, voxel size = 1.1 × 1.1 × 5.5 mm3, 30 directions. Fractional anisotropy maps and tractography were later constructed on the workstation using manufacturer's softwares. Tractography was performed by placing a region-of-interest (ROI) in an axial slice to include only brainstem, so that all fibers passing through brainstem would be demonstrated. Diffusion tensor tractography showed that brainstem was small in size, cerebellum was absent, and no fibers extended from the brainstem to either residual vermis or small cerebellar peduncles. It was apparent that the absence of the afferent and efferent fibers that would normally pass through superior, middle, and inferior cerebellar peduncles caused the small size of the brainstem. Supratentorial white matter bundles were unaffected [Figure 1c]. Evaluation of three-dimensional (3D) T2-weighted sequences showed the absence of superior cerebellar and anterior and posterior inferior cerebellar arteries; while vertebral, basilar, and posterior cerebral arteries were normal.
Figure 1.
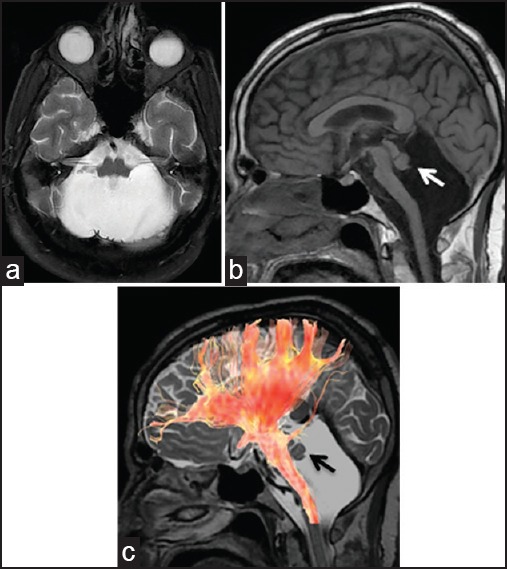
MRI of cerebellar agenesis in the 61-year-old male patient [Figure 1] and the 26-year-old female patient [Figure 2]. (a) T2-weighted axial and (b) T1-weighted sagittal images show absent cerebellum, small residual vermis (arrows), hypoplastic brainstem, and normal supratentorial anatomy. Note that the residual vermis tissue in smaller in Figure 2. (c) Diffusion tensor tractography, superimposed on 3D Cube T2 sagittal image shows that brainstem bundles are small and no fibers are extending to residual cerebellum, while supratentorial bundles are unaffected. MRI = Magnetic resonance imaging, 3D = Three-dimensional
Case 2
Total CA was diagnosed in a 26-year-old female patient. She had had a history of preterm birth (gestational age of 28 weeks) and developmental delay. She had walked and talked later than normal. Although she had been graduated from high school, she did not have employment. The examination of her motor system, including muscle strength and tone was normal without any motor deficits or abnormal movements. Examination of the cranial nerves, sensory system, and deep tendon reflexes were also normal. However, she had dysmetria, dysdiadochokinesia, dysarthria, and abnormal tandem gait. Ophthalmologic examination revealed congenital esotropia. Neuropsychiatric testing showed mild cognitive impairment (31/59), and IQ test showed mild mental retardation (between 55 and 70). Cranial MRI and diffusion tensor tractography findings were almost similar to those of case 1, although residual vermis tissue was smaller in this case [Figure 2a–c].
Figure 2.
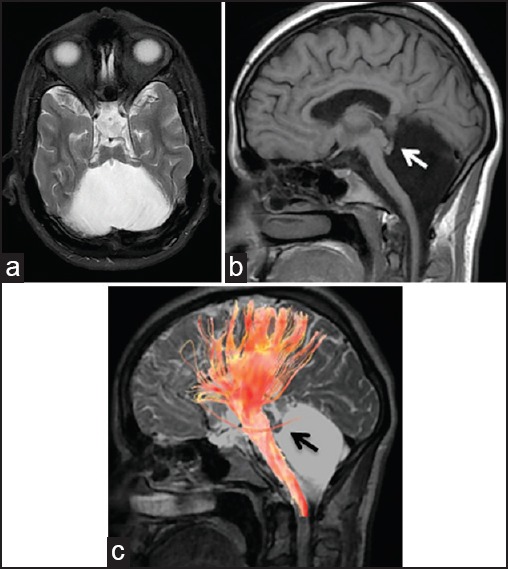
MRI of cerebellar agenesis in the 61-year-old male patient [Figure 1] and the 26-year-old female patient [Figure 2]. (a) T2-weighted axial and (b) T1-weighted sagittal images show absent cerebellum, small residual vermis (arrows), hypoplastic brainstem, and normal supratentorial anatomy. Note that the residual vermis tissue in smaller in Figure 2. (c) Diffusion tensor tractography, superimposed on 3D Cube T2 sagittal image shows that brainstem bundles are small and no fibers are extending to residual cerebellum, while supratentorial bundles are unaffected. MRI = Magnetic resonance imaging, 3D = Three-dimensional
Discussion
This study documented detailed neurologic, neuropsychiatric, and neuroimaging findings in two adult living patients with CA and supported the view that a life without a cerebellum is abnormal, although such patients can have a near normal life expectancy and lead a simple life. Using diffusion tensor tractography, it was additionally shown that supratentorial white matter bundles were unaffected and that no fibers extended from the brainstem to either residual vermis or small cerebellar peduncles.
A malformative or disruptive etiology may be responsible from CA.[15] In primary CA, there is a malformation of the developmental process; where in acquired cases, there is disruption of the cerebellum in the prenatal or perinatal period due to hemorrhage, ischemia, or other factors. CA with disruptive etiology is usually accompanied by additional brain abnormalities. Recently, mutation of Ptf1a gene, a key regulator of cerebellar neurogenesis, has been reported in a case of agenesis of the cerebellum and of the pancreas.[16] Genetic analysis was not performed in our patients.
Structural features of reported cases of CA, as assessed by anatomical dissection or MRI were almost similar to what we found in our cases: A normal sized posterior fossa, absent cerebellum, hypoplastic brainstem, and cerebellar peduncles. While in some cases there was no cerebellar remnant (complete CA), in other cases as in ours, a small remnant of paleocerebellum — a vestigial vermis — was found (near-complete CA). Absence of the cerebellum with normal development of the posterior fossa is thought to be due to the extremely early derangement of the cerebellar development during embryogenesis of the posterior fossa structures from rhombencephalon.[9] Brainstem hypoplasia is believed to be related to the loss of cerebellar radiations, including afferent and efferent fibers of the cerebellum.[17] In the diffusion tensor tractography of both of our patients, the absence of fibers from the brainstem to the residual vermis may show that the residual tissue is not functional. Posterior circulation blood vessels in both of our patients were absent. Similar finding was reported by Yu et al., supporting the assumption that the severity of posterior fossa vascular abnormalities is related to the degree of cerebellar hypoplasia.[14]
Glickstein refused the ‘oral tradition’ that people born without a cerebellum can live symptom free and claimed that CA is always associated with severe deficits in the development of normal movement.[1] Indeed, in all documented cases, the patients were late to stand, walk, and talk; however, almost all patients eventually learned to walk and speak. Some patients have never learned to read and write[11,14] (case 1), while one patient attended school,[12] and another patient was graduated from high school (case 2). One case was working in an electronic workshop,[11] while others have never been employed. Abnormal gait was present in almost all patients, although it was very mild in some cases[9,12,13](case 2). Speech was slurred and dysarthric in almost all patients. Cerebellar tests showed varying degrees of abnormalities. Abnormal eye movements or esotropia were found in some patients[10,11](case 1 and 2). Cognition was reported to be just below normal level in one patient,[13] while case 1 of our series demonstrated severe cognitive impairment, and case 2 showed mild cognitive impairment. Almost all patients presented with mild to moderate mental retardation. Other neurologic examination findings were usually normal. These findings show that cerebellum is necessary for normal motor, language, and mental development. While the cerebellum has once been known to be a center for motor coordination and execution, it is now recognized as a center for higher cognitive functions as well, including perception, language, cognition, and affection.[13] The discrepancy between the severity of morphologic abnormality and the less severe clinical presentation may be explained by the early onset of the malformation and ensuing neural reorganization, resulting in partial compensation of cerebellar functions.[9]
An interesting aspect of the disease is that various abnormalities may change over time. Tavano et al., reported that motor and language development, cognition, and affection of the patient showed a slow and stable progression up to almost adequate levels over years due to the lifelong rehabilitation program.[13] On the other hand; neurological, mental, and cognitive status of case 1 of our series deteriorated late in his life. Similar deterioration was reported in the case of Boyd during the last 9 years of his life.[6] The reason for the clinical worsening in these cases is not known, although it may be explained by age-related changes. It may be said that a rehabilitation program might be helpful during the early course of the disease, while in later ages worsening of findings might be expected.
In conclusion, patients with CA present with a variety of developmental, clinical, and mental abnormalities. Although they may lead a simple life, they suffer various degrees of cognitive, intellectual, and fine motor skill problems. These cases emphasize the role of the cerebellum in normal motor, language, and mental development.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Glickstein M. Cerebellar agenesis. Brain. 1994;117:1209–12. doi: 10.1093/brain/117.5.1209. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton RL, Grafe MR. Complete absence of the cerebellum: A report of two cases. Acta Neuropathol. 1994;88:258–61. doi: 10.1007/BF00293402. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Malik GK, Gupta A, Saksena S, Gupta RK. MR demonstration of complete cerebellar and corpus callosum agenesis. Pediatr Neurosurg. 2007;43:29–31. doi: 10.1159/000097522. [DOI] [PubMed] [Google Scholar]
- 4.Huissoud C, Rudigoz RC, Bisch C, Brahimi P, Alias F, Tixier H, et al. Complete cerebellar agenesis: A very rare abnormality of the posterior fossa. Ultrasound Obstet Gynecol. 2009;33:730–1. doi: 10.1002/uog.6402. [DOI] [PubMed] [Google Scholar]
- 5.Leestma JE, Torres JV. Unappreciated agenesis of cerebellum in an adult: Case report of a 38-year old man. Am J Forensic Med Pathol. 2000;21:155–61. doi: 10.1097/00000433-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Boyd CA. Cerebellar agenesis revisited. Brain. 2010;133:941–4. doi: 10.1093/brain/awp265. [DOI] [PubMed] [Google Scholar]
- 7.Sener RN, Jinkins JR. Subtotal agenesis of the cerebellum in an adult. MRI demonstration. Neuroradiology. 1993;35:286–7. doi: 10.1007/BF00602617. [DOI] [PubMed] [Google Scholar]
- 8.Velioglu SK, Kuzeyli K, Ozmenoglu M. Cerebellar agenesis: A case report with clinical and MR imaging findings and a review of the literature. Eur J Neurol. 1998;5:503–6. doi: 10.1046/j.1468-1331.1998.550503.x. [DOI] [PubMed] [Google Scholar]
- 9.Gardner RJ, Coleman LT, Mitchell LA, Smith LJ, Harvey AS, Scheffer IE, et al. Near-total absence of the cerebellum. Neuropediatrics. 2001;32:62–8. doi: 10.1055/s-2001-13882. [DOI] [PubMed] [Google Scholar]
- 10.Tekin D, Uysal S, Iyigun O. Primary cerebellar agenesis-Chiari IV malformation. O.M.U. Tip Dergisi. 2002;19:213–6. [Google Scholar]
- 11.Timmann D, Dimitrova A, Hein-Kropp C, Wilhelm H, Dörfler A. Cerebellar agenesis: Clinical, neuropsychological and MR findings. Neurocase. 2003;9:402–13. doi: 10.1076/neur.9.5.402.16555. [DOI] [PubMed] [Google Scholar]
- 12.Titomanlio L, Romano A, Del Giudice E. Cerebellar agenesis. Neurology. 2005;64:E21. doi: 10.1212/wnl.64.6.e21. [DOI] [PubMed] [Google Scholar]
- 13.Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–60. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- 14.Yu F, Jiang QJ, Sun XY, Zhang RW. A new case of complete primary cerebellar agenesis: Clinical and imaging findings in a living patient. Brain. 2014 doi: 10.1093/brain/awu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poretti A, Risen S, Meoded A, Northington FJ, Johnston MV, Boltshauser E, et al. Cerebellar agenesis: An extreme form of cerebellar disruption in preterm neonates. JPNR J Pediatr Neuroradiol. 2013;2:163–7. [Google Scholar]
- 16.Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–5. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- 17.Barkovich AJ, Millen KJ, Dobyns WB. A developmental classification of malformations of the brainstem. Ann Neurol. 2007;62:625–39. doi: 10.1002/ana.21239. [DOI] [PubMed] [Google Scholar]


