Sir,
Stroke-related impairments and inactivity contribute to the accelerated development of bone mass removal.[1,2,3] However, the extent of decrease in bone mineral density (BMD) after stroke and all factors that influence such rapid bone loss are still not sufficiently explored. The purpose of this study was to determine the extent of BMD reduction in patients after stroke and to identify risk factors for osteoporosis.
This research was designed as a cross-sectional study that included patients after a first stroke (≤1.5 years from a stroke; N = 40), who went through inhospital rehabilitation treatment. Data were gathered by the means of anamnesis and available medical documentation. Lunar densitometer was used to determine BMD. Dual X-ray absorptiometry (DXA) examination was performed at the beginning of rehabilitation treatment and at a 1-year follow-up. DXA measurement was performed by Hologic quantitative digital radiography (QDR) (Hologic Deutschland GmbH, Germany) 4500A densitometer at the lumbar spine L2-L4 segment in anteroposterior (AP) position, while the femur was analyzed at the femoral neck, trochanter, and Ward's triangle. Femoral BMD was analyzed on the paretic side of the body.
Bone density measurements were reported as g/cm2. T-scores (comparison with mean young adult population peak bone mass) and Z-scores (comparison with age-matched population mean bone mass) were automatically generated from the Hologic normative databases; the scores were gender- and race-matched. BMD criteria for the diagnosis of osteoporosis (BMD T-score <–2.5) and osteopenia (BMD T-score of –1 to –2.5) were used. Patients who were diagnosed with osteopenia and low serum vitamin D levels received vitamin D supplementation therapy after initial DXA examination, while osteoporotic patients received antiresorptive bone therapy (bisphosphonates).
For statistical analysis, we used the statistical program International Business Machines (IBM) Statistical Package for the Social Sciences (SPSS) Statistics 22.0. Results were presented using standard statistical measures of central tendency and range of results. To determine the difference between variables, T-test for independent samples was used.
In this study, most patients were menopausal women (82.50%; N = 33). The mean age of patients was 66.5 ± 9.8 years. The average values of height were 157.62 ± 36.41 cm, the average values of weight were 67.15 ± 13.68 kg, and the average values of body mass index (BMI) were 25.66 ± 3.98. The most prevalent impairment was right-sided hemiparesis (65%; N = 26). We also recruited 40 consecutive age- and sex-matched healthy subjects who were free from earlier fractures, chronic diseases, and medications influencing bone metabolism (e.g., glucocorticoids, anticonvulsants, and thyroxine). Healthy subjects were selected randomly from applicants for an annual health check in our hospital. For age-matching, the stroke patients and the healthy subjects were matched by the year of birth. The comparison between incidence of osteoporosis in stroke and in healthy subjects is shown in Figure 1.
Figure 1.
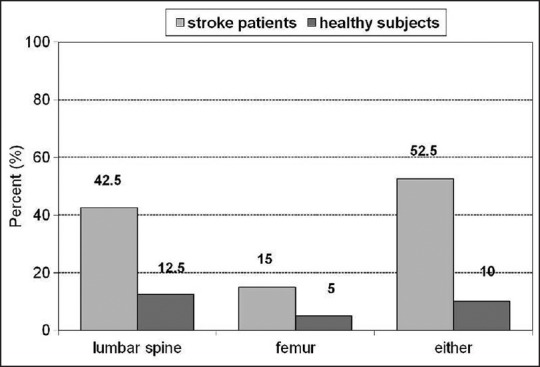
Comparison between the incidence of osteoporosis in stroke patients and in healthy subjects
The average length of time elapsed since stroke at the moment of initial evaluation was 1.5 ± 0.4 years. One year after inhospital rehabilitation, we performed control DXA examination in available patients (92.5%; N = 37), because two female patients died and one male patient refused control examination.
The most common risk factor for stroke in our research was arterial hypertension (41.97%), followed by heart disease (35.80%), heredity (9.88%), dyslipidemia, and diabetes mellitus (6.17%).
Risk factors for osteoporosis were assessed in this research as well. The largest percentage of participants in this study consisted of nonsmokers (67.50%). Positive family history for fractures was found in 2.50% of the patients. Hip fractures were the most common (12.50%), while vertebral fractures were present in 5% of patients. In our research, the largest percentage of patients used antihypertensive therapy (21.09%), followed by antidepressants (12.93%), and statins (6.80%). Only 2.5% of the patients were receiving antiresorptive bone therapy and vitamin D supplementation at the moment of initial evaluation.
The average value of DXA T-score of the lumbar spine, vertebrae L1-L4 at baseline was –1.89 ± 0.69, average value of lumbar spine BMD at baseline was 0.986 ± 0.234 [Figure 1]. According to the criteria we set, most patients (42.50%; N = 17) had osteoporosis of the lumbar spine; preserved BMD was found in 13 patients (32.50%), and 10 patients (25%) had osteopenia. No significant difference was found between the values of lumbar spine BMD and T-score at baseline and follow-up examination [Table 1]. To compare BMD values of stroke patients with population and age-matched mean bone mass, we used Z-score. The average value of DXA Z-score of the lumbar spine vertebrae L1-L4 at baseline and at follow-up are shown in Table 1.
Table 1.
Comparison between BMD, T-score, and Z-score at initial evaluation (baseline) and 12-month follow-up
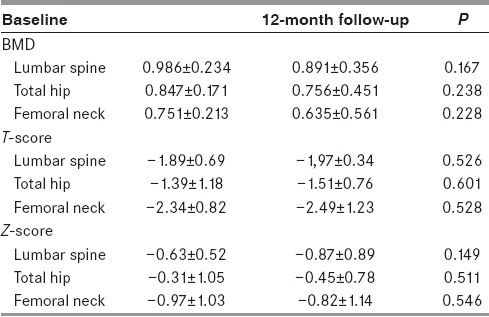
The average value of T-score of the femur (total) at baseline was –1.39 ± 1.18 and the average value of femur BMD at baseline was 0.847 ± 0.17. At initial evaluation, preserved BMD of the femur was observed in 13 patients (32.50%), while most patients had osteopenia (52.50%). The smallest number of patients had osteoporosis of the femur (15%). Baseline and follow-up values of femur BMD and T-score have shown no significant difference. Z-score values of femur total are shown in Table 1.
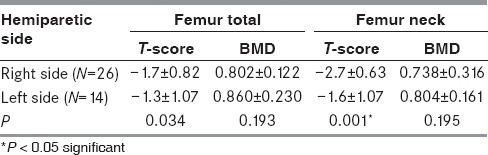
We also analyzed DXA T-scores of the femoral neck: 52.5% of patients had femoral neck osteopenia, 15.0% had osteoporosis, while 32.5% had preserved BMD. The values of femoral neck BMD, T-score, and Z-score did not significantly differ between initial evaluation and follow-up [Table 1]. In our research, we found no statistically significant difference in mean BMD and T-score between left and right femur. However, statistically significant difference in mean T-score at the femoral neck was found between the left and right sided hemiparetic patients (P< 0.001) [Table 2].
Table 2.
Comparison between mean values of DXA, BMD, and T-score of the femoral neck and femur total in left and right hemiparetic stroke patients
Results from epidemiological studies indicate an association between cerebrovascular (CV) disease and osteoporosis.[4,5] In our research, 42.50% of the stroke patients had osteoporosis of the lumbar spine and 15% had osteoporosis of the femur that further strengthens the hypothesis of a possible relationship between CV disease and osteoporosis. The results of our study show that despite the fact that the majority of patients after stroke have osteopenia or osteoporosis, a very small number of them receives treatment for the prevention of decreased BMD. Our results also demonstrate that there was a slight decrease in the values of BMD, T-scores, and Z-scores at a 12-month follow-up, but no statistically significant difference was found between those and initial values. This may imply that physical therapy, vitamin D supplementation, and antiresorptive bone therapy prevented further significant bone loss in poststroke patients, but further research is needed to confirm this hypothesis. Our study provides additional evidence that the reduction in BMD is one of the major complications of a stroke. Given that the incidence of fractures in this population is large, there is a need to perform routine DXA examinations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pang MY, Lau RW. The effects of treadmill exercise training on hip bone density and tibial bone geometry in stroke survivors: A pilot study. Neurorehabil Neural Repair. 2010;24:368–76. doi: 10.1177/1545968309353326. [DOI] [PubMed] [Google Scholar]
- 2.Marsden J, Gibson LM, Lightbody CE, Sharma AK, Siddiqi M, Watkins C. Can early onset bone loss be effectively managed in post-stroke patients. An integrative review of the evidence. Age Ageing. 2008;37:142–50. doi: 10.1093/ageing/afm198. [DOI] [PubMed] [Google Scholar]
- 3.Marzolini S, McIlroy W, Tang A, Corbett D, Craven BC, Oh PI, et al. Predictors of low bone mineral density of the stroke-affected hip among ambulatory individuals with chronic stroke. Osteoporosis Int. 2014;25:2631–8. doi: 10.1007/s00198-014-2793-3. [DOI] [PubMed] [Google Scholar]
- 4.Tomasevic-Todorovic S, Boskovic K, Filipovic K, Zvekic-Svorcan J, Grajic M, Hanna F. Bone mineral density in patients with stroke. Osteoporos Int. 2014;25:192. [Google Scholar]
- 5.Myint PK, Clark AB, Kwok CS, Loke YK, Yeong JK, Luben RN, et al. Bone mineral density and incidence of stroke: European prospective investigation into cancer-norfolk population-based study, systematic review, and meta-analysis. Stroke. 2014;45:373–82. doi: 10.1161/STROKEAHA.113.002999. [DOI] [PubMed] [Google Scholar]


