Abstract
Radiation-induced liver disease (RILD) is a major limitation of radiation therapy (RT) for the treatment of liver cancer. Emerging data indicate that hedgehog (Hh) signaling plays a central role in liver fibrosis and regeneration after liver injury. Here, we review the potential role of Hh signaling in RILD and propose the temporary use of Hh inhibition during liver RT to radiosensitize HCC tumor cells and inhibit their progression, while blocking the initiation of the radiation-induced fibrotic response in the surrounding normal liver.
Keywords: Radiation-induced liver disease, Hedgehog signaling
Classic RILD
Radiation-induced liver disease (RILD) is a major limitation of radiation therapy (RT) for the treatment of liver cancer. Classic RILD presents with hepatomegaly, anicteric ascites, and alkaline phosphatase elevated out of proportion to other liver enzymes [1], 1–3 months after liver RT. The pathological hallmark is that of venoocclusive disease (VOD) of the central and sublobular veins and centrilobular sinusoids [2, 3]. Morphologically, VOD is characterized by occlusion of the central vein lumen by erythrocytes trapped in a dense meshwork of reticulin and collagen fibers, with atrophy of centrilobular liver plates and loss of acinar zone 3 hepatocytes typically observed [2, 3]. Recently, the term sinusoidal obstructive syndrome (SOS) has been proposed as a better description of the pathology of liver injury seen after the administration of chemotherapy with or without RT [4]. In addition to endothelial cell damage, hepatic stellate cell activation is noted in patients with severe congestive changes of classic RILD [5]. Hepatic stellate cells have multiple functions, including modulating liver regeneration, secretion of lipoproteins, growth factors, and cytokines that play a key role in regulating inflammation and fibrosis. Of these cytokines, transforming growth factor-β (TGF-β) has been implicated in the perisinusoidal and hepatic fibrosis in RILD [6, 7].
Non-classic RILD
Other liver toxicities seen following liver irradiation which have been termed “non-classic RILD” include a general decline in liver function, elevation of liver enzymes, and reactivation of viral hepatitis. In the non-classic RILD syndromes, hepatocellular loss and dys-function along with hepatic sinusoidal endothelial death and stellate cell activation have also been noted. In livers with regenerating hepatocytes as in cirrhotic livers, radiation can induce mitotic catastrophe and cell death of the regenerating hepatocytes thereby causing hepatocyte injury which manifests itself with markedly elevated serum transaminases (>5 times the upper limit of normal) within 3 months of completion of hepatic RT [8]. Additionally, loss of hepatocellular regeneration capacity has been noted to be a consequence of hepatic irradiation and may render the irradiated liver incapable of the compensation that prevents irreversible hepatic failure [9]. Similarly, patients with Hepatitis B Virus (HBV) carrier status have been shown to have an increased risk of this toxicity, compared to the non-carrier group. Chou et al. [10] demonstrated that the HBV reactivation is due to a bystander effect, whereby IL-6 is released from endothelial cells after irradiation, which acts upon infected hepatocytes to stimulate HBV replication.
Hh signaling in the liver
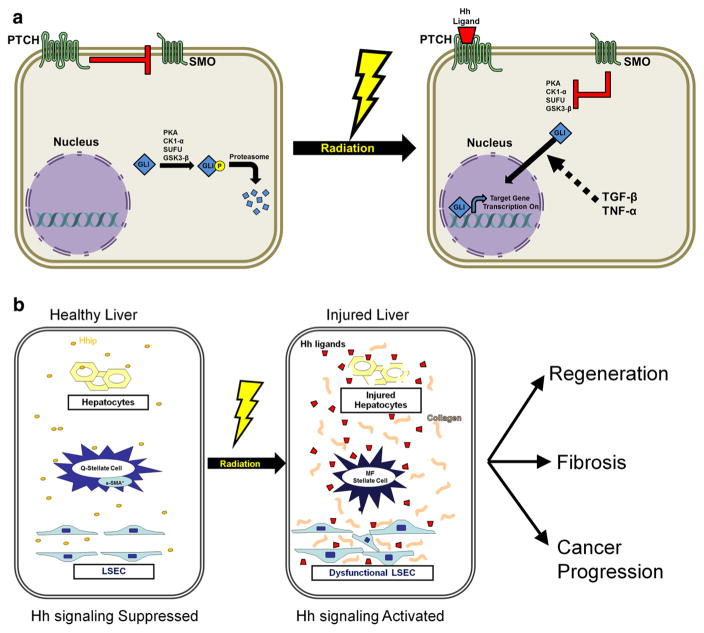
Emerging data indicate that hedgehog (Hh) signaling plays a central role in liver fibrosis and regeneration after liver injury. Healthy livers express low levels of Hh ligands and relatively high levels of Hh interacting protein (Hhip) which binds to Hh ligands, preventing them from engaging receptors on Hh-responsive target cells [11]. During liver injury, production of Hh ligands increases and Hhip is repressed, permitting ligand–receptor interaction and activation of the Hh signaling pathway [12, 13]. Binding of Hh ligands to the patched receptor on Hh responsive cells prevent the repression of smoothened (SMO). This process, known as the canonical Hh signaling pathway (Fig. 1a), affects the glioblastoma (GLI) family of transcription factors (GLI–3) which regulates the transcription of the Hh genes to influence cell viability, production and differentiation [11]. TGF-β and tumor necrosis factor-α (TNF-α) can modulate Hh signaling by promoting transcription of GLI transcription factors in a Hh and SMO independent manner [14, 15].
Fig. 1.
a Radiation induced activation of canonical and non-canonical hedgehog (Hh) signaling pathway. In non-irradiated tissue, Hh ligands are not present. In this setting, patched (PTCH) receptor inhibits a co-receptor, smoothened (SMO), which in turn fails to inhibit several intracellular kinases that phosphorylate glioblastoma (GLI) family of transcription factors and target it for ubiquitination and proteasome degradation. Radiation induces the secretion of hedgehog ligands which binds to PTCH receptors, thereby activating SMO induced inhibition of intracellular kinases, resulting in stabilization of GLI for its transcription effects, via the canonical pathway. In the non-canonical pathway, radiation induces transforming growth factor-β (TGF-β) and tumor necrosis factor-α (TNF-α), which can independently activate GLI. b Activation of Hh signaling promotes radiation induced liver injury and compensatory regeneration. Healthy livers express low levels of Hh ligands and relatively high levels of Hh interacting protein (Hhip), thereby inactivating Hh signaling. After liver irradiation, Hh ligands are secreted by injured liver cells, while Hhip secretion is repressed, permitting Hh ligand– PTCH receptor interaction and activation of the canonical Hh signaling pathway. This stimulates radiation induced fibrosis by inducing quiescent (Q) hepatic stellate cells’ transition to myofibroblastic (MF) hepatic stellate cells. Further Hh signaling in liver sinusoidal endothelial cells (LSECs) promotes radiation induced liver sinusoidal dysfunction, contributing to RILD. Hh signaling could also promote compensatory regeneration of the non-irradiated liver as well as cancer progression
Many of the cell types required for liver repair and regeneration are Hh responsive, including hepatic stellate cells [16], liver sinusoidal endothelial cells (LSECs) [17], hepatocytes and bipotent liver progenitors [11]. Activation of Hh signaling stimulates quiescent hepatic stellate cells to transition to become myofibroblastic hepatic stellate cells through a Hh dependent epithelial–mesenchymal transition (EMT)-like process [11]. Conditionally inhibiting Hh signaling in myofibroblasts after partial hepatectomy not only decreased accumulation of myofibroblasts and fibrosis, it also blocked liver progenitor accumulation, inhibited regeneration of hepatocytes and cholangiocytes, suppressed repair of liver damage, and reduced recovery of liver mass [18].
Hh signaling plays a significant role in the “capillarisation” of LSECs [17]. Healthy LSECs serve as a powerful scavenger system in the body. However, after liver injury but before development of fibrosis or hepatitis, LSECs undergo “capillarisation” which induces a change in the phenotype of LSECs to a vascular type with resulting defenestration, formation of an organized basement membrane and remodeling of the hepatic vasculature [19]. Once LSEC loses its phenotype, it promotes fibrosis as it loses its ability to inhibit the activation of hepatic stellate cells [20]. Inhibition of the Hh pathway can prevent capillarisation of LSECs both in vitro and in vivo [17]. Hh signaling pathway also plays a critical role in developing cancer stem cells leading to angiogenesis, migration, invasion, and metastasis. In one study, Hh signaling occurred in over 50 % of human HCC and the expression of GLI1 gene in tumor tissues significantly correlated with disease-free survival and overall survival [21].
Hh signaling and radiation liver injury
Given the important role of Hh signaling in different models of liver injury and the role of Hh signaling in influencing the function of hepatic stellate cells, sinusoidal endothelial cells and hepatocytes, all of which play an important role in RILD, the study by Wang et al. [22] is significant in highlighting the role of Hh signaling in hepatic radiation injury. They hypothesized that the gender-specific expression of Hh signaling may explain the different responses to radiation liver injury seen between male and female C57Bl6 mice. Although, female patients are more sensitive to alcohol-induced liver injury [23, 24], such an association has not been described in the RILD literature. The key finding of this study is that in contrast to male animals, female C57Bl6 mice had increased hepatic steatosis, apoptotic cells, and an increase in the number of Sox-9-positive and Pan-CK expressing cells (hepatic progenitor cells) in their livers in response to a single dose of hepatic irradiation of 6 Gy. Female mice, but not males, had an increase in the expression of Hh signaling molecules in their livers 1-week post irradiation. Interestingly, irradiation induced sonic Hh (SHH) but not Indian Hh (IHH) in female mice, suggesting a differential response of Hh ligands after liver injury. Finally, female mice had increased levels of TGF-β1, collagen α1 and N-cadherin in the liver, along with increased sinusoidal deposition of collagen fibrils at this time.
One of the limitations of using small animals as a model for RILD is that they do not develop the characteristic morphological changes of VOD seen in humans. Although various forms of RILD occur in small animals, whole-liver irradiation failed to produce VOD in rats [9], dogs [25] and rhesus monkeys [26]. The only animal model to develop radiation dose-dependent VOD resembling classic human RILD has been recently shown in the cynomolgus monkey using high doses of whole liver hypofractionated RT [27]. In the study by Wang et al. [22], even though the female mice receiving liver irradiation had increased peri-portal and peri-venous steatosis and increased peri-sinusoidal deposition of collagen fibrils, they still failed to show VOD and SOS. Another limitation of this study is the use of only a single relatively small dose for liver irradiation and a single follow up time of 1 week after irradiation for analysis. It is not clear that the changes seen 1 week after 6 Gy of liver irradiation would ultimately correlate with fibrosis and inhibition of regeneration. Additionally, even though male C57B16 mice compared to the female mice did not appear to exhibit any radiation changes 1 week following 6 Gy liver irradiation, it is likely that with higher dose and longer follow time the changes will be more obvious. Interestingly, the same authors recently showed that the male C57B16 mice receiving 6 Gy liver irradiation did indeed exhibit an increase in steatosis and increased Hh signaling, 6 and 10 weeks post irradiation [28].
Despite these limitations, small animal models are essential in elucidating the mechanisms of RILD. The two studies by Wang et al. [22, 28] have described the correlation of radiation-induced Hh signaling with hepatic steatosis, progenitor expansion and fibrosis and also highlighted the potential role of gender in hepatic radiation injury in C57Bl6 mice (Fig. 1). Similarly, a study by Leonard et al. [29] showed that GLI1 expression and Hh signaling pathway is activated in mouse embryonic fibroblasts and HEK293 cells activation after irradiation. However, when looking at tumor cells, irradiation of HCC cells caused the release of SHH ligand, activated Hh signaling but this protected the HCC cells against ionizing radiation [30]. Inhibition of Hh signaling in human colon carcinoma cells at the level of the GLI genes induced DNA damage in early S-phase, leading to cell death in human colon carcinoma cells [31]. Therefore, it appears that in normal, non-cancerous tissue, as in female C57Bl6 livers, embryonic mouse fibroblasts and HEK293 cells, activation of Hh signaling is associated with increased radiation injury, but in tumor cells, as in HCC and human colon carcinoma cells, increased Hh signaling led to increased radiation resistance and decreased cell death.
Can Hh inhibitors increase the therapeutic ratio of RT for the treatment of liver tumors?
Liver irradiation leads to loss of hepatocytes and non-parenchymal cell fractions leading to atrophy of the irradiated lobe and compensatory hypertrophy of the non-irradiated lobe. Following partial liver irradiation, shrinkage of the high-dose irradiated volume with a compensatory increase in liver volume is commonly seen on follow-up CT and MR imaging [32]. Hh signaling, through the canonical and non-canonical pathways (Fig. 1a), could play a significant role in both of these responses by acting as a regulator of progenitor cell growth, while simultaneously promoting liver inflammation and fibrogenic response (Fig. 1b). Additionally, Hh signaling also plays a significant role in HCC tumor progression. Therefore, inhibition of Hh signaling could represent a novel strategy to modulate the hepatic radiation response and cancer progression (Fig. 1b).
Currently, there are a number of potential therapeutic agents that have been investigated to modulate Hh signaling pathways mostly by inhibiting SMO, with promising clinical trial results in cancers that harbor activating mutations of the Hh pathway [15, 33, 34]. Moreover, the five SMO inhibitors with available clinical data to date have been well tolerated [15]. The most common reported adverse events were mild-moderate dysgeusia, muscle spasms, alopecia, anorexia and fatigue [15]. Only one study reported dose limiting hepatic toxicity in two patients [35]. One of the patients had a history of hepatitis C and alcohol use and experienced asymptomatic grade 3 AST elevation and grade 2 ALT elevation after receiving the highest dose for 25 days. The second patient experienced an asymptomatic grade 3 ALT elevation which resolved when study drug was held [35].
Given the potential for hepatic toxicity in patients with impaired liver function, caution should be exercised in optimizing the dose and timing of treatment with Hh inhibitors as prolonged use of these agents might interfere with the compensatory regeneration after hepatic radiation injury. Therefore, we propose the use of temporary Hh inhibition during liver RT to radiosensitize HCC tumor cells and inhibit their progression, while blocking the initiation of the radiation-induced fibrotic response in the surrounding normal liver. The timing and modulatory effect of Hh inhibition should be explored in carefully designed pre-clinical and clinical trials to allow for improvements in patient outcomes.
In summary, RILD remains a major limiting factor in the treatment of liver cancers with RT. Understanding the mechanisms of RILD and developing methods to ameliorate against it will be important in the future. Given the significant role of Hh signaling in liver fibrosis, regeneration after liver injury and cancer progression, further studies are needed to explore the potential role of Hh signaling pathway inhibition in improving the therapeutic effects of radiotherapy for patients with liver cancer.
Abbreviations
- EMT
Epithelial–mesenchymal transition
- GLI
Glioblastoma family
- Hh
Hedgehog
- Hhip
Hedgehog interacting protein
- IHH
Indian hedgehog
- LSECs
Liver sinusoidal endothelial cells
- RILD
Radiation-induced liver disease
- SHH
Sonic hedgehog
- SOS
Sinusoidal obstructive syndrome
- TGF-β
Transforming growth factor-β
- VOD
Veno-occlusive disease
Footnotes
Compliance with ethical requirements and Conflict of interest This article does not contain any studies with human or animal subjects. Rafi Kabarriti and Chandan Guha declare no conflict of interest.
Contributor Information
Rafi Kabarriti, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210 Street, Bronx, NY 10467, USA.
Chandan Guha, Email: cguhamd@gmail.com, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210 Street, Bronx, NY 10467, USA. Department of Pathology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, USA.
References
- 1.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 2.Ogata K, Hizawa K, Yoshida M, Kitamuro T, Akagi G, Kagawa K, et al. Hepatic injury following irradiation—a morphologic study. Tokushima J Exp Med. 1963;43:240–251. [PubMed] [Google Scholar]
- 3.Reed GB, Jr, Cox AJ., Jr The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol. 1966;48:597–611. [PMC free article] [PubMed] [Google Scholar]
- 4.DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease) Semin Liver Dis. 2002;22:27–42. doi: 10.1055/s-2002-23204. [DOI] [PubMed] [Google Scholar]
- 5.Sempoux C, Horsmans Y, Geubel A, Fraikin J, Van Beers BE, Gigot JF, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128–134. doi: 10.1002/hep.510260117. [DOI] [PubMed] [Google Scholar]
- 6.Anscher MS, Crocker IR, Jirtle RL. Transforming growth factor-beta 1 expression in irradiated liver. Radiat Res. 1990;122:77–85. [PubMed] [Google Scholar]
- 7.Anscher MS, Peters WP, Reisenbichler H, Petros WP, Jirtle RL. Transforming growth factor beta as a predictor of liver and lung fibrosis after autologous bone marrow transplantation for advanced breast cancer. N Engl J Med. 1993;328:1592–1598. doi: 10.1056/NEJM199306033282203. [DOI] [PubMed] [Google Scholar]
- 8.Cheng JC, Wu JK, Lee PC, Liu HS, Jian JJ, Lin YM, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004;60:1502–1509. doi: 10.1016/j.ijrobp.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, et al. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999;59:5871–5874. [PubMed] [Google Scholar]
- 10.Chou CH, Chen PJ, Lee PH, Cheng AL, Hsu HC, Cheng JC. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin Cancer Res. 2007;13:851–857. doi: 10.1158/1078-0432.CCR-06-2459. [DOI] [PubMed] [Google Scholar]
- 11.Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–373. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–1723. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57:1275–1282. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 14.Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, et al. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 15.Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska-Syn M, Karaca G, et al. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut. 2013;62:299–309. doi: 10.1136/gutjnl-2011-301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swiderska-Syn M, Syn WK, Xie G, Kruger L, Machado MV, Karaca G, et al. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. doi: 10.1136/gutjnl-2013-305962. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLeve LD, Wang X, Hu L, McCuskey MK, McCuskey RS. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G757–G763. doi: 10.1152/ajpgi.00017.2004. [DOI] [PubMed] [Google Scholar]
- 20.DeLeve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Che L, Yuan YH, Jia J, Ren J. Activation of sonic hedgehog signaling pathway is an independent potential prognosis predictor in human hepatocellular carcinoma patients. Chin J Cancer Res. 2012;24:323–331. doi: 10.3978/j.issn.1000-9604.2012.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Lee K, Hyun J, Lee Y, Kim Y, Jung Y. Hedgehog signaling influences gender-specific response of liver to radiation in mice. Hepatol Int. 2013;7:1065–1074. doi: 10.1007/s12072-013-9461-0. [DOI] [PubMed] [Google Scholar]
- 23.Sato N, Lindros KO, Baraona E, Ikejima K, Mezey E, Jarvelainen HA, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res. 2001;25:40S–45S. doi: 10.1097/00000374-200105051-00007. [DOI] [PubMed] [Google Scholar]
- 24.O’Shea RS, Dasarathy S, McCullough AJ Practice Guideline Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 25.Epstein RB, Min KW, Anderson SL, Syzek L. A canine model for hepatic venoocclusive disease. Transplantation. 1992;54:12–16. doi: 10.1097/00007890-199207000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Stephens LC, Peters LJ, Ang KK. Tolerance of rhesus monkey liver to ionizing radiation. Radiat Oncol Investig. 1994;1:279–284. [Google Scholar]
- 27.Yannam GR, Han B, Setoyama K, Yamamoto T, Ito R, Brooks JM, Guzman-Lepe J, et al. A nonhuman primate model of human radiation-induced venocclusive liver disease and hepatocyte injury. Int J Radiat Oncol Biol Phys. 2014;88:404–411. doi: 10.1016/j.ijrobp.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Lee Y, Kim J, Hyun J, Lee K, Kim Y, et al. Potential role of hedgehog pathway in liver response to radiation. PLoS ONE. 2013;8:e74141. doi: 10.1371/journal.pone.0074141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard JM, Ye H, Wetmore C, Karnitz LM. Sonic Hedgehog signaling impairs ionizing radiation-induced checkpoint activation and induces genomic instability. J Cell Biol. 2008;183:385–391. doi: 10.1083/jcb.200804042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YJ, Lin CP, Hsu ML, Shieh HR, Chao NK, Chao KS. Sonic hedgehog signaling protects human hepatocellular carcinoma cells against ionizing radiation in an autocrine manner. Int J Radiat Oncol Biol Phys. 2011;80:851–859. doi: 10.1016/j.ijrobp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Mazumdar T, Devecchio J, Agyeman A, Shi T, Houghton JA. Blocking Hedgehog survival signaling at the level of the GLI genes induces DNA damage and extensive cell death in human colon carcinoma cells. Cancer Res. 2011;71:5904–5914. doi: 10.1158/0008-5472.CAN-10-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herfarth KK, Hof H, Bahner ML, Lohr F, Hoss A, van Kaick G, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys. 2003;57:444–451. doi: 10.1016/s0360-3016(03)00586-8. [DOI] [PubMed] [Google Scholar]
- 33.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 34.Ng JM, Curran T. The Hedgehog’s tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimeno A, Weiss GJ, Miller WH, Jr, Gettinger S, Eigl BJ, Chang AL, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19:2766–2774. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]