Abstract
Enhanced levels of platelet/granulocyte aggregates (PGAs) are found in patients suffering from many different inflammatory vascular diseases, and their formation in animal models of vascular disease is associated with increased thromboinflammation and worsened outcomes. The complement system, a part of the innate immune system, influences PGA formation, however the mechanisms for its effects are unknown. Here, we have defined complement-mediated mechanisms that enhance PGA formation in human whole blood stimulated with thrombin receptor activating peptide (TRAP) using ex-vivo flow cytometry assays. We demonstrate that physiological properdin, a positive regulator of complement alternative pathway activity, increases PGA formation when added to TRAP-stimulated blood. All physiological properdin forms increase PGA formation, but properdin tetramers are the most efficient at increasing complement activity and PGA formation. Inhibition of endogenous properdin, either circulating in the blood or produced locally by leukocytes, impairs TRAP-mediated PGA formation to the same level as specific inhibition of either the alternative or classical pathways. In addition, blocking the interaction of C5a with its cellular receptor prevents properdin-mediated increases in PGA formation. Adding either properdin tetramers or C5a to whole blood increases CD11b expression on granulocytes and this increase is prevented by blockade of the C5a-C5a receptor axis. Finally, we demonstrate that the effects of properdin on PGA formation are tightly regulated by Factor H. Cumulatively, our data indicate that properdin enhances PGA formation via increased production of C5a, and that inhibition of properdin function has therapeutic potential to limit thromboinflammation in diseases characterized by increased PGA formation.
Introduction
Platelets are critical for vascular hemostasis and repair in response to blood vessel damage (1). Platelets also help initiate inflammatory responses that induce endothelial regeneration and vessel protection from potential invasion by microorganisms by binding and recruiting leukocytes to sites of vascular damage (1-4). Leukocytes not only promote inflammation, but have a dynamic role in the regulation of thrombosis. Tissue factor expressed on leukocyte surfaces or leukocyte-derived microparticles increases thrombus formation (5-8), whereas leukocytes limit thrombus formation by directly phagocytosing activated platelets, decreasing local levels of ADP, and preventing fibrinogen binding to platelets (9). Platelets and leukocytes interact to form stable platelet/leukocyte aggregates (PLA) (10), which involves an ordered series of events where P-selectin on activated platelets binds to P-selectin glycoprotein ligand-1 (PSGL-1) on leukocytes. This stimulates the upregulation and activation of complement receptor 3 (CR3; CD11b/CD18; Mac-1) on leukocytes, which then binds multiple ligands on platelets to form stable aggregates (11-14). Excessive PLA formation increases tissue factor expression, secretion of proinflammatory cytokines and chemokines, production of reactive oxygen species, secretion of damaging proteases, and upregulation of adhesion molecules on leukocytes (11-13). Increased levels of circulating PLAs are found in patients suffering from many inflammatory diseases, including cardiovascular, myeloproliferative, and inflammatory bowel diseases (15-20), and inhibiting PLA formation (by inhibiting P selectin or CD11b) reduces pathology and improves outcomes in various vascular injury animal models (8, 21-24). Understanding mechanisms by which PLA formation is regulated is imperative to elucidate how PLAs can increase to levels that result in pathologic thromboinflammation and to identify potential therapeutic targets.
The complement system links the innate and adaptive immune systems and helps orchestrate inflammatory reactions. Complement activity can initiate by three distinct pathways (classical, lectin, and alternative) that converge at the cleavage of the central molecule, C3, to C3b and C3a. C3b binds covalently to exposed hydroxyl- and amino-groups on cell surfaces, enabling the formation of C3/C5 convertases that amplify complement activity and lead to activation of terminal complement components to produce the anaphylatoxin C5a and the membrane attack complex (MAC; C5b-9) (25). While all 3 pathways recognize distinct molecular patterns on cell surfaces to activate, the alternative pathway (AP) mainly initiates spontaneously in the fluid phase (25, 26). Properdin, the only known positive complement regulatory molecule, stabilizes the AP convertases increasing their activity 5-10-fold (26). The AP amplifies on any surface on which C3b is bound, including C3b originally deposited by the lectin or classical pathways (CP), thus it is an essential amplification loop for all complement activity. All pathways must be tightly regulated to prevent unwanted tissue damage (25). Factor H (FH) is a key regulator of the AP (27).
Physiological properdin circulates in plasma at 4-25μg/ml as dimers (P2), trimers (P3), and tetramers (P4) of head-to-tail associations of monomeric subunits, however repeated freeze/thaw cycles can form higher order non-physiological aggregates (Pn) that bind non-specifically to surfaces and consume complement in solution (28-31). Properdin is primarily produced by leukocytes, including neutrophils, which secrete properdin in response to proinflammatory stimuli, leading to higher concentrations at inflammatory locations (26, 32, 33). AP activation on activated platelets leads to terminal complement activation, generating MAC (31), which enhances platelet activation (34, 35), and C5a. C5a contributes to formation of platelet/granulocyte aggregates (PGAs) in human whole blood stimulated with thrombin receptor-activating peptide (TRAP) (14, 36), however the complement pathways involved, as well as the role of physiological properdin in this process, are unknown. Here, we have elucidated a critical role for physiological properdin in amplifying AP and CP activity at the platelet/granulocyte interface to increase PGA formation via C5a production, with resulting C5a-mediated CD11b upregulation on granulocytes, all of which is tightly controlled by FH.
Materials and Methods
Buffers
Modified HEPES/Tyrode’s (HT) buffer (137mM NaCl, 2.8mM KCl, 1mM MgCl2, 12mM NaHCO3, 0.4mM Na2HPO4, 10mM HEPES, 0.35% BSA, 5.5mM Glucose; pH 7.4); PBS (10mM NaH2PO4, 145mM NaCl, pH 7.4).
Detection antibodies
Murine monoclonal antibodies (all IgG1): anti-human CD42b-APC (Biolegend), anti-human CD45-PE (Biolegend), anti-human/mouse C3/C3b/iC3b-FITC (Cedarlane), and anti-human CD11b-PerCP/Cy5.5 (Biolegend). IgG1 isotype controls: APC- and PerCP/Cy5.5-labeled (Biolegend), PE-labeled (Chemicon), FITC-labeled (Cedarlane).
Complement modulators
To inhibit convertase-mediated C3 activation, either the originally described C3 inhibitor compstatin (I[CVVQDWGHHRC]T-NH2; Tocris Bioscience) (37) or the ~1,000-fold more potent analog Cp20 (Ac-I[CV-1MeW-QDW-Sar-AHRC]mI-NH2; prepared by solid-phase peptide synthesis) (38) was used. At concentrations in large excess of C3 as used in our assays (50μM final), both compstatin analogs were equally effective at inhibiting TRAP-mediated PGA formation and C3 fragment deposition on granulocytes (Figure S1). Samples receiving either compstatin analog were designated ‘Comp’. PMX53 (39), rH19-20 (40), and anti-Factor B monoclonal antibody #1379 (41) were produced as previously described. We generated, purified, and characterized three mouse IgG1κ anti-properdin monoclonal antibodies: 3A3E1, 6E11A4, and 1G6D2 (manuscript in preparation). 1G6D2, which does not inhibit properdin function, was used as both a negative inhibition control and isotype control. SALO, a specific CP inhibitor, was generated as previously described (42). C5a was purchased from Complement Technologies.
Purification of properdin and separation of physiological forms
Purification of properdin from normal human plasma was carried out as previously described (30). Physiological polymeric forms of properdin (P2, P3, P4) were separated from Pns by gel filtration chromatography. Briefly, pure properdin (5mg) was loaded onto a Phenomenex Bio Sep-Sec-S4000 column (600 × 7.8mm) with a guard column (75 × 7.8mm), and eluted at a 0.5 ml/min flow rate in PBS. Purified, physiological forms of properdin were stored at 4°C and used within two weeks of separation (28, 30).
Measurement of TRAP-mediated PGA formation, C3 fragment deposition, and CD11b expression
Human whole blood was collected via venipuncture from healthy donors. The Institutional Review Board from the University of Toledo College of Medicine and Life Sciences approved the protocols, and written informed consent was obtained from all donors, in accordance with the Declaration of Helsinki. Blood was drawn into BD vacutainer tubes containing 50μg/ml lepirudin (Refludan; Bayer or Celgene, generous donation from Dr. Sanjay Ram). Blood (20μl) was immediately gently mixed with 60μl total of modified HT buffer + reagents (TRAP +/− properdin, complement modulators, and/or rH19-20), previously added to tubes at room temperature (RT). TRAP (Bachem) was used at a final concentration of 20μM, unless otherwise indicated. Complement modulators were used at the final concentrations indicated in the figure legends. Compstatin (50μM final) was used as a positive control for complete complement inhibition. A sample that was not activated with TRAP, but received compstatin immediately after blood collection (designated as NA+Comp) served as the baseline control for C3 fragment deposition and CD11b expression. The blood + reagents mixture was incubated at 37°C for 15 min. The reaction was stopped using 800μl of RBC lysis/fixation solution (Biolegend). The samples were then washed with modified HT buffer and stained with detection antibodies for 15 min at RT. At least 10,000 events (granulocytes and monocytes) were acquired using a Becton Dickinson FACSCalibur flow cytometer, and the data was analyzed using FlowJo 7.6 software (Tree Star). Granulocytes were gated based on CD45-associated fluorescence and side scatter, and the percent of gated granulocytes positive for CD42b fluorescence, as well as the C3- and CD11b-associated geometric mean fluorescence intensities (GMFI) on gated granulocytes were determined.
Analysis of C5a levels in reaction supernatants
Whole blood experiments were set up as described in the previous section, in triplicate. Following the 37°C incubation (blood + reagents), one set of tubes was immediately centrifuged at 300×g for 10 minutes at 4°C. The supernatant was then centrifuged at 13,000×g for 5 minutes at 4°C and immediately stored at −80°C until use. Supernatants were diluted 1/10 and analyzed using a C5a ELISA kit (Abcam), following manufacturer’s instructions. The remaining two sets of tubes were processed as described above to determine PGA formation, C3 fragment deposition and CD11b expression.
Statistics
The data were analyzed with GraphPad Prism 6.0 software. Cumulative raw data was analyzed by repeated measures ANOVA with Dunnett’s post-test, using the Geisser-Greenhouse correction, to compare groups to TRAP alone. Individual experiments were analyzed by one-way ANOVA with either Dunnett’s or Tukey’s post-test, depending on the experiment. Statistical tests are indicated in the figure legends.
Results
Physiological properdin forms increase platelet/granulocyte aggregate formation
Properdin circulates as dimers (P2), trimers (P3), and tetramers (P4) in an approximate 1:2:1 ratio (28). Repeated freeze-thaw cycles cause purified properdin to form Pns with the abnormal ability to activate complement in solution (28, 29) as well as bind non-specifically to surfaces (30, 43), including non-activated platelets (31). In a study conducted by Ruef et al (44), purified properdin significantly increased PLA formation in citrate-anticoagulated human whole blood. In order to accurately assess the function of purified properdin in human whole blood, it is essential to separate Pns from P2-P4s and to avoid certain anticoagulants, such as citrate, that dampen complement activation and PLA formation (45, 46). Here, we separated P2-P4s from Pns by size exclusion chromatography to determine the effects of physiological properdin on PGA formation. In our assays, lepirudin, a direct thrombin inhibitor without any complement inhibitory properties (47), was used as the anticoagulant, and human whole blood was stimulated with TRAP, an agonist that directly stimulates thrombin receptors (48).
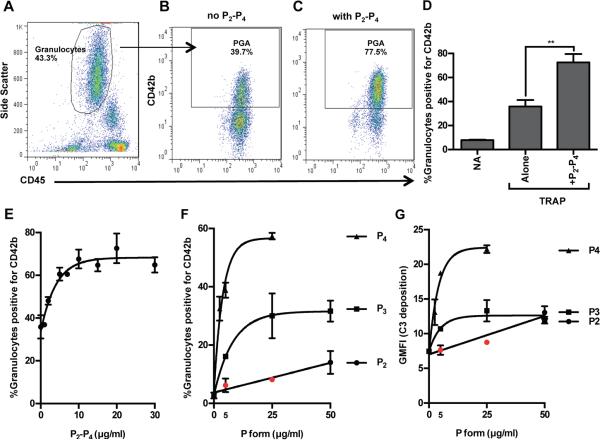
Figure 1A shows the gating strategy used to identify granulocyte populations (49). Figure 1B-C are dot plots showing an increase in the percent of granulocytes with associated CD42b fluorescence (i.e. platelets) upon addition of 25μg/ml physiological properdin forms (P2-P4) in a 1:2:1 ratio to TRAP-stimulated whole blood. Figure 1D shows that the addition of P2-P4 leads to an ~2-fold increase in PGA formation compared to TRAP-stimulated whole blood without added properdin. The effect of properdin on TRAP-mediated PGA formation is dose-dependent, as properdin caused a significant increase in PGA formation at concentrations as low as 2μg/ml and reached its maximal effects by 10μg/ml (Figure 1E).
Figure 1. Physiological properdin forms increase TRAP-mediated PGA formation.
(A-E) Lepirudin anticoagulated whole blood was incubated without (NA) or with TRAP (20μM) +/− physiological properdin dimers, trimers, and tetramers (P2-P4) in a 1:2:1 ratio (A-D, 25μg/ml; E, 0-30μg/ml). (A) Gating strategy to select granulocyte population. (B-C) Percent PGA formation in TRAP-activated granulocyte population without or with P2-P4, respectively; (D) Representative bar graph of B and C; p<0.01 (**). (E) Dose curve of effects of P2-P4 on TRAP-mediated PGA formation. (F-G) Effect of different physiological P forms on PGA formation and C3 fragment deposition on granulocytes within the same experiment. Lepirudin anticoagulated whole blood was incubated with a submaximal dose of TRAP (10μM) and increasing concentrations of individual properdin forms (P2, P3, P4; 0-50μg/ml). Samples were processed as described in Materials and Methods. p<0.05 as compared to TRAP alone (0μg/ml P form), unless indicated in red. Maximal effects of P forms on C3 fragment deposition varied depending on the donor (P4s: 1.5-3-fold, P2s and P3s: 1.2-2-fold increases over TRAP alone); one donor shown in G. (A-G) All experiments shown are representative of at least 4 independent experiments and are shown as mean and SD of duplicate observations. The data was analyzed by one-way ANOVA with Dunnett’s multiple comparison test against TRAP alone.
To determine whether the physiological properdin forms had differential effects on PGA formation, each form was individually added to whole blood stimulated with TRAP (Figure 1F-G). A lower dose of TRAP (10μM) was used in these experiments in order to increase the sensitivity of detecting properdin-mediated effects on PGA formation. Figure 1F is the representative data from 1 of 4 independent experiments conducted with different donors’ blood. The effects of properdin were dose-dependent and P4s increased PGA formation to a greater extent than P2s and P3s, requiring lower concentrations to achieve maximal effects. P3s caused greater increases in PGA formation than P2s at each concentration tested. There was considerable inter-individual variation in the maximal effects of each form on PGA formation (P4s: 2.5-14-fold, P3s: 2.5-11-fold, P2s: 2-5-fold increases in PGA over TRAP alone), but for each individual, P4s always had the greatest effect.
C3 fragments deposited on the granulocytes serve as a marker of the degree of complement activation induced or enhanced by each properdin form. Figure 1G shows P4s were the most efficient at increasing, in a dose-dependent manner, C3 fragment deposition (by ~3-fold) on granulocytes in TRAP-stimulated whole blood. P2s and P3s increased C3 fragment deposition on granulocytes to approximately the same level, but to a significantly lesser extent than P4s, even when used at concentrations 2-fold higher than the maximal P4 dose (Figure 1G). Altogether the data indicate that all physiological properdin forms increase PGA formation, but P4s cause the greatest increases and account for the majority of C3 fragments deposited on the granulocytes in TRAP-stimulated whole blood.
Inhibition of endogenous properdin reduces TRAP-induced PGA formation
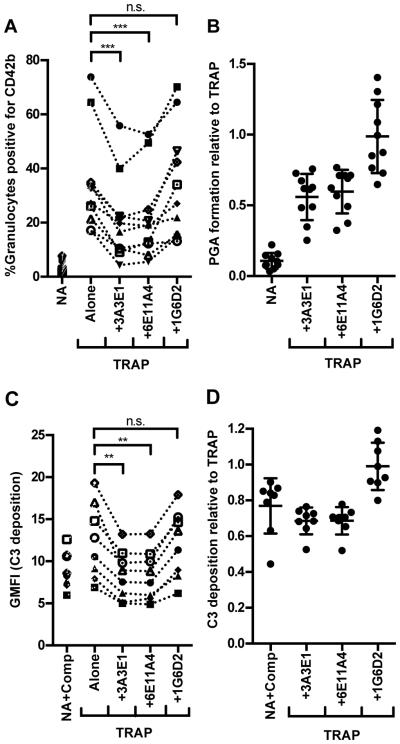
Because properdin is produced locally by leukocytes upon stimulation, its concentration in inflammatory microenvironments could be significantly higher than its plasma concentration of 4-25μg/ml (26). Therefore, we sought to determine whether endogenous properdin (produced locally by leukocytes or already circulating in the plasma) modulates the basal level of TRAP-mediated PGA formation. We incubated TRAP-stimulated whole blood with three different anti-properdin monoclonal antibodies that we recently produced and characterized: 3A3E1, 6E11A4, and 1G6D2 (manuscript in preparation). 3A3E1 and 6E11A4 inhibit AP-mediated lysis of rabbit erythrocytes by preventing properdin from binding to C3b. 1G6D2 recognizes properdin, is the same isotype as the inhibitory antibodies (IgG1), and does not inhibit AP-mediated lysis of rabbit erythrocytes. Thus, 1G6D2 was used as a negative control for assessing properdin function. Figure 2A shows that the inhibitory antibodies, 3A3E1 and 6E11A4, significantly inhibited PGA formation relative to TRAP alone, while 1G6D2 did not. Figure 2B shows the data for each donor graphed relative to the level of PGA formation induced by TRAP alone in that particular donor (such that TRAP alone = 1 for each donor). The data indicate that the addition of 3A3E1 and 6E11A4 reduced PGA formation by ~50% regardless of the level of PGA formation induced by TRAP alone in each donor’s blood (Figure 2B). A low level of C3 fragment deposition, most likely due to complement activation on granulocytes during processing (before the addition of compstatin), was detected on NA+Comp groups (Figure 2C-D; isotype control for C3 fragment detection ~0.3-0.6 relative to TRAP; not shown). C3 fragment deposition trended toward an increase on TRAP versus NA+Comp groups (Figure 2C, p=0.08), while 3A3E1 and 6E11A4 significantly reduced C3 fragment deposition by ~30% as compared to TRAP alone (Figure 2C-D). C3-covered microparticles released from granulocytes during PGA formation (50) could reduce the total amount of detectable C3 fragments on granulocytes. This, combined with the inhibition of AP activity, may explain why TRAP+3A3E1 and 6E11A4 groups had lower C3 fragment levels than the NA+Comp control. 1G6D2 did not effect C3 fragment deposition (Figure 2C-D), thus inhibition of endogenous properdin function significantly limits complement activity and PGA formation in TRAP-stimulated whole blood.
Figure 2. Inhibition of endogenous properdin function impairs TRAP-mediated PGA formation.
Lepirudin anticoagulated whole blood was incubated with TRAP (20μM) without or with 3A3E1, 6E11A4, or 1G6D2 (100μg/ml). A sample that did not receive TRAP (NA) and that was preincubated with compstatin (NA+Comp) was included for (A-B) and (C-D), respectively. PGA formation (A-B) and C3 fragment deposition on granulocytes (C-D) was determined as described in Materials and Methods. Each graph represents cumulative data from experiments carried out with different human volunteer blood donors. The mean for each group, assessed in duplicate, is graphed in (A,C), while (B,D) represent the mean for each group graphed relative to the mean of TRAP alone (assigned a value of 1) for each independent experiment, and the SD of each group is shown. (A-B) n=10; (C-D) n=8. The data was analyzed by repeated measures ANOVA with Dunnett’s multiple comparison test against TRAP alone. p<0.01 (**), p<0.001 (***) and p>0.05 (n.s.).
AP and CP activation contribute to TRAP-mediated PGA formation
Platelets can activate both the CP and AP on their surface (31, 51, 52) and secrete chondroitin sulfate, which activates the CP in the fluid phase (36, 53). Isolated neutrophils also activate the AP on their surface (32). Because the AP can amplify complement using C3b originally deposited by the CP, we next determined whether the effects of properdin on PGA formation (Figure 2) were due to AP activation alone or also due to amplification of CP activity.
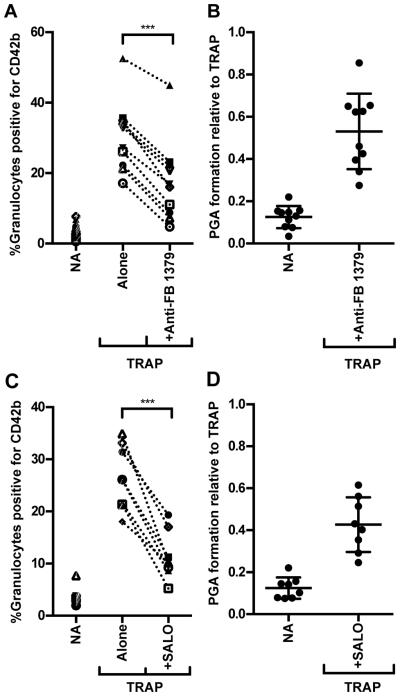
Anti-Factor B #1379, an inhibitory monoclonal antibody (41), and SALO, a salivary protein produced by the sandfly Lutzomyia longipalis (54-56) that specifically inhibits the CP (42), were used to determine the effects of AP and CP inhibition on TRAP-mediated PGA formation, respectively. Addition of either anti-Factor B #1379 or SALO to whole blood significantly impaired TRAP-mediated PGA formation in each donor’s blood by an average of ~50% (Figures 3A-B and 3C-D respectively, and Figure 4A-B), as well as C3 fragment deposition (Figure 4C-D) in a mode similar to the anti-properdin monoclonals (Figures 2 and 4). This data as well as the ability of the CP inhibitor to inhibit PGA formation and C3 fragment deposition to the same level as inhibiting all complement activity with compstatin (Figure 4), even when AP activity is intact, suggest that complement activity in TRAP-stimulated whole blood may be initiated by the CP and amplified by properdin-mediated AP convertase stabilization.
Figure 3. Both AP and CP activity contributes to TRAP-mediated PGA formation.
Lepirudin anticoagulated whole blood was incubated with TRAP (20μM) without or with anti-Factor B #1379 (100μg/ml; A-B) or SALO (2μM; C-D). PGA formation was determined as described in Materials and Methods. The mean for each group, assessed in duplicate, is graphed in (A,C), while (B,D) represent the mean for each group graphed relative to the mean of TRAP alone (assigned a value of 1) for each independent experiment, and the SD of each group is shown. (A) n=10; (B) n=8. Raw data was analyzed by repeated measures ANOVA with Dunnett’s multiple comparison test against TRAP alone. p<0.001 (***).
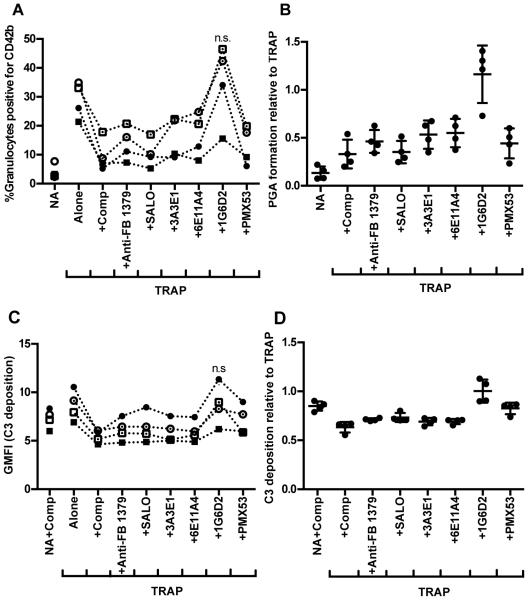
Figure 4. Inhibition of TRAP-mediated PGA formation with complement inhibitors.
Lepirudin anticoagulated whole blood was incubated without (A-B: NA, C-D: NA+Comp) or with TRAP (20μM) alone or with anti-complement reagents (Anti-properdin and anti-Factor B #1379 - 100μg/ml; SALO - 2μM; PMX53 – 16.7μM; Comp - 50 μM). Graphs include cumulative data from experiments carried out with 4 different human volunteer blood samples in which PGA formation (A-B) and C3 fragment deposition (C-D) were determined in the same experiment, as described in Materials and Methods. The mean for each group, assessed in duplicate, is graphed in (A,C), while (B,D) represent the mean for each group graphed relative to the mean of TRAP alone (assigned a value of 1) for each independent experiment, and the SD of each group is shown. The data was analyzed by repeated measures ANOVA with Dunnett’s multiple comparison test against TRAP alone. (A,C) p<0.05 compared to TRAP alone unless indicated as non-significant (n.s.).
Properdin-mediated C5a generation enhances TRAP-mediated PGA formation via upregulation of CD11b on granulocytes
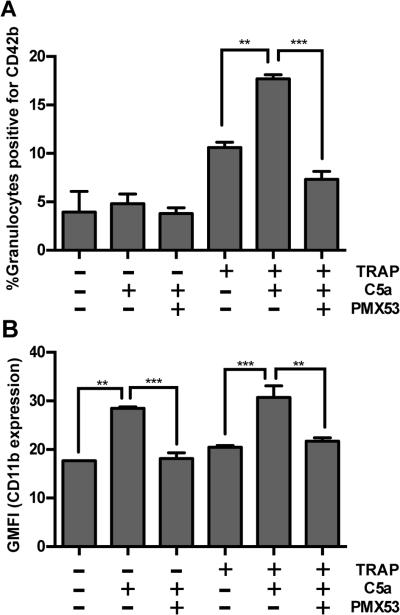
We next investigated the potential downstream effector molecules that could account for properdin’s effects on PGA formation. PMX53, a peptide that blocks the interaction of C5a with its receptor (39), was previously shown to impair TRAP-mediated PGA formation (14, 36). We compared the effects of PMX53 to the effects of our other reagents in the same donor’s blood to discern whether C5a and/or MAC generation influences PGA formation. All reagents except 1G6D2 significantly inhibited PGA formation to an average of ~50% the level seen with TRAP alone (Figure 4A-B) and reduced C3 fragment deposition on granulocytes to a level similar to the NA+Comp control (Figure 4C-D). PMX53 and inhibition of properdin (3A3E1 and 6E11A4) had equivalent inhibitory effects on PGA formation as inhibiting all complement with compstatin (Figure 4A-B), thus indicating C5a is the key complement effector molecule for PGA formation in TRAP-stimulated blood and properdin is a key enhancer of C5a generation.
To confirm the role of properdin in generating C5a at the platelet/granulocyte interface, we determined whether inhibition of C5a function with PMX53 could prevent P4-mediated increases in PGA formation. Figure 5A shows that P4s increased PGA formation ~7-fold over TRAP alone, and PMX53 completely inhibited P4-mediated enhancement of PGA formation. Compstatin, 3A3E1, and 6E11A4 had identical effects as PMX53. C5a levels in reaction supernatants were measured by ELISA (Figure 5B) and displayed a pattern similar to that of PGA formation (Figure 5A), with PMX53 being the only exception, as expected, because it inhibits C5a function rather than generation.
Figure 5. P4s increase PGA formation via C5a-mediated enhancement of CD11b expression.
Lepirudin anticoagulated whole blood was incubated with TRAP alone (10μM) or in combination with P4s (5μg/ml) and anti-complement reagents (anti-properdin - 100μg/ml; PMX53 – 16.7μM; Comp - 50μM). PGA formation (A), C5a in supernatants (B), and CD11b expression (C) were determined as described in Materials and Methods. Results shown in A and C are representative of 1 of 5 independent experiments carried out with different human volunteer blood samples, and in 2 of these 5 experiments C5a was also determined (1 representative experiment shown in B). Two additional C5a ELISAs were carried out using supernatants harvested from experiments carried out under identical conditions, but that did not include duplicate samples for flow cytometry analysis. In the 5 independent experiments, effects of P4s on PGA formation varied between 2.5-14-fold increases over TRAP alone, depending on the donor (variation not shown). Results are shown as mean and SD of duplicate observations. The data was analyzed by one-way ANOVA with Dunnett’s multiple comparison test against TRAP+P4. p<0.001 compared to TRAP+P4 unless indicated as n.s.
Finally, we aimed to determine a potential mechanism for the effects of properdin-mediated C5a generation on PGA formation. PMX53 was previously shown to inhibit CD11b (one of two chains that comprise CR3) upregulation on granulocytes in TRAP-stimulated whole blood (36). We measured CD11b expression on granulocytes in the presence of P4s and 3A3E1, 6E11A4, and PMX53, using 1G6D2 as a negative and IgG1κ isotype control antibody. 3A3E1, 6E11A4, and PMX53 completely abrogated P4-mediated increases in CD11b expression, while 1G6D2 had a partial effect (Figure 5C), suggesting that properdin-mediated C5a generation enhances PGA formation via upregulation of CD11b on granulocytes. We next assessed the direct effect of C5a on PGA formation and CD11b expression. Although C5a had no effect on PGA formation on its own (Figure 6A), C5a caused an ~1.5-fold increase in CD11b expression on granulocytes compared to the non-activated control (Figure 6B). C5a significantly increased PGA formation and CD11b expression on granulocytes in the presence of submaximal doses of TRAP (10μM) by approximately 1.5-fold (Figure 6A-B). The effects of C5a on CD11b expression (either alone or with TRAP), as well as C5a-enhancement of PGA formation in the presence of submaximal doses of TRAP, were all abrogated by PMX53 (Figure 6A-B). Together, our results indicate properdin is key in promoting the generation of C5a at the platelet/granulocyte interface, which enhances PGA formation (in the presence of platelet agonists) by binding to C5a receptor 1 (C5aR1; CD88). This C5a/C5a receptor interaction results in up-regulation of CR3 (CD11b/CD18) on granulocytes, contributing to aggregate stabilization.
Figure 6. C5a directly enhances TRAP-mediated PGA formation and CD11b expression.
Lepirudin anticoagulated whole blood was incubated without or with TRAP (10μM) +/− C5a (210nM) or C5a + PMX53 (16.7μM). Results are representative of 5 independent experiments in which PGA formation (A) and CD11b (B) expression on granulocytes were determined in the same experiment, as described in Materials and Methods. Results are shown as mean and SD of duplicate observations. The data was analyzed by one-way ANOVA with Tukey’s multiple comparison test. p<0.01 (**) and p<0.001 (***).
Properdin-mediated increases in PGA formation are tightly regulated by FH
FH regulates the AP on cell surfaces and is the primary negative AP regulator in the fluid phase. It protects cell surfaces by recognizing and binding to the combination of C3b and polyanions via mainly its C-terminal domains, 19 and 20, while regulating complement with its N-terminus (27, 40, 57). A recombinant protein composed solely of FH domains 19 and 20, designated rH19-20, competitively inhibits FH cell-surface interactions without affecting fluid-phase regulation and simulates the pathophysiological mechanisms involved in the prothrombotic disease atypical hemolytic uremic syndrome (aHUS) (40, 58-60). The contribution of FH to regulation of PGA formation is unknown.
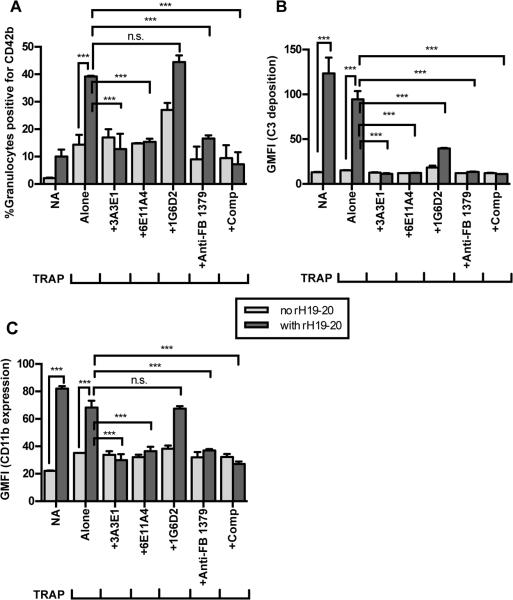
The addition of rH19-20 to TRAP-stimulated whole blood increased PGA formation and C3 fragment deposition on granulocytes compared to TRAP alone (Figure 7A-B), indicating FH cell-surface protection is key in limiting TRAP-mediated PGA formation despite the presence of membrane-bound complement regulators on granulocytes and platelets. In non-activated (NA) blood, rH19-20 increased C3 fragment deposition on granulocytes and CD11b expression (Figure 7B-C), but did not affect the formation of PGA (Figure 7A). This is in agreement with the need for initial platelet activation for PGA formation (61), and suggests that complement effector molecules produced in the absence of FH cell-surface protection do not activate platelets. Higher C3 fragment deposition and CD11b expression on granulocytes in NA vs TRAP-stimulated blood, in the presence of rH19-20, may be an indication that bound platelets mask detection of each marker or that TRAP-stimulation leads to shedding of C3- and/or CD11b-coated microparticles under these conditions. To determine if properdin activity could account for rH19-20-mediated increases in PGA formation, the TRAP-stimulated whole blood was incubated with rH19-20 in the presence of the anti-complement reagents (Figure 7A-B). 3A3E1, 6E11A4, anti-Factor B #1379, and compstatin significantly inhibited rH19-20-mediated increases in PGA formation and C3 fragment deposition to approximately the same level as NA blood, whereas 1G6D2 had no effect on PGA formation and a partial effect on C3 fragment deposition (Figure 7A-B). Partial effects of 1G6D2 on rH19-20-mediated C3 fragment deposition (Figure 7B) and P4-mediated CD11b expression (Fig. 5C) may occur due to steric hindrance of properdin function by antibody-properdin complexes formed as a result of the high antibody concentrations used in our assays. Figure 7C shows representative data indicating that inhibition of properdin, the AP, or all complement, reduces rH19-20-mediated increases in CD11b expression. These effects were observed in at least 3 of 6 experiments, except for 6E11A4 which significantly inhibited CD11b expression twice. The remaining experiments showed identical trends to figure 7C, but did not reach statistical significance. Altogether, the data indicate that FH regulates properdin-mediated increases in AP activity and subsequent PGA formation by mechanisms including, but not necessarily limited to, increased CD11b expression in TRAP-stimulated whole blood. Figure 8 shows a proposed model for the overall mechanisms of the effects of properdin on PGA formation.
Figure 7. FH controls properdin-mediated PGA formation.
Lepirudin anticoagulated whole blood was incubated with TRAP (10μM) without or with the FH competitive inhibitor rH19-20 (20μM) +/− anti-complement reagents (anti-properdin and anti-Factor B #1379 - 100μg/ml; Comp - 50μM). A sample that did not receive TRAP (NA) +/− rH19-20 was also included for each experiment. PGA formation (A), C3 fragment deposition on granulocytes (B), and CD11b expression on granulocytes (C) were determined as described in Materials and Methods. Graphs are representative of 6 independent experiments in which A-C were determined in the same experiment, although statistical significance for CD11b expression only matched the representative figure in ~3 of 6 donors. Results are shown as mean and SD of duplicate observations. The data was analyzed by one-way ANOVA with Tukey’s multiple comparison test. p<0.001 (***) and p>0.05 (n.s.).
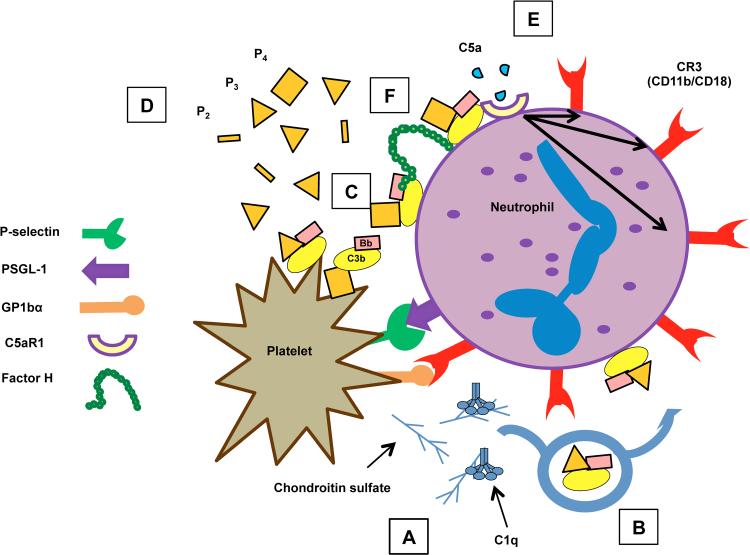
Figure 8. Model for the mechanism of properdin’s effects on PGA formation.
(A) Activated platelets initially tether to granulocytes via P-selectin/PSGL-1 interactions, where they activate the CP on their surface and/or secrete chondroitin sulfate, a known activator of the CP. (B) Properdin-enhanced AP activity amplifies CP activity initiated by platelets, leading to the deposition of C3b on the granulocyte surface. The AP can then use deposited C3b to amplify its activity directly on the granulocyte surface. The AP also activates spontaneously on (C) neutrophils and activated platelets, and (D) AP activity is enhanced by high levels of properdin oligomers (P2, P3, and especially P4), secreted from neutrophils. (E) Properdin-enhanced AP activity ultimately leads to increased levels of C5a that binds to C5a receptor 1 on neutrophils to enhance CR3 expression. (F) FH regulates AP/properdin-mediated PGA formation.
Discussion
Given that platelets have important roles in hemostasis and in the immune response against microorganisms (2), it is essential to identify novel therapeutic targets to limit thrombosis and inflammation in the vasculature without directly affecting platelet function. Granulocytes have both prothrombotic and proinflammatory potential, therefore finding ways to limit the recruitment and activation of granulocytes by platelets could lessen pathology caused by the innate immune response. Long-term blockade of all platelet/granulocyte interactions through the use of P-selectin/PSGL-1 or CR3 antagonists could potentially increase patient susceptibility to infections or even autoimmune diseases (62-64). Here, we have elucidated a role of properdin in influencing stable PGA formation, thus identifying properdin as a potential target for limiting leukocyte-mediated thromboinflammation without directly affecting platelet function or completely blocking platelet/granulocyte interactions in inflammatory vascular diseases.
The addition of physiological properdin enhanced PGA formation in TRAP-stimulated blood in a dose-dependent manner (Figure 1). P4s were the most effective at increasing TRAP-mediated PGA formation and C3 fragment deposition on granulocytes (Figure 1F-G and 5A). P4s also significantly increased C5a levels recovered in supernatants and CD11b expression on granulocytes (Figure 5B-C). The effectiveness of P4s is in agreement with their previously described high degree of native properdin activity (ability to stabilize the convertases, promoting AP activation) as compared to P2s and P3s (28). In addition, weak fluid phase complement activation by P4s (less than 7% that of aggregated/non-physiological properdin) (28) may potentially contribute to C5a generation, thus enhancing PGA formation. Properdin is produced and secreted by many different leukocytes (26), but it is unknown whether different leukocytes secrete properdin forms in the same physiological ratio found in plasma (28). For instance, T-cell derived properdin was shown to be 100-fold more active than serum-derived properdin in an AP hemolytic assay (65). Given our data, specific inhibition of P4s may have therapeutic potential, thus studies are needed to characterize the distribution of properdin forms in inflammatory microenvironments and to develop novel reagents that specifically inhibit each form. Inhibition of endogenous properdin in TRAP-stimulated whole blood with monoclonal antibodies 3A3E1 and 6E11A4 limited PGA formation (Figure 2A-B). This effect correlated with an ability to inhibit C3 fragment deposition on granulocytes (Figure 2C-D). Investigation into the mechanisms of complement activation at the platelet/granulocyte interface revealed roles for both the AP and CP (Figures 3-4). Properdin is essential for AP activation on platelets and neutrophils (31, 32) (Figure 8C-D model), and platelets also activate the CP on or near their surface (Figure 8A) (36, 51, 53). CP activity in the vicinity of the platelet or granulocyte surface could lead to the deposition of C3b on these cells, serving as a site for formation of the AP C3 convertase (C3bBb), which properdin would stabilize, enhancing AP activity (Figure 8B). Regardless of the activation mechanism, the generation of C5a is critical for PGA formation in TRAP-stimulated whole blood, resulting in enhanced CR3 expression to form stable aggregates (Figures 4-6; 8E). Inhibition of properdin was as effective at controlling PGA formation as specific inhibition of the C5a-C5aR1 axis (Figure 4A-B), reflecting the ability of the inhibitory properdin antibodies to significantly reduce C5a generation at the platelet/granulocyte interface (Figure 5B). The only complement-specific inhibitor currently in the clinic is an anti-C5 monoclonal antibody, however direct inhibition of C5 in clinical trials for myocardial infarction and coronary artery bypass surgery, two conditions associated with increased PGA formation (66, 67), had disappointing results (68, 69). Inhibition of properdin significantly reduces AP activity (C3 fragment deposition, Figure 2C-D and C5a generation, Figure 5B) at the platelet/granulocyte interface. Patients treated with properdin inhibitors could be effectively protected from neisserial infections (the main consequence of properdin deficiency) with the available tetravalent meningococcal vaccine (70). Therefore, properdin inhibitors alone or combined with C5aR1 antagonists may be an option to prevent complement-mediated enhancement of PGA formation in vascular diseases, although the therapeutic window for effects of these antagonists remain to be determined experimentally and would likely vary based on the disease context.
Host cells are normally protected from autologous AP complement activation by various redundant membrane-bound (CD55, CD46, CD59, CD35, CRIg) and fluid phase (Factors H, I) complement regulatory proteins (25, 27). Addition of the FH competitive inhibitor rH19-20 to whole blood significantly increased TRAP-mediated PGA formation, as well as C3 fragment deposition and CD11b expression (in at least 3 of 6 donors) on granulocytes (Figure 7; 8F), even though membrane-bound complement regulators are present. The inconsistency in the statistical effects of the reagents on CD11b expression may be a reflection of the sensitivity of our assay, differences in the basal activation state of neutrophils from different donors, or other undetermined sources of inter-individual variation, such as non-complement-mediated factors that govern CD11b upregulation. RH19-20-mediated increases in PGA formation and C3 fragment deposition were consistently abolished by 3A3E1 and 6E11A4 (Figure 7; 8F). This indicates that FH is essential for controlling properdin-mediated complement activity, and that properdin has a critical role at enhancing complement activity when FH regulation is absent. These data may also be relevant in aHUS-mediated thromboinflammation mechanisms.
Our study elucidated a critical role for properdin in enhancing PGA formation in whole blood stimulated with TRAP, but platelets can be activated by other agonists (1). Additional studies have demonstrated PGA formation upon activation with LPS and Shiga toxin (50, 71), shear stress (72), and conditions that simulate coronary artery bypass (67, 73). The extent of complement activation on platelets varies depending on the agonist (31, 51, 74, 75), thus the role of properdin in PGA formation may vary based on the agonist used. Because the AP amplifies complement activity initiated by all complement pathways, properdin will likely have a critical role in any effect that depends on complement activation. Altogether, our data implicates properdin as having a key role in enhancing complement activity at the platelet/granulocyte interface, and inhibition of properdin has therapeutic potential to limit thromboinflammation, through limiting PGA formation, even in the absence of FH cell-surface protection, such as that which occurs in patients with aHUS.
Supplementary Material
Acknowledgements
The authors thank Heather Emch and Sean Ehinger for excellent technical support, and Dr. Sanjay Ram for the donation of Lepirudin.
This work was supported by National Institute of Health grants R01HL112937 (V.P.F.), R01DK076690 (J.M.T.), and P01AI068730 (J.D.L.), and by American Heart Association Predoctoral Fellowship 15PRE25230012 (A.Z.B.).
Footnotes
Disclosures
V.P.F., A.Z.B., G.S., K.V.K., C.C., D.R., J.D.L., and J.G.V. declare no conflict of interest. J.M.T. receives royalties from Alexion Pharmaceuticals, Inc.
References
- 1.Quinn M, Fitzgerald D. Platelet function: assessment, diagnosis, and treatment. Humana Press; Totowa, New Jersey: 2010. [Google Scholar]
- 2.Semple JW, Freedman J. Platelets and innate immunity. Cell. Mol. Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J. Clin. Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davi G, Patrono C. Platelet activation and atherothrombosis. N. Engl. J. Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla M, Camera M, Colnago D, Marenzi G, De Metrio M, Giesen PL, Balduini A, Veglia F, Gertow K, Biglioli P, Tremoli E. Tissue factor in patients with acute coronary syndromes: expression in platelets, leukocytes, and platelet-leukocyte aggregates. Arterioscler. Thromb. Vasc. Biol. 2008;28:947–953. doi: 10.1161/ATVBAHA.107.161471. [DOI] [PubMed] [Google Scholar]
- 6.Celi A, Pellegrini G, Lorenzet R, De Blasi A, Ready N, Furie BC, Furie B. P-selectin induces the expression of tissue factor on monocytes. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maugeri N, Brambilla M, Camera M, Carbone A, Tremoli E, Donati MB, de Gaetano G, Cerletti C. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J. Thromb. Haemost. 2006;4:1323–1330. doi: 10.1111/j.1538-7836.2006.01968.x. [DOI] [PubMed] [Google Scholar]
- 8.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu Y-M, Sajer SA, Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 9.Maugeri N, Baldini M, Ramirez GA, Rovere-Querini P, Manfredi AA. Platelet-leukocyte deregulated interactions foster sterile inflammation and tissue damage in immune-mediated vessel diseases. Thromb. Res. 2012;129:267–273. doi: 10.1016/j.thromres.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Totani L, Evangelista V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler. Thromb. Vasc. Biol. 2010;30:2357–2361. doi: 10.1161/ATVBAHA.110.207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerletti C, Tamburrelli C, Izzi B, Gianfagna F, de Gaetano G. Platelet-leukocyte interactions in thrombosis. Thromb. Res. 2012;129:263–266. doi: 10.1016/j.thromres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 13.May AE, Langer H, Seizer P, Bigalke B, Lindemann S, Gawaz M. Platelet-leukocyte interactions in inflammation and atherothrombosis. Semin. Thromb. Hemost. 2007;33:123–127. doi: 10.1055/s-2007-969023. [DOI] [PubMed] [Google Scholar]
- 14.Hamad OA, Mitroulis I, Fromell K, Kozarcanin H, Chavakis T, Ricklin D, Lambris JD, Ekdahl KN, Nilsson B. Contact activation of C3 enables tethering between activated platelets and polymorphonuclear leukocytes via CD11b/CD18. Thromb. Haemost. 2015;114:1207–1217. doi: 10.1160/TH15-02-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ott I, Neumann FJ, Gawaz M, Schmitt M, Schomig A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation. 1996;94:1239–1246. doi: 10.1161/01.cir.94.6.1239. [DOI] [PubMed] [Google Scholar]
- 16.Czyz A, Kolacz E, Angerer D, Zawilska K. Expression of activation antigens on lymphocyte surface and circulating platelet-leukocyte aggregates in ischaemic heart disease. Kardiol. Pol. 2005;62:189–200. discussion 201. [PubMed] [Google Scholar]
- 17.Jensen MK, de Nully Brown P, Lund BV, Nielsen OJ, Hasselbalch HC. Increased circulating platelet-leukocyte aggregates in myeloproliferative disorders is correlated to previous thrombosis, platelet activation and platelet count. Eur. J. Haematol. 2001;66:143–151. doi: 10.1034/j.1600-0609.2001.00359.x. [DOI] [PubMed] [Google Scholar]
- 18.Elalamy I, Chakroun T, Gerotziafas GT, Petropoulou A, Robert F, Karroum A, Elgrably F, Samama MM, Hatmi M. Circulating platelet-leukocyte aggregates: a marker of microvascular injury in diabetic patients. Thromb. Res. 2008;121:843–848. doi: 10.1016/j.thromres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Tekelioglu Y, Uzun H. Circulating platelet-leukocyte aggregates in patients with inflammatory bowel disease. J. Chin. Med. Assoc. 2013;76:182–185. doi: 10.1016/j.jcma.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, Hechtman HB, Michelson AD. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 1998;31:352–358. doi: 10.1016/s0735-1097(97)00510-x. [DOI] [PubMed] [Google Scholar]
- 21.Merhi Y, Provost P, Chauvet P, Theoret JF, Phillips ML, Latour JG. Selectin blockade reduces neutrophil interaction with platelets at the site of deep arterial injury by angioplasty in pigs. Arterioscler. Thromb. Vasc. Biol. 1999;19:372–377. doi: 10.1161/01.atv.19.2.372. [DOI] [PubMed] [Google Scholar]
- 22.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 23.Smyth SS, Reis ED, Zhang W, Fallon JT, Gordon RE, Coller BS. Beta(3)-integrin-deficient mice but not P-selectin-deficient mice develop intimal hyperplasia after vascular injury: correlation with leukocyte recruitment to adherent platelets 1 hour after injury. Circulation. 2001;103:2501–2507. doi: 10.1161/01.cir.103.20.2501. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, Zago AC, Lopez J, Andre P, Plow E, Simon DI. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 25.Holers VM. Complement and its receptors: new insights into human disease. Annu. Rev. Immunol. 2014;32:433–459. doi: 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- 26.Cortes C, Ohtola JA, Saggu G, Ferreira VP. Local release of properdin in the cellular microenvironment: role in pattern recognition and amplification of the alternative pathway of complement. Front. Immunol. 2012;3:412. doi: 10.3389/fimmu.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira VP, Pangburn MK, Cortes C. Complement control protein factor H: the good, the bad, and the inadequate. Mol. Immunol. 2010;47:2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pangburn MK. Analysis of the natural polymeric forms of human properdin and their functions in complement activation. J. Immunol. 1989;142:202–207. [PubMed] [Google Scholar]
- 29.Farries TC, Finch JT, Lachmann PJ, Harrison RA. Resolution and analysis of 'native' and 'activated' properdin. Biochem. J. 1987;243:507–517. doi: 10.1042/bj2430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira VP, Cortes C, Pangburn MK. Native polymeric forms of properdin selectively bind to targets and promote activation of the alternative pathway of complement. Immunobiology. 2010;215:932–940. doi: 10.1016/j.imbio.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saggu G, Cortes C, Emch HN, Ramirez G, Worth RG, Ferreira VP. Identification of a novel mode of complement activation on stimulated platelets mediated by properdin and C3(H2O) J. Immunol. 2013;190:6457–6467. doi: 10.4049/jimmunol.1300610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camous L, Roumenina L, Bigot S, Brachemi S, Fremeaux-Bacchi V, Lesavre P, Halbwachs-Mecarelli L. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood. 2011;117:1340–1349. doi: 10.1182/blood-2010-05-283564. [DOI] [PubMed] [Google Scholar]
- 33.Wirthmueller U, Dewald B, Thelen M, Schafer MK, Stover C, Whaley K, North J, Eggleton P, Reid KB, Schwaeble WJ. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J. Immunol. 1997;158:4444–4451. [PubMed] [Google Scholar]
- 34.Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68:875–880. [PubMed] [Google Scholar]
- 35.Rinder CS, Rinder HM, Smith MJ, Tracey JB, Fitch J, Li L, Rollins SA, Smith BR. Selective blockade of membrane attack complex formation during simulated extracorporeal circulation inhibits platelet but not leukocyte activation. J. Thorac. Cardiovasc. Surg. 1999;118:460–466. doi: 10.1016/S0022-5223(99)70183-2. [DOI] [PubMed] [Google Scholar]
- 36.Hamad OA, Ekdahl KN, Nilsson PH, Andersson J, Magotti P, Lambris JD, Nilsson B. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J. Thromb. Haemost. 2008;6:1413–1421. doi: 10.1111/j.1538-7836.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- 38.Qu H, Magotti P, Ricklin D, Wu EL, Kourtzelis I, Wu YQ, Kaznessis YN, Lambris JD. Novel analogues of the therapeutic complement inhibitor compstatin with significantly improved affinity and potency. Mol. Immunol. 2011;48:481–489. doi: 10.1016/j.molimm.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. J. Med. Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J. Immunol. 2006;177:6308–6316. doi: 10.4049/jimmunol.177.9.6308. [DOI] [PubMed] [Google Scholar]
- 41.Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, Holers VM. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol. Immunol. 2005;42:87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 42.Ferreira VP, Fazito Vale V, Pangburn MK, Abdeladhim M, Ferreira Mendes-Sousa A, Coutinho-Abreu IV, Rasouli M, Brandt EA, Meneses C, Lima KF, Nascimento Araujo R, Horacio Pereira M, Kotsyfakis M, Oliveira F, Kamhawi S, Ribeiro JM, Gontijo NF, Collin N, Valenzuela JG. SALO, a novel classical pathway complement inhibitor from saliva of the sand fly Lutzomyia longipalpis. Sci. Rep. 2016;6:19300. doi: 10.1038/srep19300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal S, Ferreira VP, Cortes C, Pangburn MK, Rice PA, Ram S. An evaluation of the role of properdin in alternative pathway activation on Neisseria meningitidis and Neisseria gonorrhoeae. J. Immunol. 2010;185:507–516. doi: 10.4049/jimmunol.0903598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruef J, Kuehnl P, Meinertz T, Merten M. The complement factor properdin induces formation of platelet-leukocyte aggregates via leukocyte activation. Platelets. 2008;19:359–364. doi: 10.1080/09537100802105040. [DOI] [PubMed] [Google Scholar]
- 45.Bournazos S, Rennie J, Hart SP, Dransfield I. Choice of anticoagulant critically affects measurement of circulating platelet-leukocyte complexes. Arterioscler. Thromb. Vasc. Biol. 2008;28:e2–3. doi: 10.1161/ATVBAHA.107.153387. [DOI] [PubMed] [Google Scholar]
- 46.Mollnes TE, Garred P, Bergseth G. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin. Exp. Immunol. 1988;73:484–488. [PMC free article] [PubMed] [Google Scholar]
- 47.Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G, Videm V, Lappegard KT, Kohl J, Lambris JD. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 48.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 49.Nagasawa A, Matsuno K, Tamura S, Hayasaka K, Shimizu C, Moriyama T. The basis examination of leukocyte-platelet aggregates with CD45 gating as a novel platelet activation marker. Int. J. Lab. Hematol. 2013;35:534–541. doi: 10.1111/ijlh.12051. [DOI] [PubMed] [Google Scholar]
- 50.Stahl AL, Sartz L, Karpman D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood. 2011;117:5503–5513. doi: 10.1182/blood-2010-09-309161. [DOI] [PubMed] [Google Scholar]
- 51.Peerschke EI, Yin W, Grigg SE, Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J. Thromb. Haemost. 2006;4:2035–2042. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- 52.Speth C, Rambach G, Wurzner R, Lass-Florl C, Kozarcanin H, Hamad OA, Nilsson B, Ekdahl KN. Complement and platelets: Mutual interference in the immune network. Mol. Immunol. 2015;67:108–118. doi: 10.1016/j.molimm.2015.03.244. [DOI] [PubMed] [Google Scholar]
- 53.Hamad OA, Nilsson PH, Lasaosa M, Ricklin D, Lambris JD, Nilsson B, Ekdahl KN. Contribution of chondroitin sulfate A to the binding of complement proteins to activated platelets. PLoS One. 2010;5:e12889. doi: 10.1371/journal.pone.0012889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tavares NM, Silva RA, Costa DJ, Pitombo MA, Fukutani KF, Miranda JC, Valenzuela JG, Barral A, de Oliveira CI, Barral-Netto M, Brodskyn C. Lutzomyia longipalpis saliva or salivary protein LJM19 protects against Leishmania braziliensis and the saliva of its vector, Lutzomyia intermedia. PLoS Negl. Trop. Dis. 2011;5:e1169. doi: 10.1371/journal.pntd.0001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, Silva C, de Oliveira CI, Miranda JC, Elnaiem DE, Kamhawi S, Valenzuela JG, Brodskyn CI. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7845–7850. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.da Silva RA, Tavares NM, Costa D, Pitombo M, Barbosa L, Fukutani K, Miranda JC, de Oliveira CI, Valenzuela JG, Barral A, Soto M, Barral-Netto M, Brodskyn C. DNA vaccination with KMP11 and Lutzomyia longipalpis salivary protein protects hamsters against visceral leishmaniasis. Acta Trop. 2011;120:185–190. doi: 10.1016/j.actatropica.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferreira VP, Herbert AP, Cortes C, McKee KA, Blaum BS, Esswein ST, Uhrin D, Barlow PN, Pangburn MK, Kavanagh D. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J. Immunol. 2009;182:7009–7018. doi: 10.4049/jimmunol.0804031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banda NK, Mehta G, Ferreira VP, Cortes C, Pickering MC, Pangburn MK, Arend WP, Holers VM. Essential role of surface-bound complement factor H in controlling immune complex-induced arthritis. J. Immunol. 2013;190:3560–3569. doi: 10.4049/jimmunol.1203271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Renner B, Ferreira VP, Cortes C, Goldberg R, Ljubanovic D, Pangburn MK, Pickering MC, Tomlinson S, Holland-Neidermyer A, Strassheim D, Holers VM, Thurman JM. Binding of factor H to tubular epithelial cells limits interstitial complement activation in ischemic injury. Kidney Int. 2011;80:165–173. doi: 10.1038/ki.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda K, Thurman JM, Tomlinson S, Okamoto M, Shiraishi Y, Ferreira VP, Cortes C, Pangburn MK, Holers VM, Gelfand EW. The critical role of complement alternative pathway regulator factor H in allergen-induced airway hyperresponsiveness and inflammation. J. Immunol. 2012;188:661–667. doi: 10.4049/jimmunol.1101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Izzi B, Pampuch A, Costanzo S, Vohnout B, Iacoviello L, Cerletti C, de Gaetano G. Determinants of platelet conjugate formation with polymorphonuclear leukocytes or monocytes in whole blood. Thromb. Haemost. 2007;98:1276–1284. [PubMed] [Google Scholar]
- 62.Ley K. The role of selectins in inflammation and disease. Trends Mol. Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 63.Kum WW, Lee S, Grassl GA, Bidshahri R, Hsu K, Ziltener HJ, Finlay BB. Lack of functional P-selectin ligand exacerbates Salmonella serovar typhimurium infection. J. Immunol. 2009;182:6550–6561. doi: 10.4049/jimmunol.0802536. [DOI] [PubMed] [Google Scholar]
- 64.Rosetti F, Mayadas TN. The many faces of Mac-1 in autoimmune disease. Immunol. Rev. 2016;269:175–193. doi: 10.1111/imr.12373. [DOI] [PubMed] [Google Scholar]
- 65.Schwaeble W, Dippold WG, Schafer MK, Pohla H, Jonas D, Luttig B, Weihe E, Huemer HP, Dierich MP, Reid KB. Properdin, a positive regulator of complement activation, is expressed in human T cell lines and peripheral blood T cells. J. Immunol. 1993;151:2521–2528. [PubMed] [Google Scholar]
- 66.Maugeri N, Rovere-Querini P, Slavich M, Coppi G, Doni A, Bottazzi B, Garlanda C, Cianflone D, Maseri A, Mantovani A, Manfredi AA. Early and transient release of leukocyte pentraxin 3 during acute myocardial infarction. J. Immunol. 2011;187:970–979. doi: 10.4049/jimmunol.1100261. [DOI] [PubMed] [Google Scholar]
- 67.Rinder CS, Bonan JL, Rinder HM, Mathew J, Hines R, Smith BR. Cardiopulmonary bypass induces leukocyte-platelet adhesion. Blood. 1992;79:1201–1205. [PubMed] [Google Scholar]
- 68.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J. Immunol. 2013;190:3839–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verrier ED, Shernan SK, Taylor KM, Van de Werf F, Newman MF, Chen JC, Carrier M, Haverich A, Malloy KJ, Adams PX, Todaro TG, Mojcik CF, Rollins SA, Levy JH, P.-C. Investigators Terminal complement blockade with pexelizumab during coronary artery bypass graft surgery requiring cardiopulmonary bypass: a randomized trial. JAMA. 2004;291:2319–2327. doi: 10.1001/jama.291.19.2319. [DOI] [PubMed] [Google Scholar]
- 70.Mathew S, Overturf GD. Complement and properidin deficiencies in meningococcal disease. Pediatr. Infect. Dis. J. 2006;25:255–256. doi: 10.1097/01.inf.0000209215.65445.04. [DOI] [PubMed] [Google Scholar]
- 71.Stahl AL, Sartz L, Nelsson A, Bekassy ZD, Karpman D. Shiga toxin and lipopolysaccharide induce platelet-leukocyte aggregates and tissue factor release, a thrombotic mechanism in hemolytic uremic syndrome. PLoS One. 2009;4:e6990. doi: 10.1371/journal.pone.0006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu H, Varon D, Hjemdahl P, Savion N, Schulman S, Li N. Platelet-leukocyte aggregation under shear stress: differential involvement of selectins and integrins. Thromb. Haemost. 2003;90:679–687. doi: 10.1160/TH03-05-0274. [DOI] [PubMed] [Google Scholar]
- 73.Lappegard KT, Fung M, Bergseth G, Riesenfeld J, Lambris JD, Videm V, Mollnes TE. Effect of complement inhibition and heparin coating on artificial surface-induced leukocyte and platelet activation. Ann. Thorac. Surg. 2004;77:932–941. doi: 10.1016/S0003-4975(03)01519-4. [DOI] [PubMed] [Google Scholar]
- 74.Yin W, Rubenstein DA. Dose effect of shear stress on platelet complement activation in a cone and plate shearing device. Cell. Mol. Bioeng. 2009;2:274–280. [Google Scholar]
- 75.Shanmugavelayudam SK, Rubenstein DA, Yin W. Effects of physiologically relevant dynamic shear stress on platelet complement activation. Platelets. 2011;22:602–610. doi: 10.3109/09537104.2011.585257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.