Abstract
Since publication of the first Guidelines to Prevent Perinatal Group B Strep (GBS) disease in 1996, the incidence and mortality from early onset sepsis (EOS), and particularly GBS, the leading cause of EOS, has drastically decreased. In 2010, the Centers for Disease Control (CDC) provided updated Guidelines for the prevention of perinatal Group B streptococcal disease. In 2012, the AAP Committee on Fetus and Newborn (COFN) provided a Clinical Report that provided a thorough review of EOS and voiced overall support of the 2010 CDC Guidelines. In addition, the COFN authors suggested an approach different from the 2010 CDC Guidelines for at-risk asymptomatic infants. The COFN also ventured into the uncertain territory of recommending longer duration of empirical antibiotic treatment for asymptomatic infants with negative cultures, but abnormal CBC and/or CRP values. With the current focus on antibiotic stewardship, the 2012 COFN Clinical Report algorithms evoked questions from the Neonatology community. The COFN has recently responded with modified recommendations for empirical antibiotic duration and explanations for the recommendations for use of antibiotics in subgroups of infants for whom the CDC did not recommend starting antibiotics. Our goal in this article is to review the 2010 CDC and 2012 COFN guidelines and COFN's recently published guideline modifications and discuss mechanisms that may reduce the number of term and near term infants to be started on antibiotics.
Defining the problem: Early Onset Sepsis
Epidemiologists define early onset sepsis (EOS) as culture positive infections occurring the first 3 postnatal days. ([1, 2]) The CDC defines early onset group B strep (GBS) disease as blood or cerebral spinal fluid culture-proven infection occurring in the first 7 postnatal days ([3, 4]). The NICHD definition of EOS also requires that the infection be treated with antibiotics for 5 or more continuous days.([2]) However EOS is defined, the obstetric and pediatric communities have collaborated to greatly reduce risk of the major cause of EOS in term infants, Group B streptococci (GBS; Streptococci agalactaie) since the first CDC Guidelines to reduce risk were published in 1996. ([3]) At the time the first guidelines emerged, the incidence of EOS in the U.S. was 3 - 4 cases per 1000 liveborn infants.[5] With Guideline modifications in 2002 and 2010 strongly recommending universal screening, fine-tuning of culture methods and intrapartum antimicrobial prophylaxis (IAP) drug choice when the mother is penicillin allergic, the incidence of EOS has fallen to 0.3/1,000. ([2]; [4]; [6]) GBS remains the leading cause of EOS in term infants, while E coli is most prevalent among premature infants. ([2], [7])
At a population level, the 2 – 10-fold reduction in prevalence of EOS since 1996 is remarkable.([8], [2], [9]) The guidelines have saved lives. However, the guideline-based strategies that have led to this reduction have contributed to 30% of mothers in the U.S. receiving antibiotics during labor.([8];[10]; [11]) On the neonatal side, single center experiences and population estimates based on clinicians following the Guidelines since the first were published indicate that 15 – 20% of term infants (over 500,000 infants per year in the US), most of whom are asymptomatic, are evaluated with screening blood tests for EOS and many also receive empirical antibiotics. ([11]; [12]; [13])
Risks of antibiotics, why be cautious?
Adherence to the CDC Guidelines has resulted in significant decreases in EOS, but antibiotic exposures, in the absence of an identified infection to treat, do not appear to be totally without risk. The emerging evidence for risk provides a rationale for identifying mechanisms to limit antibiotic exposure initiation to infants at highest risk while missing extremely few if any infants with evolving infection, and limiting the duration of antibiotics for those whose evolving clinical picture indicates an extremely low likelihood of infection. Aminoglycosides are among the most commonly used antimicrobials for prevention and empirical treatment of EOS, and have potential to cause renal and ototoxicity. ([14], [15]) Among premature infants, duration of the initial empirical course is associated with later onset infection, necrotizing enterocolitis and death. ([16], [17],[18])
In a Swedish cohort, antibiotic exposure in the neonatal period was associated with almost triple the odds of later wheezing in infants ≥ 33 weeks. ([19]) In a Dutch cohort use of neonatal antibiotics was associated with changes to the microbiome, which in turn were associated with atopic symptoms (eczema and wheeze). ([20], [21]) While the information linking antibiotic exposure to wheezing and atopy via the microbiome is intriguing and biologically plausible, and animal studies have demonstrated the strong influence of neonatal antibiotics on later gut microbiome and respiratory outcomes ([22], [23]), authors of meta-analyses of the cohort studies associating neonatal antibiotic exposures with later wheezing in children find that the associations are subject to bias, and recommend caution before justifying limitation of antibiotics for the purpose of avoiding asthma at the current stage of evidence accumulation. ([24], [25]) More immediately, clinicians and the community at large share the concern that overall use of antibiotics contributes to development of resistant organisms, making careful and selective use of antibiotics to the highest risk patients a universal goal. Antibiotic stewardship is the third of four core activities identified by the CDC to limit development of antimicrobial resistant organisms: 1) prevent infections, preventing spread; 2) tracking resistance patterns; 3) improving use of antibiotics; 4) developing new antibiotics and diagnostic tests.[26]
Review of past and current CDC Guidelines
The third and most recent iteration of the CDC Guidelines to prevent GBS perinatal disease was published in 2010. ([4]) The initial and subsequent CDC Guidelines were the combined efforts of CDC and numerous professional societies and experts in obstetrics, pediatrics and microbiology. ([3]) The first Guidelines suggested obstetric caregivers choose between a solely risk-factor based approach to use of intrapartum antibiotic prophylaxis (IAP) or a universal screening of mothers plus risk factors based approach to use of IAP. In 2002 the CDC recommended universal culture-based screening of all pregnant women at 35 -37 weeks' gestation to optimize the identification of women who should receive intrapartum antibiotic prophylaxis (IAP). ([27])
The third and most recent version of the CDC Guidelines has been endorsed by the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, the American College of Nurse-Midwives, the American Academy of Family Physicians, and the American Society for Microbiology. ([4]) Most of the changes between the second and third version of the Guidelines deal with the antepartum approach to the mother and the laboratory methods used for identification of GBS and testing for antimicrobial sensitivities. While the initial CDC Guidelines recommended penicillin as the ideal choice for IAP for GBS because penicillin has a narrower spectrum of antimicrobial activity and therefore might be less likely to select for resistant organism than ampicillin, one clinical trial found that penicillin and ampicillin administered intravenously intrapartum were associated equally with the presence of ampicillin-resistant gram-negative organisms on postpartum vaginal-perineal culture ([4]; [28]). The CDC now defines adequate IAP as > 4 hours of IV penicillin, ampicillin or cefazolin before delivery. [(4)]
The 2010 CDC Guidelines also include a revised algorithm for management of newborns with respect to risk for early-onset GBS disease, that, if followed, could reduce antibiotic exposures among asymptomatic infants with risk factors compared with the earlier Guideline. (Figure 1) The 2010 Guidelines state that the algorithm applies to all newborns, not just term and near term infants. One very strong point made in the Guidelines is that infants with signs of sepsis should receive a full diagnostic evaluation and receive antibiotic therapy pending the results. ([4]) The full diagnostic evaluation includes a blood culture, a CBC including differential and platelet count, a chest radiograph if respiratory signs are present, and a lumbar puncture if the newborn is stable enough to tolerate the procedure and sepsis is highly suspected. Empirical therapy should include antimicrobial agents active against GBS (including intravenous ampicillin) as well as other organisms that might cause neonatal sepsis, such as E. coli.
Figure 1. CDC 2012 Algorithm for secondary prevention of early-onset group B streptococcal (GBS) disease among newborns.
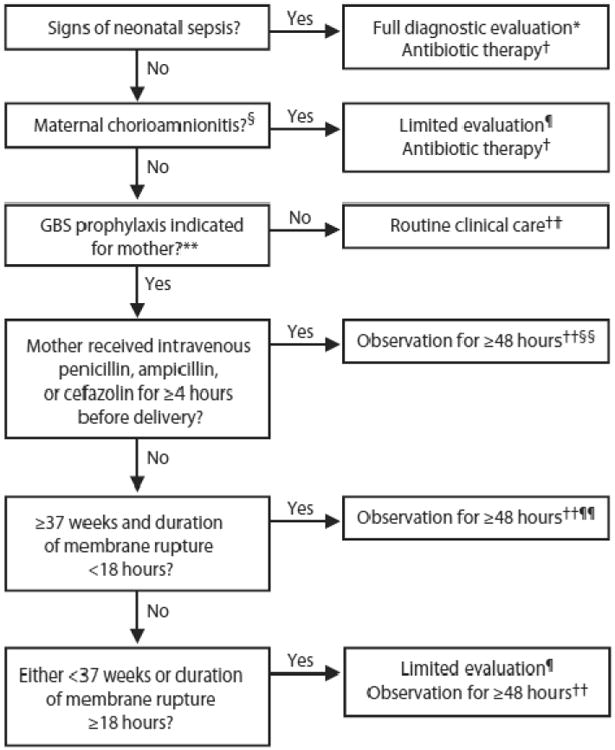
From Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59: 1-36; with permission.
* Full diagnostic evaluation includes a blood culture, a complete blood count (CBC) including white blood cell differential and platelet counts, chest radiograph (if respiratory abnormalities are present), and lumbar puncture (if patient is stable enough to tolerate procedure and sepsis is suspected).
† Antibiotic therapy should be directed toward the most common causes of neonatal sepsis, including intravenous ampicillin for GBS and coverage for other organisms (including Escherichia coli and other gram-negative pathogens) and should take into account local antibiotic resistance patterns.
§ Consultation with obstetric providers is important to determine the level of clinical suspicion for chorioamnionitis. Chorioamnionitis is diagnosed clinically and some of the signs are nonspecific.
¶ Limited evaluation includes blood culture (at birth) and CBC with differential and platelets (at birth and/or at 6--12 hours of life).
†† If signs of sepsis develop, a full diagnostic evaluation should be conducted and antibiotic therapy initiated.
§§ If ≥37 weeks' gestation, observation may occur at home after 24 hours if other discharge criteria have been met, access to medical care is readily available, and a person who is able to comply fully with instructions for home observation will be present. If any of these conditions is not met, the infant should be observed in the hospital for at least 48 hours and until discharge criteria are achieved.
¶¶ Some experts recommend a CBC with differential and platelets at age 6--12 hours.
Well-appearing infants whose mothers had been identified as having chorioamnionitis, a difficult clinical diagnosis to make, but one associated with 2 – 3 fold increase in odds of EOS ([12];[29]), should have diagnostic tests including a CBC and a blood culture and be started on empirical antibiotics while awaiting culture results. In the 2010 CDC algorithm, the asymptomatic infants whose mothers were diagnosed with chorioamnionitis are the only asymptomatic infants that are to receive empirical antibiotics. The CDC Guidelines acknowledge the poor positive predictive value of the CBC indices, particulary when the CBC is obtained at birth compared with results from samples obtained between 6 and 12 postnatal hours, but even results obtained at 6 to 12 hours are poor predictors of positive cultures. ([11];[30];[31]) While the CDC recommends the CBCs and differentials and platelet counts be examined, they provide no guidance on normal ranges or advise on what clinicians should do with the results. For newborns whose mothers had chorioamnionitis and are started on empirical antibiotics, no guidance on how the results should influence duration of antibiotics if the culture remains negative is provided. ([4])
For infants with risk factors other than chorioamnionitis, the 2002 CDC Guideline recommended broad use of a “limited evaluation” which included a blood culture at birth and a complete blood count with differential and platelet count at birth and/or at 6 – 12 postnatal hours. All infants born to mothers with inadequate IAP were to have the limited evaluation. In the 2010 Guideline the CDC recommends that well-appearing infants ≥ 37 weeks gestation whose mothers had an indication for GBS prophylaxis but received no or inadequate IAP can be managed with observation alone for ≥48 hours, without diagnostic tests. For infants with inadequate IAP who are <37 weeks gestational age or for infants of any gestational age whose membranes were ruptured ≥ 18 hours before delivery, the CDC recommends observation for ≥ 48 hours plus the limited evaluation, but no empirical treatment unless there are clinical signs of sepsis. If signs develop, a full evaluation should be undertaken. For well-appearing late preterm infants 35 - 36 weeks gestation whose mothers received adequate IAP, the 2010 CDC Guideline does not recommend diagnostic evaluations. Evidence for these last two recommendations, both regarding preterm infants, arises from “opinions of respected authorities based on clinical or laboratory experience, descriptive studies, or reports of expert committees”. ([4])
AAP Committee on Fetus and the Newborn (COFN)
In 2011, the AAP published an overall agreement with the CDC Guidelines [32] While the 2011 AAP paper reviewed and summarized the CDC Guidelines, in 2012, the AAP COFN took a step further and published a “clinical report” with a goal to “provide a practical and, when possible, evidence-based approach to the management of infants with suspected or proven early-onset sepsis.”. ([33]) The report provided a valuable and thorough review of the background behind the CDC guidelines to reduce GBS EOS, information about the accuracy, reliability and validity of the various diagnostic strategies for EOS, as well as a review of treatment strategies for EOS. The COFN report included recommendations for use of diagnostic tests and empirical treatment also included important variations from the CDC guidelines and made a first attempt at giving guidance for duration of empirical antibiotics in infants without signs of infection whose mothers had risk factors, but whose cultures remained negative.
The first departure from the CDC guidelines was the COFN recommendation that preterm infants born to mothers with chorioamnionitis, rupture of membranes ≥ 18 hours, or inadequate IAP, have laboratory exams drawn plus empirical treatment for a minimum of 72 hours. The COFN based this variation, which increased the number of infants to be treated compared with the CDC, on the higher risk of infection in premature infants compared to term infants when any of these risk factors were present. ([34])
In addition to the guidance for starting empirical antibiotics in premature infants, the COFN provided two algorithms to apply to asymptomatic infants whose mothers had risk factors for EOS, one for infants < 37 weeks gestation, and one for infants ≥ 37 weeks whose mothers had chorioamnionitis. In the algorithm for premature infants, those with maternal risk factors (chorioamnionitis or prolonged rupture of membranes ≥ 18 hours or inadequate IAP) should have screening laboratory exams including blood culture at birth and a CBC and differential plus/minus a c-reactive protein (CRP) between 6 and 12 postnatal hours, and initiation of empirical antibiotics. If the culture was positive, antibiotics should continue, and if the blood culture was negative and the infant remained well, antibiotics should stop. If the blood culture was negative, the infant was well, but the 6 – 12 postnatal hour laboratory exams were abnormal, COFN recommended continuation of empirical antibiotics, without a recommended duration. Similarly, for infants ≥ 37 weeks gestation whose mothers were diagnosed with chorioamnionitis, cultures were to be drawn at birth, screening tests should be drawn between 6 and 12 postnatal hours, and empirical antibiotics started, similar to the 2010 CDC Guidelines. Antibiotics would be continued in well-appearing infants if the culture was positive or if the culture was negative but the laboratory data was abnormal and the mother had received antibiotics during labor and delivery. As with the premature infants with abnormal screening tests, neither duration of this continuation of empirical antibiotic treatment nor guidance on degree of variance from normal to define abnormal screening laboratory results were provided. [(33)]
Immediate Response to the COFN 2012 Guidelines
The pediatric community responded to the COFN clinical report with 4 letters to the editor. ([9]; [35]; [36];[37]) The writers pointed out the approach to the asymptomatic premature infant whose mother had chorioamnionitis, prolonged rupture of membranes, or inadequate IAP differed from the CDC approach which included only screening laboratories. The COFN strategy would lead to more empirical antiobiotic use than following the CDC Guideline. The writers also expressed concern that the COFN suggestions would increase duration of empirical antimicrobial courses in well-appearing infants based on tests with poor positive predictive value. The writers also pointed out the potential of biomarker tests other than the CBC and differential and CRP that could have value. ([9]; [35]; [36];[37]) One writer offered the suggestion of assessing maternal temperature instead of trying to define chorioamnionitis, referring to development of an online EOS risk calculator based on assessment of 350 cases of EOS matched 3:1 with controls taken from a population of over 600,000 infants born at ≥ 34 weeks at 14 California and Massachusetts hospitals. ([9], [11])
In the immediate response to these letters, the COFN authors identified the number of days of empirical antibiotics an asymptomatic infant born to a woman treated with antibiotics for chorioamnionitis or for EOS risk factors should receive if the laboratory studies were abnormal as a major area of uncertainty in the prevention of EOS. [(38)] They modified their stance on continuing antibiotics when results were abnormal and maintained their stance on treating premature infants with mothers with risk factors. Modifying the stance on continuation of antibiotics when laboratory results are abnormal they stated: “We would not treat a well-appearing, asymptomatic term infant with a negative blood culture longer than 48 – 72 hours, whose mother was treated for chorioamnionitis, even when the infant's laboratory data are abnormal.” They cited the 2010 CDC Guidelines report as providing rationale for this approach, i.e., a normal physical examination at 48 – 72 hours in an otherwise well infant should exclude the possibility of early-onset sepsis. ([4]) For premature infants, the COFN justified the recommendation for empirical therapy initiation for asymptomatic preterm infants whose mothers had risk factors with the fact that the preterm infants are at higher risk of EOS than term infants with maternal risk factors. ([34]) The COFN response to the query on potential biomarkers was that more study was needed before widespread adoption, and while they liked the potential to utilize the risk of EOS calculator which included maternal temperature during labor to quantify risk rather than the categorical definition of chorioamnionitis, and acknowledged its use would likely decrease the number of sepsis evaluations in the late preterm and term populations,([11]) they felt it should be validated in more studies. In summary, they said revised COFN algorithms would be forthcoming.[(38)]
The Next Round
In 2013, before publication of the revised COFN algorithms published in June 2014, Brady and Polin wrote independently with the purpose “to clarify AAP policy” on EOS, acknowledging the differences between the 2010 CDC Guidelines and the 2012 COFN Clinical Report. [(33);(39)] They reinforced the revised guidance on antibiotic duration provided in the response to the letters that followed the 2012 COFN Clinical Report's guidance which emphasized continuing empirical antibiotics based on persistently abnormal physical exam findings rather than laboratory values. ([39]) They pointed out that the 2012 COFN clinical report noted that in some situations, approaches that differed from the 2010 CDC Guidelines could be altered, and may depend on local practice and resources. The 2013 Commentary by Brady and Polin states that COFN and CDC Guidelines concur in two situations: 1) symptomatic infants should be treated with broad-spectrum antiobiotics; 2) healthy appearing term and preterm infants whose mothers had chorioamnionitis should have a blood culture at birth, have empirical treatment started, and have laboratory exams drawn between 6 and 12 postnatal hours (a CBC with differential +/- CRP), but the CDC did not offer guidance on what to do with the laboratory results. [(39)]
The COFN and CDC still differed in the approach to two situations. First, for well-appearing term infants whose mother did not have chorioamnionitis, but who had an indication for IAP and were inadequately treated: CDC and COFN agree that these infants can be observed without additional testing if ROM is < 18 hours. The COFN believes infants 35 and 36 weeks gestation can be treated similarly. Differences between CDC and COFN arise if ROM is ≥ 18 hours and IAP is inadequate. The CDC Guidelines recommend a limited evaluation which would include a CBC with differential at birth or 6 to 12 postnatal hours, and hospital observation for 48 hours.[(4)] The COFN recommends observation for 48 hours, without the laboratory tests, unless close observation is not possible.[(39)] The second area of discrepancy is for well-appearing infants < 37 weeks gestation whose mothers, did not have suspected chorioamnionitis, but had another indication, but did not receive adequate IAP. The CDC recommended a limited evaluation (blood culture and CBC) and observation in hospital for 48 hours, while the COFN recommended a CBC plus/minus CRP obtained between 6 to 12 postnatal hours, and only obtaining a blood culture if antibiotics were to be started because of abnormal laboratory values. This statement seems to differ from the algorithm in the 2012 COFN Clinical Report, which provides guidance for infants < 37 weeks with inadequate IAP, including obtaining laboratory tests, a blood culture, starting empirical antibiotics (while obtaining the culture and awaiting laboratory results) and continuing antibiotics if the laboratory results were abnormal. ([34]; [39])
The final ‘recommendation’ in the 2013 Brady and Polin Commentary points out that the CDC did not address duration of empirical antibiotic treatment. [(39)] The COFN made their initial recommendations based on laboratory testing results, and then reiterated the COFN written response to the community comments on their Clinical Report: “healthy-appearing infants without evidence of bacterial infection should receive broad-spectrum antimicrobial agents for no more than 48 hours. In small premature infants some may continue antibiotics for up to 72 hours while awaiting bacterial culture results.” ([39] There was no further discussion or definition of levels of laboratory to consider abnormal, or whether the abnormal laboratories should be considered evidence of infection that could drive clinicians to continue empirical treatment, and how many more days empirical antibiotics should be continued.
Impact on Practice and the Latest Word from COFN
Clinicians may feel the need to use the CBC and differential and other biomarker tests such as CRP because of the potential for false negative results from blood cultures, especially in cases where blood sample volume is low or when intrapartum antibiotics have been administered. ([34];[40]) When laboratory values, particularly the CBC, differential and CRP are measured in cases of asymptomatic infants born to mothers with risk factors, the prevalence of abnormal results can be quite high. Jackson reported on 2427 neutrophil counts and Immature to Total neutrophil (I:T) ratios obtained during the first 24 postnatal hours from 856 infants born to mothers diagnosed with chorioamnionitis. Ninety-seven percent of symptomatic infants had abnormal neutrophil counts, and 99% of the asymptomatic infants had an abnormal neutrophil count, immature neutrophil count, or I:T ratio. ([41])
More recently, Kiser et al reported their center's experience managing infants whose mothers had chorioamnionitis. Their local guideline resembled the algorithm for management of infants born to mothers with chorioamnionitis provided by COFN in 2012, with inclusion of continuing antibiotics to 7 days in asymptomatic infants who had abnormal CBC or CRP results. Of 554 infants studied 4 (0.7%) had positive cultures, 22 (4%) were treated for sepsis based on clinical signs without a positive culture. One hundred twelve (20.2%) asymptomatic infants were treated with prolonged antibiotics based on abnormal laboratory data. Most of the infants also had spinal taps, and none had a positive cerebrospinal fluid culture. ([42])
Following up on the pledge to provide revised algorithms, and concurrent in the issue of Pediatrics that included Kiser et al's report, Drs. Polin, Watterburg, Benitz and Eichenwald wrote a commentary on the “Conundrum of Early-Onset Sepsis”.([43]) The authors acknowledge that deciding how best to evaluate and treat an infant at risk for EOS is exemplary of every clinician's never-ending dilemma of dealing with the real world where decisions for individual patients are made absent informative results from high-quality randomized trials. To that point, they summarize Kiser's finding that based on an algorithm similar to COFN's 2012 algorithm, and consistent with Jackson's earlier report, many asymptomatic term infants born to women with chorioamnionitis would receive prolonged antibiotic courses, counter to COFN's revised statement that healthy-appearing infants with negative cultures should receive no more than 48 – 72 hours. ([39], [42], [43]) They further clarified that healthy appearing infants with negative cultures should have antibiotics stopped ‘even when the infant's laboratory results are abnormal.”([38];[43]) For Kiser's cohort, this could have decreased the percentage of infants treated for >48 hours from 24% to 4%. [43] Polin et al go on to make several conclusions from the accumulating evidence and experience, including that the physical exam is as good or better than most laboratory tests in “ruling in or ruling out sepsis,” and that “commonly used laboratory tests have a limited positive predictive accuracy and should never be used as a rationale to continue treatment in an otherwise healthy term infant at 48 to 72 hours of life. [43] Interestingly, they cite the 2012 Clinical report ([33]) for the “laboratory tests should never be used” conclusion, when that report included the algorithm that guided clinicians to continue treatment if the laboratory values collected between 6 and 12 postnatal hours were abnormal.
The authors of this most recent commentary then make three suggestions for management of newborns suspected of EOS: 1) antibiotics may be discontinued by 48 hours in well-appearing term newborns born to women with chorioamnionitis; 2) longer (to 72 hours) empirical treatment might be considered for premature infants, or infants with abnormal screening studies; and 3) lumbar punctures are recommended in cases where the blood culture is positive, the infant does not improve on appropriate antimicrobial coverage or the clinician views the infant as having a high probability of sepsis on the basis of clinical signs or abnormal laboratory data.([43]) So with this latest update, these authors allow (without recommending) the laboratory values to influence the decision to treat longer rather than 48 hours, and whether a spinal tap is done or not.
Where do we go next?
The COFN panel acknowledges that they are breaking new ground with their evolving recommendations on duration of empirical therapy, and the lack of strong data to support their decisions. ([43]) We await either cohort study data or randomized trials of 2012 COFN clinical report approaches, like Kiser's cohort study, versus 2012 COFN ‘In reply’ approaches which, for the most part, disregard the ancillary tests. Choosing an outcome of greatest importance that is feasibly measured (more likely hospital readmissions rather than wheezing at school age), but has a low incidence (4 of 404 readmitted for fever or suspected sepsis in Jackson's cohort study) will make such a study challenging. For a study to have 90% power to detect a difference between a 1% re-hospitalization rate for suspected infection in infants managed with a strategy of giving empirical antibiotics for longer than 2 -3 days versus 3% among infants managed with an approach that stopped empirical antibiotics after 48 hours regardless of laboratory values like the more recent modified recommendation ([38];[43]) with an alpha of 0.05, 1028 infants would be needed for each study arm. Not insurmountable, but this type of study may be hard to do given strength of entrenched local opinion on a specific clinical approach and the challenges of achieving equipoise among clinicians and families agreeing to enroll their newborn into the study and not entrusting their own doctor with the decision.
In addition to the duration of antibiotics questions, alternatives to the CDC and COFN algorithms to obtain diagnostic tests based on risk factors inclusive of chorioamnionitis and start treatment warrant some discussion. Chorioaminionitis can be a subjective definition, and obstetricians wishing to maximize likelihood that mothers and infants stay together and get discharged to home in the first postnatal day may avoid classification of a mother as having chorioamnionitis. [9, 12]Escobar and Puopolo acknowledge this clinical reality, and propose that we adopt a multi-factor assessment approach that includes the objective measure of maximum maternal temperature in labor in calculating odds ratios for sepsis in infants. Clinicians could use the calculated odds ratio based on the web-available tool that is the product of their cohort study to develop an individualized approach for each infant, incorporating known, objective maternal risk factors. This clinical tool is available as an online tool: http://www.dor.kaiser.org/external/DORExternal/research/InfectionProbabilityCalculator.aspx ([6]; [9]; [12])
Finally, all the authors of the commentaries, guidelines, and clinical reports agree that infants with signs of infection deserve treatment, even when intrapartum antibiotics were used. Escobar reported much higher odds of EOS among symptomatic vs. asymptomatic infants evaluated for EOS, but the asymptomatic infants with risk factors still had higher odds of EOS than the overall population rate. ([12]) That said, most clinicians would agree that for term and later preterm infants, there is some tolerable duration of signs to resolve that would allow for not treating, especially in the absence of risk factors. In the last report by Polin et al, they say, “Symptomatic neonates without risk factors for infection (who improve over the first 6 hours of life) may not require treatment, but must be monitored closely.”[43] Severity of the clinical signs must also be considered, and shorter duration tolerated before empirical antibiotics started when variation from expected norms are extreme.
Conclusion
Since their inception in 1996, the Guidelines aimed at preventing perinatally acquired GBS, and indirectly EOS, have led to significant reduction in EOS and EOS related mortality. In their 2010 iteration, the CDC Guidelines have narrowed the categories of infants that receive empirical antibiotics from prior versions, and continue to recommend laboratory tests in evaluation of infants at risk for EOS. COFN has taken steps into the less-charted waters of duration of empirical antimicrobial therapy for suspected EOS, but more recently have reconsidered reliance of abnormal laboratory test results to drive duration of empirical antibiotics beyond 48 – 72 postnatal hours. Undoubtedly, the CDC and COFN guidelines will continue to adapt to emerging evidence on the contributions of novel biomarkers as we learn more about the intricacies of the newborns response to infection ([44];[45];[46]). While we await the future guidelines and better predictive value from novel biomarker tests and combinations of risk factors and biomarker levels, following COFNs’ most recent compilation of recommendations, basing initiation of treatment on presence and persistence of signs of infection, and basing duration of treatment primarily on presence of signs in the absence of positive cultures seems reasonable. The ancillary tests may inform clinicians and provide rationales for closer observation, and even longer empirical treatment when clinical signs are equivocal or complete resolution is delayed.
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manual of Operations Vermont Oxford Network Database. Part 2 Data Definitions and Data Forms for Infants Born in 2013. [accessed October 5, 2014];2013 cited; Available from: https://public.vtoxford.org//wp-content/uploads/2014/03/Manual-of-Operations-Part-2-17_1.pdf.
- 2.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E.coli disease continues. Pediatrics. 2011;127:817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevention of perinatal group B streptococcal disease: a public health perspective. CDC Morbidity and Mortality Weekly Report. 1996;45 [PubMed] [Google Scholar]
- 4.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 5.Schuchat A, Zywicki SS, Dinsmoor MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics. 2000;105:21–6. doi: 10.1542/peds.105.1.21. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyay S, Puopolo KM. Risk assessment in neonatal early onset sepsis. Semin Perinatol. 2012;36:408–15. doi: 10.1053/j.semperi.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauserman MS, Laughon MM, Hornik CP, et al. Group B Streptococcus and Escherichia coli infections in the intensive care nursery in the era of intrapartum antibiotic prophylaxis. Pediatr Infect Dis J. 2013;32:208–12. doi: 10.1097/INF.0b013e318275058a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatr Infect Dis J. 2011;30:937–41. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puopolo KM. Response to the American Academy of Pediatrics, Committee on the Fetus and Newborn statement, “management of neonates with suspected or proven early-onset bacterial sepsis”. Pediatrics. 2012;130:e1054–5. doi: 10.1542/peds.2012-2302C. author reply e5-7. [DOI] [PubMed] [Google Scholar]
- 10.Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009;360:2626–36. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 11.Puopolo KM, Draper D, Wi S, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011;128:e1155–63. doi: 10.1542/peds.2010-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escobar GJ, Li DK, Armstrong MA, et al. Neonatal sepsis workups in infants >/=2000 grams at birth: A population-based study. Pediatrics. 2000;106:256–63. doi: 10.1542/peds.106.2.256. [DOI] [PubMed] [Google Scholar]
- 13.Mukhopadhyay S, Eichenwald EC, Puopolo KM. Neonatal early-onset sepsis evaluations among well-appearing infants: projected impact of changes in CDC GBS guidelines. J Perinatol. 2013;33:198–205. doi: 10.1038/jp.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh EM, Hornik C, Clark RH, Laughon MM Best Pharmaceuticals for Children Act—Pediatric Trials Network. Medication use in the neonatal intensive care unit. American Journal of Perinatology. 2014;31:811–22. doi: 10.1055/s-0033-1361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCracken GH., Jr Aminoglycoside toxicity in infants and children. Am J Med. 1986;80:172–8. doi: 10.1016/0002-9343(86)90497-3. [DOI] [PubMed] [Google Scholar]
- 16.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–7. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–5. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alm B, Erdes L, Mollborg P, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics. 2008;121:697–702. doi: 10.1542/peds.2007-1232. [DOI] [PubMed] [Google Scholar]
- 20.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 21.Penders J, Thijs C, van den Brandt PA, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–7. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011;127:1087–94. doi: 10.1016/j.jaci.2011.02.015. quiz 95-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell SL, Gold MJ, Willing BP, et al. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes. 2013;4:158–64. doi: 10.4161/gmic.23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heintze K, Petersen KU. The case of drug causation of childhood asthma: antibiotics and paracetamol. Eur J Clin Pharmacol. 2013;69:1197–209. doi: 10.1007/s00228-012-1463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penders J, Kummeling I, Thijs C. Infant antibiotic use and wheeze and asthma risk: a systematic review and meta-analysis. Eur Respir J. 2011;38:295–302. doi: 10.1183/09031936.00105010. [DOI] [PubMed] [Google Scholar]
- 26.Antibiotic resistance threats in the United States. CDC Morbidity and Mortality Weekly Report. 2013 [cited; http://www.cdc.gov/drugresistance/threat-report-2013/index.html]. Available from: October 5, 2014.
- 27.Prevention of perinatal group B streptococcal disease: revised guidelines from CDC. CDC Morbidity and Mortality Weekly Report. 2002;51 [PubMed] [Google Scholar]
- 28.Edwards RK, Clark P, Sistrom CL, Duff P. Intrapartum antibiotic prophylaxis 1: relative effects of recommended antibiotics on gram-negative pathogens. Obstet Gynecol. 2002;100:534–9. doi: 10.1016/s0029-7844(02)02096-3. [DOI] [PubMed] [Google Scholar]
- 29.Alexander JM, McIntire DM, Leveno KJ. Chorioamnionitis and the prognosis for term infants. Obstet Gynecol. 1999;94:274–8. doi: 10.1016/s0029-7844(99)00256-2. [DOI] [PubMed] [Google Scholar]
- 30.Christensen RD, Rothstein G, Hill HR, Hall RT. Fatal early onset group B streptococcal sepsis with normal leukocyte counts. Pediatr Infect Dis. 1985;4:242–5. doi: 10.1097/00006454-198505000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Newman TB, Puopolo KM, Wi S, et al. Interpreting complete blood counts soon after birth in newborns at risk for sepsis. Pediatrics. 2010;126:903–9. doi: 10.1542/peds.2010-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker CJ, Byington CL, Polin RA. Policy statement-Recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics. 2011;128:611–6. doi: 10.1542/peds.2011-1466. [DOI] [PubMed] [Google Scholar]
- 33.Polin RA. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129:1006–15. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 34.Ottolini MC, Lundgren K, Mirkinson LJ, et al. Utility of complete blood count and blood culture screening to diagnose neonatal sepsis in the asymptomatic at risk newborn. Pediatr Infect Dis J. 2003;22:430–4. doi: 10.1097/01.inf.0000068206.11303.dd. [DOI] [PubMed] [Google Scholar]
- 35.Cotten CM, Benjamin DK, Jr, Smith PB, et al. Empirical antibiotic therapy for suspected early-onset bacterial sepsis. Pediatrics. 2012;130:e1052–3. doi: 10.1542/peds.2012-2302A. [DOI] [PubMed] [Google Scholar]
- 36.Sukumar M. Need clarification on “abnormal labs”. Pediatrics. 2012;130:e1055. doi: 10.1542/peds.2012-2302D. [DOI] [PubMed] [Google Scholar]
- 37.Sise ME, Parravicini E, Barasch J. Urinary neutrophil gelatinase associated lipocalin identifies neonates with high probability of sepsis. Pediatrics. 2012;130:e1053–4. doi: 10.1542/peds.2012-2302B. [DOI] [PubMed] [Google Scholar]
- 38.Polin RA on behalf of the Committee on Fetus and Newborn. In reply. Pediatrics. 2012;130:e1055–e1057. [Google Scholar]
- 39.Brady MT, Polin RA. Prevention and management of infants with suspected or proven neonatal sepsis. Pediatrics. 2013;132:166–8. doi: 10.1542/peds.2013-1310. [DOI] [PubMed] [Google Scholar]
- 40.Schelonka RL, Chai MK, Yoder BA, et al. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129:275–8. doi: 10.1016/s0022-3476(96)70254-8. [DOI] [PubMed] [Google Scholar]
- 41.Jackson GL, Engle WD, Sendelbach DM, et al. Are complete blood cell counts useful in the evaluation of asymptomatic neonates exposed to suspected chorioamnionitis? Pediatrics. 2004;113:1173–80. doi: 10.1542/peds.113.5.1173. [DOI] [PubMed] [Google Scholar]
- 42.Kiser C, Nawab U, McKenna K, Aghai ZH. Role of guidelines on length of therapy in chorioamnionitis and neonatal sepsis. Pediatrics. 2014;133:992–8. doi: 10.1542/peds.2013-2927. [DOI] [PubMed] [Google Scholar]
- 43.Polin RA, Watterberg K, Benitz W, Eichenwald E. The conundrum of early-onset sepsis. Pediatrics. 2014;133:1122–3. doi: 10.1542/peds.2014-0360. [DOI] [PubMed] [Google Scholar]
- 44.Srinivasan L, Harris MC. New technologies for the rapid diagnosis of neonatal sepsis. Curr Opin Pediatr. 2012;24:165–71. doi: 10.1097/MOP.0b013e3283504df3. [DOI] [PubMed] [Google Scholar]
- 45.Wynn JL, Cvijanovich NZ, Allen GL, et al. The influence of developmental age on the early transcriptomic response of children with septic shock. Mol Med. 2011;17:1146–56. doi: 10.2119/molmed.2011.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah BA, Padbury JF. Neonatal sepsis: an old problem with new insights. Virulence. 2014;5:170–8. doi: 10.4161/viru.26906. [DOI] [PMC free article] [PubMed] [Google Scholar]


