Abstract
Despite the defined function of the β-catenin pathway in thymocytes, its functional role in peripheral T cells is poorly understood. We report that in a mouse model, β-catenin protein is constitutively degraded in peripheral T cells. Introduction of stabilized β-catenin into primary T cells inhibited proliferation and cytokine secretion after TCR stimulation and blunted effector cell differentiation. Functional and biochemical studies revealed that β-catenin selectively inhibited linker for activation of T cells phosphorylation on tyrosine 136, which was associated with defective phospholipase C-γ1 phosphorylation and calcium signaling but normal ERK activation. Our findings indicate that β-catenin negatively regulates T cell activation by a previously undescribed mechanism and suggest that conditions under which β-catenin might be inducibly stabilized in vivo would be inhibitory for T cell-based immunity.
The β-catenin pathway has been implicated in hematopoietic stem cell self-renewal capacities and other developmental pathways in vivo (1). Cytoplasmic β-catenin is included in a destruction complex formed by the adenomatous polyposis coli, the axis inhibition protein 1, the casein kinase 1, and glycogen synthase kinase 3β (GSK-3β) (2). When included in this complex, β-catenin is constitutively phosphorylated on serine 33 and 37 and on threonine 41 by the GSK-3β and targeted for degradation by the proteasome (3). Activation of the Wnt signaling cascade after the binding of Wnt ligands on Frizzled receptors at the membrane results in the phosphorylation of GSK-3β and the inhibition of its kinase activity. As a consequence, β-catenin protein can accumulate in the cytoplasm and translocate to the nucleus where it binds Lef/Tcf family proteins and facilitates their transcriptional activation (4, 5). In addition to this role in gene transcription, β-catenin also interacts with E-cadherin and has been reported to regulate cell-surface proximal signals and adhesion (6). Therefore, β-catenin can exert both transcriptional and non-transcription-based cellular regulation.
An important role for the β-catenin pathway in thymic development has been suggested by the study of Wnt and Tcf1 or Lef1 gene-deficient mice. Specifically, double mutant mice exhibit profound defects in T cell maturation in the thymus (7–9). Similarly, thymi of Wnt1 × Wnt4 double mutant mice and Wnt3A−/− mice showed low cellularity and a strong reduction in cell numbers (10, 11). However, the exact role played by β-catenin in this process has been more controversial (12). The expression of a nondegradable form of β-catenin resulted in the transition fromCD4−CD8− (double-negative) to CD4+CD8+ (double-positive) thymocytes in the absence of pre-TCR signaling (13) and enhanced generation of mature thymocytes (14). In addition, T lineage-specific deletion of β-catenin was reported to impair T cell development resulting in a reduced number of splenic T cells (15). Also, β- and γ-catenin were directly implicated in T cell development because the inhibition of interaction between these proteins with Lcf/lef transcription factors resulted in a major block in the transition from the CD4−CD8− to the CD4+ CD8+ stage (16). However, deletion of β-catenin in bone marrow (BM) progenitors did not induce any detectable perturbation of the hematopoietic system, including the lymphoid lineage. In particular, T cell development and repopulation was totally normal in lethally irradiated mice that received BM progenitors deleted for β-catenin (17). Moreover, simultaneous deletion of β- and γ-catenin in BM progenitors resulted in the same observations, excluding the possibility of a compensatory function by γ-catenin (18, 19).
In contrast with at least some data regarding the contribution of β-catenin to thymocyte development, the role of β-catenin in peripheral T cell function is largely unknown. It has been reported that the activation of the β-catenin pathway through exposure to Wnt3a regulates T cell transmigration in human peripheral T cells (20). However, retroviral transduction of regulatory T cells (Tregs) to express a nondegradable form of β-catenin resulted in enhanced survival of those cells by increasing the expression of Bcl-XL (21), suggesting a potential role for β-catenin in Treg function and therefore implying a potential immune inhibitory role for peripheral immune responses. In addition, it has been shown that the TCF-1 transcription factor can induce GATA-3 expression required for the differentiation of CD4+ T cells into the Th2 fate (22). Finally, recent data suggested that activation of β-catenin pathway through pharmacologic inhibition of GSK-3β in CD8+ T cells resulted in generation of a T cell memory stem cells phenotype harboring increased antitumor activity (23). However, we have recently analyzed this phenomenon through direct manipulation of β-catenin expression in primary T cells and found no effect on T cell memory phenotype, suggesting that GSK-3β may regulate T cell differentiation independently of β-catenin (24, 25). Despite these few studies, the overall function of β-catenin in regulation of peripheral T cell activation remains poorly understood, motivating direct investigation of the functional consequences of β-catenin stabilization in peripheral T cells.
Signaling via the TCR for Ag has been intensively studied, and many key biochemical events are well defined (26). Central to TCR-mediated T cell activation is the adapter protein linker for activation of T cells (LAT), which is phosphorylated by the tyrosine kinase Zap70. This generates docking sites for multiple downstream signaling molecules, including Gads, Grb2/SOS, Vav, SLP76, Cbl-b, and phospholipase C-γ1 (PLC-γ1) (27). Four tyrosine residues have been characterized as being phosphorylated after TCR engagement and responsible for LAT binding activity. In particular, the Y132/Y136 (human/mouse) residue constitutes the unique binding site for PLC-γ1 (28). When bound to LAT and tyrosine-phosphorylated, PLC-γ1 is activated and mediates the hydrolysis of PIP2 to generate diacylglycerol (DAG) and inositol trisphosphate, which activate the protein kinase C and calcium (Ca2+) pathways, respectively.
The relative paucity of information regarding the functional role of β-catenin in peripheral T cells prompted us to study this pathway using careful genetic manipulation directly in the peripheral T cell compartment. To analyze functional effects in primary T cells rather than T cell lines, mice transgenic for the coxsackie and adenovirus receptor (CAR) in the T cell compartment were used (29), which enable high-efficiency transduction using adenoviral vectors without cellular activation. We found that β-catenin was constitutively degraded in primary T cells. Introduction of a stabilized β-catenin inhibited T cell activation through a mechanism reflected by defective LAT phosphorylation at tyrosine 136, which was associated with defective PLC-γ1 phosphorylation and calcium mobilization. This was associated with functional inhibition of IL-2 production and effector cell differentiation. Our results suggest that β-catenin may be a negative regulator of peripheral T cell activation.
Materials and Methods
Mice
Mice expressing the extracellular domain of CAR have been described (29). C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen-free conditions in a specific pathogen-free animal facility at The University of Chicago (Chicago, IL), and experimental procedures involving mice were approved by the institutional animal care and use committee.
Reagents and tissue culture
Lactacystin and the inhibitor of GSK-3, SB216763, were from Sigma (St. Louis, MO). To inhibit GSK-3 kinase activity, T cells were treated 4 h with the drug before being used for further experiments. T cells were cultured in complete DMEM medium supplemented with 10% FCS, 1% penicillin, 1% streptomycin, MOPS, 2-mercaptoethanol, and 1% nonessential amino acids in an 8% CO2 incubator at 37°C.
Adenoviral vectors
The complementary DNA (cDNA) encoding mutated β-catenin was a gift from B. Vogelstein and K. Kinzler (The Johns Hopkins University, Baltimore, MD). Construction of a recombinant adenoviral vector containing the gene expression unit ofβ-catenin under the ubiquitin promoter was performed with a two-cosmid system that has been described (30). Adenoviral vectors coding for the Cre recombinase (Cre) and an adenoviral vector without a coding cDNA (empty adenoviral vector [EV]) were previously described (31).
Adenoviral transduction of CAR transgenic T cells
Primary total T cells and CD4+ or CD8+ T cells were isolated from splenocytes by negative selection using Ab cocktails (Miltenyi Biotec, Auburn, CA). T cells from CAR transgenic (Tg) mice were transduced with adenoviral vectors as described previously (31). Transduced CAR Tg T cells were rested overnight and used for experiments the next day. For the CAR Tg/conditional β-catenin knockout experiments, cells were transduced with adeno-Cre, rested overnight, and cultured 4 d at 106 cells/ml in complete medium supplemented with 3 ng/ml IL-7 (R&D Systems) to allow time for gene deletion before being used for further experiments, as described previously (31). Some cell toxicity has been observed during transduction with high doses of adenoviral vectors (multiplicity of infection >100), which is first normalized by using cells transduced with an empty vector that has the same toxicity as the β-catenin or the Cre one. Second, before using the cells for the different assays, they are renumbered after the overnight resting period that follows the cell transduction, a time that does not show any consequent toxicity.
T cell activation
Primary T cells were activated with beads (Dynal, Carlsbad, CA) coated with anti-CD3 (145-2C11) at 1 µg/ml and anti-CD28 (PV1) 1 µg/ml or PMA (10 ng/ml) and ionomycin (2 µM) and analyzed as described previously for cytokine production and proliferation (32). For biochemical analysis, T cells were stimulated as indicated, and the reaction was stopped by addition of ice-cold Dulbecco’s PBS.
Immunoblot analyses
Cells were lysed in 0.5% (v/v) Triton X-100 lysis buffer, and immunoblot analysis was performed as described (33). Abs to the following targets were used: β-catenin (9562 or 9582; Cell Signaling, Danvers, MA), total ERK (13-6200; Invitrogen, Carlsbad, CA), phosphorylated ERK (9101; Cell Signaling), Cre (69050; Novagen, San Diego, CA), phosphotyrosine (4G10, 05-1050; Millipore, Billerica, MA), phosphorylated and total Zap70 (2717 and 2705; Cell Signaling), phosphorylated and total PLC-γ1 (2821 and 2822; Cell Signaling), Cbl-b (sc-8006; Santa Cruz Biotechnology, Santa Cruz, CA), phosphorylated and total Akt (9271 and 9272; Cell Signaling), phosphorylated LAT (Tyr132/136; Ab4476-50; Abcam (Cambridge, MA); Tyr171; Ab73205-100; Abcam; Tyr191; 04-467; Millipore; Tyr226; Ab14520-50; Abcam), and total LAT (06-807; Millipore). Anti-rabbit and anti-mouse HRP-conjugated Abs were from GE Healthcare (Piscataway, NJ). Images were assembled using Adobe Photoshop software.
Quantitative RT-PCR
Total RNA was isolated by using RNeasy Mini column purification (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The RNA was DNase I-treated, and cDNA was synthesized from 1 µg total RNA using MLV-Reverse Transcriptase (Invitrogen) according to the manufacturer’s directions. Each gene was evaluated using a specific primer/probe set purchased from Applied Biosystems and labeled with FAM dye. ΔCT of a particular gene was normalized against the ΔCT of 18S. Data were analyzed using SDS Software (Applied Biosystems, Carlsbad, CA).
Flow cytometry
Abs against the following molecules coupled to the indicated fluorochromes were purchased from BD Pharmingen (BD, San Diego, CA) or eBioscience (eBio, San Diego, CA): FITC anti-CD8 (53-6.7; BD), PerCP anti-CD4 (RM4-5; BD), PE anti-CD3 (2C11; BD), Cy-Chrome anti-TCRβ (H57-597; BD), PE anti-CD25 (PL61, BD), PE anti-CD28 (37.51, eBio), and PE anti–IL-17 (eBio17B7; eBio). In general, 106 cells pretreated or not with GolgiPlug (BD) were blocked with the anti-FcR mAb 2.4G2, stained with the indicated Abs or appropriate isotype controls for 15 min at 4°C, and then washed. For intracellular staining, cells were fixed, permeabilized (PermFix; BD), and stained in saponin-containing buffer following the manufacturer’s instructions. Cells were subsequently washed twice in PBS containing 1% FCS and resuspended for FACS analysis. Flow cytometry was performed on the FACSCanto cytometer using BD FACSDiva software. Data analysis was performed using FlowJo software.
Calcium measurement
Intracellular calcium concentration was determined using two visible-wavelength calcium-sensitive dyes, Fluo-3-acetoxymethyl ester and fura red acetoxymethyl ester, as previously described (34). Fluo-3 fluorescence increases whereas fura red fluorescence decreases upon binding to calcium. The fluo-3/fura red ratio is a good indication of intracellular calcium levels. Briefly, T cells were labeled with fluo-3 and fura red (Invitrogen). The cells were then incubated with biotinylated anti-CD3 (2C11; 5 µg/ml) and washed. Cells were kept at 37°C until analysis. The ratio of fluo-3/fura red was measured over time before and after stimulation of the cells with streptavidin.
T cell differentiation into effector subsets
CD4+ cells were incubated for 3 d in plates coated with anti-CD3 (2C11; 1 µg/ml for Th0, Th1, and Th2 or 5 µg/ml for Th17) and anti-CD28 (PV1; 1 µg/ml) in either neutral (Th0) conditions (IL-2, 10 U/ml), Th1-polarizing conditions (IL-2, 10 U/ml; IL-12, 5 ng/ml; anti–IL-4, 10 µg/ml), Th2-polarizing conditions (IL-2, 10 U/ml; IL-4, 10 ng/ml; anti–IFN-γ, 10 µg/ml), or Th17-polarizing conditions (IL-2, 10 U/ml; TGF-β, 0.5 ng/ml; IL-6, 20 ng/ml). Polarized cells were then activated with PMA and ionomycin for 4 h in presence of GolgiPlug (BD) (Th17) or rested overnight before being stimulated with anti-CD3 and anti-CD28 (Th1 and Th2 conditions).
Immunofluorescence microscopy
Cells were incubated for 5 min at 37°C on poly-l-lysine–coated coverslips and then fixed for 20 min in 3% paraformaldehyde in PBS as described (35). The samples were quenched with 50 mM NH4Cl/PBS, permeabilized and blocked with a solution of PSG (PBS, 0.01% saponin, 0.25% fish skin gelatin, and 0.1% NaN3; all from Sigma) for 30 min and then incubated for 1 h with anti–β-catenin Ab (2677; Cell Signaling), washed five times in PSG, and then incubated for 1 h with anti-mouse Texas red Ab (Invitrogen). The coverslips were then washed again in PSG, rinsed in ddH2O, and mounted with Vectashield (Vector Laboratories Burlingame, CA). Cells were observed using the Leica SP2 Laser Scanning Confocal microscope (Bannockburn, IL) and images processed using Adobe Photoshop.
Statistics
Statistical analysis of mean results was done using unpaired t test with Welch correction if needed. Error bars reflect the SD. Statistical significance was set at p < 0.05 or p < 0.01.
Results
β-catenin is constitutively degraded in peripheral T cells
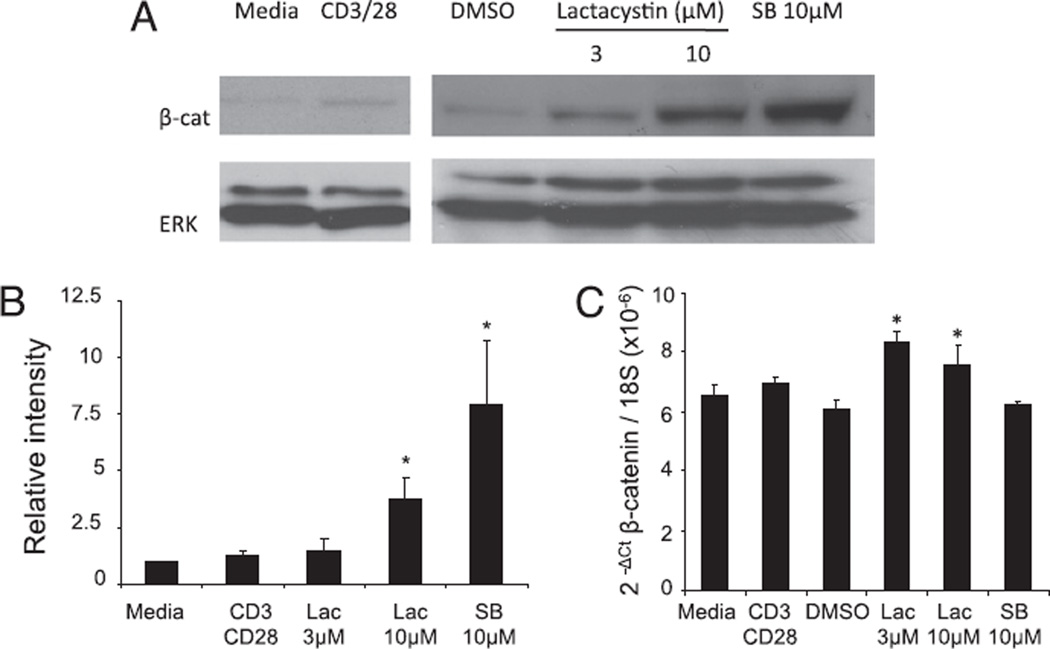
As a first step to explore the role of β-catenin in peripheral T cells, we analyzed the expression of the endogenous β-catenin protein in T cell subsets freshly isolated from mouse spleen and lymph nodes. Western blot analysis revealed that β-catenin was essentially undetectable in untreated T cells, and stimulation via the TCR complex resulted in only minimally detected expression (Fig. 1A). Absence of β-catenin protein was not due to lack of β-catenin mRNA, which was easily detected by quantitative RT-PCR (Fig. 1C). Phosphorylation of the β-catenin protein by the kinase GSK-3β is known to induce its ubiquitination and degradation by the proteasome. To test if the absence of β-catenin protein was due to constitutive degradation, we treated the cells with a proteasome inhibitor (lactacystin) or with a specific inhibitor of the GSK-3β kinase activity (SB216763). Both treatments induced a strong increase (4- and 8-fold, respectively) in detectable β-catenin protein expression compared with basal level as measured by Western blotting (Fig. 1A, 1B), whereas mRNA expression tested by real-time RT-PCR was constant after SB216763 treatment or only minimally increased after lactacystin treatment (Fig. 1C). TCR ligation also resulted in substantial β-catenin accumulation after treatment with lactacystin, confirming that degradation is still dominant (data not shown). These results indicate that the β-catenin gene is transcribed in peripheral T cells but that the protein is constitutively degraded.
FIGURE 1.
β-Catenin is constitutively degraded in T cells. Purified splenic T cells were pretreated with lactacystin (Lac) or SB216763 (SB) or activated with anti-CD3 and CD28 for 6 h and lysed. A, β-Catenin expression was assessed by SDS-PAGE and immunoblotting with Abs to the indicated proteins. A representative of three individual experiments is shown. B, The expression of β-catenin protein was quantified by densitometry and expressed as a ratio to the basal expression level. C, β-Catenin mRNA expression was measured by real-time RT-PCR and normalized to 18S expression. The average of two separate experiments is shown. *p < 0.05.
Introduction of nondegradable β-catenin inhibits T cell activation
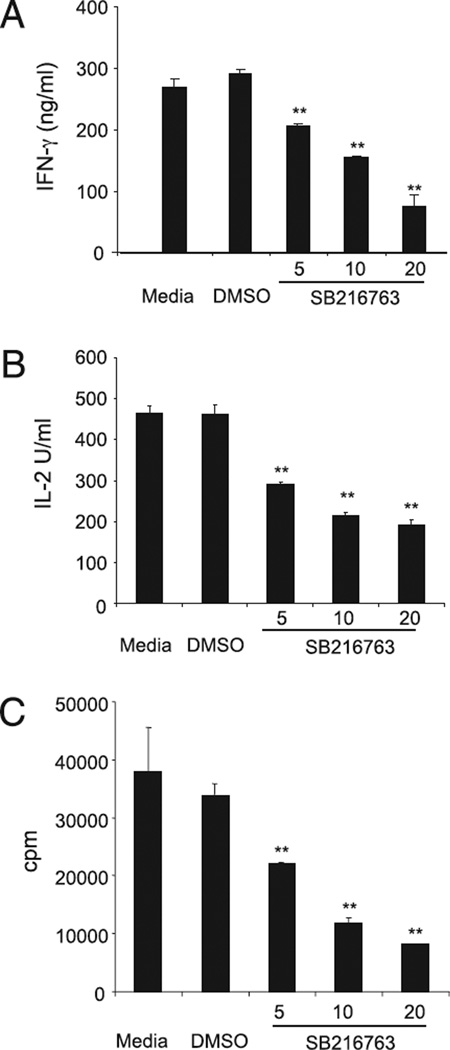
To begin to investigate the functional consequences of β-catenin stabilization in peripheral T cells, we pretreated primary splenic T cells with the GSK-3β inhibitor SB216763 and assessed cytokine production and proliferation in response to anti-CD3/anti-CD28 stimulation. Both cytokine secretion (Fig. 2A, 2B) and proliferation (Fig. 2C) were highly reduced, suggesting that stabilization of the β-catenin pathway could have an inhibitory effect on T cell function. Minimal effects resulting in a small reduction of IL-2 secretion and a slight increase of IFN-γ was seen when PMA plus ionomycin was used as a stimulus, arguing for a dominant inhibitory effect on proximal TCR signaling (Supplemental Fig. 1). No significant effect of the drug treatment (up to 20 µM) was observed on T cell survival (data not shown).
FIGURE 2.
GSK-3β inhibition interferes with T cell activation. Purified peripheral T cells were stimulated with anti-CD3 and anti-CD28 mAbs in the presence of increasing concentrations of the GSK-3β inhibitor. A and B, Secretion of IFN-γ (A) and IL-2 (B) was measured by ELISA 16 h poststimulation. C, [3H]Thymidine incorporation was measured 48 h poststimulation. A representative of two independent experiments is shown. **p< 0.01.
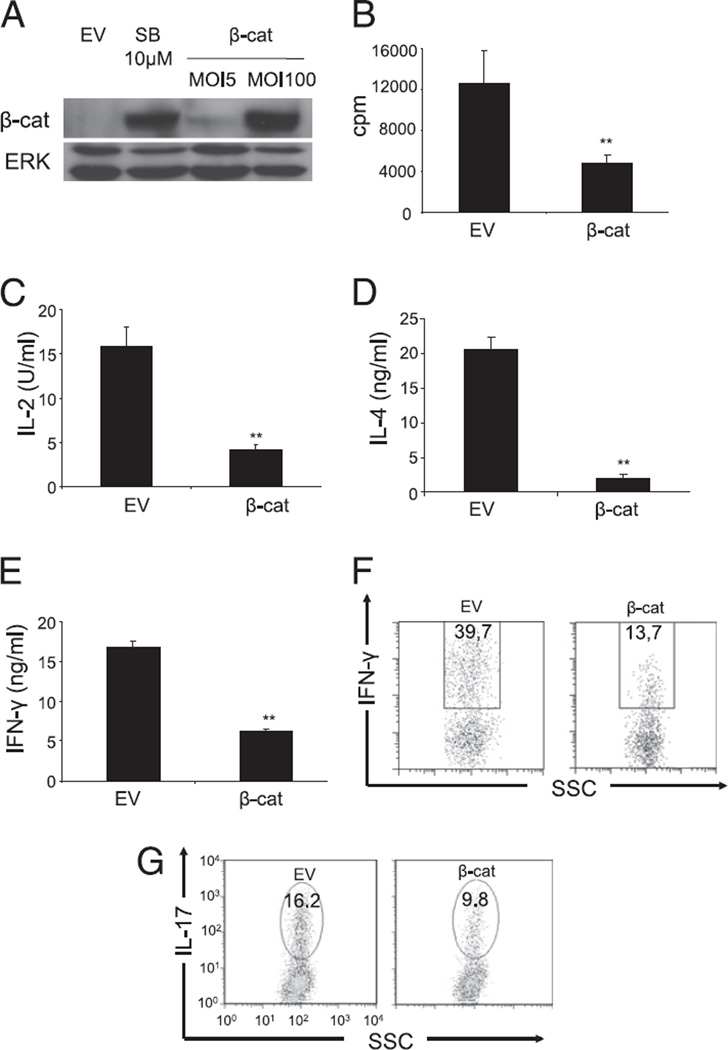
GSK-3β regulates multiple downstream signaling molecules in addition to β-catenin, and pharmacologic inhibitors can have unexpected off-target effects. Therefore, we decided to engineer an adenoviral vector coding for a nondegradable β-catenin protein containing an amino acid substitution in position 33 (S33Y) that prevents its phosphorylation and consequent degradation. Transduction of CAR Tg T cells with adenoviral vectors has been used successfully in our laboratory (30) to manipulate genetically resting T cells without a need for cellular activation. To express protein expression level of β-catenin comparable with the endogenous levels observed in T cells after the inhibition of GSK-3β, several concentrations of vector were explored (Fig. 3A). Proliferation and IL-2 production were then assessed in response to anti-CD3/CD28 stimulation. T cells expressing stabilized β-catenin showed reduced proliferation and IL-2 production compared with that of T cells transduced with an EV (Fig. 3B, 3C). Similar results were observed with either naive or in vitro-primed effector T cells and in both the CD4+ and CD8+ T cell subpopulations (data not shown).
FIGURE 3.
Expression of a nondegradable β-catenin protein inhibits effector functions of T cells. Purified peripheral T cells from CAR Tg mice were transduced with an EV or an adenoviral vector coding for a nondegradable form of the β-catenin (β-cat). A, Transduced cells were lysed, and β-catenin expression was assessed by SDS-PAGE and immunoblotting with Abs to the indicated proteins. A representative of six independent experiments is shown. B and C, Transduced cells were left untreated or stimulated with anti-CD3 and CD28, and [3H]thymidine incorporation was measured 48 h poststimulation (B), and IL-2 secretion was measured by ELISA 16 h poststimulation (C). An average of six independent experiments is shown. D–F, CD4+ T cells were cultured under Th1 and Th2 conditions for 3 d and were transduced either with EV or β-cat. IL-4 produced by Th2 differentiated T cells (D) was measured by ELISA and IFN-γ produced by Th1 cells was measured by ELISA (E) or flow cytometry (F) 16 h after anti-CD3 and CD28 stimulation. G, CD4+ T cells were transduced with EV or β-cat and differentiated under Th17 conditions. IL-17 production was measured by flow cytometry 4 h after stimulation with PMA and ionomycin in presence of an inhibitor of the Golgi transporter. A representative of two independent experiments is shown. **p < 0.01.
The effect of introducing nondegradable β-catenin in CD4+ T cells differentiated under Th1 and Th2 conditions also was examined, and a significant reduction in both IL-4 (Fig. 3D) and IFN-γ (Fig. 3E, 3F) secretion was observed. Similarly, introduction of stabilized β-catenin during Th17 differentiation generated a marked reduction in IL-17 secretion compared with that of EV-treated T cells (Fig. 3G). Thus, these results suggest that the presence of stabilized β-catenin in primary T cells is generally inhibitory for multiple T cell functions.
Despite the minimal detection of stabilized β-catenin in primary T cells in vitro, it was of interest to determine whether deletion of β-catenin might alter T cell function. To this end, we used a model system generated by crossing β-catenin conditional knockout mice, containing Loxp sites in introns 1 and 6, with CAR Tg mice, a system that we have shown allows for deletion of targeted alleles using a Cre adenovirus in vitro (31). We first confirmed that β-catenin was deleted in the T cells treated with the adenovirus encoding Cre compared with T cells treated with empty virus, in the presence of the GSK-3β inhibitor (Supplemental Fig. 2A). β-Catenin can only be detected in the presence of a GSK-3 inhibitor because of minimal protein expression at baseline. Upon β-catenin deletion, a minimal increase in IL-2 production and proliferation in response to CD3/CD28 ligation was observed in vitro (Supplemental Fig. 2B, 2C). This is not unexpected, as only minimal β-catenin protein was detected under the in vitro conditions. For primary T cells, therefore, it is likely that alternative ligands induce meaningful β-catenin stabilization that were not included in this in vitro culture system.
Expression of stabilized β-catenin in T cells inhibits proximal TCR signaling
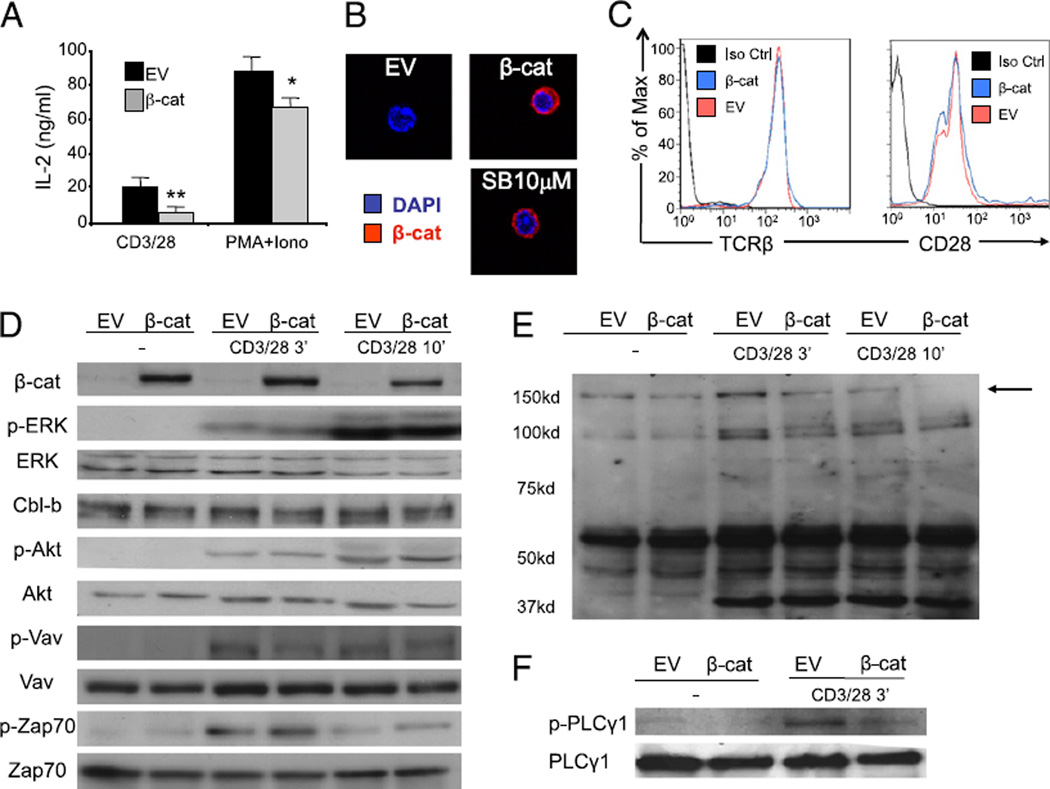
To begin to understand the mechanism of inhibition of T cell activation by nondegradable β-catenin protein, we examined IL-2 secretion by T cells stimulated by PMA and ionomycin to bypass proximal TCR signaling. In fact, IL-2 production in response to PMA/ionomycin was minimally affected by stabilized β-catenin, arguing that β-catenin is likely inhibiting early TCR signaling events (Fig. 4A). These results also suggest that β-catenin is not directly repressing IL-2 gene transcription. Because β-catenin theoretically could regulate cellular function either by translocating to the nucleus and influencing transcription or through direct protein–protein interactions, we examined the cellular localization of stabilized β-catenin protein in primary T cells by confocal microscopy. Notably, the β-catenin protein expressed after treatment with GSK-3β inhibitor or through adenoviral transduction was found to be predominately cytosolic (Fig. 4B). This result suggested that β-catenin may be functioning by directly interfering with TCR-based signaling. As a crude attempt to block transcriptional effects of β-catenin, we treated transduced cells with cycloheximide to prevent production of new proteins. However, this approach also blocked expression of the transduced β-catenin protein, making the results uninterpretable (data not shown). We also performed gene expression profiling of β-catenin–transduced T cells compared with control cells in search of candidate genes that might be induced by β-catenin and inhibitory for TCR signaling. However, no clear candidates were identified (data not shown).
FIGURE 4.
Expression of stabilized β-catenin in T cells inhibits proximal TCR signaling. Purified peripheral T cells from CAR Tg mice were transduced with an EV or an adenoviral vector coding for a nondegradable form of the β-catenin (β-cat). A, Transduced cells were left untreated or stimulated with either anti-CD3 and CD28 or PMA and ionomycin, and IL-2 production was measured by ELISA 16 h later. An average of four independent experiments is shown. B, EV or β-cat transduced T cells were analyzed by confocal immunofluorescence microscopy to determine the localization of β-catenin. The nuclear dye DAPI is shown in blue and β-catenin in red. A representative image of highly positive cells is shown (original magnification ×400). C, Transduced cells were analyzed by flow cytometry for the expression of CD28 and TCRβ. A representative of two independent experiments is shown. D–F, Cells were activated with anti-CD3 and anti-CD28 for indicated time points, and cell lysates were blotted with selected phospho-specific and total Abs as indicated (D, F) or for total phospho-tyrosine expression (E). A representative of three individual experiments is shown. *p < 0.05; **p < 0.01.
We therefore focused on potential defects in proximal TCR signaling in the presence of stabilized β-catenin. Flow cytometry revealed that surface expression of TCRβ and CD28 were not reduced in T cells expressing nondegradable β-catenin (Fig. 4C). Examination of the phosphorylation status of specific target proteins activated in the TCR signaling cascade revealed no difference in the induction of p-Zap70, p-Akt, p-Vav, or p-Erk after CD3/CD28 ligation (Fig. 4D). Expression of the negative regulator Cbl-b also was not increased. To broaden the set of candidate signaling proteins affected, global phosphotyrosine Western blotting was performed. This analysis revealed that a single major tyrosine phosphorylated protein of ~150 kDa was blunted in the presence of nondegradable β-catenin (Fig. 4E). As this band probably corresponds with PLC-γ1, a phospho-specific Ab for PLC-γ1 was used. These results confirmed the inhibition of PLC-γ1 phosphorylation in T cells expressing nondegradable β-catenin (Fig. 4F) (5-fold increase of phospho-PLC-γ1 in control cells versus 1.5-fold in β-catenin–expressing cells; p < 0.05). These results suggest that the presence of stabilized β-catenin does not inhibit all early phosphorylation events downstream from TCR signaling but rather is relatively selective for blunting PLC-γ1 phosphorylation.
β-Catenin stabilization in T cells prevents the activation of the LAT/PLC-γ1/Ca2+ cascade after TCR signaling
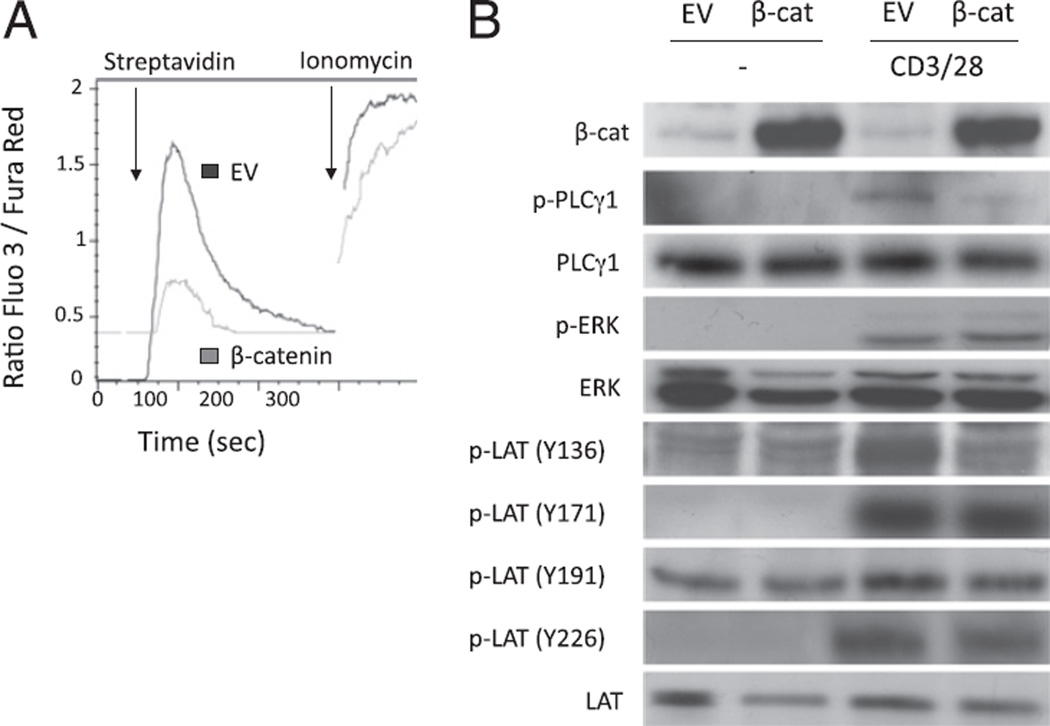
Diminished PLC-γ1 phosphorylation would predict decreased enzymatic activity and blunted rise of intracellular free calcium. We therefore examined anti-CD3–induced Ca2+ flux in EV or β-catenin–transduced T cells. As expected, Ca2+ release induced after TCR stimulation was substantially blunted in T cells expressing stabilized β-catenin, whereas calcium elevation was clearly observed after ionomycin treatment (Fig. 5A).
FIGURE 5.
Expression of stabilized β-catenin in T cells prevents activation of the LAT/PLC-γ1/Ca2+ pathway after TCR ligation. Purified peripheral T cells from CAR Tg mice were transduced with an EV or an adenoviral vector coding for a nondegradable form of the β-catenin (β-cat). A, To measure the Ca2+ release after TCR stimulation, transduced cells were stained with fluo-3 and fura red dye and incubated with a biotinylated anti-CD3 Ab. Cells were kept at 37°C and analyzed by flow cytometry for the fluo-3 and fura red expression over time before and after activation of the cells with streptavidin. Ionomycin was used as a positive control. A representative of three individual experiments is shown. B, Cells were activated with anti-CD3 and anti-CD28 for 5 min, and cell lysates were blotted with specific Abs as indicated. A representative of three individual experiments is shown.
It was curious that PLC-γ1 phosphorylation was diminished whereas ZAP70 phosphorylation, as well as phosphorylation of other ZAP70 targets, such as Vav, were intact. In addition, it was apparently paradoxical that calcium flux, which is dependent upon PLC-γ1–induced generation of inositol trisphosphate, was diminished whereas ERK activation, which can result from PLC-γ1– induced generation of DAG and Ras activation via GRP1, was preserved. A potential explanation could be a selective phosphorylation defect in the tyrosine 136 residue of LAT, which would result in defective recruitment of PLC-γ1 into the activation complex. In such a scenario, Grb2/Sos recruitment via other LAT phosphorylation sites would be preserved, thus allowing for continued activation of the Ras/ERK cascade. To test this hypothesis, we used phospho-specific Abs specific for the four phosphotyrosines in LAT reported to be induced after anti-CD3/CD28 ligation. Notably, a significant decrease in phosphorylation at tyrosine 136 of LAT was indeed observed (Fig. 5B; 2.4-fold induction of phospho-LAT [Y136] expression in control cells versus 1.1-fold in β-catenin–expressing cells; p < 0.05). In contrast, phosphorylation at tyrosines 171, 191, and 226 was relatively preserved in the cells expressing a stabilized β-catenin. Together, these results argue that stabilized β-catenin selectively inhibits the LAT/PLC-γ1/Ca2+ pathway in peripheral T cells, resulting in blunted T cell activation and effector cell differentiation.
Discussion
Despite evidence that the β-catenin pathway plays an important role during thymocyte development, its function in circulating T cells is poorly understood. We observed that β-catenin was constitutively degraded in peripheral conventional T cells and that the expression of a nondegradable β-catenin protein was inhibitory for T cell activation by selectively blocking the LAT/PLC-γ1/Ca2+ pathway. Although it has been suggested that β-catenin might promote expression of Cbl-b (21), we could not confirm this in our study at the protein level in primary T cells. In addition, the signaling defect in T cells expressing stable β-catenin does not correspond with what we and others have reported to be regulated by Cbl-b, which includes altered Akt and Vav phosphorylation (36). Together, our data suggest that expression of stabilized β-catenin is a negative regulator of activation of peripheral T cells by a novel mechanism.
In contrast with our observations in peripheral T cells, it was shown recently that the expression of nondegradable β-catenin in double-positive thymocytes can increase the phosphorylation of Jnk and p38 after CD3 and CD28 stimulation (37). This difference could result from a different cellular context between thymocytes and peripheral T cells or could be the result of differential timing for the overexpression of β-catenin, as the thymic phenotype could reflect compensatory changes in the double-positive cells that survive and accumulate despite β-catenin expression. Nevertheless, IL-2 secretion by β-catenin overexpressing thymocytes was still reduced, confirming what we have observed for peripheral T cells expressing a stabilized β-catenin protein (21).
It is of interest that the defective PLC-γ1 activation and calcium flux, in concert with diminished phosphorylation of tyrosine 136 in LAT, upon expression of stabilized β-catenin generates a similar phenotype to T cells expressing a LAT mutant at position 136 (38). T cells expressing a Y136F LAT exhibited reduced TCR-mediated activation of PLC-γ1, Ca2+ release, and IL-2 production along with intact TCR-induced ERK activation. A similar phenotype was described either in T cells from LAT-deficient mice or in the LAT-deficient Jurkat cell line J.CaM2.5 (39, 40) engineered to express the LAT homologue adapter linker for activation of B cells/non-T cell activation linker (LAB/NTAL). This protein contains a similar domain structure as LAT but is expressed mainly in B but not in T cells and lacks the region corresponding with tyrosine 136 in LAT. Together, these data are consistent with the notion that the presence of stabilized β-catenin phenocopies expression of tyrosine 136 mutants of LAT.
It is not yet clear if expression of stable β-catenin directly gives rise to this selective LAT tyrosine phosphorylation defect or if this mechanism is dependent on Wnt/β-catenin target gene expression. Our gene expression profiling of β-catenin–transduced cells did not reveal any obvious candidates that could inhibit TCR signaling. It does not appear that ZAP70 itself is functionally impaired, as tyrosine phosphorylation of ZAP70 was intact as was phosphorylation of other putative ZAP70 substrates, including Vav. Moreover, phosphorylation at other tyrosines in LAT (Y171, Y191, and Y226) was also intact. The preservation of ERK phosphorylation in T cells expressing stabilized β-catenin could be consistent with a conserved ability of LAT to recruit Grb2 and SOS on these other phosphorylated tyrosines (28, 41, 42). Because it was shown in Jurkat cells that the LAT–Grb2–SOS complex by itself is not able to compensate for RasGRP1 deficiency (43), a low-level residual PLC-γ1 might provide enough DAG to contribute to downstream activation of ERK.
The armadillo repeat region of β-catenin forming a superhelix motif with a long positively charged groove (44) could theoretically interact with a negatively charged protein like LAT (45). However, using immunoprecipitation experiments, we were not able to demonstrate a detectable interaction between β-catenin and either LAT or PLC-γ1. Thus, if there is a direct protein interaction, it does not appear to be stable in standard lysis buffers. It was also conceivable that β-catenin might induce a phosphatase that selectively dephosphorylated LAT at tyrosine 136. However, exposure of β-catenin–expressing T cells to sodium vanadate did not restore LAT phosphorylation. Therefore, identification of the final molecular mechanism of this novel signaling defect will require significant further investigation.
It was recently observed that expression of stabilized β-catenin in Tregs via retroviral transduction increased Treg survival (21). These results suggest that β-catenin may contribute to the functional phenotype of Tregs. Notably, we have found that Tregs constitutively express stabilized β-catenin and have generated preliminary data that conditional deletion of β-catenin from Tregs in vitro led to a markedly decreased cell survival (G. Driessens and T.F. Gajewski, unpublished observations). These data support the notion that β-catenin may be functionally important in the Treg lineage and suggest that future studies conditionally deleting β-catenin selectively in peripheral Tregs in vivo will be of interest.
In contrast, conditional deletion of β-catenin in conventional T cells had minimal effects on CD3/CD28-induced activation in vitro, consistent with minimal detected expression of β-catenin in that T cell subset. It will nonetheless be of interest to determine the effect of β-catenin deletion on conventional T cell function in vivo, as it is expected there will be a condition when β-catenin is indeed stabilized during systemic immune responses that are not reproduced using reductionist systems in vitro. As suggested by the expression of the nondegradable protein, β-catenin could play an inhibitory role on T cell activation once activated in peripheral T cells. Teleologically, it would be surprising that T cells expend energy to express and then subsequently degrade β-catenin protein constitutively without a biologic need to stabilize it under some condition in vivo. It will be of interest to study the expression of Wnt family members and the potential inhibitory activity on T cells localized in specific microenvironments, particularly within tumors that produce Wnt ligand(s).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 CA118153 and R21 AI79373. G.D. is a fellow of the Leukemia and Lymphoma Society.
We thank B. Vogelstein and K. Kinzler (The Johns Hopkins University) for kindly providing the cDNA encoding nondegradable β-catenin.
Abbreviations used in this article
- BM
bone marrow
- CAR
coxsackie and adenovirus receptor
- cDNA
complementary DNA
- Cre
Cre recombinase
- DAG
diacylglycerol
- eBio
eBioscience
- EV
empty adenoviral vector
- GSK-3β
glycogen synthase kinase 3β
- Lac
lactacystin
- LAT
linker for activation of T cells
- PLC-γ1
phospholipase C-γ1
- SB
SB216763
- Tg
transgenic
- Treg
regulatory T cell
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur. J. Immunol. 2008;38:1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dualkinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 3.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 2001;20:4935–4943. doi: 10.1093/emboj/20.17.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat. Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 6.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J. Cell. Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 8.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J. Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 9.Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, Grosschedl R. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity. 1998;8:11–20. doi: 10.1016/s1074-7613(00)80454-9. [DOI] [PubMed] [Google Scholar]
- 10.Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur. J. Immunol. 2002;32:967–971. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Luis TC, Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T, Heuvelmans S, De Krijger RR, van Dongen JJ, Staal FJ. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 12.Staal FJ, Luis TC. Wnt signaling in hematopoiesis: crucial factors for self-renewal, proliferation, and cell fate decisions. J. Cell. Biochem. 2010;109:844–849. doi: 10.1002/jcb.22467. [DOI] [PubMed] [Google Scholar]
- 13.Gounari F, Aifantis I, Khazaie K, Hoeflinger S, Harada N, Taketo MM, von Boehmer H. Somatic activation of beta-catenin bypasses pre-TCR signaling and TCR selection in thymocyte development. Nat. Immunol. 2001;2:863–869. doi: 10.1038/ni0901-863. [DOI] [PubMed] [Google Scholar]
- 14.Mulroy T, Xu Y, Sen JM. Beta-catenin expression enhances generation of mature thymocytes. Int. Immunol. 2003;15:1485–1494. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat. Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 16.Pongracz JE, Parnell SM, Jones T, Anderson G, Jenkinson EJ. Overexpression of ICAT highlights a role for catenin-mediated canonical Wnt signalling in early T cell development. Eur. J. Immunol. 2006;36:2376–2383. doi: 10.1002/eji.200535721. [DOI] [PubMed] [Google Scholar]
- 17.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 19.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 20.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007;26:227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat. Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 22.Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat. Immunol. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driessens G, Zheng Y, Gajewski TF. Beta-catenin does not regulate memory T cell phenotype. Nat. Med. 2010;16:513–514. doi: 10.1038/nm0510-513. author reply 514–515. [DOI] [PubMed] [Google Scholar]
- 25.Gattinoni L, Ji Y, Restifo NP. Reply to “β-catenin does not regulate memory T cell phenotype”. Nat. Med. 2010;16:514–515. doi: 10.1038/nm0510-513. [DOI] [PubMed] [Google Scholar]
- 26.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu. Rev. Immunol. 2002;20:371–394. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 27.Aguado E, Martínez-Florensa M, Aparicio P. Activation of T lymphocytes and the role of the adapter LAT. Transpl. Immunol. 2006;17:23–26. doi: 10.1016/j.trim.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J. Biol. Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 29.Wan YY, Leon RP, Marks R, Cham CM, Schaack J, Gajewski TF, DeGregori J. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc. Natl. Acad. Sci. USA. 2000;97:13784–13789. doi: 10.1073/pnas.250356297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Zeng W, Murakawa M, Freeman MW, Seed B. Episomal segregation of the adenovirus enhancer sequence by conditional genome rearrangement abrogates late viral gene expression. J. Virol. 2000;74:11296–11303. doi: 10.1128/jvi.74.23.11296-11303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zha Y, Shah R, Locke F, Wong A, Gajewski TF. Use of Creadenovirus and CAR transgenic mice for efficient deletion of genes in postthymic T cells. J. Immunol. Methods. 2008;331:94–102. doi: 10.1016/j.jim.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivas FV, O’Herrin S, Gajewski TF. CD28 is not required for c-Jun N-terminal kinase activation in T cells. J. Immunol. 2001;167:3123–3128. doi: 10.4049/jimmunol.167.6.3123. [DOI] [PubMed] [Google Scholar]
- 34.June CH, Moore JS. Measurement of intracellular ions by flow cytometry. Curr. Protoc. Immunol. 2004;Chapter 5(Unit 5.5) doi: 10.1002/0471142735.im0505s64. [DOI] [PubMed] [Google Scholar]
- 35.Cannon JL, Labno CM, Bosco G, Seth A, McGavin MH, Siminovitch KA, Rosen MK, Burkhardt JK. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity. 2001;15:249–259. doi: 10.1016/s1074-7613(01)00178-9. [DOI] [PubMed] [Google Scholar]
- 36.Zha Y, Gajewski TF. An adenoviral vector encoding dominant negative Cbl lowers the threshold for T cell activation in post-thymic T cells. Cell. Immunol. 2007;247:95–102. doi: 10.1016/j.cellimm.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kovalovsky D, Yu Y, Dose M, Emmanouilidou A, Konstantinou T, Germar K, Aghajani K, Guo Z, Mandal M, Gounari F. Beta-catenin/Tcf determines the outcome of thymic selection in response to alpha-betaTCR signaling. J. Immunol. 2009;183:3873–3884. doi: 10.4049/jimmunol.0901369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacaná E, Menon RK, Shores EW, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 39.Brdicka T, Imrich M, Angelisová P, Brdicková N, Horváth O, Spicka J, Hilgert I, Lusková P, Dráber P, Novák P, et al. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 2002;196:1617–1626. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen E, Zhu M, Craven B, Zhang W. Linker for activation of B cells: a functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-gamma1 binding. J. Immunol. 2004;172:6810–6819. doi: 10.4049/jimmunol.172.11.6810. [DOI] [PubMed] [Google Scholar]
- 41.Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. J. Biol. Chem. 2001;276:29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 42.Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping theZap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem. J. 2001;356:461–471. doi: 10.1042/0264-6021:3560461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber AH, Nelson WJ, Weis WI. Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 1997;90:871–882. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- 45.Wange RL. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci. STKE. 2000;2000:re1. doi: 10.1126/stke.2000.63.re1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.