Abstract
Myeloid cells have diverse roles in regulating immunity, inflammation, and extracellular matrix (ECM) turnover. To accomplish these tasks, myeloid cells carry an arsenal of metalloproteinases, which include the matrix metalloproteinases (MMPs) and the adamalysins. These enzymes have diverse substrate repertoires, and are thus involved in mediating proteolytic cascades, cell migration and cell signaling. Dysregulation of metalloproteinases contributes to pathogenic processes, including inflammation, fibrosis and cancer. Metalloproteinases also have important non-proteolytic functions in controlling cytoskeletal dynamics during macrophage fusion and enhancing transcription to promote anti-viral immunity. This review highlights the diverse contributions of metalloproteinases to myeloid cell functions.
Keywords: matrix metalloproteinase (MMP), adamalysin, proteolysis, non-proteolytic functions, inflammation, cancer
INTRODUCTION
Myeloid cells play significant roles in tissue remodeling, including the turnover of extracellular matrix (ECM), regulation of inflammation and progression of cancer. Proteinases are important mediators of these processes and myeloid cells are major sources of proteinases, including the matrix metalloproteinases (MMPs). Indeed, the first cellular sources of MMPs, collagenase (MMP8) and gelatinase B (MMP9), were discovered in neutrophils (1, 2). Papers published in the 1970s and 1980s demonstrated that macrophages also secreted collagenolytic (MMP8), gelatinolytic (MMP9) and elastinolytic (MMP12) metalloproteinases that degrade components of the ECM in the extracellular, pericellular and lysosomal compartments (3-7). Taken together these early discoveries suggested that metalloproteinases produced by myeloid cells have a major role in the remodeling of the microenvironment.
The metalloproteinase family includes the MMPs, the adamalysins, which include ADAMs (a disintegrin and metalloproteinases) and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs), as well as the astacins. These multi-modular enzymes share a common, highly conserved motif (HEXXHXXGXXH) containing three histidine residues that coordinate a zinc ion at the catalytic site, which is critical for carrying out hydrolysis of protein substrates. Together, metalloproteinases can cleave nearly all components of ECM (8) and are widely expressed during normal development as well as in disease states such as fibrosis, regeneration and cancer (reviewed in (9)). We focus this report on the MMPs because they have been more actively studied, and to a lesser extent, discuss the role of adamalysins in myeloid cells.
MEMBERS OF THE METALLOPROTEINASE FAMILY
The MMP family of endopeptidases includes 23 human and 24 mouse members. The soluble and cell surface bound MMPs process substrates through proteolysis, and clip short fragments from cytokines, chemokines and growth factors to alter their bioactive properties. In addition, they actively participate in ectodomain shedding of cell surface receptors, which affects the protein composition of the plasma membrane and the cellular interactions with growth factors and the microenvironment. After cleavage of cell surface receptors by MMPs, the intracellular and intramembrane portions of these receptors can undergo further cleavage to release proteins that have additional intracellular functions, allowing MMPs to serve as crucial regulators of cell signaling.
Structurally, MMPs consist of an amino-terminal signal peptide required for extracellular secretion, a pro-peptide domain required for activation, a catalytic domain responsible for proteolysis, a linker peptide (hinge region) and a carboxy-terminal hemopexin (HPX) domain required for recognizing protein partners and determining substrate specificity (Figure 1). MMPs are initially expressed and secreted as zymogens, which are enzymatically inactive (also called pro-MMPs). This is achieved by the interaction of a cysteine residue of the pro-peptide domain with the zinc-binding site within the catalytic domain (termed the cysteine switch), which characterizes proteinases as metalloproteinases. Disrupting the cysteine-zinc interaction, either chemically (by reactive oxygen species, or oxidized glutathione), or proteolytically by removing the pro-peptide domain, activates the MMP (10). In vivo, this activation is mediated by membrane-tethered MMPs (MT-MMPs) and other serine proteinases such as plasmin and neutrophil elastase. Once activated, MMPs bind and recognize substrates via the HPX domain, and enzymatically cleave these substrates, causing degradation and/or generating new biologically active fragments.
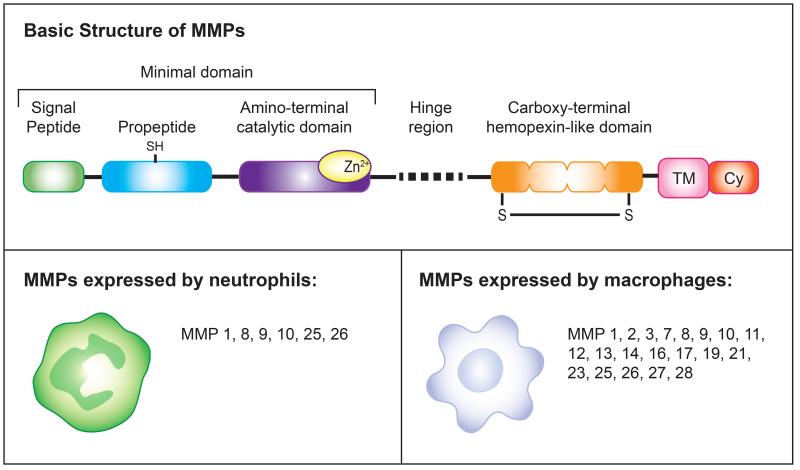
Figure 1. Matrix metalloproteinase structure and expression in myeloid cells.
Top panel. MMPs have a basic structure comprised of functional subdomains. All MMPs have a “minimal domain” comprised of an amino-terminal signal sequence that directs them to the endoplasmic reticulum, a pro-peptide domain with a cysteine that provides a zinc-interacting thiol (SH) group to maintain them as inactive zymogens, and a catalytic domain with three histidines that form a zinc-binding site (Zn). Most MMPs contain additional domains, the most common of which is the carboxy-terminal hemopexin-like domain which mediates interactions with TIMPs, cell-surface molecules and proteolytic substrates. This domain is composed of a four β-propeller structure and contains a disulfide bond (S-S) between the first and the last subdomain. Membrane-type MMPs (MT-MMPs) have an additional single-span transmembrane domain (TM) and a very short cytoplasmic domain (Cy).
Bottom panels. Expression of MMPs in myeloid cells. Neutrophils and macrophages release a variety of proteinases into the extracellular space during diverse biological processes including infection, tumorigenesis, and tissue repair. While neutrophils and macrophages are able to express several MMPs, the specific MMPs expressed by each cell type depends on the tissue microenvironment. In addition, both neutrophils and macrophages express a number of ADAM and ADAMTS proteins (not depicted here) that are important for their function and regulating inflammation and signaling.
The diversity of MMPs within the genome allows this family of proteinases to have a broad range of substrates. MMPs have traditionally been classified based on their ECM substrate profile as well as structural similarities. These include collagenases (MMP1, MMP8, MMP13 and MMP19), gelatinases (MMP2 and MMP9), matrilysins (MMP7 and MMP26) and stromelysins (MMP3, MMP10 and MMP11). In addition, there are four transmembrane (MT)-MMPs: MMP14 (MT1-MMP), MMP15 (MT2-MMP), MMP16 (MT3-MMP) and MMP24 (MT5-MMP) and two glycosylphosphatidylinositol (GPI)-anchored MMPs: MMP17 (MT4-MMP) and MMP25 (MT6-MMP) (11), that have critical roles in myeloid cell migration and processing chemokines (reviewed in (12)). However, these traditional MMP subgroups may not reflect the metalloproteinase’s dominant role in vivo or its most important set of substrates since many MMPs have multiple and context-dependent substrates. For example, MMP14 is a membrane-tethered MMP that is a collagenase but also activates other MMPs such as MMP2; MMP9 cleaves gelatin as well as other ECM proteins such as type IV collagen and non-ECM proteins such as VEGF sequestered in the ECM.
The adamalysins include ADAM and ADAMTS proteins (13). To date, 21 ADAM genes have been identified but only 12 encode proteolytic proteins, indicating that many ADAMs have non-proteolytic functions (14). Those with proteolytic activities are called sheddases because of their major role in cleaving transmembrane protein ectodomains (15). In lieu of the hemopexin domain, ADAMs instead contain a cysteine-rich region, a series of epidermal growth factor (EGF)-like repeats and a disintegrin domain, which mediate cell-ECM interactions by binding integrins that allow ADAMs to bind and interact with substrates on neighboring cells. Most ADAMs are membrane-anchored and function in the pericellular space. In contrast, ADAMTS proteins are secreted proteinases with thrombospondin type I-like repeats and cleave many ECM proteins including aggrecan (16).
Given their potential to have broad effects on the structural and biochemical milieu of the microenvironment, tight regulation of metalloproteinase activity must be achieved. Not surprisingly, metalloproteinases are regulated at multiple levels, including RNA transcription, protein synthesis, secretion and intracellular trafficking, localization, zymogen activation and inhibition by extracellular inhibitors (11). For example, metalloproteinases can be compartmentalized in intracellular or extracellular locations, such as in granules or sequestered by ECM components such as glycoasaminoglycans. In addition, MMPs are tightly regulated in the extracellular space (17). MT-MMPs, for example, are internalized by clathrin-dependent and independent modes of endocytosis and transported to lysosomes or recycled back to the cell surface (18, 19). MMPs are cleared from extracellular fluids by binding to low-density lipoprotein receptor-related protein (LRP)-1, leading to internalization and degradation (20, 21). Thus, extracellular and pericellular control are important mechanisms by which MMPs are locally regulated.
Importantly, MMPs and adamalysins are also regulated by tissue inhibitors of metalloproteinases (TIMPs), which are the most important endogenous inhibitors of metalloproteinase activity (22). The TIMP family consists of four members (TIMP1 - 4), which can reversibly bind and inhibit the activity of all MMPs and ADAMs. TIMPs contain an amino-terminal domain that specifically folds within itself and wedges into the active site of MMPs to inhibit these proteins (23). TIMP3 is sequestered in the ECM, whereas the other TIMPs are soluble in vivo. Not surprisingly, TIMP levels are also regulated by endocytosis by binding LRP-1, demonstrating that endocytic mechanisms play an important role in regulating metalloproteinase activity (24, 25).
Relative concentrations of metalloproteinase to TIMP levels determine overall proteolytic activity, with higher MMP/ADAM levels favoring proteolysis and higher TIMP levels favoring inhibition. In neutrophils, MMP9 is stored in tertiary granules for immediate release following stimulation with interleukin-8 (IL-8) or tumor necrosis factor (TNF) (26). Interestingly, neutrophils lack TIMP1, which allows these cells to release MMP9 rapidly in an active form upon stimulation, consistent with their roles as first responders to infection or tissue damage and control processes such as angiogenesis (27). The regulation of metalloproteinase expression and activity is therefore critical in determining myeloid cell function.
FUNCTIONS OF METALLOPROTEINASES IN MYELOID CELLS
Metalloproteinases are important in myeloid cell migration
Because metalloproteinases degrade and remodel the ECM, they play a significant role in facilitating cell migration through basement membranes and the interstitium. Collectively, MMPs are able to degrade components of the ECM including collagens, laminin, fibronectin, and fibrin, as well as aggrecan, perlecan and nidogen. In addition, MMPs also regulate the turnover of ECM proteins (such as fibronectin) through endocytosis, as a separate mechanism to remodel the ECM (28). Expression of both secreted and membrane-bound MMPs is a common mechanism by which cells loosen the ECM in order to move through the dense deposits of ECM proteins. Mast cells, for example, utilize MMP9 to migrate through tissue, which can be induced by pro-inflammatory cytokines such as tumor-necrosis factor (TNF)-α (29). MMP2 and MMP9 synergistically promote neutrophil infiltration, as knockout mice deficient in Mmp2 and Mmp9 have impaired neutrophil influx in multiple infection and inflammation models, including influenza virus infection and acute pancreatitis (30, 31).
In addition to cleaving ECM components, metalloproteinases also regulate chemokine-induced migration. For example, macrophage-derived MMP12 cleaves and inactivates CXC-chemokine ligand 2 (CXCL2) and CXCL3, which reduces the influx of neutrophils and other macrophages, thereby attenuating the acute immune response (32). Furthermore, metalloproteinases such as MMP14 can cleave cell surface adhesion molecules such as CD44 and syndecan to promote migration. Finally, the adamalysins also regulate migration, for example, in acute lung inflammation; deletion of Adam10 in myeloid cells results in reduced lipopolysaccharide (LPS)-induced pulmonary inflammation and decreased pulmonary edema (33). Taken together, these examples illustrate that metalloproteinases can regulate myeloid cell migration by cleaving ECM components, as well as chemokines and cell surface proteins.
Metalloproteinases are involved in regulating inflammation and fibrosis
ADAM17 was discovered in 1997 as an enzyme that releases the membrane-bound TNF-α precursor into its soluble form (34, 35). This discovery provided the first evidence for the physiologic activity of ADAMs. ADAM17, also known as the TNF-α-converting enzyme (TACE), is ubiquitously expressed. Increased ADAM17 catalytic activity has been associated with a variety of inflammatory diseases characterized by elevated levels of tissue TNF-α including rheumatoid arthritis, inflammatory bowel disease, and osteoarthritis (14). ADAM17 is the principal enzyme responsible for the release of soluble TNF-α from myeloid cells during endotoxin-mediated shock (36). ADAM17 also cleaves the TNF receptor 1 (TNFR1) and interleukin-6 receptor (IL-6R) to control downstream inflammatory signaling, as well as adhesion proteins such as L-selectin (reviewed in (37)). In addition, MMPs contribute to pathologic inflammatory conditions, including arthritis in which local macrophages express higher levels of MMP2, MMP9, MMP13 and MMP14, ultimately leading to destruction of cartilage and bone. Alveolar macrophages also express higher amounts of MMP1 and MMP12 in emphysema, and their expression levels correlate with the severity of pulmonary disease. MMP12 is responsible for generating elastin fragments that promote inflammatory cell recruitment (38). These studies demonstrate that myeloid cell-derived metalloproteinases are important components that initiate and sustain inflammation.
Conversely, metalloproteinases are also involved in dampening the inflammatory response. For example, ADAM17 down-regulates macrophage colony-stimulating factor receptor (M-CSFR) in macrophages undergoing activation (39). In addition, ADAM8, which is expressed in lung bronchial epithelial cells and myeloid cells such as eosinophils, monocytes, macrophages, and dendritic cells, protects against allergic airway inflammation. This is mediated by the ability of ADAM8 to activate the apoptotic pathway (40). MMPs like MMP12 also regulate the immune response by cleaving and inactivating chemokines. Following corneal injury, for example, MMP12 alters levels of CXCL1 and CCL2, which facilitates wound repair by regulating leukocyte infiltration and angiogenesis (41). Taken together, these studies highlight the role of metalloproteinases in regulating both physiologic and pathologic systemic immune responses.
Metalloproteinases in myeloid cells have non-proteolytic functions
Although the majority of research on MMPs has primarily focused on their catalytic activities, recent studies suggest that other domains also play critical roles. For example, MMP12 in macrophages exhibits anti-bacterial properties important in clearing gram-positive and gram-negative bacterial infections, and this activity lies within the carboxy-terminal and not the catalytic domain (42). Remarkably, MMP12 also has anti-viral properties by functioning as a transcription factor intracellularly to promote interferon alpha (IFNα) expression. Interestingly, its protease function is also important in the extracellular space to dampen IFNα signaling, which provides an auto-feedback mechanism (Figure 2) (43).
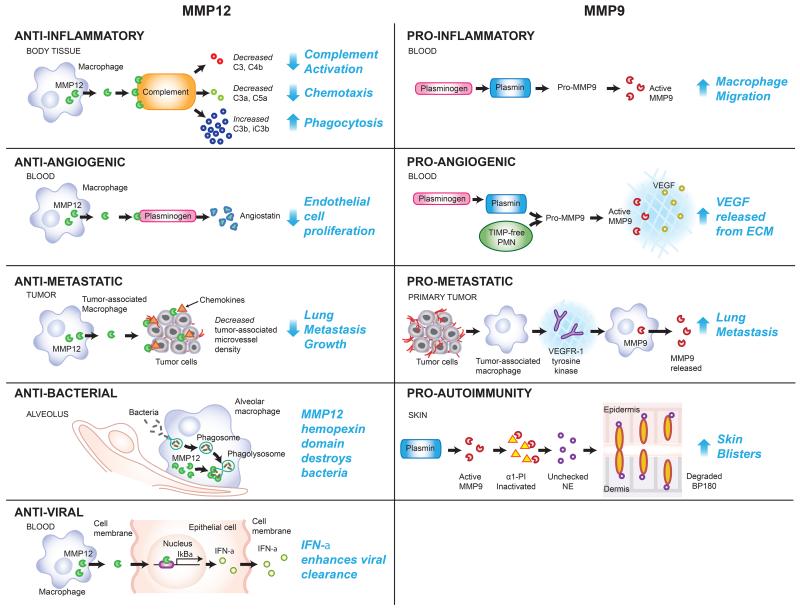
Figure 2. Functions of MMP12 and MMP9 in biological processes.
MMP12 and MMP9 have contrasting roles in the processes of inflammation, angiogenesis, and metastasis with MMP12 inhibiting these processes and MMP9 promoting these processes. MMP12 protects against inflammation by cleaving complements C3 and C4b and reducing complement activation, cleaving complements C3a and C5a and reducing neutrophil recruitment, and creating cleaved forms of C3b and iC3b which are potent phagocytosis enhancers (65). In contrast, MMP9 promotes inflammation by stimulating macrophage migration and infiltration upon being activated by plasminogen (66). MMPs also promote autoimmune disease. For example, in the skin disease bullous pemphigoid, MMP9 activated by plasmin proteolytically inactivates α1-proteinase inhibitor (α1-PI), the physiological inhibitor of neutrophil elastase (NE), which allows unrestrained activity of NE (67). NE degrades BP180, which results in dermal-epidermal separation (68, 69). MMP12 inhibits angiogenesis through its cleavage of plasminogen to generate angiostatin which results in decreased endothelial cell proliferation (70). MMP9, however, promotes angiogenesis through the release of VEGF into the extracellular matrix following activation by plasmin or upon secretion from TIMP-free neutrophils (27, 50). Lung metastatic growth is reduced by MMP12 produced by tumor-associated macrophages, which interact with chemokines to decrease tumor-associated microvessel density (58). Lung metastasis is increased by MMP9 produced by tumor-associated macrophages via VEGFR-1/Flt-1 tyrosine kinase and MMP9 levels in the lungs of patients with distant tumors are significantly elevated compared to the lungs control patients (71). Finally, in addition to the above functions, MMP12 has direct direct anti-microbial activity through its hemopexin domain by disrupting bacterial membranes in phagolysosomes (42). MMP12 also enhances anti-viral clearance by binding to the promoter of the gene encoding IκBα (NFKBIA) and enhancing the production of IκBα, which promotes interferon (IFN)-α secretion from the cell (43). Together, these examples illustrate the diverse functions of MMPs in myeloid cells.
MMP14 also regulates macrophage gene expression by localizing in the nucleus and having transcription-factor like activity. MMP14 triggers PI3Kδ signaling to regulate the Mi-2/NuRD nucleosome remodeling complex, which controls the expression of immune response genes (44). In addition, MMP14 regulates myeloid cell migration by controlling Rac1 activity via its cytoplasmic tail, which is necessary for macrophage fusion during osteoclast and giant-cell formation. Again, this function appears to be independent of its catalytic activity (45). These unexpected findings illustrate that MMPs have multiple activities independent from their catalytic domains. Future work aimed at identifying MMPs with alternative functions, and characterizing additional MMPs with transcription factor-like activities will yield insights in how these metalloproteinases regulate myeloid cell function.
Metalloproteinases supplied by myeloid cells promote and restrict cancer
Metalloproteinases are aberrantly expressed during all stages of tumorigenesis (reviewed in (46)), whereby altered proteolysis leads to unregulated cell growth, tissue remodeling, invasion, and ultimately, metastasis. MMPs are prominently expressed in myeloid cells responding to progressing tumors (47, 48).
In addition to their role in ECM turnover and cancer cell migration, MMPs regulate signaling pathways that control tumor growth, inflammation and angiogenesis (Figure 2). For example, MMP9 is upregulated in invasive human papilloma virus (HPV)-induced skin cancer. Mice lacking MMP9 have decreased keratinocyte proliferation and incidence of invasive tumors. Interestingly, MMP9 is predominantly expressed in neutrophils, macrophages, and mast cells, rather than in the tumor cells. Transplanting Mmp9-deficient mice with MMP9-sufficient bone marrow cells restores skin cancer progression in the mouse model (49). Interestingly, in models of pancreatic islet carcinoma, MMP2 and MMP9 from infiltrating myeloid cells stimulate tumor cell growth and release vascular endothelial growth factor (VEGF), one of the main drivers of angiogenesis, from the ECM (50). These findings suggest that myeloid cell-derived MMPs promote tumor growth, not only by structurally reorganizing the ECM, but also by releasing growth factors from the ECM that directly stimulate cell growth or enhance angiogenesis. Myeloid cells can also affect the vascular permeability of tumors and tumor responsiveness to chemotherapeutics like doxorubicin in an MMP-dependent manner (51), and myeloid cell-derived MMPs are involved in preparing the metastatic niche to facilitate colonization of new organs by circulating tumor cells (52, 53). Whether MMPs might have non-proteolytic roles in promoting cancer remains to be determined. One possibility is that MMPs might regulate self-renewal pathways (54) or enhance invasion (55), which may be important in maintaining the cancer stem cell niche or promoting metastasis.
Finally, MMPs can also restrict tumor progression. Loss of function mutations in MMP8, which is highly expressed in neutrophils, increases melanoma progression, while inactivating ADAMTS15 mutations are found in human colorectal cancer samples; expression of the wild-type metalloproteinase restricts tumor growth and progression (56, 57). MMP12 from macrophages also suppresses growth of lung metastases, and is associated with decreased tumor-associated blood vessel density in vivo (58). In addition, conditional inactivation of ADAM10, a major proteinase that regulates Notch cleavage and activation in hematopoietic stem cells, results in a myeloproliferative disorder characterized by splenomegaly and increased numbers of myeloid cells (59). Together, these studies highlight the remarkable contributions of MMPs and adamalysins to both promoting and restricting cancer progression and metastasis.
CONCLUSIONS AND FUTURE RESEARCH DIRECTIONS
As we have discussed, metalloproteinases produced by myeloid cells have broad roles in development and disease, and their perturbation may be therapeutically beneficial (Figure 2). Most MMP inhibitors, however, have been non-specific and have focused only on targeting the MMP catalytic domain. Given their expanding non-proteolytic roles, it will be important to develop MMP inhibitors that target different domains and functions. It is also important to consider that some metalloproteinases restrict disease progression and so their pan-inhibition may result in worse outcomes. These issues may help explain why initial clinical trials with MMP inhibitors were disappointing in cancer patients (60). In addition, the hemopexin domain is an important determinant of stem cell function and cell invasion (54, 55). Inhibitors that selectively target specific metalloproteinases and domains, therefore, need to be developed and investigated for therapeutic potential (61). Non-catalytic inhibitors may need to be used in combination with catalytic inhibitors for complete MMP inhibition. Since MMP hemopexin domains are unique, this strategy may also increase specificity. In addition, antibodies that specifically recognize certain MMP conformations or domains will also help elucidate MMP function. This would allow targeting of specific pathogenic configurations without affecting its other roles.
A more comprehensive understanding of the various functions of myeloid-derived MMPs and adamalysins will also be important, as well as the ability to control metalloproteinase activation, localization and expression. Given MMP12 and MMP14’s surprising transcription factor activity, it will be important to characterize their gene targets, which will likely be cell-type specific, and to determine whether other MMPs or adamalysins might also have transcriptional activity. It will also be crucial to elucidate how the transcriptional complex is assembled and what other proteins might be within that complex in order to better understand what novel functions are conferred. In addition, the complete substrate repertoire of myeloid-cell derived metalloproteinases in specific physiologic and pathologic conditions will be important to determine through proteomic approaches (62). Because MMPs have unique functions depending on their location, identifying drugs that inhibit the extracellular but not the intracellular activity (or vice versa) may be necessary. Finally, utilizing microRNAs that target MMPs, ADAMs, and ADAMTS may increase specificity and be a useful strategy to modulate their expression.
Myeloid cells regulate the local microenvironment via MMPs and adamalysins. Depending on what substrates or protein-binding partners are in the vicinity, MMPs can facilitate different, sometimes opposite, biological processes (Figure 2). Thus, appreciating the spatial context of myeloid cells and their secreted metalloproteinases and understanding the pericellular activity of these metalloproteinases are critical. Better methods, especially imaging tools, to assess MMP and adamalysin function in vivo and in real-time are needed to fully appreciate their complexity. Recent advancements in MMP imaging have come forward (47, 63). For example, the development of dynamic high-resolution multimodal microscopy, which uses a green fluorescent protein (GFP)–tagged proteinase in combination with a fluorophore-labeled ECM polymer (63) can reveal proteinase localization and activity in live cells. Finally, it will be critical to understand the timing that different myeloid metalloproteinases act during physiologic and pathologic processes. Because the same metalloproteinase may play opposite roles during the early versus late stages, it is important to consider the timing of using MMP and adamalysin inhibitors (64). These exciting concepts in metalloproteinase biology underscore their complex roles in physiology, immunity and diseases mediated by myeloid cells and offer critical insights into strategies and considerations for the design of more effective therapeutics.
ACKNOWLEDGEMENTS
We thank Suling Wang for assistance with preparing and illustrating the figures. This study was supported by funds from the National Institutes of Health, USA (R01 CA057621 and U01 ES019458 to Z.W. and R01 EY022739 to MFC and P30 EY002162).
REFERENCES
- 1.Lazarus GS, Brown RS, Daniels JR, Fullmer HM. Human granulocyte collagenase. Science. 1968;159:1483–5. doi: 10.1126/science.159.3822.1483. [DOI] [PubMed] [Google Scholar]
- 2.Sopata I, Dancewicz AM. Presence of a gelatin-specific proteinase and its latent form in human leucocytes. Biochim Biophys Acta. 1974;370:510–23. doi: 10.1016/0005-2744(74)90112-0. [DOI] [PubMed] [Google Scholar]
- 3.Gordon S, Werb Z. Secretion of macrophage neutral proteinase is enhanced by colchicine. Proc Natl Acad Sci U S A. 1976;73:872–6. doi: 10.1073/pnas.73.3.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werb Z, Bainton DF, Jones PA. Degradation of connective tissue matrices by macrophages. III. Morphological and biochemical studies on extracellular, pericellular, and intracellular events in matrix proteolysis by macrophages in culture. J Exp Med. 1980;152:1537–53. doi: 10.1084/jem.152.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werb Z, Banda MJ, Jones PA. Degradation of connective tissue matrices by macrophages. I. Proteolysis of elastin, glycoproteins, and collagen by proteinases isolated from macrophages. J Exp Med. 1980;152:1340–57. doi: 10.1084/jem.152.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werb Z, Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975;142:361–77. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werb Z, Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975;142:346–60. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87:5578–82. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marco M, Fortin C, Fulop T. Membrane-type matrix metalloproteinases: key mediators of leukocyte function. J Leukoc Biol. 2013;94:237–46. doi: 10.1189/jlb.0612267. [DOI] [PubMed] [Google Scholar]
- 13.Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272:556–62. doi: 10.1074/jbc.272.1.556. [DOI] [PubMed] [Google Scholar]
- 14.Lisi S, D’Amore M, Sisto M. ADAM17 at the interface between inflammation and autoimmunity. Immunol Lett. 2014;162:159–169. doi: 10.1016/j.imlet.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–41. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 16.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–7. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K, Murphy G, Troeberg L. Extracellular regulation of metalloproteinases. Matrix Biol. 2015;44-46C:255–263. doi: 10.1016/j.matbio.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Uekita T, Itoh Y, Yana I, Ohno H, Seiki M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J Cell Biol. 2001;155:1345–56. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccard H, Van den Steen PE, Opdenakker G. Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J Leukoc Biol. 2007;81:870–92. doi: 10.1189/jlb.1006629. [DOI] [PubMed] [Google Scholar]
- 20.Barmina OY, Walling HW, Fiacco GJ, Freije JM, Lopez-Otin C, Jeffrey JJ, Partridge NC. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J Biol Chem. 1999;274:30087–93. doi: 10.1074/jbc.274.42.30087. [DOI] [PubMed] [Google Scholar]
- 21.Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276:15498–503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- 22.Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13:649–65. doi: 10.1038/nri3499. [DOI] [PubMed] [Google Scholar]
- 23.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Scilabra SD, Troeberg L, Yamamoto K, Emonard H, Thogersen I, Enghild JJ, Strickland DK, Nagase H. Differential regulation of extracellular tissue inhibitor of metalloproteinases-3 levels by cell membrane-bound and shed low density lipoprotein receptor-related protein 1. J Biol Chem. 2012;288:332–42. doi: 10.1074/jbc.M112.393322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thevenard J, Verzeaux L, Devy J, Etique N, Jeanne A, Schneider C, Hachet C, Ferracci G, David M, Martiny L, Charpentier E, Khrestchatisky M, Rivera S, Dedieu S, Emonard H. Low-density lipoprotein receptor-related protein-1 mediates endocytic clearance of tissue inhibitor of metalloproteinases-1 and promotes its cytokine-like activities. PLoS One. 2014;9:e103839. doi: 10.1371/journal.pone.0103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masure S, Proost P, Van Damme J, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. Eur J Biochem. 1991;198:391–8. doi: 10.1111/j.1432-1033.1991.tb16027.x. [DOI] [PubMed] [Google Scholar]
- 27.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–7. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi F, Sottile J. MT1-MMP regulates the turnover and endocytosis of extracellular matrix fibronectin. J Cell Sci. 2011;124:4039–50. doi: 10.1242/jcs.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Girolamo N, Indoh I, Jackson N, Wakefield D, McNeil HP, Yan W, Geczy C, Arm JP, Tedla N. Human mast cell-derived gelatinase B (matrix metalloproteinase-9) is regulated by inflammatory cytokines: role in cell migration. J Immunol. 2006;177:2638–50. doi: 10.4049/jimmunol.177.4.2638. [DOI] [PubMed] [Google Scholar]
- 30.Bradley LM, Douglass MF, Chatterjee D, Akira S, Baaten BJ. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog. 2012;8:e1002641. doi: 10.1371/journal.ppat.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awla D, Abdulla A, Syk I, Jeppsson B, Regner S, Thorlacius H. Neutrophil-derived matrix metalloproteinase-9 is a potent activator of trypsinogen in acinar cells in acute pancreatitis. J Leukoc Biol. 2012;91:711–9. doi: 10.1189/jlb.0811443. [DOI] [PubMed] [Google Scholar]
- 32.Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–64. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- 33.Pruessmeyer J, Hess FM, Alert H, Groth E, Pasqualon T, Schwarz N, Nyamoya S, Kollert J, van der Vorst E, Donners M, Martin C, Uhlig S, Saftig P, Dreymueller D, Ludwig A. Leukocytes require ADAM10 but not ADAM17 for their migration and inflammatory recruitment into the alveolar space. Blood. 2014;123:4077–88. doi: 10.1182/blood-2013-09-511543. [DOI] [PubMed] [Google Scholar]
- 34.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 35.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 36.Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP. Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol. 2007;179:2686–9. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 37.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–7. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Houghton AM, Quintero PA, Perkins DL, Kobayashi DK, Kelley DG, Marconcini LA, Mecham RP, Senior RM, Shapiro SD. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–9. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rovida E, Paccagnini A, Del Rosso M, Peschon J, Dello Sbarba P. TNF-alpha-converting enzyme cleaves the macrophage colony-stimulating factor receptor in macrophages undergoing activation. J Immunol. 2001;166:1583–9. doi: 10.4049/jimmunol.166.3.1583. [DOI] [PubMed] [Google Scholar]
- 40.Knolle MD, Nakajima T, Hergrueter A, Gupta K, Polverino F, Craig VJ, Fyfe SE, Zahid M, Permaul P, Cernadas M, Montano G, Tesfaigzi Y, Sholl L, Kobzik L, Israel E, Owen CA. Adam8 limits the development of allergic airway inflammation in mice. J Immunol. 2013;190:6434–49. doi: 10.4049/jimmunol.1202329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan MF, Li J, Bertrand A, Casbon AJ, Lin JH, Maltseva I, Werb Z. Protective effects of matrix metalloproteinase-12 following corneal injury. J Cell Sci. 2013;126:3948–60. doi: 10.1242/jcs.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houghton AM, Hartzell WO, Robbins CS, Gomis-Ruth FX, Shapiro SD. Macrophage elastase kills bacteria within murine macrophages. Nature. 2009;460:637–41. doi: 10.1038/nature08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchant DJ, Bellac CL, Moraes TJ, Wadsworth SJ, Dufour A, Butler GS, Bilawchuk LM, Hendry RG, Robertson AG, Cheung CT, Ng J, Ang L, Luo Z, Heilbron K, Norris MJ, Duan W, Bucyk T, Karpov A, Devel L, Georgiadis D, Hegele RG, Luo H, Granville DJ, Dive V, McManus BM, Overall CM. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat Med. 2014;20:493–502. doi: 10.1038/nm.3508. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu-Hirota R, Xiong W, Baxter BT, Kunkel SL, Maillard I, Chen XW, Sabeh F, Liu R, Li XY, Weiss SJ. MT1-MMP regulates the PI3Kdelta.Mi-2/NuRD-dependent control of macrophage immune function. Genes Dev. 2012;26:395–413. doi: 10.1101/gad.178749.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalo P, Guadamillas MC, Hernandez-Riquer MV, Pollan A, Grande-Garcia A, Bartolome RA, Vasanji A, Ambrogio C, Chiarle R, Teixido J, Risteli J, Apte SS, del Pozo MA, Arroyo AG. MT1-MMP is required for myeloid cell fusion via regulation of Rac1 signaling. Dev Cell. 2010;18:77–89. doi: 10.1016/j.devcel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohela M, Casbon AJ, Olow A, Bonham L, Branstetter D, Weng N, Smith J, Werb Z. Intravital imaging reveals distinct responses of depleting dynamic tumor-associated macrophage and dendritic cell subpopulations. Proc Natl Acad Sci U S A. 2014;111:E5086–95. doi: 10.1073/pnas.1419899111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, Passegue E, Werb Z. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–90. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, Fein M, Rasch MG, Tan YX, Qiu J, Park J, Sinha P, Bissell MJ, Frengen E, Werb Z, Egeblad M. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, Shi H, Luo Y. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 2009;69:7529–37. doi: 10.1158/0008-5472.CAN-08-4382. [DOI] [PubMed] [Google Scholar]
- 54.Kessenbrock K, Dijkgraaf GJ, Lawson DA, Littlepage LE, Shahi P, Pieper U, Werb Z. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell. 2013;13:300–13. doi: 10.1016/j.stem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Correia AL, Mori H, Chen EI, Schmitt FC, Bissell MJ. The hemopexin domain of MMP3 is responsible for mammary epithelial invasion and morphogenesis through extracellular interaction with HSP90beta. Genes Dev. 2013;27:805–17. doi: 10.1101/gad.211383.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, Lopez-Otin C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nature genetics. 2003;35:252–7. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 57.Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, Davis S, Wang C, Cronin JC, Agrawal NS, Lin JC, Westbroek W, Hoogstraten-Miller S, Molinolo AA, Fetsch P, Filie AC, O’Connell MP, Banister CE, Howard JD, Buckhaults P, Weeraratna AT, Brody LC, Rosenberg SA, Samuels Y. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nature genetics. 2009;41:518–20. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–55. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 59.Yoda M, Kimura T, Tohmonda T, Uchikawa S, Koba T, Takito J, Morioka H, Matsumoto M, Link DC, Chiba K, Okada Y, Toyama Y, Horiuchi K. Dual functions of cell-autonomous and non-cell-autonomous ADAM10 activity in granulopoiesis. Blood. 2011;118:6939–42. doi: 10.1182/blood-2011-06-357210. [DOI] [PubMed] [Google Scholar]
- 60.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 61.Remacle AG, Golubkov VS, Shiryaev SA, Dahl R, Stebbins JL, Chernov AV, Cheltsov AV, Pellecchia M, Strongin AY. Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012;72:2339–49. doi: 10.1158/0008-5472.CAN-11-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tam EM, Morrison CJ, Wu YI, Stack MS, Overall CM. Membrane protease proteomics: Isotope-coded affinity tag MS identification of undescribed MT1-matrix metalloproteinase substrates. Proc Natl Acad Sci U S A. 2004;101:6917–22. doi: 10.1073/pnas.0305862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 64.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, 3rd, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3:e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bellac CL, Dufour A, Krisinger MJ, Loonchanta A, Starr AE, Auf dem Keller U, Lange PF, Goebeler V, Kappelhoff R, Butler GS, Burtnick LD, Conway EM, Roberts CR, Overall CM. Macrophage matrix metalloproteinase-12 dampens inflammation and neutrophil influx in arthritis. Cell Rep. 2014;9:618–32. doi: 10.1016/j.celrep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008;118:3012–24. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102:647–55. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 68.Shimanovich I, Mihai S, Oostingh GJ, Ilenchuk TT, Brocker EB, Opdenakker G, Zillikens D, Sitaru C. Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J Pathol. 2004;204:519–27. doi: 10.1002/path.1674. [DOI] [PubMed] [Google Scholar]
- 69.Liu Z, Li N, Diaz LA, Shipley M, Senior RM, Werb Z. Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J Clin Invest. 2005;115:879–87. doi: 10.1172/JCI23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–52. [PubMed] [Google Scholar]
- 71.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]