Abstract
The bioavailability of members of the transforming growth factor β (TGF-β) family is controlled by a number of mechanisms. Bona fide TGF-β is sequestered into the matrix in a latent state and must be activated before it can bind to its receptors. Here, we review the molecules and mechanisms that regulate the bioavailability of TGF-β and compare these mechanisms with those used to regulate other TGF-β family members. We also assess the physiological significance of various latent TGF-β activators, as well as other extracellular modulators of TGF-β family signaling, by examining the available in vivo data from knockout mouse models and other biological systems.
When TGF-β is secreted from cells, it is sequestered in the matrix in a latent state and must be activated before it can bind to its receptors. The activities of other TGF- β family members are also modulated extracellularly.
The bioavailability of transforming growth factor β (TGF-β) family ligands is not only regulated by the release of the growth factor from cells, but it is also controlled by extracellular mechanisms that modulate, inhibit, activate, or enhance the binding of these signaling molecules to their receptors.
This is especially true of the bona fide TGF-β dimer; as when TGF-β is secreted from cells it is tightly bound in a latent complex consisting of its dimeric pro-peptide (referred to as latency-associated peptide [LAP]), and a latent TGF-β-binding protein (LTBP). This tripartite complex of TGF-β, LAP, and LTBP is called the large latent complex (LLC). Within this complex, LAP confers latency to the cytokine and the LTBP functions to direct and sequester the growth factor into the extracellular matrix (ECM) and assists in the conversion of the latent TGF-β to its active form, a process known as activation.
In the last decade, it has become clear that the properties of the TGF-β LLC are critical in modulating the action of the cytokine and that controlled release of TGF-β from the ECM by activation is central to understanding TGF-β signaling in a larger biological context. Here, we review the extracellular regulation of TGF-β and examine the available in vivo data to place these regulatory mechanisms in a physiological context.
BIOSYNTHESIS AND LATENCY OF TGF-β
TGF-β Processing, Latency, and Interaction with LTBPs
TGF-β is initially translated as a ∼50-kDa pro-protein containing both growth factor and LAP. This pro-protein is vectorially discharged into the endoplasmic reticulum where it dimerizes and folds (Fig. 1, lower right). Within the endoplasmic reticulum, the dimeric pro-TGF-β is linked to a single LTBP by a pair of disulfide bonds between LTBP and LAP (Miyazono et al. 1991; Gleizes et al. 1996; Saharinen et al. 1996). The pro-peptide (LAP) is subsequently cleaved from the mature cytokine in the trans-Golgi by furin or furin-type enzymes (Fig. 1, lower left) (Dubois et al. 1995; Hyytiainen et al. 2004). The complex of TGF-β and LAP is referred to as the small latent complex (SLC).
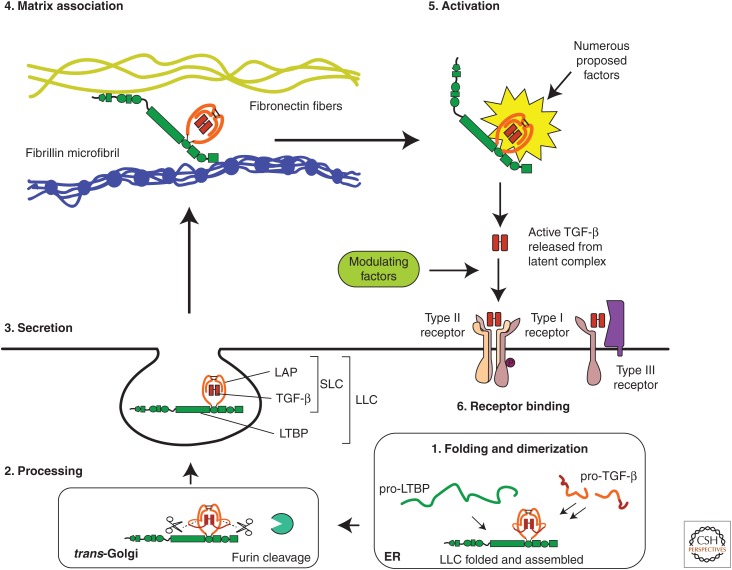
Figure 1.
Scheme for the secretion and extracellular regulation of transforming growth factor β (TGF-β). Starting at the bottom right (1), TGF-β and latent TGF-β-binding protein (LTBP) are translated into the endoplasmic reticulum (ER) where pro-TGF-β dimerizes and is then disulfide bonded to LTBP to form a ternary complex. The TGF-β dimer is cleaved from its pro-peptide (latency-associated peptide [LAP]) in the trans-Golgi network, but TGF-β and LAP remain strongly associated via noncovalent interactions forming the large latent complex (LLC) (lower left) (2). Once secreted (middle left) (3), the LTBP may bind various matrix fibers that sequester latent TGF-β until it is released by an activator (upper left) (4). The latent complex is then activated (upper right) (5), by one of several potential mechanisms, releasing the mature TGF-β. Active TGF-β may bind to cell-surface receptors (lower right) (6), although other factors may also bind the active growth factor at this stage and either inhibit or promote receptor binding.
One of the remarkable properties of the TGF-β latent complex is the fact that the interaction of LAP with the growth factor is of such high affinity that all TGF-β isoforms are secreted as part of a latent complex, either LLC or SLC. The one known exception is Camurati–Engelmann disease, in which patients have specific mutations in TGF-β1 LAP or in the TGF-β1 signal peptide that yield increased levels of secreted, constitutively active TGF-β that may be the cause of the bone thickening observed in this rare condition (Saito et al. 2001; Janssens et al. 2003).
LTBPs may serve a chaperone-like function for pro-TGF-β by enhancing its folding and secretion (Miyazono et al. 1991). Without LTBP, the cysteines in LAP that bind LTBP may form incorrect disulfide bonds, and this complex would be degraded within the cell (Brunner et al. 1989). This may account for the enhanced secretion of TGF-β in the presence of LTBP (Miyazono et al. 1991; Rifkin 2005) and is consistent with the heightened secretion of TGF-β1 C33S compared with wild-type protein (Annes et al. 2004). However, some cell types, such as the osteosarcoma line UMR-106, produce only the SLC, and the ROS 17/2.8 line produces a mixture of both SLC and LLC (Dallas et al. 1994). Significant amounts of mature TGF-β are also secreted when HEK-293T cells are transfected with TGF-β expression constructs alone (Walton et al. 2010). Thus, the pro-TGF-β dimer can properly fold and be processed on its own, but covalent interaction with an LTBP may still enhance the rate of folding and secretion.
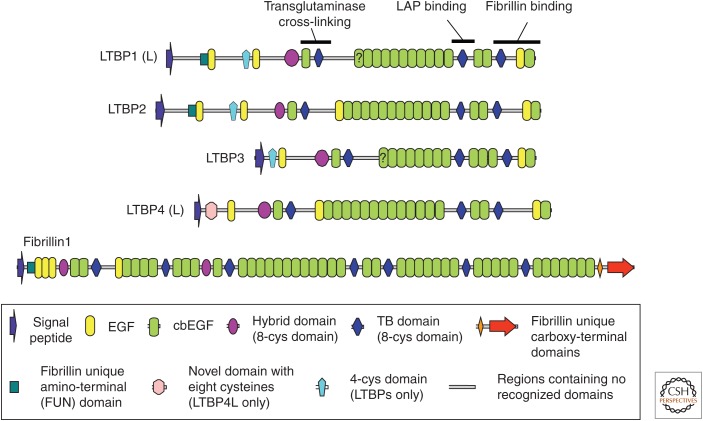
Unlike the TGF-β LAP interactions, which are noncovalent, the binding of LAP with LTBP is mediated by a pair of disulfide bonds between the most amino-terminal cysteines in LAP and a unique cysteine pair in LTBP. The LTBPs are large multidomain glycoproteins that consist of tandem arrays of calcium-binding epidermal growth factor–like domains (cbEGFs), 8-Cys type domains (which can be subcategorized into TGF-β-binding [TB] domains and hybrid domains), and regions with no clear domain homology that introduce flexibility into these proteins (Fig. 2) (Robertson et al. 2014). LTBPs bind LAP through a pair of cysteines in the third 8-Cys domain (or second TB domain) (Gleizes et al. 1996; Saharinen et al. 1996), but within the LTBP family (LTBP-1, -2, -3, and -4), there is variability with respect to the ability to bond to LAP (Saharinen and Keski-Oja 2000). LTBP-1 and -3 bind well to all three TGF-β isoforms, as shown by cotransfection assays, whereas LTBP-2 does not bind at all, and LTBP-4 binds only to TGF-β1-LAP and does so inefficiently. LTBP-4-binding to LAP may be enhanced in an amino-terminally extended LTBP-4 splice form, but the nature and significance of this binding are unclear (Kantola et al. 2010).
Figure 2.
The multidomain structure of latent transforming growth factor β (TGF-β)-binding proteins (LTBPs). Domain structures of the four LTBPs found in the human genome, along with human fibrillin-1 for comparison. Some regions associated with specific functions are highlighted in LTBP-1. The key at the bottom of the figure describes the different domain types. L in LTBP-1 and -4 denotes the long form. The question marks on domains in LTBP-1L and LTBP-3 denote that it is unclear from their sequences whether these EGF domains bind calcium. EGF, Epidermal growth factor domain; cbEGF, calcium-binding epidermal growth factor domain.
The binding of LTBP to the TGF-β pro-peptide is primarily determined by the availability at the protein surface of a cysteine pair in the third 8-Cys (second TB) domain. LTBP-1, -3, and -4 each contain a dipeptide insertion found only in TB domains that bind to LAP (Saharinen and Keski-Oja 2000). The dipeptide is missing in the third 8-Cys domain of LTBP-2 and in all other 8-Cys domains of the LTBPs and the structurally related fibrillins. Addition of the dipeptide into the LTBP-2 third 8-Cys domain converts a nonbinding domain into a LAP-binding domain. The initial docking interaction between LAP and LTBP involves a number of acidic and basic residues, the importance of which has been highlighted by mutagenesis studies (Chen et al. 2005; Walton et al. 2010). The higher number of negatively charged residues arranged around the reactive cysteines may explain the more effective binding of the third 8-Cys domains of LTBP-1 and -3 to SLC compared with LTBP-4. Secretion of LTBP-3 is dependent on association with TGF-β (Penttinen et al. 2002), but LTBP-1, -2, and -4 are secreted efficiently without bound TGF-β (Saharinen et al. 1999).
The potential functions of all four LTBPs in vivo have been investigated using LTBP-null mice (Robertson et al. 2015). LTBP-1 and -4 exist as both long (L) and short (S) forms generated by the use of separate promoters and initiation codons. Deleting exons specific to the generation of the long form of LTBP-1 in mice results in cardiac outflow tract defects that cause embryonic lethality, potentially a result of decreased levels of TGF-β (Todorovic et al. 2007, 2011). Interestingly, deleting an exon shared by both long and short forms of LTBP-1 resulted in only minor craniofacial abnormalities (Drews et al. 2008). The lack of a more severe phenotype appears to be a result of compensatory splicing around the deleted exon (Todorovic and Rifkin 2012), and a study of mice with conditional deletion of exon 8, which prevents expression of both isoforms of LTBP-1, showed similar lethal cardiovascular defects to those seen with LTBP-1L-null mice (Horiguchi et al. 2015). Gene targeting of LTBP-2 on the other hand results in only minor defects, such as disorganization and detachment of the ciliary zonules (Inoue et al. 2014). These results are consistent with LTBP-2’s inability to bind LAP and suggest LTBP-2 may be important for microfibril assembly.
LTBP-3-null mice are viable but display bone defects, including osteopetrosis and premature ossification of the skull synchondroses (Dabovic et al. 2002; Colarossi et al. 2005), phenotypes that indicate reduced TGF-β signaling. Interestingly, morpholino gene silencing of LTBP-3 in zebrafish causes cardiovascular defects not seen in the mouse model (Zhou et al. 2011). These defects were phenotypically similar to those caused by inhibition of TGF-β signaling and were rescued by expression of a constitutively active TGF-β receptor, demonstrating the importance of LTBP-3 in facilitating TGF-β action.
Studies of mice with a disrupted Ltbp4 gene revealed multiple developmental defects including impaired alveolar septation, cardiomyopathy, and rectal prolapse (Sterner-Kock et al. 2002), phenotypes reflected in human patients with LTBP-4 deficiency (Urban et al. 2009). Reduced deposition of extracellular TGF-β and reductions in phospho-Smad2 in various tissues were also seen in this study. However, detailed examination of the lungs of Ltbp4-null animals revealed a surprising increase in phospho-Smad2 (Dabovic et al. 2009). Furthermore, breeding these mice onto a Tgfb2−/− background rescued the alveolar septation defect (Dabovic et al. 2009), suggesting that this defect was actually caused by increased TGF-β signaling. This rescue effect was specific to TGF-β2 as a Tgfb1−/− or Tgfb3−/− background did not rescue the lung defect (Dabovic et al. 2014). To investigate the importance of the LTBP-4–LAP interaction, a mouse was generated in which the cysteines (Cys 1235 and 1260) in LTBP-4 responsible for putative binding to LAP were mutated to serine to inhibit disulfide bond formation. These mice appeared phenotypically normal (Dabovic et al. 2014), which suggests that sequestering TGF-β via LAP is not a significant function of LTBP-4. Additional evidence shows that the primary role of LTBP-4 appears to be the regulation of elastic fiber assembly through its interactions with fibulin-5 and fibrillin (Noda et al. 2013; Dabovic et al. 2014). This makes LTBP-1 and LTBP-3 the LTBPs of greatest interest when considering the extracellular pool of latent TGF-β.
Latency is dependent solely on the presence of LAP as mutant TGF-β1 LAP (C33S) that cannot bind LTBP still maintains TGF-β in the latent state (Gentry and Nash 1990; Annes et al. 2004). This SLC is efficiently secreted without LTBP (Yoshinaga et al. 2008). However, the Cys33 residue in LAP plays an important role in TGF-β1 function in vivo, as mice with the C33S TGF-β1 mutation develop inflammation that is similar to that observed in TGF-β1-null mice (although not as severe), implying defective activation of SLC (Yoshinaga et al. 2008). These mutant mice also develop gastrointestinal tumors (Yoshinaga et al. 2008; Shibahara et al. 2012). If the primary function of this cysteine is binding to LTBP, these results reflect the crucial importance of SLC–LTBP interactions for proper extracellular TGF-β function.
Substitution of Cys223 and Cys225 in TGF-β1 LAP with serines, on the other hand, prevents the dimerization of LAP and results in the secretion of constitutively active TGF-β (Brunner et al. 1989). This mutant form of TGF-β has been used in many transgenic studies that show the destructive potential of the release of constitutively active TGF-β and highlight the importance of latency in proper TGF-β function (Sellheyer et al. 1993; Sanderson et al. 1995). However, although these transgenic studies provide useful information on the effects of a generalized abundance of active TGF-β, they are less informative for examining the more targeted mechanisms of latent TGF-β localization and activation that occur in normal tissues.
Interactions between the LLC and the ECM
Besides promoting effective secretion of latent TGF-β from cells, another major function of LTBPs is localization of latent TGF-β to the ECM (Fig. 1, upper left) (Koli et al. 2005). This feature has many important implications for latent TGF-β activation and TGF-β bioavailability.
The best-studied interactions of an LTBP with the ECM are those of LTBP-1. Initial studies using immunofluorescence to monitor binding of recombinant fragments to matrix showed that LTBP-1 possessed two ECM-binding regions at the amino terminus and one at the carboxyl terminus (Unsold et al. 2001). Further experiments showed specific interactions with fibronectin (Dallas et al. 2005a; Fontana et al. 2005; Kantola et al. 2008) and fibrillin fibers (Isogai et al. 2003; Ono et al. 2009; Massam-Wu et al. 2010), as well as the formation of transglutaminase cross-links with the ECM (Nunes et al. 1997). The specific ECM component to which LTBP-1 is cross-linked was not determined.
The last three carboxy-terminal domains of LTBP-1 interact with the amino terminus of fibrillin (Isogai et al. 2003; Ono et al. 2009), and this region of LTBP-1 contains flexible linkers that may facilitate this interaction (Robertson et al. 2014). The carboxyl termini of LTBP-2 and LTBP-4 are closely homologous with the carboxyl terminus of LTBP-1 and interact with fibrillin in a similar manner (Isogai et al. 2003; Ono et al. 2009). LTBP-3, on the other hand, has strikingly different sequence features at its carboxyl terminus (Robertson et al. 2011, 2014), and a recombinant fragment of this region of LTBP-3 does not interact with the fibrillin amino terminus in solid phase assays, suggesting that the LTBP-3 carboxyl terminus may have distinct properties (Isogai et al. 2003). The interaction of LTBP-1 with fibronectin has not been well characterized, and, in some assays, this interaction is mediated by heparan sulfate proteoglycans (Chen et al. 2007). Fibronectin is essential for the incorporation of LTBP-1 into extracellular fibers in cell culture. Studies conducted by Ono et al. showed LTBP-1 ECM incorporation by murine neonatal fibroblasts (cultured for 13 days) was dependent on fibrillin-1 (Ono et al. 2009). However, more recently, Zilberberg et al. (2012) showed that fibrillin-1 is not essential for incorporation of LTBP-1 into the ECM of aortic smooth muscle cells or primary lung fibroblasts. But fibrillin-1 is still required for matrix incorporation of LTBP-3 and LTBP-4 in these cultures (Zilberberg et al. 2012), suggesting a link between LTBP-3 and the fibrillin matrix, although no direct interaction between these molecules has yet been shown. The assembly of fibrillin fibers is also dependent on fibronectin in most cell cultures (Kinsey et al. 2008; Sabatier et al. 2009), making it difficult to tease apart the relative importance of the fibrillin and fibronectin networks in LTBP incorporation and TGF-β regulation.
The functional significance of TGF-β’s ECM localization is still unclear. Some clues may derive from studies of Marfan syndrome (MFS), a genetic condition caused by mutations in the fibrillin-1 gene resulting in a number of symptoms, including tall stature, arachnodactyly, ectopia lentis, and aortic dilatation. Fibrillin is an important structural component of the ECM (Jensen et al. 2012), but interestingly the reduction of fibrillin-1 levels in MFS also leads to increased levels of active TGF-β (Neptune et al. 2003). Defects in MFS mice, such as impaired alveolar septation, mitral valve prolapse, and aortic dissection, can be prevented by inhibition of TGF-β signaling (Judge et al. 2004; Ng et al. 2004; Habashi et al. 2006, 2011; Cohn et al. 2007). One hypothesis is that an insufficiency of fibrillin-1 fibers leads to a shortage of available sites to anchor latent TGF-β in the matrix, resulting in excess soluble LLC available for activation. An alternative hypothesis is based on the observation that fibronectin contributes to latent TGF-β activation by integrins by providing cell-ECM traction (see below). Therefore, perturbing the strength or structure of the ECM by reducing fibrillin-1 levels in MFS could directly affect latent TGF-β activation via traction, rather than passively releasing more latent cytokine from the matrix. It is also conceivable that perturbations of LTBP–fibrillin interactions are not directly involved in MFS pathology and that cellular responses to a defective matrix actually cause the increase in TGF-β activity. Additionally, direct inhibition of TGF-β in young mice can actually worsen the aortic phenotype (Cook et al. 2015), whereas loss of LTBP3 in fibrillin-deficient mice protects them from developing aortic aneurysms (Zilberberg et al. 2015). Both studies show that the relationship between fibrillin and TGF-β may be more complex and nuanced than first thought.
Disruptions of the ECM associated with enhanced levels of active TGF-β have been reported in a variety of genetic diseases of connective tissue, including multiple myopathic states (Cohn et al. 2007), fibromuscular dysplasia (Ganesh et al. 2014), and geleophysic and acromicric dysplasias (Le Goff et al. 2011; Le Goff and Cormier-Daire 2012). This may indicate that a primary response to abnormal matrix is the activation of latent TGF-β. One report of particular interest shows dysregulated TGF-β signaling in mouse models of osteogenesis imperfecta (OI), a brittle bone disease usually caused by mutations in type I collagen genes (Grafe et al. 2014). Mouse models of both dominant and recessive forms of OI showed increases in TGF-β signaling, and bone density was significantly improved by treatment with the TGF-β neutralizing antibody 1D11 (Grafe et al. 2014). These observations reinforce the idea of latent TGF-β activation as a process responsive to generalized perturbations in matrix structure and integrity.
Recently, LTBP-1 has been shown to bind and colocalize with extracellular deposits of Notch3 present in the cerebral arterioles of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) patients, and although the precise implications for TGF-β signaling are unclear, this further highlights the potential sensitivity of the LLC to extracellular perturbations (Kast et al. 2014).
Other Sources of Latent TGF-β
As well as LTBPs, latent TGF-β binds covalently to the trans-membrane leucine-rich repeat protein glycoprotein-A repetitions predominant protein (GARP) (Wang et al. 2012). GARP expression appears restricted to regulatory T cells and platelets (Wang et al. 2008; Stockis et al. 2009; Tran et al. 2009), whereas LTBPs are expressed ubiquitously (Rifkin 2005). GARP-like leucine-rich repeat proteins do not appear until the evolution of bony fish (Robertson and Rifkin 2013), although TGF-β and LTBPs evolved earlier, as they are found in sea urchins, acorn worms, and lancelets (Robertson et al. 2011; Robertson and Rifkin 2013), suggesting that LTBPs may have more general functions in TGF-β biology, with GARP activities restricted to T-cell and platelet-based functions. In fact, use of anti-GARP antibodies has been reported to suppress the activity of regulatory T-cells and aid with anticancer immunotherapy (Cuende et al. 2015).
E-selectin-ligand-1 binds intracellular pro-TGF-β (Olofsson et al. 1997); however, its role appears to be that of a negative regulator of TGF-β by inhibiting pro-TGF-β intracellular processing and secretion (Yang et al. 2010). The existence of other extracellular latent TGF-β complexes, independent of LTBPs, cannot be ruled out. Mice lacking expression of TGF-β1 and TGF-β3 (Mu et al. 2008), or TGF-β2 and TGF-β3 (Dunker and Krieglstein 2002) display significant deleterious effects in embryonic development, but double-null mutations of LTBP-3 and LTBP-4 are viable after birth and do not show clear phenotypic similarities with TGF-β-null mutations (Dabovic et al. 2011). As previously mentioned LTBP-1-null mice display lethal defects in embryonic heart development (Todorovic et al. 2007, 2011; Horiguchi et al. 2015), but direct phenotypic similarities with TGF-β-null mice are not clearly apparent. Inactivation of both LTBP-1 and LTBP-3 in the same mice has not yet been reported, but these may be the most informative for testing the overall significance of LTBPs in TGF-β regulation in vivo.
Latency and ECM Interactions of Other TGF-β Family Members
TGF-β is not alone in its localization to ECM fibers; many other members of the TGF-β family and their pro-peptides form associations with ECM components. Fibrillin, in particular, interacts directly with the pro-peptides of a number of TGF-β family members, including bone morphogenetic protein 2 (BMP-2), BMP-4, BMP-7, BMP-10, and growth and differentiation factor 5 (GDF-5) (Sengle et al. 2008a). Surface plasmon resonance studies showed that the pro-peptides of these growth factors interact with the amino-terminal regions of fibrillin-1 and -2 with similar affinities (Gregory et al. 2005; Sengle et al. 2008a), and some pro-peptides associate with other regions of the fibrillin molecule. The binding sites for BMP-7 and BMP-5 have been localized more precisely in the fibrillin amino terminus and have been shown to be dependent on the presence of the fibrillin unique amino-terminal (FUN) domain (Sengle et al. 2008a, 2011; Yadin et al. 2013). However, not all pro-domains of the TGF-β family members interact with the amino terminus of fibrillin. For example, the myostatin (GDF-8) pro-domain does not bind this region, but it does associate with the ECM via perlecan domain V glycosaminoglycan (GAG) side chains (Sengle et al. 2011).
Although the interactions between latent growth factors and fibrillin have been clearly shown in vitro, their in vivo significance is unclear. BMP-7 and BMP-4 colocalize with fibrillin-1 in tissue sections (Gregory et al. 2005; Sengle et al. 2008a), suggesting that these interactions occur in vivo, but their importance is unknown. Functional hypotheses might be proposed similar to those suggested for LLC binding to fibrillin; for example, BMP binding to fibrillin may localize and direct growth factor signals to certain regions of the ECM, and so determine specific features of tissue development (Ramirez and Rifkin 2009). TGF-β, BMP-10, and myostatin are latent when complexed with their pro-domains and unable to induce signaling without the help of an activator, for example, BMP-1 can activate BMP-10 by cleaving its pro-domain (Lee and McPherron 2001; Sengle et al. 2011). However, BMP-4, -5, -7, and -9 pro-domain complexes are not latent, and their pro-domains can be displaced from the growth factor allowing these molecules to bind to their receptors without activation (Brown et al. 2005; Sengle et al. 2008b, 2011). The fibrillin interaction might confer “latency” to growth factors not rendered latent by their pro-peptides alone, as localization to the ECM may shield the growth factors from cell-surface receptors. Signaling then could be regulated by displacement of the growth factor from fibrillin or contact of fibrillin microfibrils with the cell. Alternatively, fibrillin interaction could promote growth factor activity by preventing inhibitor binding.
Increased BMP activity in fibrillin-1 deficient osteoblasts is consistent with the hypothesis that fibrillin may inhibit BMPs activity (Nistala et al. 2010), but whether this involves a direct mechanism of fibrillin-binding BMP pro-domains was not tested.
Clues to the in vivo mechanisms by which fibrillins regulate BMP activity are also sparse but there are some suggestive data. For example, fibrillin-2-null mice display syndactyly, whereas BMP-7-deficient mice display polydactyly (Arteaga-Solis et al. 2001). Heterozygous Bmp7+/− or Fbn2+/− mice are normal, but a combined heterozygous deficiency of BMP-7 and fibrillin-2 yields mice with both polydactyly and syndactyly. These enhanced digit abnormalities in double heterozygotes may indicate a form of epistasis. In fibrillin-2 deficient mice transcription of several genes, including Msx, was rendered insensitive to implanted BMP-4 beads. These results suggest that fibrillin-2 and BMPs play interrelated roles in specific developmental pathways, but the full significance of their interaction is unclear.
In summary, there are several pieces of in vivo and in vitro evidence connecting the ECM and signaling by other molecules of the TGF-β family, but the precise mechanisms at work are not yet understood.
ACTIVATION OF LATENT TGF-β
The latent TGF-β complex can be considered as a sensor that remains in the ECM until triggered by a specific signal to release TGF-β (Annes et al. 2003); a process referred to as activation (Fig. 1, upper right). A number of different latent TGF-β activators have been described, ranging from extremes of pH, to proteases, to cell-surface integrins.
ACTIVATION BY PROTEINS
Integrins
Cell-surface integrins are well-established activators of latent TGF-β (Munger et al. 1999; Annes et al. 2002; Mu et al. 2002). Integrins αvβ6 and αvβ8 are the best described activators, but other αv integrins, such as αvβ1, αvβ3, and αvβ5, have also been implicated as interacting with LAP and activating latent TGF-β (Munger et al. 1998; Lu et al. 2002; Ludbrook et al. 2003; Wipff et al. 2007; Wipff and Hinz 2008; Munger and Sheppard 2011; Tatler et al. 2011; Hinz 2013; Sarrazy et al. 2014).
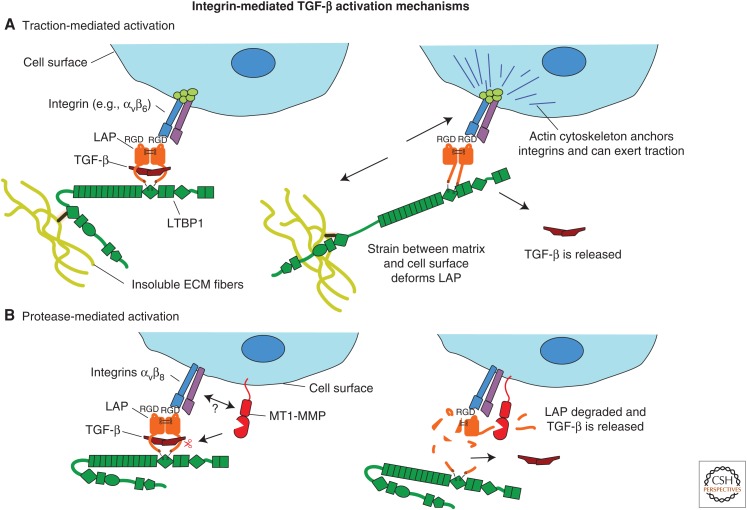
The best-studied mechanism for integrin-mediated activation of latent TGF-β is direct activation by traction between cells and matrix (Fig. 3A). Early studies showed that overexpression of β6 integrin-activated latent TGF-β1, even in the presence of protease inhibitors (Munger et al. 1999). Further studies showed that latent TGF-β1 activation by αvβ6 was dependent on covalent attachment of LAP to LTBP-1 (Annes et al. 2004), the presence of specific ECM-binding regions in the hinge domain of LTBP-1 (Annes et al. 2004), and the presence of a fibronectin matrix (Fontana et al. 2005). The integrin-binding RGD (Arg-Gly-Asp) sequence in LAP and the cytoplasmic sequences of β6 integrin that anchor it to the actin cytoskeleton were also required for TGF-β activation (Munger et al. 1999; Annes et al. 2004). These results are consistent with an activation mechanism whereby the conformation of LAP anchored to the ECM by LTBP and bound to the cell surface by integrins is distorted by traction between matrix and cells, liberating active TGF-β (Annes et al. 2004; Fontana et al. 2005; Wipff et al. 2007). This mechanism has been further elaborated by experiments describing the integrin-dependent release of active TGF-β by contracting myofibroblast cytoskeletons (Wipff et al. 2007), as well as from cell-free matrix in a force-dependent manner by ferromagnetic beads coated with either integrins or anti-LAP antibodies (Buscemi et al. 2011). Indeed, the state of the latent complex may be sensitized for activation by previous modifications of the matrix (Klingberg et al. 2014). The traction mechanism is supported by the crystal structure of TGF-β bound to LAP, which shows that Cys33, required for LTBP attachment, and the RGD motif are on opposite sides of the molecule, with TGF-β sandwiched between two arms of a LAP “straightjacket” (Shi et al. 2011). This straightjacket can be deformed by traction applied to the molecule, as shown by atomic force microscopy experiments (Buscemi et al. 2011).
Figure 3.
Proposed mechanisms of latent transforming growth factor β (TGF-β) activation by integrins. Two mechanisms have been proposed by which integrins activate latent TGF-β. (A) Traction between cells and extracellular matrix (ECM) is transmitted to latency-associated peptide (LAP) via latent TGF-β-binding proteins (LTBPs) bound to the matrix and cell-surface integrins bound to the cell’s actin cytoskeleton. This deforms LAP and releases active TGF-β. (B) Integrins bind LAP at the cell surface and this may make LAP more accessible to proteases, such as membrane-type matrix metalloproteinase 1 (MT1-MMP), that cleave LAP and release the active growth factor, by localizing it and recruiting proteases into its vicinity, or by changing the structure of LAP so that it is rendered more protease sensitive.
The mechanism of latent TGF-β activation by αvβ8 is more obscure. Latent TGF-β activation by this integrin was reported to be blocked by the presence of matrix metalloproteinase (MMP) inhibitors and did not require the cytoplasmic domain of αvβ8, indicating a traction-independent mechanism (Mu et al. 2002). This suggests an alternative protease-dependent mechanism, perhaps involving recruitment of MMPs by integrins to facilitate LAP cleavage and release of TGF-β (Fig. 3B). Alternatively, binding by the integrin may induce changes in LAP structure that promote protease cleavage. MT1-MMP (membrane-type matrix metalloproteinase 1) was suggested as a potential candidate protease, as it both conferred the ability to activate latent TGF-β, when transfected into the αvβ8-expressing cancer line H1264 and colocalizes with integrin αvβ8 at substrate contacts (Mu et al. 2002). However, as described below, inactivation of MMPs does not produce a TGF-β, null-like phenotype.
As well as activating latent TGF-β within the LLC, both integrins αvβ6 and αvβ8 can activate latent TGF-β bound to GARP on the surface of cells expressing this complex (Wang et al. 2012; Edwards et al. 2014). This form of latent TGF-β may play an important role in regulating immune responses, as GARP is primarily expressed by T-cells.
The critical nature of integrin-mediated latent TGF-β activation in vivo was illustrated by a mouse model in which the integrin-binding RGD motif in TGF-β1-LAP was replaced with an RGE sequence (Yang et al. 2007; Aluwihare et al. 2009). These mice phenocopy TGF-β1-null mice and display severe multiorgan inflammation and absence of epidermal Langerhans cells. As the mutant TGF-β is secreted normally, this phenocopy shows that the integrin-binding RGD motif in TGF-β1 LAP is essential for latent TGF-β1 activation. TGF-β3 also contains an RGD motif, the importance of which has not been conclusively tested in vivo. However, mice with null mutations in both αvβ6 and αvβ8 integrins display cleft palate, also observed in TGF-β3 knockout mice. Pharmacological inhibition of αvβ6 in mice lacking αvβ8 causes inflammation similar to that seen in TGF-β1-deficient mice (Yang et al. 2007; Aluwihare et al. 2009). These observations suggest that integrins play a key role in the in vivo activation of both TGF-β1 and 3. Replacement of the TGF-β3 coding sequence with a TGF-β1 sequence into the Tgfb3 locus partially rescues the palate closure defect seen in TGF-β3-null mice (Yang and Kaartinen 2007), indicating that TGF-β1 LAP shares some critical properties with TGF-β3 LAP, as latent TGF-β1 can be appropriately activated in place of TGF-β3 in this context.
In addition to being important for latent TGF-β activation during development, integrins also play a significant role in diseases like fibrosis, in which TGF-β is a major mediator (Leask and Abraham 2004). Conditional deletion of the αv integrin gene significantly attenuated hepatic, pulmonary, and renal fibrosis in mouse models (Henderson et al. 2013), and deletion of integrin β6 helps protect against radiation-induced lung fibrosis (Puthawala et al. 2008). TGF-β is also an important mediator of immune tolerance, and conditional deletion of β8 integrin in dendritic cells causes severe inflammatory bowel disease and autoimmunity (Travis et al. 2007). Integrin β8 expressed by effector regulatory T cells is also important for suppressing aberrant T-cell-mediated inflammation (Worthington et al. 2015), consistent with the role of this integrin in activating TGF-β and promoting immune tolerance. Integrin β8 expressed by lung fibroblasts also plays a critical role in activating TGF-β to regulate dendritic cell trafficking in lung tissue, which can in turn contribute to fibrosis and inflammation (Kitamura et al. 2011). In addition, integrin β8 expressed on dendritic cells regulates maturation of TH17 cells, which contribute to autoimmunity (Melton et al. 2010), and integrin β8-mediated TGF-β activation also inhibits epithelial proliferation in bronchial tissue (Fjellbirkeland et al. 2003). Therapeutic strategies using monoclonal antibodies to target TGF-β activation by integrins have shown promise in treating fibroinflammatory airway disease in animal models (Minagawa et al. 2014).
As well as playing roles in fibrosis and inflammation, TGF-β activation by integrin β8 plays a role in wound closure in vitro (Neurohr et al. 2006) and may act as an angiogenic control switch regulated by perivascular astrocytes (Cambier et al. 2005). Specific expression of integrin β8 by nonmyelinating Schwann cells in bone marrow may assist in maintaining dormancy in hematopoietic stem cells by generating a highly localized niche of active TGF-β near blood vessels (Yamazaki et al. 2011).
Unlike TGF-β1 and 3, TGF-β2 lacks an RGD motif and was not activated in experiments testing latent TGF-β activation by specific integrins (Annes et al. 2002). TGF-β2 contains other conserved motifs in the RGD region that are not found in TGF-β1 and -β3 (Robertson and Rifkin 2013), which suggests the possibility that TGF-β2 is the target of specific activators that bind TGF-β2 LAP in a similar fashion to integrin binding to TGF-β1 and TGF-β3 LAP RGD.
Proteases
A number of proteases of various classes can activate latent TGF-β in vitro, including cysteine proteases like calpain (Abe et al. 1998), aspartyl proteases like cathepsin D (Lyons et al. 1988), and numerous serine and metalloproteases (Maeda et al. 2001; Jenkins 2008). However, the relevance of these assorted proteases for latent TGF-β activation in vivo is unclear, as mice deficient in individual proteases fail to display TGF-β, null-like phenotypes. Although this may seem to suggest a minor biological role for protease-mediated activation, it may also reflect the general ability of multiple proteases to activate latent TGF-β, creating significant redundancy in vivo.
Many of the MMPs, including MMP2, MMP9, MMP3, MMP13, and MMP14 (MT1-MMP) (Jenkins 2008), activate latent TGF-β in vitro. MMP2 and MMP9 activate latent TGF-β when localized by CD44 to the surface of tumor cells during angiogenesis (Yu and Stamenkovic 2000), and MMP3 from chondrocyte-derived matrix vesicles activates latent TGF-β1 (Maeda et al. 2001). Interestingly, although MMP2 is present in these chondrocyte vesicles, it did not activate TGF-β in this context.
Mice deficient in many of these proteases have been generated, but none clearly show TGF-β-related phenotypes. Inactivation of MMP3 yields mice with no visible abnormalities (Mudgett et al. 1998; Johnson et al. 2011), and loss of MMP13 yields only mild skeletal defects in mice (Stickens et al. 2004). Mice lacking expression of both MMP2 and MMP9 are viable (Garg et al. 2009), as are mice lacking MMP9 and MMP13 expression (Stickens et al. 2004), although these mice are runted because of defects in their growth plates. MT1-MMP (MMP14)-deficient mice suffer from osteopenia, dwarfism, and fibrosis, but this is likely a result of deficiencies in collagen turnover and not because of insufficient TGF-β activation (Holmbeck et al. 1999). MMP2- and MT1-MMP-deficient mice display respiratory failure, abnormal blood vessel development, immature muscle fibers, and die immediately after birth (Oh et al. 2004). However, none of these phenotypes are reminiscent of defective TGF-β signaling.
The BMP-1/tolloid MMPs also play a role in latent TGF-β activation. However, rather than directly degrading LAP, they catalyze the cleavage of LTBP-1 from the ECM, releasing latent TGF-β bound to LTBP-1, which requires activation by other factors, such as MMPs (Ge and Greenspan 2006). Mice with null mutations in BMP-1 and the closely related tolloid1 (Tll1) protease display excessive accumulation of LTBP-1 in embryonic tissues and reduced phospho-Smad2/3 staining, suggesting defective activation of latent TGF-β (Ge and Greenspan 2006). Mice deficient in BMP-1 or Tll1 expression die before birth and these proteins process a variety of other matrix and signaling molecules; therefore, it is difficult to attain a clear assessment of their importance for latent TGF-β activation in vivo (Pappano et al. 2003).
Various serine proteases, including kallikreins and plasmin, activate TGF-β (Lyons et al. 1990). Plasmin mediates TGF-β activation in cocultures of endothelial cells and pericytes (Antonelli-Orlidge et al. 1989; Sato and Rifkin 1989), in cultures of endothelial cells treated with retinoids (Kojima and Rifkin 1993), and on the surface of activated macrophages (Nunes et al. 1995; Yehualaeshet et al. 1999; Jenkins 2008). Activation in endothelial-pericyte cocultures was inhibited by antibodies to LTBP-1 (Flaumenhaft et al. 1993) and inhibitors of the mannose-6-phosphate receptor (Dennis and Rifkin 1991). Activation by macrophages was dependent on plasmin and latent TGF-β bound at the cell surface by thrombospondin1 (TSP1) and CD36 (Yehualaeshet et al. 1999; Jenkins 2008). Despite the importance of plasmin in several TGF-β-activating cell systems, mice with targeted deletion of the plasminogen gene show no clear developmental abnormalities and are viable (Bugge et al. 1995), which rules out a nonredundant role for this protease in latent TGF-β activation.
Kallikreins are a large family of serine proteases, several of which activate latent TGF-β. Kallikreins appear to play a role in TGF-β-mediated immunosuppression in seminal plasma (Emami and Diamandis 2010) and lipopolysaccharide impairment of liver regeneration (Akita et al. 2002). Plasma kallikrein (PLK) cleaves TGF-β1 LAP between Arg58 and Leu59. Antibodies have been raised against the resulting LAP peptides and used to show that the specific PLK-LAP cleavage products are significantly more abundant in the liver tissue of patients with hepatic fibrosis (Hara et al. 2014). Prostate-specific antigen (PSA), a member of the kallikrein family, activates TGF-β2 but not TGF-β1 (Dallas et al. 2005b). The large number of kallikrein proteases again provides significant potential redundancy and, at present, only limited data on kallikrein-null mice are available (Bergaya et al. 2001).
Although many proteases activate latent TGF-β in a variety of contexts, most of these studies have been conducted in vitro, and obtaining clear in vivo data on the role of proteases in latent TGF-β activation is difficult for the reasons discussed above. One way to gain future insight into the significance of protease-mediated latent TGF-β activation in vivo might be to identify specific cleavage sites in LAP or LTBP responsible for activation, and generate mice with mutations replacing these sites with protease-insensitive sequences.
Deglycosylation
Deglycosidases, including endoglycosidase F (Miyazono and Heldin 1989) and influenza neuramidase, activate latent TGF-β (Schultz-Cherry and Hinshaw 1996; Carlson et al. 2010). The LAPs of human TGF-β1 and -β2 possess three potential N-glycosylation sites, whereas TGF-β3 LAP has only two sites. It is not clear which sites are important for activation by deglycosidases or what the activation mechanism may be. One possibility is that removing sugar groups exposes protease-sensitive sites, as activation by neuramidase is at least partially protease dependent (Carlson et al. 2010). The in vivo role of deglycosylation-driven latent TGF-β activation also remains undefined.
Other Protein Factors
As well as the molecules discussed above, a diverse range of other proteins have been implicated as latent TGF-β activators.
TSP-1 has been the subject of particularly intense study with respect to latent TGF-β activation. Recombinant TSP-1 activates latent TGF-β when added to cell cultures or purified latent TGF-β in vitro (Murphy-Ullrich and Poczatek 2000). TSP-1 interacts with both LAP and active TGF-β in copurification and pull-down assays (Murphy-Ullrich et al. 1992; Yang et al. 1997; Ribeiro et al. 1999). A KRFK peptide that mimics the RFK sequence between the first and second TSP-1 domains activates latent TGF-β (Schultz-Cherry et al. 1994, 1995) and inhibits TSP-1 binding. The ability of an LSKL peptide to inhibit this activation further suggested that interactions between the RFK motif of TSP-1 and the LSKL motif in the amino terminus of LAP were responsible for TGF-β release (Ribeiro et al. 1999). Consistent with this hypothesis is the observation that TSP-2 does not activate latent TGF-β, as the RFK motif is absent from TSP-2 (Schultz-Cherry et al. 1995).
Although these are interesting results, some groups have failed to observe latent TGF-β activation in response to TSP-1 (Grainger and Frow 2000), and surface plasmon resonance studies failed to show TSP-1 binding to either LAP, TGF-β, or SLC (Bailly et al. 1997). Moreover, platelet α-granules activate latent TGF-β and are a rich source of TSP-1, but α-granules derived from TSP-1-null platelets activate latent TGF-β just as effectively as those from wild-type platelets (Abdelouahed et al. 2000).
TSP-1-null mice suffer from inflammation of the lung and pancreas as well as various other defects (Lawler et al. 1998). The sites of inflammation overlap, in part, with those observed in the multiorgan inflammation phenotype of TGF-β1 knockout mice (Kulkarni et al. 1993; Crawford et al. 1998). Reduced inflammation was observed in Tsp1−/− animals treated with the latent TGF-β-activating KRFK peptide, whereas treating wild-type mice with the LSKL peptide that inhibits activation resulted in increased inflammation (Crawford et al. 1998). However, Tsp1−/− mice fail to mimic all of the features of TGF-β1-null mice, such as cardiac inflammation. Tsp1−/− animals also display phenotypes, like kyphosis, not seen in TGF-β1-null animals. Moreover, although TSP-1 and the KRFK peptide-activated latent TGF-β2 in vitro (Ribeiro et al. 1999), TSP-1-null animals do not display any of the serious defects seen in TGF-β2 knockout mice. This leaves the debate over the exact role of TSP-1 in vivo unresolved, but rules out TSP-1 as a central activator of all three TGF-β isoforms.
F-spondin (or spondin-1) increases TGF-β activity in cartilage explants (Attur et al. 2009). An antibody targeted to the F-spondin thrombospondin type 1 repeats blocks this activation, suggesting parallels with activation by TSP-1. However, more information is needed on whether F-spondin activates latent TGF-β by binding directly to LAP or if some other mechanism is at work in these cultures.
Neuropilin (Nrp) is a cell-surface protein reported to act as an activator of latent TGF-β on the surface of T cells (Glinka and Prud’homme 2008; Glinka et al. 2011) and in some tumor lines (Glinka et al. 2011). The mechanism by which Nrp activates latent TGF-β remains undefined, but in solid-phase assays Nrp binds mature TGF-β1, LAP, and both type I and II TGF-β receptors (Glinka et al. 2011). Nrp may initiate intracellular signaling events and could act as a co-receptor for TGF-β (Glinka and Prud’homme 2008; Glinka et al. 2011; Grandclement et al. 2011).
Pregnancy specific glycoprotein 1 (PSG1) is another protein with no known enzymatic activity recently shown to activate both latent TGF-β1 and 2. PSG1 also inhibits dextran sodium sulfate–induced colitis in a TGF-β-dependent manner (Blois et al. 2013). However, both the molecular mechanism and in vivo significance of this activator remain unknown. Tenascin-X has also been reported to promote epithelial to mesenchymal transition by activating TGF-β through interactions between its fibrinogen-like domain and the SLC. These interactions may deform LAP and also rely on cell adhesion via integrin α11β1 to promote activation (Alcaraz et al. 2014).
Activation by Physicochemical Factors
Latent TGF-β can be activated by exposure to specific physical or chemical conditions, including detergents, ionizing, and ultraviolet (UV) radiation (Barcellos-Hoff et al. 1994; Barcellos-Hoff 1996; Ehrhart et al. 1997; Wang and Kochevar 2005; Anscher et al. 2006; Biswas et al. 2007), reactive oxygen species (ROS) (Barcellos-Hoff and Dix 1996), heat (Brown et al. 1990), physical shear (Ahamed et al. 2008), and extremes of pH (Lyons et al. 1988).
Not all of these conditions are encountered in vivo, but some may be biologically relevant. For example, ROS activate latent TGF-β both in cell cultures and in cell-free systems (Barcellos-Hoff and Dix 1996), and ROS are proposed to mediate latent TGF-β activation by asbestos, which can lead to mesothelioma in exposed lungs (Pociask et al. 2004). ROS produced by HIV-infected regulatory T cells are also proposed to activate latent TGF-β and lead to immunosuppression (Amarnath et al. 2007). ROS may mediate latent TGF-β activation caused by ionizing (Barcellos-Hoff 1996) and UV-B radiation (Wang and Kochevar 2005). One study suggested that ROS activation was specific to TGF-β1 and relied on the presence of Met253 (Jobling et al. 2006), but this residue is not well conserved (Robertson and Rifkin 2013) and the exact mechanism by which ROS activate latent TGF-β is unclear. One possibility is that ROS catalyze direct breaks in the backbone of LAP to release the active growth factor (Barcellos-Hoff and Dix 1996), but it is not known if LAP is particularly sensitive to ROS-driven scission.
Latent TGF-β activation by low pH could play a physiological role in some circumstances. Osteoclasts reduce extracellular pH to ∼4.5 during bone resorption (Teitelbaum 2000), and these cells activate latent TGF-β (Oreffo et al. 1989). Tumor cells also significantly lower the pH of their microenvironment (Jullien et al. 1989). However, whether the drop in pH is both necessary and sufficient for latent TGF-β activation in this later context has not been clearly shown. Lactic acid also promotes TGF-β activity at physiological concentrations and may be a player in idiopathic pulmonary fibrosis (Kottmann et al. 2012). But, although the ability of lactate to up-regulate α-smooth muscle actin was dependent on TGF-β signaling and the lowered pH of the medium, studies of lactic acid in other contexts have shown that lactate up-regulates genes that may affect TGF-β bioavailability (Seliger et al. 2013). The effects of physiological levels of lactic acid on latent TGF-β in a cell free system have not yet been reported.
Physical shear or stirring can activate a significant percentage of the latent TGF-β present in platelet releasate (Ahamed et al. 2008). This reaction is dependent on LTBP binding to LAP, as activation is not seen with the releasate from C33S TGF-β1 mice (Ahamed et al. 2008). The full activation mechanism is not known, but it is inhibited by the thiol isomerase-binding peptide mastoparan and appears to involve disulfide exchange (Brophy et al. 2013).
Although there is compelling evidence for many physicochemical factors playing important roles in TGF-β biology, the current lack of detailed activation mechanisms and specific residues to target for knockin studies prevent us from testing their true significance in vivo.
ACTIVATION OF TGF-β—AN EVOLUTIONARY PERSPECTIVE
The range of latent TGF-β activation mechanisms may seem daunting, especially when trying to consider what the trigger for TGF-β activity may be in different tissue contexts. In vivo studies knocking out specific activators or key activation sequences cast some light on their relative significance. However, these experiments are difficult and time consuming and so there remain many potential activators whose significance has not yet been tested.
An alternative approach to qualitatively gauge the significance of various latent TGF-β activators is to examine the evolutionary time frames in which they emerge and to analyze the conservation of residues critical to specific activation mechanisms.
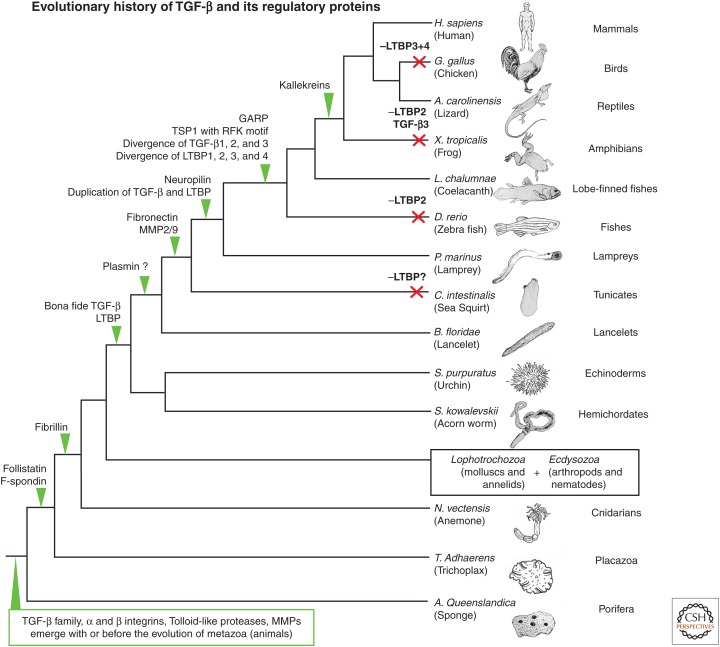
This evolutionary analysis has been performed for a variety of established latent TGF-β activators and has provided a number of insights (Fig. 4) (Robertson et al. 2011; Robertson and Rifkin 2013). One interesting observation is that bona fide TGF-β seems to have coevolved with LTBPs and that the key sequence features for the attachment of LTBP and LAP are present in the most evolutionary distant TGF-β and LTBP sequences, including those in sea urchins, lancelets, and acorn worms (Robertson et al. 2011; Robertson and Rifkin 2013). Additionally, integrin-binding RGD motifs are also present in these evolutionary distant TGF-β sequences and are highly conserved through to humans (except in TGF-β2, where they are consistently absent).
Figure 4.
Evolution of transforming growth factor β (TGF-β) and its extracellular regulators. Phylogenetic tree highlighting the evolutionary relationships between a selection of organisms with sequenced genomes. Green arrows show where specific proteins appear to have evolved based on their presence or absence from available genome and other sequence libraries. Red crosses indicate where specific genes appear to have been lost. The variable quality of some shotgun genome sequences means that the absence of a gene from a specific species is not always definitive, as some genes may be missed or fragmented in the available sequence data. This introduces some uncertainty to the evolutionary story, but ambiguity is kept to a minimum by searching through the many different genomes available. GARP, Glycoprotein A repetitions predominant protein; TSP1, thrombospondin 1; LTBP, TGF-β-binding protein; MMP, matrix metalloproteinase; H. sapiens, Homo sapiens; G. gallus, Gallus gallus; A. carolinensis, Anolis carolinensis; X. tropicalis, Xenopus tropicalis; L. chalumnae, Latimeria chalumnae; D. rerio, Danio rerio; P. marinus, Perkinsus marinus; C. intestinalis, Ciona intestinalis; B. floridae, Branchiostoma floridae; S. purpuratus, Strongylocentrotus purpuratus; S. kowalevskii, Saccoglossus kowalevskii; N. vectensis, Nematostella vectensis; T. Adhaerens, Trichoplax Adhaerens; A. Queenslandica, Amphimedon Queenslandica.
These facts suggest that all elements required for traction-induced latent TGF-β activation by integrins were present soon after “true” TGF-β diverged from the rest of the TGF-β family. If integrins were initially the main activators of latent TGF-β, this would be consistent with their continued in vivo significance, as observed in mouse models (Yang et al. 2007; Aluwihare et al. 2009). On the other hand, the evolutionary data do not support an early evolving function for TSP-1, plasmin, or many other specific proteases, which in many cases do not seem to emerge until well after the LTBP–TGF-β complex first appeared (Robertson and Rifkin 2013).
If we make the assumption that the earliest evolving latent TGF-β activators have remained important throughout evolution, traction-mediated activation by integrins may continue to be an important mechanism for releasing TGF-β in many tissue contexts. Therefore, we propose that future studies examining the role of TGF-β in development and homeostasis should give careful consideration the dynamic relationship between cells and their biomechanical environment.
POSTACTIVATION BIOAVAILABILITY OF TGF-β AND OTHER TGF-β FAMILY MEMBERS
One generalization that distinguishes bona fide TGF-β from many other members of the family, such as the BMPs and GDFs, is that TGF-β bioavailability is primarily regulated by binding and release from LAP, which locks up TGF-β before it leaves the cell. However, the biological regulators characterized for BMPs and GDFs generally bind to the active forms of these growth factors after they have been secreted. The release of certain BMPs and GDFs by specific cells can generate morphogen gradients, and their antagonists help to localize and confine the activity of these growth factors once in the extracellular space. These regulation mechanisms have been discussed in more detail elsewhere (Hinck et al. 2016; Kim et al. 2016), but they are mentioned here in passing to highlight key conceptual differences between signaling by bona fide TGF-β and signaling by other members of the family.
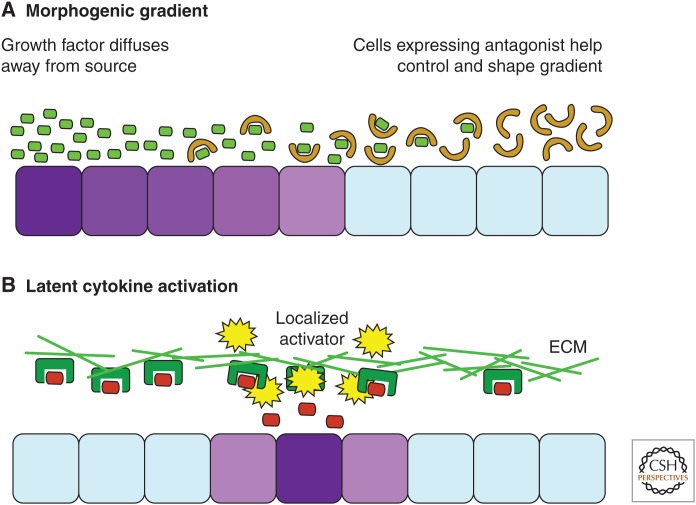
The simplified concepts of growth-factor signaling by localized activation or morphogen gradients are shown in Figure 5. In a morphogen gradient, the active growth factor is released by a specific cellular source and its concentration reduces as it diffuses away from that source. Further control may also be introduced by antagonists that prevent the diffusing growth factors from binding their receptors and add further 3D structure to the growth-factor gradient. The active concentration of a growth factor in any given region of the morphogen gradient can provide individual cells with an indication of their relative position within a developing organism, which may help define their niches within a tissue. In the localized activation model, on the other hand, the growth factor is predistributed throughout the ECM in its latent state. Its local concentration is determined by the rate of activation rather than its rate of release by cells and its diffusion from a central source. One might speculate that this mode of activation may allow bona fide TGF-β to act as a more direct readout of local extracellular events rather than a long distance signal of developmental status.
Figure 5.
Conceptual mechanisms for the extracellular regulation of transforming growth factor β (TGF-β) and bone morphogenetic protein (BMP)/growth and differentiation factor (GDF) compared. Growth factors may convey information to cells in a number of ways. Shown here are some hypothetical schemes for ways in which active growth factors may be distributed in the extracellular matrix (ECM). Cells are colored purple to indicate their responsiveness to growth-factor signaling, or light blue if unresponsive. (A) Here, cells are provided with a measure of their position relative to the source of the growth factor, as its concentration will decrease when diffusing away from the site of release. Antagonists further control this gradient, which becomes even more relevant when considering the three dimensions of a developing tissue. Growth factors are light green and antagonists light brown. (B) Here, the growth factor does not come from a localized source but is distributed through the matrix in a latent state. The local concentration of growth factor is determined by the availability of activators to release it from this state. Also, depending on how quickly the growth factor is cleared from the extracellular space, it may not have time to diffuse far, which could limit gradient formation. Growth factors are red, whereas the ECM and proteins that anchor them are dark green. These different concepts could be important when considering growth-factor signaling from a systems biology perspective.
Although we currently consider factors that regulate TGF-β latency and activation to be the key controllers of its signaling, there are also a variety of proteins that bind and regulate active TGF-β after its release from LLC. For example, the proteoglycan decorin binds active TGF-β (Hildebrand et al. 1994; Schonherr et al. 1998) and appears to inhibit TGF-β signaling in many contexts (Isaka et al. 1996; Teicher et al. 1997), although early studies of decorin suggested that it could enhance TGF-β bioactivity in the bone matrix (Takeuchi et al. 1994). Decorin can inhibit TGF-β signaling through calcium-dependent phosphorylation of Smad2 at Ser240 (Abdel-Wahab et al. 2002), and may interface with TGF-β signaling on a more complex level than just binding the active growth factor. In the bone marrow stroma, both decorin and biglycan appear to work together to inhibit TGF-β signaling and maintain sufficient osteoblast proliferation (Bi et al. 2005). Other molecules that may interact with active TGF-β include collagen IV in the basement membrane (Paralkar et al. 1991), human IgG (Stach and Rowley 1993; Bouchard et al. 1995), and the cell-surface proteins β glycan (also known as TGF-β type III receptor) and endoglin, which may play a role as TGF-β co-receptors.
It is important to consider that TGF-β is a “sticky” molecule that associates nonspecifically with hydrophobic surfaces. TGF-β is active at very low concentrations and copurifies with a number of His-tagged proteins when they are expressed and purified from HEK293 cells (Kaur and Reinhardt 2012), as well as with gelatin-purified fibronectins (Fava and McClure 1987). These properties further complicate interpretation of in vitro experiments, and underline the importance of in vivo studies to confirm proposed biochemical mechanisms.
To ensure that the TGF-β signal does not persist after activation, mature TGF-β is rapidly cleared from the extracellular space. α2-Macroglobulin binds TGF-β and may mediate its endocytosis (LaMarre et al. 1990, 1991). However, α2-macroglobulin knockout mice have no clear defects to suggest excessive accumulation of active TGF-β in tissues (Umans et al. 1995), and so the full mechanisms by which active TGF-β is cleared from the ECM remain a mystery.
Although the biological data on how active TGF-β is regulated after its release from LAP remain patchy, antagonists of other members of the TGF-β family have been well described and shown to play significant roles in vivo. Myostatin (GDF-8) and activin, for example, are more closely related to TGF-β than many other TGF-β family members (Burt and Law 1994; Herpin et al. 2004), and although not much is known about the significance of their pro-peptides in their regulation, follistatin and follistatin-related proteins play a significant role in controlling these two growth factors by binding and blocking their interaction with receptors (Walton et al. 2011). The interplay of myostatin and follistatin has been particularly well examined in the context of muscle growth, where loss of myostatin causes widespread muscle hypertrophy and hyperplasia (McPherron et al. 1997). Transgenic overexpression of follistatin in skeletal muscle causes similar muscle overgrowth (Lee and McPherron 2001), but follistatin also stimulates muscle hypertrophy through myostatin-independent mechanisms (Winbanks et al. 2012). Follistatin-null mice display multiple developmental defects, reflecting its important role in regulating bioavailability of both activin and myostatin (Matzuk et al. 1995).
A diverse array of other proteins is hypothesized to regulate the bioavailability of TGF-β family members by binding and sequestration. Unlike TGF-β, many BMPs are biologically active when they are secreted from cells, and form morphogenic gradients (Gazzerro and Canalis 2006). A number of BMP antagonists share little clear sequence homology, but several contain a “cystine-knot” structural motif, which is a highly versatile fold that often forms dimers and is found in a diverse selection of ECM proteins. For example, cystine-knot motifs also comprise the growth factor domains of the TGF-β family (Vitt et al. 2001), suggesting a common, if extremely distant, evolutionary ancestor for both the TGF-β family and many of its antagonists. Noggin, chordin, and DAN family proteins all interact with multiple members of the BMP family and are crucial in the biology of these signaling proteins.
There are clearly a large number of both TGF-β family members and their antagonists. The full significance of this diversity is far from well understood, and the ability of multiple TGF-β family members to bind multiple antagonists and receptors introduces significant potential for redundancy, multifunctionality, and the formation of complex protein gradients in a developing organism. This complexity may, in turn, play an important role in directing the elaborate patterns adopted by our organs and tissues during development.
CONCLUDING REMARKS
The bioavailability of TGF-β and TGF-β family members is regulated in complex and diverse ways. It is rarely appropriate to consider these growth factors as simple autocrine or paracrine signals sent from one cell to another. Instead, they must be considered in the broader structural context of the ECM, both in terms of how they may be sequestered, stored, and concentrated although interactions with insoluble matrix components, and also how these growth factors will be regulated by gradients and localization of specific inhibitors and activators.
This field poses many complex challenges for future study, which will require extensive and coordinated efforts to decipher the key mechanisms at work and their significance in health and disease. However, the considerable therapeutic potential of modulating TGF-β family signaling in an extracellular context makes this research a particularly pressing endeavor.
ACKNOWLEDGMENTS
We acknowledge B. Dabovic, M. Horiguchi, and L. Zilberberg for their insightful comments, and funding from National Institutes of Health (NIH) Grants R01 CA034282 (Regulation of TGF-β Activity in the Lung by LTBP-4) and P01 AR49698 (Cell Signaling in Marfan Syndrome) to D.B.R.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
- Abdelouahed M, Ludlow A, Brunner G, Lawler J. 2000. Activation of platelet-transforming growth factor β-1 in the absence of thrombospondin-1. J Biol Chem 275: 17933–17936. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab N, Wicks SJ, Mason RM, Chantry A. 2002. Decorin suppresses transforming growth factor-β-induced expression of plasminogen activator inhibitor-1 in human mesangial cells through a mechanism that involves Ca2+-dependent phosphorylation of Smad2 at serine-240. Biochem J 362: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Oda N, Sato Y. 1998. Cell-associated activation of latent transforming growth factor-β by calpain. J Cell Physiol 174: 186–193. [DOI] [PubMed] [Google Scholar]
- Ahamed J, Burg N, Yoshinaga K, Janczak CA, Rifkin DB, Coller BS. 2008. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-β1. Blood 112: 3650–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita K, Okuno M, Enya M, Imai S, Moriwaki H, Kawada N, Suzuki Y, Kojima S. 2002. Impaired liver regeneration in mice by lipopolysaccharide via TNF-α/kallikrein-mediated activation of latent TGF-β. Gastroenterology 123: 352–364. [DOI] [PubMed] [Google Scholar]
- Alcaraz LB, Exposito JY, Chuvin N, Pommier RM, Cluzel C, Martel S, Sentis S, Bartholin L, Lethias C, Valcourt U. 2014. Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-β. J Cell Biol 205: 409–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. 2009. Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci 122: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarnath S, Dong L, Li J, Wu Y, Chen W. 2007. Endogenous TGF-β activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25− T cells. Retrovirology 4: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Rifkin DB, Munger JS. 2002. The integrin αVβ6 binds and activates latent TGFβ3. FEBS Lett 511: 65–68. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. 2003. Making sense of latent TGF-β activation. J Cell Sci 116: 217–224. [DOI] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. 2004. Integrin αVβ6-mediated activation of latent TGF-β requires the latent TGF-β-binding protein-1. J Cell Biol 165: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anscher MS, Thrasher B, Rabbani Z, Teicher B, Vujaskovic Z. 2006. Antitransforming growth factor-β antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys 65: 876–881. [DOI] [PubMed] [Google Scholar]
- Antonelli-Orlidge A, Saunders KB, Smith SR, D’Amore PA. 1989. An activated form of transforming growth factor β is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci 86: 4544–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F. 2001. Regulation of limb patterning by extracellular microfibrils. J Cell Biol 154: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attur MG, Palmer GD, Al-Mussawir HE, Dave M, Teixeira CC, Rifkin DB, Appleton CT, Beier F, Abramson SB. 2009. F-spondin, a neuroregulatory protein, is up-regulated in osteoarthritis and regulates cartilage metabolism via TGF-β activation. FASEB J 23: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly S, Brand C, Chambaz EM, Feige JJ. 1997. Analysis of small latent transforming growth factor-β complex formation and dissociation by surface plasmon resonance. Absence of direct interaction with thrombospondins. J Biol Chem 272: 16329–16334. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH. 1996. Latency and activation in the control of TGF-β. J Mamm Gland Biol Neoplasia 1: 353–363. [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Dix TA. 1996. Redox-mediated activation of latent transforming growth factor-β1. Mol Endocrinol 10: 1077–1083. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. 1994. Transforming growth factor-β activation in irradiated murine mammary gland. J Clin Invest 93: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergaya S, Meneton P, Bloch-Faure M, Mathieu E, Alhenc-Gelas F, Levy BI, Boulanger CM. 2001. Decreased flow-dependent dilation in carotid arteries of tissue kallikrein-knockout mice. Circ Res 88: 593–599. [DOI] [PubMed] [Google Scholar]
- Bi Y, Stuelten CH, Kilts T, Wadhwa S, Iozzo RV, Robey PG, Chen XD, Young MF. 2005. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem 280: 30481–30489. [DOI] [PubMed] [Google Scholar]
- Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. 2007. Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest 117: 1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blois SM, Sulkowski G, Tirado-Gonzalez I, Warren J, Freitag N, Klapp BF, Rifkin D, Fuss I, Strober W, Dveksler GS. 2013. Pregnancy-specific glycoprotein 1 (PSG1) activates TGF-β and prevents dextran sodium sulfate (DSS)-induced colitis in mice. Mucosal Immunol 7: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Galinha A, Tartour E, Fridman WH, Sautes C. 1995. A transforming growth factor β-like immunosuppressive factor in immunoglobulin G-binding factor. J Exp Med 182: 1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy TM, Coller BS, Ahamed J. 2013. Identification of the thiol isomerase-binding peptide, mastoparan, as a novel inhibitor of shear-induced transforming growth factor β1 (TGF-β1) activation. J Biol Chem 288: 10628–10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Wakefield LM, Levinson AD, Sporn MB. 1990. Physicochemical activation of recombinant latent transforming growth factor-β’s 1, 2, and 3. Growth Factors 3: 35–43. [DOI] [PubMed] [Google Scholar]
- Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L, Singh M, Tsareva T, Parice Y, Mahoney A, et al. 2005. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem 280: 25111–25118. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Marquardt H, Malacko AR, Lioubin MN, Purchio AF. 1989. Site-directed mutagenesis of cysteine residues in the pro region of the transforming growth factor β1 precursor. Expression and characterization of mutant proteins. J Biol Chem 264: 13660–13664. [PubMed] [Google Scholar]
- Bugge TH, Flick MJ, Daugherty CC, Degen JL. 1995. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproduction. Genes Dev 9: 794–807. [DOI] [PubMed] [Google Scholar]
- Burt DW, Law AS. 1994. Evolution of the transforming growth factor-β superfamily. Prog Growth Factor Res 5: 99–118. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. 2011. The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol 21: 2046–2054. [DOI] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. 2005. Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: An angiogenic control switch. Am J Pathol 166: 1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CM, Turpin EA, Moser LA, O’Brien KB, Cline TD, Jones JC, Tumpey TM, Katz JM, Kelley LA, Gauldie J, et al. 2010. Transforming growth factor-β: Activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog 6: e1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ali T, Todorovic V, O’Leary JM, Kristina Downing A, Rifkin DB. 2005. Amino acid requirements for formation of the TGF-β-latent TGF-β-binding protein complexes. J Mol Biol 345: 175–186. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sivakumar P, Barley C, Peters DM, Gomes RR, Farach-Carson MC, Dallas SL. 2007. Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-β (TGF-β) by modulating assembly of latent TGF-β-binding protein-1. J Biol Chem 282: 26418–26430. [DOI] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, et al. 2007. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nat Med 13: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colarossi C, Chen Y, Obata H, Jurukovski V, Fontana L, Dabovic B, Rifkin DB. 2005. Lung alveolar septation defects in Ltbp-3-null mice. Am J Pathol 167: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JR, Clayton NP, Carta L, Galatioto J, Chiu E, Smaldone S, Nelson CA, Cheng SH, Wentworth BM, Ramirez F. 2015. Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler Thromb Vasc Biol 35: 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. 1998. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 93: 1159–1170. [DOI] [PubMed] [Google Scholar]
- Cuende J, Lienart S, Dedobbeleer O, van der Woning B, De Boeck G, Stockis J, Huygens C, Colau D, Somja J, Delvenne P, et al. 2015. Monoclonal antibodies against GARP/TGF-β1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Sci Transl Med 7: 284ra256. [DOI] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Colarossi C, Obata H, Zambuto L, Perle MA, Rifkin DB. 2002. Bone abnormalities in latent TGF-β-binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-β bioavailability. J Cell Biol 156: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis EC, Rifkin DB. 2009. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-β activity. J Cell Physiol 219: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Choi J, Davis EC, Sakai LY, Todorovic V, Vassallo M, Zilberberg L, Singh A, Rifkin DB. 2011. Control of lung development by latent TGF-β-binding proteins. J Cell Physiol 226: 1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabovic B, Robertson IB, Zilberberg L, Vassallo M, Davis EC, Rifkin DB. 2014. Function of latent TGF-β-binding protein 4 and fibulin 5 in elastogenesis and lung development. J Cell Physiol 230: 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas SL, Park-Snyder S, Miyazono K, Twardzik D, Mundy GR, Bonewald LF. 1994. Characterization and autoregulation of latent transforming growth factor β (TGF-β) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGF-β-binding protein. J Biol Chem 269: 6815–6821. [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. 2005a. Fibronectin regulates latent transforming growth factor-β (TGF-β) by controlling matrix assembly of latent TGF-β-binding protein-1. J Biol Chem 280: 18871–18880. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Zhao S, Cramer SD, Chen Z, Peehl DM, Bonewald LF. 2005b. Preferential production of latent transforming growth factor β-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J Cell Physiol 202: 361–370. [DOI] [PubMed] [Google Scholar]
- Dennis PA, Rifkin DB. 1991. Cellular activation of latent transforming growth factor β requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci 88: 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews F, Knobel S, Moser M, Muhlack KG, Mohren S, Stoll C, Bosio A, Gressner AM, Weiskirchen R. 2008. Disruption of the latent transforming growth factor-β-binding protein-1 gene causes alteration in facial structure and influences TGF-β bioavailability. Biochim Biophys Acta 1783: 34–48. [DOI] [PubMed] [Google Scholar]
- Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. 1995. Processing of transforming growth factor β1 precursor by human furin convertase. J Biol Chem 270: 10618–10624. [DOI] [PubMed] [Google Scholar]
- Dunker N, Krieglstein K. 2002. Tgfβ2−/− Tgfβ3−/− double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol (Berl) 206: 73–83. [DOI] [PubMed] [Google Scholar]
- Edwards JP, Thornton AM, Shevach EM. 2014. Release of active TGF-β1 from the latent TGF-β1/GARP complex on T regulatory cells is mediated by integrin β8. J Immunol 193: 2843–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart EJ, Segarini P, Tsang ML, Carroll AG, Barcellos-Hoff MH. 1997. Latent transforming growth factor β1 activation in situ: Quantitative and functional evidence after low-dose γ-irradiation. FASEB J 11: 991–1002. [DOI] [PubMed] [Google Scholar]
- Emami N, Diamandis EP. 2010. Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGF β1 in semen. Biol Chem 391: 85–95. [DOI] [PubMed] [Google Scholar]
- Fava RA, McClure DB. 1987. Fibronectin-associated transforming growth factor. J Cell Physiol 131: 184–189. [DOI] [PubMed] [Google Scholar]
- Fjellbirkeland L, Cambier S, Broaddus VC, Hill A, Brunetta P, Dolganov G, Jablons D, Nishimura SL. 2003. Integrin αvβ8-mediated activation of transforming growth factor-β inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol 163: 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaumenhaft R, Abe M, Sato Y, Miyazono K, Harpel J, Heldin CH, Rifkin DB. 1993. Role of the latent TGF-β-binding protein in the activation of latent TGF-β by co-cultures of endothelial and smooth muscle cells. J Cell Biol 120: 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Chen Y, Prijatelj P, Sakai T, Fassler R, Sakai LY, Rifkin DB. 2005. Fibronectin is required for integrin αvβ6-mediated activation of latent TGF-β complexes containing LTBP-1. FASEB J 19: 1798–1808. [DOI] [PubMed] [Google Scholar]
- Ganesh SK, Morissette R, Xu Z, Schoenhoff F, Griswold BF, Yang J, Tong L, Yang ML, Hunker K, Sloper L, et al. 2014. Clinical and biochemical profiles suggest fibromuscular dysplasia is a systemic disease with altered TGF-β expression and connective tissue features. FASEB J 28: 3313–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. 2009. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol 296: G175–G184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzerro E, Canalis E. 2006. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord 7: 51–65. [DOI] [PubMed] [Google Scholar]
- Ge G, Greenspan DS. 2006. BMP1 controls TGFβ1 activation via cleavage of latent TGF-β-binding protein. J Cell Biol 175: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry LE, Nash BW. 1990. The pro-domain of pre-pro-transforming growth factor β1 when independently expressed as a functional binding protein for the mature growth factor. Biochemistry 29: 6851–6857. [DOI] [PubMed] [Google Scholar]
- Gleizes PE, Beavis RC, Mazzieri R, Shen B, Rifkin DB. 1996. Identification and characterization of an eight-cysteine repeat of the latent transforming growth factor-β-binding protein-1 that mediates bonding to the latent transforming growth factor-β1. J Biol Chem 271: 29891–29896. [DOI] [PubMed] [Google Scholar]
- Glinka Y, Prud’homme GJ. 2008. Neuropilin-1 is a receptor for transforming growth factor β-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol 84: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Y, Stoilova S, Mohammed N, Prud’homme GJ. 2011. Neuropilin-1 exerts co-receptor function for TGF-β-1 on the membrane of cancer cells and enhances responses to both latent and active TGF-β. Carcinogenesis 32: 613–621. [DOI] [PubMed] [Google Scholar]
- Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, et al. 2014. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med 20: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger DJ, Frow EK. 2000. Thrombospondin 1 does not activate transforming growth factor β1 in a chemically defined system or in smooth-muscle-cell cultures. Biochem J 350: 291–298. [PMC free article] [PubMed] [Google Scholar]
- Grandclement C, Pallandre JR, Valmary Degano S, Viel E, Bouard A, Balland J, Remy-Martin JP, Simon B, Rouleau A, Boireau W, et al. 2011. Neuropilin-2 expression promotes TGF-β1-mediated epithelial to mesenchymal transition in colorectal cancer cells. PLoS ONE 6: e20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KE, Ono RN, Charbonneau NL, Kuo CL, Keene DR, Bachinger HP, Sakai LY. 2005. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J Biol Chem 280: 27970–27980. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, et al. 2006. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. 2011. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Kirita A, Kondo W, Matsuura T, Nagatsuma K, Dohmae N, Ogawa S, Imajoh-Ohmi S, Friedman SL, Rifkin DB, et al. 2014. LAP degradation product reflects plasma kallikrein-dependent TGF-β activation in patients with hepatic fibrosis. Springerplus 3: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, et al. 2013. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med 19: 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin A, Lelong C, Favrel P. 2004. Transforming growth factor-β-related proteins: An ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol 28: 461–485. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. 1994. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J 302: 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck AP, Mueller TD, Springer T. 2016. Structural basis of growth factor–receptor, growth factor–prodomain, growth factor–modulator interactions, and functional implications for signaling. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a022103. [DOI] [Google Scholar]
- Hinz B. 2013. It has to be the αv: Myofibroblast integrins activate latent TGF-β1. Nat Med 19: 1567–1568. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, et al. 1999. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99: 81–92. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Todorovic V, Hadjiolova K, Weiskirchen R, Rifkin DB. 2015. Abrogation of both short and long forms of latent transforming growth factor-β-binding protein-1 causes defective cardiovascular development and is perinatally lethal. Matrix Biol 43: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytiainen M, Penttinen C, Keski-Oja J. 2004. Latent TGF-β-binding proteins: Extracellular matrix association and roles in TGF-β activation. Crit Rev Clin Lab Sci 41: 233–264. [DOI] [PubMed] [Google Scholar]
- Inoue T, Ohbayashi T, Fujikawa Y, Yoshida H, Akama TO, Noda K, Horiguchi M, Kameyama K, Hata Y, Takahashi K, et al. 2014. Latent TGF-β-binding protein-2 is essential for the development of ciliary zonule microfibrils. Hum Mol Genet 23: 5672–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA. 1996. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med 2: 418–423. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. 2003. Latent transforming growth factor β–binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 278: 2750–2757. [DOI] [PubMed] [Google Scholar]
- Janssens K, ten Dijke P, Ralston SH, Bergmann C, Van Hul W. 2003. Transforming growth factor-β1 mutations in Camurati–Engelmann disease lead to increased signaling by altering either activation or secretion of the mutant protein. J Biol Chem 278: 7718–7724. [DOI] [PubMed] [Google Scholar]
- Jenkins G. 2008. The role of proteases in transforming growth factor-β activation. Int J Biochem Cell Biol 40: 1068–1078. [DOI] [PubMed] [Google Scholar]
- Jensen SA, Robertson IB, Handford PA. 2012. Dissecting the fibrillin microfibril: Structural insights into organization and function. Structure 20: 215–225. [DOI] [PubMed] [Google Scholar]
- Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, Taylor SE, Ledbetter S, Lawrence CM, Rifkin DB, Barcellos-Hoff MH. 2006. Isoform-specific activation of latent transforming growth factor β (LTGF-β) by reactive oxygen species. Radiat Res 166: 839–848. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Dwivedi A, Somerville M, George SJ, Newby AC. 2011. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc Biol 31: e35–e44. [DOI] [PubMed] [Google Scholar]
- Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. 2004. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 114: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien P, Berg TM, Lawrence DA. 1989. Acidic cellular environments: Activation of latent TGF-β and sensitization of cellular responses to TGF-β and EGF. Int J Cancer 43: 886–891. [DOI] [PubMed] [Google Scholar]
- Kantola AK, Keski-Oja J, Koli K. 2008. Fibronectin and heparin-binding domains of latent TGF-β-binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res 314: 2488–2500. [DOI] [PubMed] [Google Scholar]
- Kantola AK, Ryynanen MJ, Lhota F, Keski-Oja J, Koli K. 2010. Independent regulation of short and long forms of latent TGF-β-binding protein (LTBP)-4 in cultured fibroblasts and human tissues. J Cell Physiol 223: 727–736. [DOI] [PubMed] [Google Scholar]
- Kast J, Hanecker P, Beaufort N, Giese A, Joutel A, Dichgans M, Opherk C, Haffner C. 2014. Sequestration of latent TGF-β-binding protein 1 into CADASIL-related Notch3-ECD deposits. Acta Neuropathol Commun 2: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Reinhardt DP. 2012. Immobilized metal affinity chromatography co-purifies TGF-β1 with histidine-tagged recombinant extracellular proteins. PLoS ONE 7: e48629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Sheppard D, Chapman HA. 2016. TGF-β signaling and tissue fibrosis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. 2008. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci 121: 2696–2704. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, Goodsell A, Publicover J, Reichardt L, Jablons D, et al. 2011. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J Clin Invest 121: 2863–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg F, Chow ML, Koehler A, Boo S, Buscemi L, Quinn TM, Costell M, Alman BA, Genot E, Hinz B. 2014. Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol 207: 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Rifkin DB. 1993. Mechanism of retinoid-induced activation of latent transforming growth factor-β in bovine endothelial cells. J Cell Physiol 155: 323–332. [DOI] [PubMed] [Google Scholar]
- Koli K, Hyytiainen M, Ryynanen MJ, Keski-Oja J. 2005. Sequential deposition of latent TGF-β-binding proteins (LTBPs) during formation of the extracellular matrix in human lung fibroblasts. Exp Cell Res 310: 370–382. [DOI] [PubMed] [Google Scholar]
- Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, Honnons S, Jones C, Isern NG, Hu JZ, et al. 2012. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med 186: 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. 1993. Transforming growth factor β1-null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci 90: 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMarre J, Wollenberg GK, Gauldie J, Hayes MA. 1990. α2-Macroglobulin and serum preferentially counteract the mitoinhibitory effect of transforming growth factor-β2 in rat hepatocytes. Lab Invest 62: 545–551. [PubMed] [Google Scholar]
- LaMarre J, Hayes MA, Wollenberg GK, Hussaini I, Hall SW, Gonias SL. 1991. An α2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor-β1 in mice. J Clin Invest 87: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. 1998. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 101: 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff C, Cormier-Daire V. 2012. From tall to short: The role of TGFβ signaling in growth and its disorders. Am J Med Genet C Semin Med Genet 160C: 145–153. [DOI] [PubMed] [Google Scholar]
- Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, Zylberberg L, Collod-Beroud G, Bonnet D, Alanay Y, et al. 2011. Mutations in the TGF-β-binding protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am J Hum Genet 89: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. 2004. TGF-β signaling and the fibrotic response. FASEB J 18: 816–827. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. 2001. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci 98: 9306–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM. 2002. Integrin α8β1 mediates adhesion to LAP-TGFβ1. J Cell Sci 115: 4641–4648. [DOI] [PubMed] [Google Scholar]
- Ludbrook SB, Barry ST, Delves CJ, Horgan CM. 2003. The integrin αvβ3 is a receptor for the latency-associated peptides of transforming growth factors β1 and β3. Biochem J 369: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RM, Keski-Oja J, Moses HL. 1988. Proteolytic activation of latent transforming growth factor-β from fibroblast-conditioned medium. J Cell Biol 106: 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RM, Gentry LE, Purchio AF, Moses HL. 1990. Mechanism of activation of latent recombinant transforming growth factor β1 by plasmin. J Cell Biol 110: 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Dean DD, Gay I, Schwartz Z, and Boyan BD. 2001. Activation of latent transforming growth factor β1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J Bone Miner Res 16: 1281–1290. [DOI] [PubMed] [Google Scholar]
- Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, McGovern A, Baldock C, Shuttleworth CA, Kielty CM. 2010. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF-β. J Cell Sci 123: 3006–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. 1995. Multiple defects and perinatal death in mice deficient in follistatin. Nature 374: 360–363. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. 1997. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387: 83–90. [DOI] [PubMed] [Google Scholar]
- Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. 2010. Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest 120: 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa S, Lou J, Seed RI, Cormier A, Wu S, Cheng Y, Murray L, Tsui P, Connor J, Herbst R, et al. 2014. Selective targeting of TGF-β activation to treat fibroinflammatory airway disease. Sci Transl Med 6: 241ra279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Heldin CH. 1989. Role for carbohydrate structures in TGF-β1 latency. Nature 338: 158–160. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Olofsson A, Colosetti P, Heldin CH. 1991. A role of the latent TGF-β1-binding protein in the assembly and secretion of TGF-β1. EMBO J 10: 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. 2002. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol 157: 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. 2008. TGFβ1 and TGFβ3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev 125: 508–516. [DOI] [PubMed] [Google Scholar]
- Mudgett JS, Hutchinson NI, Chartrain NA, Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D, Shen CF, et al. 1998. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum 41: 110–121. [DOI] [PubMed] [Google Scholar]
- Munger JS, Sheppard D. 2011. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol 3: a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Giancotti FG, Rifkin DB. 1998. Interactions between growth factors and integrins: Latent forms of transforming growth factor-β are ligands for the integrin αvβ1. Mol Biol Cell 9: 2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. 1999. The integrin αvβ6 binds and activates latent TGF-β1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Poczatek M. 2000. Activation of latent TGF-β by thrombospondin-1: Mechanisms and physiology. Cytokine Growth Factor Rev 11: 59–69. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Schultz-Cherry S, Hook M. 1992. Transforming growth factor-β complexes with thrombospondin. Mol Biol Cell 3: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. 2003. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411. [DOI] [PubMed] [Google Scholar]
- Neurohr C, Nishimura SL, Sheppard D. 2006. Activation of transforming growth factor-β by the integrin αvβ8 delays epithelial wound closure. Am J Respir Cell Mol Biol 35: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, et al. 2004. TGF-β-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 114: 1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Carta L, Ono RN, Sengle G, Arteaga-Solis E, Levasseur R, Ducy P, et al. 2010. Fibrillin-1 and -2 differentially modulate endogenous TGF-β and BMP bioavailability during bone formation. J Cell Biol 190: 1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Dabovic B, Takagi K, Inoue T, Horiguchi M, Hirai M, Fujikawa Y, Akama TO, Kusumoto K, Zilberberg L, et al. 2013. Latent TGF-β-binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proc Natl Acad Sci 110: 2852–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I, Shapiro RL, Rifkin DB. 1995. Characterization of latent TGF-β activation by murine peritoneal macrophages. J Immunol 155: 1450–1459. [PubMed] [Google Scholar]
- Nunes I, Gleizes PE, Metz CN, Rifkin DB. 1997. Latent transforming growth factor β–binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor β. J Cell Biol 136: 1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Takahashi R, Adachi E, Kondo S, Kuratomi S, Noma A, Alexander DB, Motoda H, Okada A, Seiki M, et al. 2004. Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene 23: 5041–5048. [DOI] [PubMed] [Google Scholar]
- Olofsson A, Hellman U, Ten Dijke P, Grimsby S, Ichijo H, Moren A, Miyazono K, Heldin CH. 1997. Latent transforming growth factor β complex in Chinese hamster ovary cells contains the multifunctional cysteine-rich fibroblast growth factor receptor, also termed E-selectin-ligand or MG-160. Biochem J 324: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono RN, Sengle G, Charbonneau NL, Carlberg V, Bachinger HP, Sasaki T, Lee-Arteaga S, Zilberberg L, Rifkin DB, Ramirez F, et al. 2009. Latent transforming growth factor β–binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J Biol Chem 284: 16872–16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreffo ROC, Mundy GR, Seyedin SM, Bonewald LF. 1989. Activation of the bone-derived latent TGF-β complex by isolated osteoclasts. Biochem Biophys Res Commun 158: 817–823. [DOI] [PubMed] [Google Scholar]
- Pappano WN, Steiglitz BM, Scott IC, Keene DR, Greenspan DS. 2003. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol Cell Biol 23: 4428–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar VM, Vukicevic S, Reddi AH. 1991. Transforming growth factor β type 1 binds to collagen IV of basement membrane matrix: Implications for development. Dev Biol 143: 303–308. [DOI] [PubMed] [Google Scholar]
- Penttinen C, Saharinen J, Weikkolainen K, Hyytiainen M, Keski-Oja J. 2002. Secretion of human latent TGF-β-binding protein-3 (LTBP-3) is dependent on co-expression of TGF-β. J Cell Sci 115: 3457–3468. [DOI] [PubMed] [Google Scholar]
- Pociask DA, Sime PJ, Brody AR. 2004. Asbestos-derived reactive oxygen species activate TGF-β1. Lab Invest 84: 1013–1023. [DOI] [PubMed] [Google Scholar]
- Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, et al. 2008. Inhibition of integrin αvβ6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 177: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. 2009. Extracellular microfibrils: Contextual platforms for TGF-β and BMP signaling. Curr Opin Cell Biol 21: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. 1999. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-β. J Biol Chem 274: 13586–13593. [DOI] [PubMed] [Google Scholar]
- Rifkin DB. 2005. Latent transforming growth factor β (TGF-β)-binding proteins: Orchestrators of TGF-β availability. J Biol Chem 280: 7409–7412. [DOI] [PubMed] [Google Scholar]
- Robertson IB, Rifkin DB. 2013. Unchaining the beast; insights from structural and evolutionary studies on TGF-β secretion, sequestration, and activation. Cytokine Growth Factor Rev 24: 355–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I, Jensen S, Handford P. 2011. TB domain proteins: Evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem J 433: 263–276. [DOI] [PubMed] [Google Scholar]
- Robertson IB, Handford PA, Redfield C. 2014. NMR spectroscopic and bioinformatic analyses of the LTBP1 C-terminus reveal a highly dynamic domain organisation. PLoS ONE 9: e87125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. 2015. Latent TGF-β-binding proteins. Matrix Biol 47: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. 2009. Fibrillin assembly requires fibronectin. Mol Biol Cell 20: 846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen J, Keski-Oja J. 2000. Specific sequence motif of 8-Cys repeats of TGF-β-binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-β. Mol Biol Cell 11: 2691–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen J, Taipale J, Keski-Oja J. 1996. Association of the small latent transforming growth factor-β with an eight cysteine repeat of its binding protein LTBP-1. EMBO J 15: 245–253. [PMC free article] [PubMed] [Google Scholar]
- Saharinen J, Hyytiainen M, Taipale J, Keski-Oja J. 1999. Latent transforming growth factor-β-binding proteins (LTBPs)—Structural extracellular matrix proteins for targeting TGF-β action. Cytokine Growth Factor Rev 10: 99–117. [DOI] [PubMed] [Google Scholar]
- Saito T, Kinoshita A, Yoshiura K, Makita Y, Wakui K, Honke K, Niikawa N, Taniguchi N. 2001. Domain-specific mutations of a transforming growth factor (TGF)-β1 latency-associated peptide cause Camurati–Engelmann disease because of the formation of a constitutively active form of TGF-β1. J Biol Chem 276: 11469–11472. [DOI] [PubMed] [Google Scholar]
- Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. 1995. Hepatic expression of mature transforming growth factor β1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci 92: 2572–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, Caldarone CA, Hinz B. 2014. Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc Res 102: 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. 1989. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: Activation of a latent transforming growth factor-β1-like molecule by plasmin during co-culture. J Cell Biol 109: 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr E, Broszat M, Brandan E, Bruckner P, Kresse H. 1998. Decorin core protein fragment Leu155–Val260 interacts with TGF-β but does not compete for decorin binding to type I collagen. Arch Biochem Biophys 355: 241–248. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Hinshaw VS. 1996. Influenza virus neuraminidase activates latent transforming growth factor β. J Virol 70: 8624–8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Lawler J, Murphy-Ullrich JE. 1994. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-β. J Biol Chem 269: 26783–26788. [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. 1995. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J Biol Chem 270: 7304–7310. [DOI] [PubMed] [Google Scholar]
- Seliger C, Leukel P, Moeckel S, Jachnik B, Lottaz C, Kreutz M, Brawanski A, Proescholdt M, Bogdahn U, Bosserhoff AK, et al. 2013. Lactate-modulated induction of THBS-1 activates transforming growth factor (TGF)-β2 and migration of glioma cells in vitro. PLoS ONE 8: e78935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellheyer K, Bickenbach JR, Rothnagel JA, Bundman D, Longley MA, Krieg T, Roche NS, Roberts AB, Roop DR. 1993. Inhibition of skin development by overexpression of transforming growth factor β1 in the epidermis of transgenic mice. Proc Natl Acad Sci 90: 5237–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. 2008a. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem 283: 13874–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Lyons KM, Bachinger HP, Sakai LY. 2008b. A new model for growth factor activation: Type II receptors compete with the prodomain for BMP-7. J Mol Biol 381: 1025–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Sasaki T, Sakai LY. 2011. Prodomains of transforming growth factor β (TGF-β) superfamily members specify different functions: Extracellular matrix interactions and growth factor bioavailability. J Biol Chem 286: 5087–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. 2011. Latent TGF-β structure and activation. Nature 474: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara K, Ota M, Horiguchi M, Yoshinaga K, Melamed J, Rifkin DB. 2012. Production of gastrointestinal tumors in mice by modulating latent TGF-β1 activation. Cancer Res 73: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach RM, Rowley DA. 1993. A first or dominant immunization. II: Induced immunoglobulin carries transforming growth factor β and suppresses cytolytic T cell responses to unrelated alloantigens. J Exp Med 178: 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, et al. 2002. Disruption of the gene encoding the latent transforming growth factor β–binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev 16: 2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z. 2004. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 131: 5883–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockis J, Colau D, Coulie PG, Lucas S. 2009. Membrane protein GARP is a receptor for latent TGF-β on the surface of activated human Treg. Eur J Immunol 39: 3315–3322. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Kodama Y, Matsumoto T. 1994. Bone matrix decorin binds transforming growth factor-β and enhances its bioactivity. J Biol Chem 269: 32634–32638. [PubMed] [Google Scholar]
- Tatler AL, John AE, Jolly L, Habgood A, Porte J, Brightling C, Knox AJ, Pang L, Sheppard D, Huang X, et al. 2011. Integrin αvβ5-mediated TGF-β activation by airway smooth muscle cells in asthma. J Immunol 187: 6094–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher BA, Ikebe M, Ara G, Keyes SR, Herbst RS. 1997. Transforming growth factor-β1 overexpression produces drug resistance in vivo: Reversal by decorin. In Vivo 11: 463–472. [PubMed] [Google Scholar]
- Teitelbaum SL. 2000. Bone resorption by osteoclasts. Science 289: 1504–1508. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Rifkin DB. 2012. LTBPs, more than just an escort service. J Cell Biochem 113: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic V, Frendewey D, Gutstein DE, Chen Y, Freyer L, Finnegan E, Liu F, Murphy A, Valenzuela D, Yancopoulos G, et al. 2007. Long form of latent TGF-β-binding protein 1 (Ltbp1L) is essential for cardiac outflow tract septation and remodeling. Development 134: 3723–3732. [DOI] [PubMed] [Google Scholar]
- Todorovic V, Finnegan E, Freyer L, Zilberberg L, Ota M, Rifkin DB. 2011. Long form of latent TGF-β-binding protein 1 (Ltbp1L) regulates cardiac valve development. Dev Dyn 240: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. 2009. GARP (LRRC32) is essential for the surface expression of latent TGF-β on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci 106: 13445–13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. 2007. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umans L, Serneels L, Overbergh L, Lorent K, Van Leuven F, Van den Berghe H. 1995. Targeted inactivation of the mouse α2-macroglobulin gene. J Biol Chem 270: 19778–19785. [DOI] [PubMed] [Google Scholar]
- Unsold C, Hyytiainen M, Bruckner-Tuderman L, Keski-Oja J. 2001. Latent TGF-β-binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J Cell Sci 114: 187–197. [DOI] [PubMed] [Google Scholar]
- Urban Z, Hucthagowder V, Schurmann N, Todorovic V, Zilberberg L, Choi J, Sens C, Brown CW, Clark RD, Holland KE, et al. 2009. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet 85: 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitt UA, Hsu SY, Hsueh AJ. 2001. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol 15: 681–694. [DOI] [PubMed] [Google Scholar]
- Walton KL, Makanji Y, Chen J, Wilce MC, Chan KL, Robertson DM, Harrison CA. 2010. Two distinct regions of latency-associated peptide coordinate stability of the latent transforming growth factor-β1 complex. J Biol Chem 285: 17029–17037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KL, Makanji Y, Harrison CA. 2011. New insights into the mechanisms of activin action and inhibition. Mol Cell Endocrinol 359: 2–12. [DOI] [PubMed] [Google Scholar]
- Wang H, Kochevar IE. 2005. Involvement of UVB-induced reactive oxygen species in TGF-β biosynthesis and activation in keratinocytes. Free Radic Biol Med 38: 890–897. [DOI] [PubMed] [Google Scholar]
- Wang R, Wan Q, Kozhaya L, Fujii H, Unutmaz D. 2008. Identification of a regulatory T cell specific cell-surface molecule that mediates suppressive signals and induces Foxp3 expression. PLoS ONE 3: e2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhu J, Dong X, Shi M, Lu C, Springer TA. 2012. GARP regulates the bioavailability and activation of TGF-β. Mol Biol Cell 23: 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winbanks CE, Weeks KL, Thomson RE, Sepulveda PV, Beyer C, Qian H, Chen JL, Allen JM, Lancaster GI, Febbraio MA, et al. 2012. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J Cell Biol 197: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Hinz B. 2008. Integrins and the activation of latent transforming growth factor β1—An intimate relationship. Eur J Cell Biol 87: 601–615. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. 2007. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol 179: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington JJ, Kelly A, Smedley C, Bauche D, Campbell S, Marie JC, Travis MA. 2015. Integrin αvβ8-mediated TGF-β activation by effector regulatory T cells is essential for suppression of T-cell-mediated inflammation. Immunity 42: 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadin DA, Robertson IB, McNaught-Davis J, Evans P, Stoddart D, Handford PA, Jensen SA, Redfield C. 2013. Structure of the fibrillin-1 N-terminal domains suggests that heparan sulfate regulates the early stages of microfibril assembly. Structure 21: 1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. 2011. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147: 1146–1158. [DOI] [PubMed] [Google Scholar]
- Yang LT, Kaartinen V. 2007. Tgfb1 expressed in the Tgfb3 locus partially rescues the cleft palate phenotype of Tgfb3 null mutants. Dev Biol 312: 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Dignam JD, Gentry LE. 1997. Role of carbohydrate structures in the binding of β1-latency-associated peptide to ligands. Biochemistry 36: 11923–11932. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. 2007. Absence of integrin-mediated TGFβ1 activation in vivo recapitulates the phenotype of TGF-β1-null mice. J Cell Biol 176: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Mendoza-Londono R, Lu H, Tao J, Li K, Keller B, Jiang MM, Shah R, Chen Y, Bertin TK, et al. 2010. E-selectin ligand-1 regulates growth plate homeostasis in mice by inhibiting the intracellular processing and secretion of mature TGF-β. J Clin Invest 120: 2474–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehualaeshet T, O’Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. 1999. Activation of rat alveolar macrophage-derived latent transforming growth factor β1 by plasmin requires interaction with thrombospondin-1 and its cell-surface receptor, CD36. Am J Pathol 155: 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K, Obata H, Jurukovski V, Mazzieri R, Chen Y, Zilberberg L, Huso D, Melamed J, Prijatelj P, Todorovic V, et al. 2008. Perturbation of transforming growth factor (TGF)-β1 association with latent TGF-β-binding protein yields inflammation and tumors. Proc Natl Acad Sci 105: 18758–18763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. 2000. Cell-surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176. [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cashman TJ, Nevis KR, Obregon P, Carney SA, Liu Y, Gu A, Mosimann C, Sondalle S, Peterson RE, et al. 2011. Latent TGF-β-binding protein 3 identifies a second heart field in zebrafish. Nature 474: 645–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Courousse T, Sakai LY, Rifkin DB. 2012. Specificity of latent TGF-β-binding protein (LTBP) incorporation into matrix: Role of fibrillins and fibronectin. J Cell Physiol 227: 3828–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg L, Phoon CK, Robertson I, Dabovic B, Ramirez F, Rifkin DB. 2015. Genetic analysis of the contribution of LTBP-3 to thoracic aneurysm in Marfan syndrome. Proc Natl Acad Sci 112: 14012–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]