Abstract
Background
Persistent infection with high-risk human papillomavirus (HPV) has been recognized as a major cause of cervical cancer. Distribution of HPV genotypes may differ according to the geographic region and the severity of the cervical lesion. Determining HPV genotypes’ specific distribution is useful for HPV surveillance and control programs. However, little is known about the distribution of HPV genotypes in Iranian women.
Objectives
The aim of this study was to determine the distribution of HPV genotypes in Iranian women with different grades of cervical lesions.
Patients and Methods
From 2011 to 2013, a total of 436 Iranian women with convenience sampling strategy were included in this cross-sectional study. In detail, 287 women negative for intraepithelial lesion or malignancy, 32 with atypical squamous cells of undetermined significance (ASCUS), 50 with low-grade squamous intraepithelial lesion (LSIL), 44 with high-grade squamous intraepithelial lesion (HSIL), and 23 with cervical cancer were evaluated in this investigation. HPV genotypes were determined by INNO-LiPA HPV Genotyping Extra assay.
Results
In total, HPV infection was detected in 45.4% of the cases. The most common high-risk HPV (HR-HPV) genotype was HPV-16 (32.8%), followed by HPV-53 (9.1%). Within low-risk (LR-HPV) genotypes HPV-6 (22.2%) and HPV-44 (6.1%) were the most prevalent. HPV-16 was the predominant genotype in cases with cervical cancer (56.5%), ASCUS (34.4%), and HSIL (34.1%). HPV-6 was the most common genotype in normal cases (9.1%) and LSIL patients (18%). The prevalence of HPV positivity was significantly higher in cases with high-grade lesions (≥ HSIL) (64.2%) than in normal/LSIL (37.3%) (P = 0.033). The rate of HR-HPV infection was significantly higher in ≥ HSIL cases (61.2%) than normal/LSIL (27.9%) (P = 0.003).
Conclusions
This study describes robust information on the distribution of HPV genotypes among Iranian women with and without cervical lesions. The present data may be of importance for designing future public health strategies, including HPV vaccination programs.
Keywords: Papillomavirus Infections, Cervical Cancer, Iran
1. Background
Cervical cancer is the second-most common malignancy among women worldwide accounting for over half a million new cases and more than 350,000 deaths annually (1). Infection with human papilloma virus (HPV) is considered the major cause of cervical intraepithelial neoplasia and cervical cancer (2). More than 40 distinct HPV genotypes can infect the anogenital mucosa. They are categorized as ‘‘high-risk’’ HPV (HR-HPV) and ‘‘low-risk’’ HPV (LR-HPV) genotypes according to their oncogenic potential (3, 4), with HR-HPV genotypes detectable in nearly 90% of cervical cancer specimens (2, 5). HPV-16 and HPV-18 are the most common HR-HPV genotypes, accounting for nearly 50% and 20% of cervical cancers worldwide, respectively. Other prevalent HR-HPV genotypes (HPV-52, -31, -33, and -45) are responsible for approximately 30% of cervical cancer cases (4, 6).
Despite the fact that HPV-16 is the most frequent genotype worldwide, several lines of evidence have revealed that the distribution of HPV genotypes may differ according to several factors, including the geographic region and the severity of the cervical lesion (4, 7, 8). Therefore, HPV genotyping should be included in HPV infection-monitoring programs. The World Health Organization has demanded the HPV genotype distribution, along with determining of the incidence of cervical intraepithelial neoplasia and cervical cancer (9).
The determining of HPV genotypes’ specific distribution is important for screening and diagnostic plans. Moreover, with the advent of effective prophylactic vaccines against HPV-16 and HPV-18, understanding of the geographical prevalence of HPV genotypes seems to be necessary for monitoring the potential changes of HPV genotype prevalence after the introduction of the vaccination. However, little is known about the distribution of HPV genotypes in Iranian women according to cervical lesion grade. This void motivated us to embark on the present study.
2. Objectives
Since the data about HPV genotype distribution is valuable for planning future public health strategies, the primary aim of the current study was to determine the prevalence of HPV genotypes both as single or multiple infections in Iranian women. The secondary aim was to evaluate the association of HPV genotypes with the severity of the cervical lesion.
3. Patients and Methods
3.1. Cervical Samples
The current cross-sectional study’s cervical specimens were obtained from women who visited two referral and governmental centers for cervical cancer screening in Tehran, Iran, between 2011 and 2013. Center 1 was the gynecology oncology department of Imam hospital complex affiliated with Tehran University of Medical Sciences, and Center 2 was the cancer institute of Imam hospital complex affiliated with Tehran University of Medical Sciences.
The convenience sampling strategy was used, which is a nonprobability sampling technique, and all of the admitted women were evaluated. A total of 491 women who were referred during this period were primarily included. Of these, 436 were considered eligible for the study based on the following eligibility criteria: a) desire to participate in this investigation, b) age ≥ 18 years old, c) of Iranian nationality, and d) no prior hysterectomy. Out of 436 eligible samples, 346 were cervical exfoliated cells from women attending Center 1, and 90 were cervical biopsies from women with history of cervical abnormality attending Center 2. Cervical exfoliated cells were collected by a cytobrush into the PreservCyt transport medium (Cytyc Corporation, Boxborough, MA) for cytology and HPV analysis. Cervical biopsies were collected in 0.1 M phosphate buffered saline solution.
Obtained cervical cells were diagnosed cytologically according to the Bethesda system (10), and cervical biopsies were subjected to histological analysis. The mean and median ages of the participants were 35.15 ± 10.05 (range 18 - 72) and 32, respectively.
The present study was done according to the Declaration of Helsinki and relevant local regulations. The study-related protocols were approved by the Ethical Committee of Tehran University of Medical Sciences (code and date of ethical approval: 92-01-30-21080, April 8, 2011), and written informed consent was obtained from each subject. The privacy of research participants was respected in this study.
3.2. DNA Extraction and HPV Genotyping
Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The quality of extracted DNA was assessed with a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, USA), followed by a beta-globin PCR analysis (11). For HPV detection, beta-globin-positive samples were subjected to a consensus primer, GP5+/GP6+ mediated PCR (11). Subsequent HPV genotyping was carried out using the reverse-hybridization-based INNO-LiPA HPV Genotyping Extra assay (Innogenetics NV, Ghent, Belgium) according to the manufacturer’s instructions. INNO-LiPA Extra is a line-probe assay that allows simultaneous identification of 28 different HPV genotypes (12). This assay has higher analytical sensitivity in comparison with the genotyping methods based on GP5+/GP6+ over a broad range of HPVs, particularly in the presence of multiple HPV genotypes (13, 14). Moreover, INNO-LiPA has indicated highly similar genotyping results to the Linear Array (15, 16).
3.3. Statistical Analysis
Data analysis was performed using SPSS version 16 software (SPSS Inc., Chicago, IL, USA). The associations between categorical variables were examined using a chi-square test or Fisher exact test, whichever was applicable. Normal distribution of the variables was analyzed by Kolmogorov–Smirnov test. Comparison of median age among HPV risk category and cervical lesion category was performed using the Kruskal-Wallis test. A P value of ≤ 0.05 was considered to be statistically significant.
4. Results
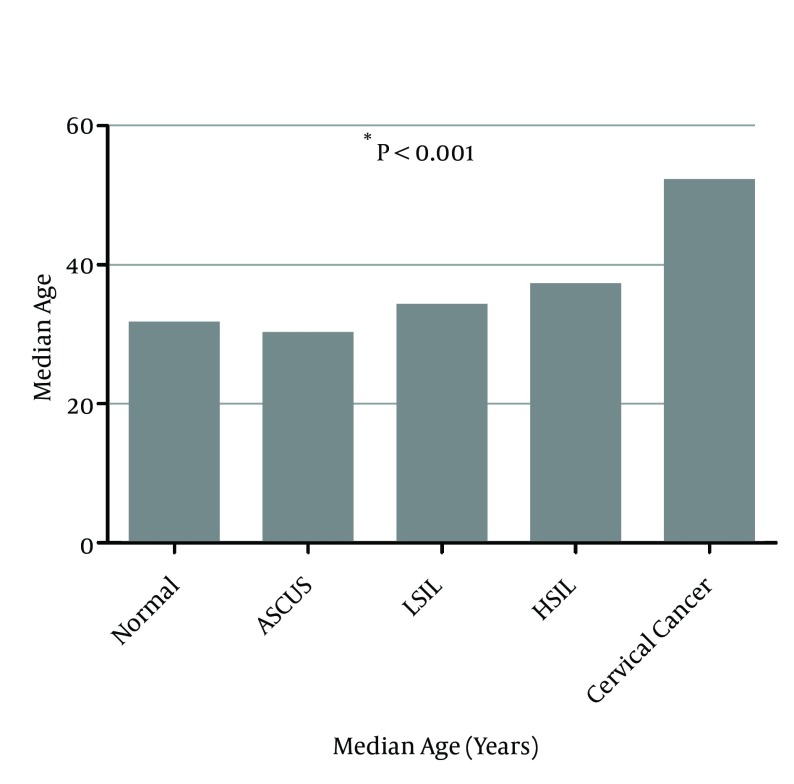
According to cytological and histological analysis, 436 cervical samples were classified as follows: 287 cases (65.8%) negative for intraepithelial lesion or malignancy, 32 cases (7.3%) with atypical squamous cells of undetermined significance (ASCUS), 50 cases (11.5%) with low-grade squamous intraepithelial lesion (LSIL), 44 cases (10.1%) with high-grade squamous intraepithelial lesion (HSIL), and 23 cases (5.3%) with cervical cancer. The median age was 31.5 years for normal women, 30 years for cases with ASCUS, 34 years for cases with LSIL, 37 years for cases with HSIL, and 52 years for cases with cervical cancer (Figure 1). The difference of median age among distinct cervical lesion grade was statistically significant (P < 0.001).
Figure 1. Association Between Age and Cervical Lesion Grade.
* The P value was determined by the Kruskal-Wallis test comparing the median age between all of the groups.
All samples were positive for the beta-globin gene and, therefore, considered appropriate for the HPV DNA detection by PCR. Overall, DNA testing was positive in 198 cases (45.4%). Among HPV positive samples, 157 cases (79.3%) were positive for HR-HPV genotypes. The most common HR-HPV genotype was HPV-16 (32.8%) followed by HPV-53 (9.1%). Low-risk HPV genotypes were detected in 66 (33.3%) of the women who tested positive for HPV infection. The most common LR genotype was HPV-6 (22.2%) followed by HPV-44 (6.1%) (Table 1).
Table 1. HPV Genotype Distribution According to Cervical Lesion Grade.
| Normal | ASCUS | LSIL | HSIL | Cervical Cancer | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single | Mul-tiple | Single | Mul-tiple | Single | Mul-tiple | Single | Mul-tiple | Single | Mul-tiple | ||
| HR-HPV | |||||||||||
| 16 | 6 (2.1) | 12 (4.2) | 5 (15.6) | 6 (18.8) | 4 (8) | 4 (8) | 13 (29.5) | 2 (4.6) | 12 (52.2) | 1 (4.3) | 65 (32.8) |
| 18 | 2 (0.7) | 3 (1.4) | NA | NA | NA | 1 (2) | 1 (2.3) | NA | 3 (13) | NA | 10 (5) |
| 26 a | 1 (0.3) | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1 (0.5) |
| 31 | 1 (0.3) | 10 (3.5) | NA | 3 (9.4) | NA | 2 (4) | 1 (2.3) | NA | NA | NA | 17 (8.6) |
| 33 | 2 (0.7) | 1 (0.3) | NA | NA | 1 (2) | NA | NA | NA | NA | NA | 4 (2) |
| 35 | NA | 2 (0.7) | 1 (3.1) | 1 (3.1) | NA | NA | NA | NA | NA | NA | 4 (2) |
| 39 | 2 (0.7) | 7 (2.4) | NA | 2 (6.2) | 1 (2) | 2 (4) | 1 (2.3) | 1 (2.3) | 1 (4.3) | NA | 17 (8.6) |
| 45 | NA | 3 (1.4) | 1 (3.1) | 2 (6.2) | NA | 1 (2) | NA | NA | NA | 1 (4.3) | 8 (4) |
| 51 | 2 (0.7) | 7 (2.4) | 1 (3.1) | 2 (6.2) | NA | 2 (4) | 1 (2.3) | NA | NA | NA | 15 (7.6) |
| 52 | 2 (0.7) | 8 (2.8) | NA | NA | NA | 2 (4) | 1 (2.3) | NA | NA | NA | 13 (6.7) |
| 53 a | 3 (1.4) | 4 (1.4) | 1 (3.1) | 2 (6.2) | 1 (2) | 4 (8) | 2 (4.6) | 1 (2.3) | NA | NA | 18 (9.1) |
| 56 | 2 (0.7) | 1 (0.3) | 2 (6.2) | 4 (12.5) | 1 (2) | 4 (8) | NA | NA | NA | NA | 14 (7.1) |
| 58 | 2 (0.7) | 1 (0.3) | 1 (3.1) | NA | NA | 1 (2) | NA | NA | 1 (4.3) | NA | 6 (3) |
| 59 | 1 (0.3) | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1 (0.5) |
| 66 a | 5 (1.7) | 5 (1.7) | NA | 3 (9.4) | 1 (2) | NA | 1 (2.3) | NA | NA | NA | 15 (7.6) |
| 68 | 2 (0.7) | 3 (1.4) | NA | 2 (6.2) | 2 (4) | 1 (2) | NA | NA | NA | NA | 10 (5) |
| 73 | 1 (0.3) | NA | 1 (3.1) | NA | NA | NA | NA | NA | NA | NA | 2 (1) |
| 82 | 2 (0.7) | 3 (1.4) | NA | NA | NA | NA | NA | NA | NA | NA | 5 (2.5) |
| LR-HPV | |||||||||||
| 6 | 20 (7) | 6 (2.1) | 4 (12.5) | 1 (3.1) | 2 (4) | 7 (14) | 1 (2.3) | NA | 1 (4.3) | 1 (4.3) | 44 (22.2) |
| 11 | 2 (0.7) | 1 (0.3) | NA | 1 (3.1) | NA | 2 (4) | NA | NA | NA | NA | 6 (3) |
| 40 | 1 (0.3) | 2 (0.7) | NA | NA | NA | 1 (2) | NA | NA | NA | NA | 4 (2) |
| 43 | 1 (0.3) | NA | NA | NA | NA | NA | NA | NA | NA | NA | 1 (0.5) |
| 44 | 1 (0.3) | 9 (3.1) | 1 (3.1) | 1 (3.1) | NA | NA | NA | NA | NA | NA | 12 (6.1) |
| 54 | NA | 3 (1.4) | NA | 1 (3.1) | NA | 1 (2) | NA | 1 (2.3) | NA | NA | 6 (3) |
| UR-HPV | |||||||||||
| 69/71 | NA | 5 (1.7) | NA | 1 (3.1) | NA | 1 (2) | NA | NA | NA | NA | 7 (3.5) |
| 74 | NA | 1 (0.3) | NA | NA | NA | NA | NA | NA | NA | NA | 1 (0.5) |
| Total HPV+ | 101 (35.2) | 29 (90.6) | 25 (50) | 24 (54.5) | 19 (82.6) | 198 (45.4) | |||||
| Total HPV− | 186 (64.8) | 3 (9.4) | 25 (50) | 20 (45.5) | 4 (17.4) | 238 (54.6) | |||||
| Total | 287 | 32 | 50 | 44 | 23 | 436 | |||||
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; HR-HPV, high-risk HPV; HSIL, high-grade squamous intraepithelial lesion; LR-HPV, low-risk HPV; LSIL, low-grade squamous intraepithelial lesion; NA, not available; UR-HPV, unknown-risk HPV.
aProbable high-risk (pHR) genotypes according to Munoz et al. (2).
Out of 198 HPV-positive women, multiple-HPV-genotypes infection were detected in 67 (33.8%), of which 37 cases (55.2%) had co-infection with 2 genotypes; 21 (31.3%) cases were multiple infected with 3 genotypes; 8 (11.2%) cases were multiple infected with 4 genotypes; and only 1 case (1.5%) was multiple infected with 5 genotypes. There was no significant correlation between multiple-HP-genotypes infection and the severity of cervical lesion.
Genotype distribution according to cervical lesion grade is demonstrated in Table 1. The predominant genotype detected in high-grade lesions was HPV-16 and accounted for 15 cases with HSIL (34.1%) and 13 patients with cervical cancer (56.5%). Also, HPV-16 was the most frequent genotype within ASCUS patients, being present in 11 cases (34.4%). The most common genotype in normal cases and LSIL patients was HPV-6, which was detected in 26 normal cases (9.1%) and 9 LSIL subjects (18%). Within the cervical cancer samples, only 6 HPV genotypes were detected: HPV-16, HPV-18, HPV-39, HPV-45, HPV-58, and HPV-6. Unknown-risk HPV (UR-HPV) genotypes, including HPV-69/71 and HPV-74, were detected in 8 (4%) cases, mostly being found in normal samples.
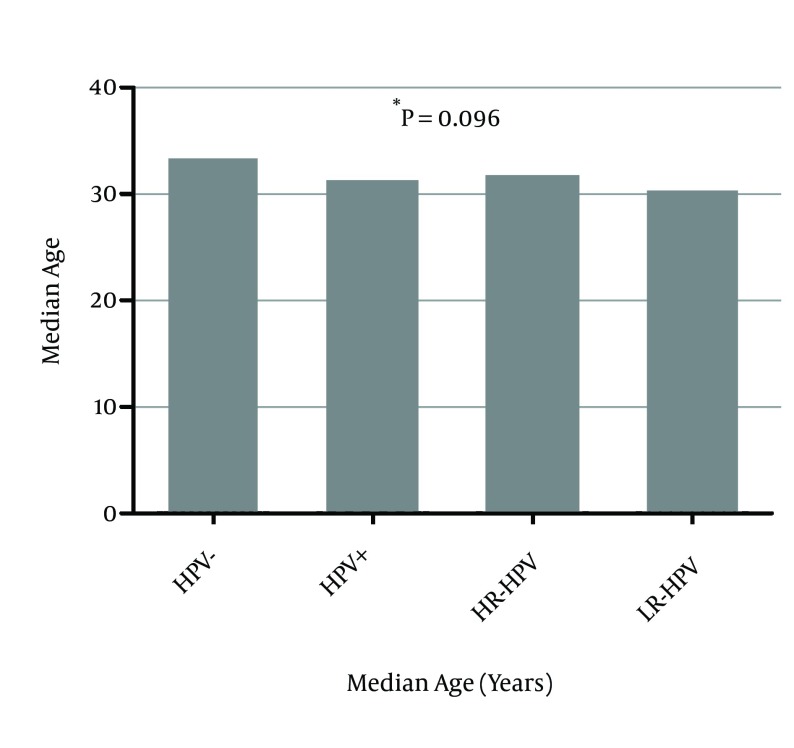
Figure 2 shows the association of age with HPV risk category including HPV negative, HPV positive, infection with LR-HPV, and infection with HR-HPV. There was no significant difference in median age of patients according to HPV risk category (P = 0.096). The distribution of HPV risk category according to cervical lesion grade categorized as normal/LSIL and ≥ HSIL is shown in Table 2. The prevalence of HPV positivity was significantly higher in ≥ HSIL cases (64.2%) than normal/LSIL (37.3%) (P = 0.033). Additionally, high-grade lesions (≥ HSIL) were significantly associated with HPV risk category, as the rate of HR-HPV infection was significantly higher in this category (61.2%) than normal/LSIL group (27.9%) (P = 0.003).
Figure 2. Association Between Age and HPV Risk Category.
* The P value was determined by the Kruskal-Wallis test, comparing the median age between all of the groups.
Table 2. Distribution of HPV Risk Category According to Cervical Lesion Gradea.
| Risk Category | Cervical Lesion Grade | P Value | |
|---|---|---|---|
| Normal/LSIL (N = 337) | ≥ HSIL (N = 67) | ||
| HPV - | 211 (62.2) | 24 (35.8) | 0.033 |
| HPV + | 126 (37.3) | 43 (64.2) | NA |
| HR-HPV | 94 (27.9) | 41 (61.2) | 0.003 |
| LR-HPV | 54 (16) | 4 (6) | NA |
Abbreviations: ≥ HSIL, high-grade squamous intraepithelial lesion or cervical cancer; NA, not available; Normal/LSIL, normal or low-grade squamous intraepithelial lesion.
aData are presented as No. (%).
5. Discussion
Cervical cancer remains a significant public health problem worldwide. Infection with HPV is the causative agent of cervical premalignant and malignant lesions. Several international meta-analyses have determined the prevalence of HPV genotypes in different grades of cervical lesions (17, 18); however little is known about the data of Iranian samples. The present study describes the distribution of HPV genotypes in cervical samples collected from Iranian women with different degrees of cervical lesion.
In this study, 45.4% of samples tested positive for HPV infection. A total of 27 different HPV genotypes were detected, including 18 HR-HPV, 6 LR-HPV, and 3 UR-HPV genotypes. In line with previous reports, HPV-16 was the most frequent genotype (8, 18, 19).
In women with normal cytology, the frequency of HPV infection was 35.2%, notably higher than that reported in Asia (14.4%) (20). Considering single and multiple infections, the most prevalent genotype in normal samples was HPV-6 (9.1%). HPV-16 was the second-most detected genotype (6.3%) within this group, higher than what has been estimated worldwide (2.6%) (7, 8) and in Asia (2.6%) (20). A population-based study carried out in Iran also reported HPV-16 as the most common genotype among women without an intraepithelial lesion or malignancy (1.8%) (21). Considering HPV-16 has a greater tendency to persist and induce malignant lesions than other HR-HPV genotypes, the high prevalence of HPV-16 observed in women with normal cytology could indicate a risk factor for cervical cancer development in the Iranian women (22-24).
The prevalence of HPV infection in women with LSIL was 50%, a rate which was within the range observed in a meta-analysis for Asia (33.3% in India to 74.6% in Korea and Japan) by Bao et al. (20). Despite the well-established role of HPV infection in the development of squamous intraepithelial lesions, this infection has not been observed in a fraction of women with cervical lesions. However, there is no evidence to support the existence of HPV-negative cervical lesions as a specific biologic entity associated with the risk of cervical cancer. Such findings seem to be attributable to cytological misinterpretations or false-negative results of HPV testing (25). After HPV-6 (18%), HPV-16 was the most frequent genotype (16%) among women with LSIL, similar with the value observed in Africa (16.3%) (18) and slightly lower than that of Asia (20%) (20). In the present study, HPV-53 (10%) and HPV-56 (10%) occupied the third place. In Clifford’s worldwide meta-analysis, HPV-16 (26.3%) was the most common, and HPV-31 (11.5%) and HPV-51 (10.6%) were the second and third prevalent genotypes in patients with LSIL (18). Similarly, in Bao’s meta-analysis from Asia, the most commonly detected genotype among women with LSIL was HPV-16 (20%), but the second and third places were occupied by HPV-58 (7.9%) and HPV-52 (7.3%), respectively (20). These discrepancies may be due to the differences in HPV genotyping protocols employed and the geographical variations in the distribution of HPV genotypes.
The rate of HPV positivity was 54.5% in women with HSIL, which is lower than that reported worldwide (84.2 %) and in Asia (81%) (6, 20). In this study, HPV-16 was found in 34.1% of women with HSIL, which is consistent with the prevalence of HPV-16 in HSIL patients from Asia (33.1%) (20). Less frequent were the infections with HPV-53 (6.8%) and HPV-39 (4.5%), which is incompatible with the data of Bao et al. (20) where HPV-58 (11.8%) and HPV-52 (10.6%) were the second- and third-most frequent genotypes. There was also such discrepancy in previous studies, suggesting that more investigation is required at the regional level and that differences in the frequency of HPV genotypes can be seen, particularly in cancer precursor lesions (19, 20, 26).
A total of 82.6% of women with cervical cancer were positive for HPV infection, a rate similar to previous reports worldwide (86.6%) and in Asia (85.9%). In the current study, 69.5% of women with cervical cancer were infected with HPV-16 (56.5%) and HPV-18 (13%), which is in agreement with a previous report from Asia in which the prevalence of HPV-16 and HPV-18 in cervical cancer samples was 52.4% and 14.5%, respectively (20). Similarly, the global prevalence of these two oncogenic genotypes was 54.3% and 12.6%, respectively (6). Moreover, in the study by Khodakarami et al. (21), the majority of Iranian women with cervical cancer harbor HPV-16 (60%) and HPV-18 (22.2%), and the remaining minority tested positive for HPV-31, HPV-45, and HPV-58. In this study, HPV-39, HPV-45, and HPV-58 also were detected among patients with cervical cancer, which is comparable to Khodakarami et al. (21), suggesting that these genotypes are the most common in Iranian women with cervical cancer. Considering the majority of cervical cancers are infected with HPV-16 and HPV-18, which are vaccine-targeted HPV genotypes, current prophylactic vaccines would be able to prevent approximately 70% of cervical cancer in Iranian women.
Regarding the incidence rate of multiple HPV genotypes infection, 33.8% of HPV-positive cases were infected with two or more genotypes. Several previous studies have indicated a different prevalence of multiple HPV genotypes infection, varying from less than 10% to more than 80%. These variations may be due to differences in geographic and ethnic characteristics, sexual behavior, and the HPV genotyping methods (27-29). The results of the present study failed to find an association between multiple HPV genotypes infection and an increased risk for squamous intraepithelial lesions and cervical cancer. So far, there is no general agreement on the potential implication of multiple HPV genotypes infection in the development of high-grade squamous intraepithelial lesions. Some reports show a correlation between an elevated risk of cervical carcinogenesis and multiple HPV genotypes infection (30, 31), while the findings of several studies support the lack of this association (32-35).
As expected, HPV infection prevalence was significantly higher in the high-grade lesions (≥ HSIL) than in normal cases and women with LSIL. In a similar manner, the frequency of infection with HR-HPV genotypes was notably higher in the ≥ HSIL category compared to the normal/LSIL group.
In contrast with the similar studies in Iran that had weaknesses including narrow sample size (36), evaluation of only cervical cancer specimens (37), and detection of few HPV genotypes (38), the current investigation included a larger number of cervical samples with different grades of cervical lesion and evaluated 28 different HPV genotypes, providing valuable data on the epidemiology of HPV infection in Iran. However, this study had some limitations, including its cross-sectional design and lack of representation of the general population.
In conclusion, the present study describes robust information on the distribution of HPV genotypes among Iranian women with and without cervical lesions. The five most prevalent genotypes were HPV-16, HPV-6, HPV-53, HPV-31, and HPV-39. The high prevalence of HPV-16 was seen in normal cases and may be a risk factor for cervical cancer development in the Iranian women. Finally, the relatively high frequency of HPV infection, especially HPV-16, among Iranian women reported here suggests the requirement for HPV surveillance and control programs in Iran, including vaccination and screening of HPV infection with sensitive methods.
Acknowledgments
The authors would like to thank the directors and staff of the Gynecology oncology department and cancer institute of Imam hospital complex affiliated with Tehran University of Medical Sciences for their assistance in sample collection.
Footnotes
Authors’ Contribution:Mostafa Salehi-Vaziri, Farzin Sadeghi, and Hossein Keyvani developed the study concept, performed experimental protocols, and prepared the manuscript. Firoozeh Sadat Hashemi, Hayedeh Haeri, Farah Bokharaei-Salim, and Seyed Hamidreza Monavari carried out administrative, technical, and material support.
Funding/Support:This work was supported through a grant from the Tehran University of Medical Sciences (Project code: 92-01-30-21080).
References
- 1.Shepherd JH. Cervical cancer. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):293–309. doi: 10.1016/j.bpobgyn.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Meijer CJ, Snijders PJ, Castle PE. Clinical utility of HPV genotyping. Gynecol Oncol. 2006;103(1):12–7. doi: 10.1016/j.ygyno.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 5.Evans MF, Adamson CS, Schned LM, St John TL, Leiman G, Ashikaga T, et al. HPV is detectable in virtually all abnormal cervical cytology samples after reinvestigation of HPV negatives with multiple alternative PCR tests. Diagn Mol Pathol. 2010;19(3):144–50. doi: 10.1097/PDM.0b013e3181c1482c. [DOI] [PubMed] [Google Scholar]
- 6.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 8.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26 Suppl 10:K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 9.Brotherton JM, Kaldor JM, Garland SM. Monitoring the control of human papillomavirus (HPV) infection and related diseases in Australia: towards a national HPV surveillance strategy. Sex Health. 2010;7(3):310–9. doi: 10.1071/SH09137. [DOI] [PubMed] [Google Scholar]
- 10.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 11.Eghbali SS, Amirinejad R, Obeidi N, Mosadeghzadeh S, Vahdat K, Azizi F, et al. Oncogenic human papillomavirus genital infection in southern Iranian women: population-based study versus clinic-based data. Virol J. 2012;9:194. doi: 10.1186/1743-422X-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poljak M, Kocjan BJ. Commercially available assays for multiplex detection of alpha human papillomaviruses. Expert Rev Anti Infect Ther. 2010;8(10):1139–62. doi: 10.1586/eri.10.104. [DOI] [PubMed] [Google Scholar]
- 13.Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37(8):2508–17. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hesselink AT, van Ham MA, Heideman DA, Groothuismink ZM, Rozendaal L, Berkhof J, et al. Comparison of GP5+/6+-PCR and SPF10-line blot assays for detection of high-risk human papillomavirus in samples from women with normal cytology results who develop grade 3 cervical intraepithelial neoplasia. J Clin Microbiol. 2008;46(10):3215–21. doi: 10.1128/JCM.00476-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Hamont D, van Ham MA, Bakkers JM, Massuger LF, Melchers WJ. Evaluation of the SPF10-INNO LiPA human papillomavirus (HPV) genotyping test and the roche linear array HPV genotyping test. J Clin Microbiol. 2006;44(9):3122–9. doi: 10.1128/JCM.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castle PE, Porras C, Quint WG, Rodriguez AC, Schiffman M, Gravitt PE, et al. Comparison of two PCR-based human papillomavirus genotyping methods. J Clin Microbiol. 2008;46(10):3437–45. doi: 10.1128/JCM.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87(11):796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 18.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1157–64. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza LP, Arbiza J, Paez M, Kasamatsu E, Castro A, Gimenez G, et al. Distribution of human papillomavirus genotypes in Paraguayan women according to the severity of the cervical lesion. J Med Virol. 2011;83(8):1351–7. doi: 10.1002/jmv.22112. [DOI] [PubMed] [Google Scholar]
- 20.Bao YP, Li N, Smith JS, Qiao YL, Accpab members. Human papillomavirus type distribution in women from Asia: a meta-analysis. Int J Gynecol Cancer. 2008;18(1):71–9. doi: 10.1111/j.1525-1438.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- 21.Khodakarami N, Clifford GM, Yavari P, Farzaneh F, Salehpour S, Broutet N, et al. Human papillomavirus infection in women with and without cervical cancer in Tehran, Iran. Int J Cancer. 2012;131(2):E156–61. doi: 10.1002/ijc.26488. [DOI] [PubMed] [Google Scholar]
- 22.Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97(14):1066–71. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 23.Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102(19):1478–88. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102(5):315–24. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuna RE, Wang SS, Rosenthal DL, Jeronimo J, Schiffman M, Solomon D, et al. Determinants of human papillomavirus-negative, low-grade squamous intraepithelial lesions in the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions triage study (ALTS). Cancer. 2005;105(5):253–62. doi: 10.1002/cncr.21232. [DOI] [PubMed] [Google Scholar]
- 26.Ramas V, Mirazo S, Bonilla S, Mendoza L, Lago O, Basiletti J, et al. Human papillomavirus genotypes distribution in cervical samples from Uruguayan women. J Med Virol. 2013;85(5):845–51. doi: 10.1002/jmv.23479. [DOI] [PubMed] [Google Scholar]
- 27.Trottier H, Mahmud S, Costa MC, Sobrinho JP, Duarte-Franco E, Rohan TE, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1274–80. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 28.Spinillo A, Dal Bello B, Gardella B, Roccio M, Dacco MD, Silini EM. Multiple human papillomavirus infection and high grade cervical intraepithelial neoplasia among women with cytological diagnosis of atypical squamous cells of undetermined significance or low grade squamous intraepithelial lesions. Gynecol Oncol. 2009;113(1):115–9. doi: 10.1016/j.ygyno.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt M, Dondog B, Waterboer T, Pawlita M, Tommasino M, Gheit T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J Clin Microbiol. 2010;48(1):143–9. doi: 10.1128/JCM.00991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasagawa T, Basha W, Yamazaki H, Inoue M. High-risk and multiple human papillomavirus infections associated with cervical abnormalities in Japanese women. Cancer Epidemiol Biomarkers Prev. 2001;10(1):45–52. [PubMed] [Google Scholar]
- 31.van der Graaf Y, Molijn A, Doornewaard H, Quint W, van Doorn LJ, van den Tweel J. Human papillomavirus and the long-term risk of cervical neoplasia. Am J Epidemiol. 2002;156(2):158–64. doi: 10.1093/aje/kwf013. [DOI] [PubMed] [Google Scholar]
- 32.Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92(6):464–74. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 33.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venturoli S, Ambretti S, Cricca M, Leo E, Costa S, Musiani M, et al. Correlation of high-risk human papillomavirus genotypes persistence and risk of residual or recurrent cervical disease after surgical treatment. J Med Virol. 2008;80(8):1434–40. doi: 10.1002/jmv.21198. [DOI] [PubMed] [Google Scholar]
- 35.Sandri MT, Riggio D, Salvatici M, Passerini R, Zorzino L, Boveri S, et al. Typing of human papillomavirus in women with cervical lesions: prevalence and distribution of different genotypes. J Med Virol. 2009;81(2):271–7. doi: 10.1002/jmv.21382. [DOI] [PubMed] [Google Scholar]
- 36.Mortazavi S, Zali M, Raoufi M, Nadji M, Kowsarian P, Nowroozi A. The Prevalence of Human Papillomavirus in Cervical Cancer in Iran. Asian Pac J Cancer Prev. 2002;3(1):69–72. [PubMed] [Google Scholar]
- 37.Haghshenas M, Golini-Moghaddam T, Rafiei A, Emadeian O, Shykhpour A, Ashrafi GH. Prevalence and type distribution of high-risk human papillomavirus in patients with cervical cancer: a population-based study. Infect Agent Cancer. 2013;8(1):20. doi: 10.1186/1750-9378-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghaffari SR, Sabokbar T, Mollahajian H, Dastan J, Ramezanzadeh F, Ensani F, et al. Prevalence of human papillomavirus genotypes in women with normal and abnormal cervical cytology in Iran. Asian Pac J Cancer Prev. 2006;7(4):529–32. [PubMed] [Google Scholar]